Abstract
Biomarkers in urine can provide useful information about the bioactivation of chemical carcinogens and can be used to investigate the chemoprotective properties of dietary nutrients. N-nitrosoproline (NPRO) excretion has been used as an index for endogenous nitrosation. In vitro and animal studies have reported that compounds in garlic may suppress nitrosation and inhibit carcinogenesis. We present a new method for extraction and sensitive detection of both NPRO and N-acetyl-S-allylcysteine from urine. The latter is a major metabolite of S-allyl cysteine which is abundant in garlic. Urine was acidified and the organic acids extracted by reversed phase extraction (RP-SPE) and use of a polymeric weak anion exchange (WAX-SPE) resin. NPRO was quantified by isotope dilution gas chromatography-mass spectrometry using 13C5NPRO and N-nitrosopipecolic acid (NPIC) as internal standards. This method was used to analyze urine samples from a study that was designed to test whether garlic supplementation inhibits NPRO synthesis. Using this method, 2.4 to 46 ng of NPRO per mL urine was detected. The method is straightforward, reliable and can be performed with readily available GC/MS instruments. N-acetyl-S-allylcysteine was quantified in the same fraction and detectable at levels of 4.1 to 176.4 ng per mL of urine. The results suggest that 3 to 5 grams of garlic supplements inhibited NPRO synthesis to an extent similar to a 0.5 g dose of ascorbic acid or a commercial supplement of aged garlic extract. Urinary NPRO concentration was inversely associated with the N-acetyl-S-allylcysteine concentration. It is possible that allyl sulfur compounds found in garlic may inhibit nitrosation in humans. .
Keywords: biomarkers, allyl sulfur compounds, nitrosation, nutrition, cancer
Introduction
The nitrosoproline (NPRO) load test was developed to monitor in vivo nitrosation capacity, a process considered to play a role in the formation of carcinogenic nitrosamines. Nitrosamines can form in the stomach when nitrate and amine-rich foods are consumed together (1). Although nitrosamines are highly mutagenic, the role of nitrate exposure in causing certain cancers has been debated (2). Nitrosamine excretion is correlated with nitrate levels in drinking water and salivary nitrate levels (3). Interstingly, it has been suggested that exposure to nitrate from drinking water, but not vegetable sources, is closely correlated with cancer risk(4). This may be due to the concomitant presence of ascorbic acid (vitamin C) in vegetables. For example, it has been shown that low ascorbic acid intake is significantly associated with increased risk of colorectal cancer in subjects highly exposed to nitrate from well water (5). Ingested nitrate is mainly excreted in urine without causing adverse effects, but approximately 20% of the absorbed dose of nitrate is metabolized to nitrite by bacteria that reside in the mouth and stomach, and 25% of the dose undergoes enterosalivary recirculation (6). At low pH, nitrite can be converted to nitrous acid, which in reaction with oxygen forms the nitrosating intermediates dinitrogen trioxide (N2O3) and nitrosylonium ion (NO+) (7). N2O3 and NO+ are potent nitrosating molecules (8). In the stomach, ascorbic acid rapidly reacts with N2O3 to generate nitric oxide (NO)(6,9). Therefore, it is possible that phytonutrients in garlic may have similar antioxidant effects as vitamin C, inhibiting N-nitrosamine production from nitrate exposure (10).
NPRO formation has been used as a biomarker to study potential dietary interventions for preventing formation of nitrosamines after nitrate ingestion. Recent studies suggest that nitrite has important vasodilatory effects by mediating nitric oxide signaling under hypoxic conditions and that NO can circulate by forming conjugates with heme, thiols (GSNO), and secondary amines (GNNO) (9, 11). Since NPRO formation is a product of the reaction between proline and nitrite, it is possible that NPRO could be developed as a biomarker of the systemic reserve of N-nitrosation capacity (12). Moreover, NPRO is a non-carcinogenic nitrosaminoacid (13, 14).
Gas chromatography-thermal energy analysis (GC-TEA) has been used to quantify NPRO concentrations in urine (12, 15). This detector pyrolyzes the nitrosamine and releases nitric oxide, which is detected by a chemiluminescent detector. The advantage of TEA is that it is highly sensitive and selective towards nitroso-containing molecules; however, the main disadvantage is that it is not sensitive to other compounds of potential interest, and the availability of these detectors has become quite limited. Garland et al. (15) previously described an isotope dilution method employing gas chromatography, negative chemical ionization mass spectrometry (GC-NCI-MS) for the detection of basal NPRO excretion following derivatization with pentafluorobenzyl bromide (PFBBr). However, this method required a thin layer chromatographic step which was time-consuming and restricted the analysis to only NPRO-PFB conjugates. Therefore, there is a need for a robust analytical method that could be used to analyze NPRO with a similar low limit of detection (0.1ng NPRO/ml urine), but also used for the detection of other compounds that are of interest.
Herein, we describe a rapid stable isotope dilution method for the quantification of NPRO by GC-NCI-MS. This analytical method was applied to a pilot study to determine if consumption of daily dosages of encapsulated raw garlic or Kyolic, a commercial aged garlic supplement, can decrease NPRO urinary excretion similar to a 0.5 gram dose of vitamin C. These treatment groups were compared to a control group that received a placebo. Finally, N-acetyl-S-allylcysteine, which is the major metabolite of S-allyl cysteine, was quantified as a marker of absorption of a garlic-derived thiol compounds concomitant with the NPRO analysis (16).
Materials and Methods
Human Subjects Protocol
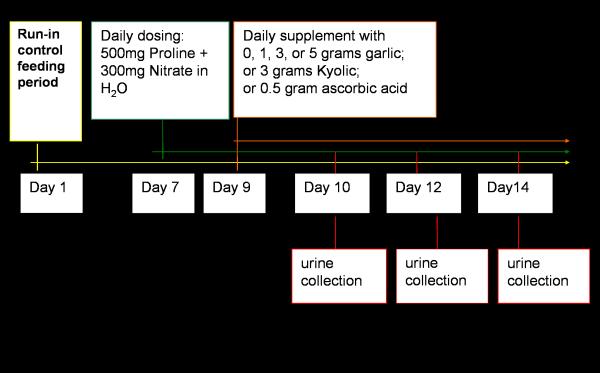
The study protocol for human subjects was approved by the Institutional Review Board of The Pennsylvania State University. All subjects gave signed informed consent for participation in this study. The diet was prepared and served by the nutritional sciences research staff at Penn State University. Figure 1 outlines the dietary feeding protocol. Subjects received a 1-week run-in diet (3 meals per day), which was devoid of foods high in nitrate or containing garlic. On the morning of the first treatment day all subjects drank a bolus dose of 300 mg of sodium nitrate dissolved in 500 ml water followed by 500 mg of l-proline dissolved in 500 ml water. A 24-hour urine sample was collected the following morning. The next day subjects were randomized into one of 5 treatment arms or the control group. Subjects in the treatment arms received a capsule containing 1, 3, or 5 grams of fresh garlic, 3 grams of aged-garlic extract (Kyolic), or 500mg ascorbic acid. The control group received a placebo capsule. Subjects continued to receive nitrate and proline as well as the treatment for seven days. Urine was collected every other day for seven days.
Figure 1.
Feeding study design - nitrate dosing began on day 7 following a run-in diet and continued for seven days. Subjects in the treatment arms received supplementation on day 9 and continued for five days. Urine was collected on day 10, day 12, and day 14.
Collection and storage of samples
Urine specimens were collected into 250 ml Nalgene containers, and 25g of ammonium sulfamate was added to each container. The urine was stored at -20°C for several years prior to analysis. Under these conditions, NPRO has been found to be quite stable (17). The urine samples were thawed and aliquotted into 50 mL volumes for storage and the total volume of the urine samples was measured. Additional, multiple aliquots of 5mL were prepared for extraction and analysis.
Nitrosoproline extractions from urine
Authentic standards of nitrosoproline, 13C5-N-nitrosoproline, and N-nitrosopipecolinic acid (NPIC) were obtained from Dr. Shantu Amin at the Penn State School of Medicine Department of Pharmacology (Hershey, PA) and the NCI Chemical Carcinogen Reference Standards Repository program. Purity of the compounds was assessed using LC-MS and found to be >99.0%. Purified standards were maintained at - 20°C prior to use. Stock solutions of 13C5NPRO and NPIC were prepared in anhydrous ethanol and maintained at 4°C during the analysis period. Each 5 ml aliquot of urine was spiked with 250ng of 13C5NPRO (50ng/ml), and vigorously mixed with 3.8 ml of 0.9M HCl in a 10 ml glass test tube. Sample clean-up was performed on a Sep-Pak Vac tC18 reversed-phase SPE cartridge (500mg/6ml) (Waters Corp., Westfield, MA), using an 18-port vacuum manifold (Alltech Associates, Deerfield, MI). The column was conditioned with 3 ml methanol followed by 3 ml 50mM formic acid in water (pH 3). The acidified urine samples were applied to the sorbent bed and the breakthrough volume was discarded. The acidic compounds were eluted using 6 ml of 100% HPLC grade water (Mallinckrodt, Hanover, Germany). A 3 ml volume of 0.1M sodium phosphate buffer (pH 6.5) was added to the collected aqueous fraction and mixed vigorously. A polymeric weak anion exchange (Strata X-AW) SPE cartridge (60mg/3ml) was used to further clean-up the sample and exchange solvents prior to derivatization (Phenomenex, Torrance, CA). This column was conditioned with 3 ml of methanol containing 2% formic acid followed by 3 ml of deionized HPLC grade water. The buffered sample was added to the SPE cartridge, and potential interfering compounds were subsequently washed away with 3 ml of water followed by 3 ml methanol. The column was dried under a vacuum of 10 inches Hg for 2 minutes. The acidic compounds were then eluted with 6 ml of methanol containing 2.5% ammonium hydroxide. The eluent was dried to approximately 0.5 ml under nitrogen at 40°C. The sample was transferred to a 1.8 ml brown borosilicate glass vial, where it was dried to completion. Derivatization was carried out on the dried sample by adding 200 ul of a 0.25% N,N-diisopropylethylamine (DIPEA) and 200 ul of 0.25% pentafluorobenzyl bromide (PFBBr) followed by heating at 65°C in aluminum blocks for 3 hours (Pierce, Rockford, IL). The derivatized samples were dried under nitrogen at 65°C. The derivatives were reconstituted in a final volume of 200 ul isooctane for analysis by GC-MS.
Gas chromatography/mass spectrometry protocols
An injector with autosampler was used to introduce 1 ul of derivatized sample into an Agilent 6890 gas chromatograph (GC) that was interfaced with a 5970N mass selective detector (Agilent Technologies, Wilmington, DE). Injections were made in the cool-on-column injection mode with the inlet programmed to track the oven temperature. There was a 3.5 minute solvent delay before turning on the mass selective detector (MSD). The flow rate of helium through the column was constantly maintained at 1 ml/min with an average linear velocity of 38 cm/s. The starting inlet pressure was 10.04 psi. Separations were performed on a 30 meter DB-5 column, with a 0.25 mm i.d. and 0.25 um film thickness (J&W Scientific, Rancho Cordova, CA). The GC temperature protocol was 100°C for 1 minute and the temperature was increased to 280°C at a linear rate of 10°C/min. The final oven temperature was held for 5.5 minutes. The method cycle time was 24.5 minutes with a 5 minute equilibration period following each run.
Molecules eluting from the column were ionized and detected in the negative chemical ionization (NCI) mode using methane as the reagent gas. The vacuum manifold pressure was 2.0x10-4 Torr with the reagent gas mass flow control set at 40 (flow rate=2 ml/min). The MSD transfer line temperature was held constant at 280°C, the source temperature was 150°C, and the quad temperature was 106°C. For quantitative determination of each urine sample, both full scan and selective ion monitoring (SIM) were performed. The full scan traces were collected for mass-to-charge ratios (m/z) between 50-300 amu. In a separate run, ion traces were set to selectively monitor ions at m/z=143 amu (NPRO, MW=144g/mole), m/z=148 amu (13C5NPRO, MW=149 g/mole), and m/z=157 amu (NPIC, MW=158 g/mole). The dwell time for each ion was 100 milliseconds.
Quantification of urinary nitrosoproline
The urinary NPRO concentration was estimated using relative response factors. Standard curves were run to determine the relative response ratio of unlabeled NPRO to 13C5 NPRO. Varying concentrations of NPRO (0.05pg/ul to 250pg/ul) were spiked with 6.25pg/ul 13C5NPRO, the expected final concentration of 13C5 NPRO following the extraction and dilution steps outlined above. The response ratios were plotted versus amount ratios; a second set of standard curves was generated daily by adding derivatized NPIC (50 pg/ul) to varying concentrations of 13C5NPRO (0.05pg/ul to 250 pg/ul). Response factors were calculated from the linear regression slope of the response ratio (y-axis) versus the amount ratio (x-axis); these were used to verify the analytical precision as well as calculate the concentration of NPRO in the urine specimens.
Synthesis and quantification of urinary N-acetyl-S-allylcysteine
N-acetyl-S-allylcysteine was synthesized according to the method described by Jandke et al.(18). Briefly, 159.5 mg (1 mmol) of N-acetyl cysteine was weighed and stirred for 10 min. at room temperature with 115.8 mg (1 mmol) allyl bromide in 10 ml water (Sigma-Aldrich, St. Louis, USA). Sodium hydroxide (2 M) was added dropwise to reach a final pH of 12.0. Approximately 60 ml anhydrous ethanol was added to the solution which was continuously stirred for 4 hours at room temperature. The solution was dried under a stream of N2 gas at 40°C. Twenty milliliters of water was added to the dried residue and 2 M HCl was added dropwise to reach pH 2.0. This solution was extracted 2 times with 40 ml volumes of ethyl acetate. The organic layer was collected and dried under N2 gas at room temperature. The residue was redissolved in 55 ml ethyl acetate and esterified with 0.010 ml of 6.8 M PFBBr along with an equimolar volume of DIPEA (0.124 ml). This reaction was carried out at room temperature overnight. The esterified residue was dried under N2 gas at room temperature, and brought up in a final volume of 22.5 ml of ethyl acetate. A standard curve was generated for external calibration. The standard curve was linear over a concentration range of 0.1 ng/ul to 56 ng/ul.
Statistical analysis
Differences across groups were assessed using the non-parametric Kruskal-Wallis test. Spearman’s rank test was used to test correlations. Linear regression was used to examine the association between the excretion of N-acetyl-S-allylcysteine and NPRO.
Results
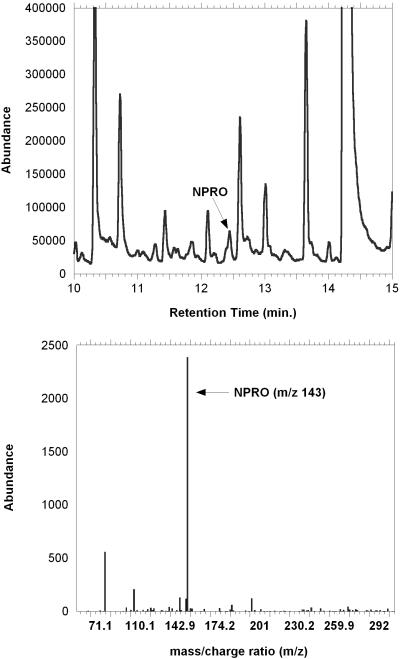
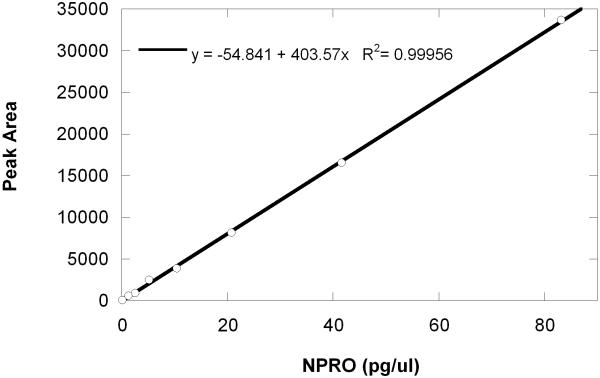
Figure 2 shows a total ion current (TIC) chromatogram collected for a five minute period of the run that contains the NPRO peak. The average retention times for NPRO, 13C5NPRO, and NPIC were 12.466 min. (+/-SD=0.007), 12.450 min. (+/-SD=0.003), and 12.888 min. (+/-SD=0.004), respectively. For quantitative determinations, the maximum difference in the retention times of the molecular ions was restrained to less than +/-0.02 minutes, which is three times the standard of deviation from the mean (n=3). Since the NPRO and 13C5NPRO peaks were nearly indistinguishable chromatographically, concentrations of endogenous NPRO in the sample were measured by estimation of ratios of molecular ion abundance for the labeled standard (m/z 148) that was added to the abundance of the molecular ion (m/z 143) that was present at the same retention time. An example of a typical standard curve for 0.1 to 80 pg/ml concentrations is shown in Figure 3.
Figure 2.
Total ion chromatogram (TIC) for a 5ml urine sample prepared as described in the methods. Panel (A) shows a 5 minute window of the ion current intensity with 20pg/ul NPRO spiked into the sample. The peak for NPRO is identified with an arrow. Panel (B) shows the mass spectra of a 2pg/ul quantity of NPRO standard. Quantitation was carried out on the molecular ion (m/z 143) at a retention time of 12.466 minutes.
Figure 3.
Representative linear standard curve of the NPRO-PFB response for a concentration range of 0.1-80 pg/ul. The linear regression equation for this standard curve was: y=-54.8 + 403.6(x). The R2 for the fit was 0.9996.
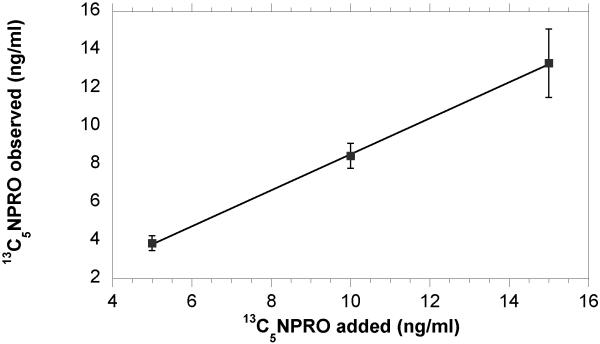
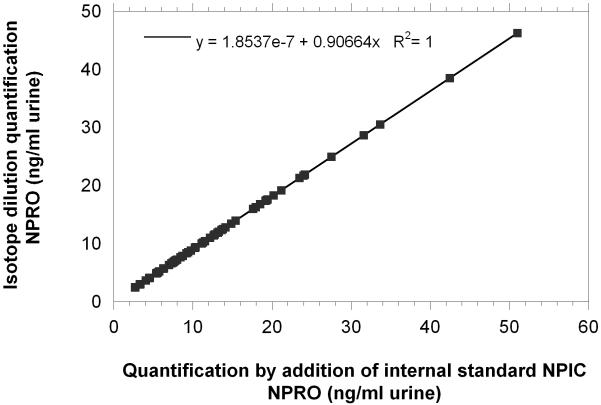
The Residual Standard Deviation (RSD) for repeated injections was 10% for 2.3 pg 13C5NPRO (n=8) and was 3% for injections of 20 pg 13C5NPRO (n=4). The precision and recovery of the method was analyzed by performing the extraction (n=3) of 5 ml urine samples that contained 25 ng, 50 ng, and 75 ng of 13C5NPRO (Fig. 4). The recovery of standard was 77%, 84% and 88%, respectively. The availability of two internal standards made it possible to compare NPIC (m/z 157) with 13C5NPRO for validation of the internal method. As seen in figure 5, the quantification of NPRO using either internal standards was in excellent agreement.
Figure 4.
The graph shows the concentration of 13C5 NPRO that was added at 5, 10 and 15 ng/ml of urine (x-axis) versus the 13C5 NPRO concentration (ng/ml) found following extraction and derivatization (y-axis). The percent return from these levels was 77, 84 and 89%, respectively. Data points and error bars are the mean (± SD) of 3 replicate measurements. The regression coefficient for linear fit was 0.944 and the goodness-of-fit for the regression model was R2= 0.9997.
Figure 5.
The graph shows the correlations between the NPRO concentrations quantified by the internal standard method versus the isotope dilution method for each sample. The regression coefficient was 0.9 and the adjusted R2 equaled 1.
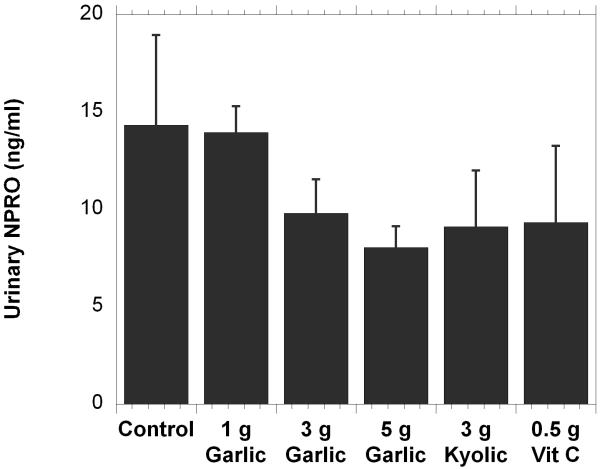
The method was then applied to the analysis of samples from the human feeding study. In that study each subject’s urinary NPRO excretion was evaluated at three different times after initiation of treatment. There was no significant difference between any of the treatment group concentrations over time; therefore, all three measurements for each person were averaged and treated as a single measurement. Comparisons were made between these averages for each of the treatment groups and the control groups (Fig. 6). Although we observed lower mean NPRO concentrations in the 3 and 5 gram garlic groups, Kyolic group and vitamin C group (when compared to the control group) the differences in NPRO concentrations between any of the groups was not statistically significant. High within-group variation in concentrations of NPRO and the low subject numbers contributed to the high standard error of these measurements.
Figure 6.
The graph shows the mean (±SEM) of the NPRO output in each of the groups. The concentrations were averaged for each subject. The groups, identified on the x-axis, are as follows: control (no supplement); 1 gram garlic supplement; 3 gram garlic supplement; 5 gram garlic supplement; 3 gram Kyolic supplement; 0.5 gram ascorbic acid supplement. The NPRO concentration (ng/ml) is on the y-axis.
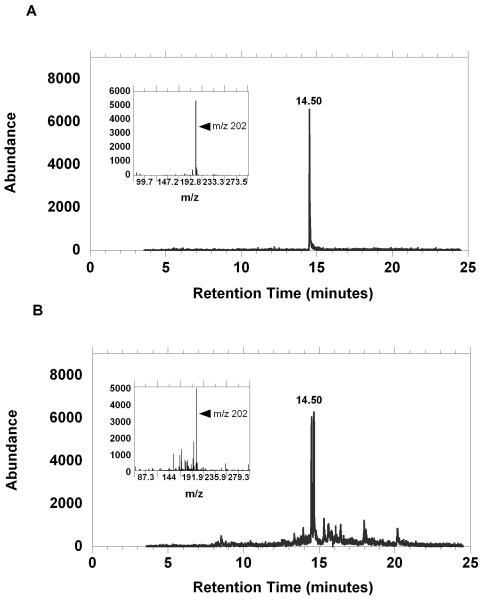
We next determined the concentration of N-acetyl S-allylcysteine in urine as a biomarker of effective garlic intake. The quantity of N-acetyl-S-allylcysteine in urine was estimated by mass spectrometry. As can be seen from figure 7, the abundance of the extracted ion current at an m/z 202 from the 90pg/ul standard (upper panel) was nearly identical to that of the quantity detected in one of the 5 gram fresh garlic supplement urine specimens. Interestingly, the double peak that is seen in the urine sample may represent a racemization-product of the N-acetyl transferase enzymes (18).
Figure 7.
The abundance of the extracted ion current for N-acetyl-S-allylcysteine (y-axis), and the mass spectra (inset) for the molecular ion m/z=202 is shown. Panel (A) shows the EIC of approximately 90ng/ul of synthesized standard. Panel (B) shows a similar concentration quantified in 5 ml of extracted urine which was collected from a subject that consumed a 5 gram quantity of a fresh garlic supplement for one day.
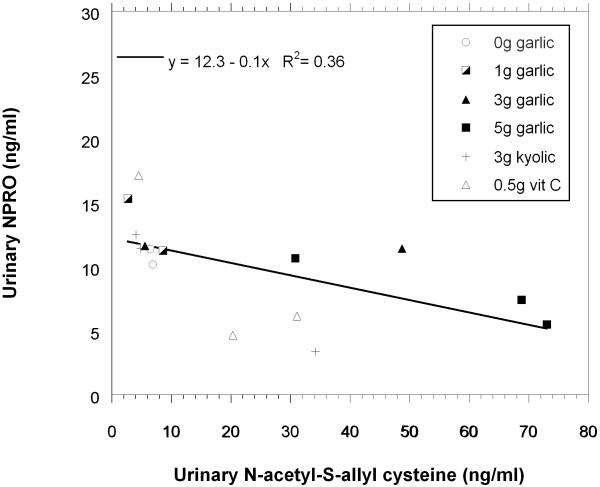
Linear regression was used to analyze associations between the concentrations of N-acetyl-S-allylcysteine and NPRO excreted in urine (Fig. 8). The data points are identified by group. From the regression model, a 100 pg/ml decrease of NPRO concentration was found to be significantly associated (p<0.02) with each 1ng/ml unit increase in urinary N-acetyl-S-allylcysteine. The data were skewed so a non-parametric Spearman rank correlation test was also conducted. Using this test, the correlation coefficient was -0.80 and the p-value < 0.003. In five subjects, the N-acetyl-S-allylcysteine was below the limit of quantification; those data points were not included in this analysis.
Figure 8.
The graph plots the average total NPRO concentrations versus the average total concentration of N-acetyl-S-allylcysteine concentrations that were excreted in the urine samples of each subject. The regression line is for all the data points.
Discussion
The purpose of the current study was to develop a protocol using mass-spectrometry that is as sensitive as GC-TEA but also allows other urinary metabolites to be analyzed concurrently. Solid state extraction is a practical clean-up method for urinary NPRO analysis, and the negative chemical GC-MS method adapted from the Garland et al. yields excellent quantitative precision and sensitivity(19). Negative chemical ionization offers a highly sensitive mode for MS analysis because interference from background ions is reduced and the molecular ion can be determined since molecule fragmentation is minimal. When esterification reagents such as PFBBr are used, derivatized molecules form highly electronegative molecular ions. However, in order to obtain sufficient sensitivity, urine samples (especially those which have been frozen and thawed) must be cleaned-up adequately and purified. The Garland study applied a laborious thin-layer chromatography step for this purpose; therefore, a solid phase extraction protocol was developed to isolate the total organic acid fraction in our urine samples. An initial reversed phase extraction was successful in removing both non-polar molecules and other inorganic ions and collecting the organic acids. A second anion exchange extraction was used to elute the organic acids contained in the aqueous fraction. These molecules are selectively displaced from the polymeric WAX resin by the eluting solvent. This clean-up procedure allowed the sufficient lowering of background such that GCMS electron capture detection sensitivity became comparable to that which has been previously reported for thermal energy analysis.
The results of the feeding study suggest that accurate determinations of NPRO and N-acetyl-s-allylcysteine can be made simultaneously. The mean concentrations of these biomarkers have an inverse correlation with each other. This suggests that the ingestion of s-allylcysteine (SAC) may mitigate nitrosation reactions in the stomach and may be an important bioactive component of garlic (20). Certain antioxidants, such as ascorbic acid are known to mediate the toxicokinetics of nitrate metabolism (21). For example, 300 mg ascorbic acid has been shown to significantly inhibit gastric formation of NPRO in numerous studies (22). However, the role of nitrate/nitrite interconversion is complex and can generate nitric oxide as an end-product which results in increased gastric perfusion and vasodilation. Similar to ascorbic acid, SAC is thought to inhibit conversion of nitrous acid to dinitrogen trioxide or nitrosylonium ion. These compounds may also interfere with genotoxicity by inhibiting the bioactivation of pro-carcinogens, or by reducing the DNA damage caused by reactive intermediates. SAC has been shown to directly inhibit nitrosation of N-morpholine and to prevent metabolic activation of N-nitrosomorpholine through inhibition of the enzyme P4502E1 (23). Moreover, these compounds have been shown to induce phase II detoxification enzymes such as quinone reductase and glutathione-S-transferases, further reinforcing their potent antioxidant activity. S-allylcysteine and N-acetyl-S-allylcysteine have both been shown to mitigate oxidative and nitrosative stress (24, 25).
The feeding study suggests that intake of 5 g garlic reduces the quantity of NPRO excreted. N-acetyl-S-allylcysteine was significantly inversely correlated with NPRO. Intra-group variance was high, making statistical judgments across days unreliable in this small group of subjects. To address these issues, data from all three measurements for each subject were averaged for group-wise comparisons. The largest decrease in NPRO formation, compared to controls, was in the 5 gram fresh garlic group. Interestingly, these subjects also excreted the highest concentrations of N-acetyl-S-allylcysteine (Figure 7). Although the bioavailability of S-allycysteine is 98% in rats, the conversion rate to N-acetyl-S-allylcysteine is highly variable in humans, and may have considerable dependence on N-acetyltransferase isoforms or exposure to inducers that may alter the disposition of N-acetyl-S-allylcysteine (26). The half-life can range from not detectable to 5 hrs with considerable intra-individual variation. However, subjects in the Vitamin C group also appear to have detectable levels of N-acetyl-S-allylcysteine, which raises some concern as to whether subjects adhered to the dietary protocol throughout the course of the study. There are other exposures, such as allyl chloride, that can generate N-acetyl-S-allylcysteine, but exposure to allyl halides is expected to be rare except in certain occupations (27, 28). Moreover, researchers have documented that consumption of onions may also generate N-acetyl-S-allylcysteine (18).
We describe a sensitive and accurate method for combined determination of NPRO and N-acetyl-S-allylcysteine. The method makes possible determinations of biomarkers of garlic intake and the potential for using these biomarkers in future investigations to examine the biological and toxicological role of nitrosation. Our controlled feeding study did suggest that garlic may play some role in inhibiting NPRO formation and that the excretion of N-acetyl-S-allylcysteine, one of the major excreted metabolites of S-ally-cysteine (SAC) in garlic, is inversely correlated with NPRO excretion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohshima H, Bartsch H. Quantitative estimation of endogenous N-nitrosation in humans by monitoring N-nitrosoproline in urine. Methods Enzymol. 1999;301:40–9. doi: 10.1016/s0076-6879(99)01067-8. [DOI] [PubMed] [Google Scholar]
- 2.Mensinga TT, Speijers GJ, Meulenbelt J. Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev. 2003;22:41–51. doi: 10.2165/00139709-200322010-00005. [DOI] [PubMed] [Google Scholar]
- 3.van Maanen JM, Welle IJ, Hageman G, Dallinga JW, Mertens PL, Kleinjans JC. Nitrate contamination of drinking water: relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N-nitrosamines. Environ Health Perspect. 1996;104:522–8. doi: 10.1289/ehp.96104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- 5.De Roos AJ, Ward MH, Lynch CF, Cantor KP. Nitrate in public water supplies and the risk of colon and rectum cancers. Epidemiology. 2003;14:640–9. doi: 10.1097/01.ede.0000091605.01334.d3. [DOI] [PubMed] [Google Scholar]
- 6.Mowat C, McColl KE. Alterations in intragastric nitrite and vitamin C levels during acid inhibitory therapy. Best Pract Res Clin Gastroenterol. 2001;15:523–37. doi: 10.1053/bega.2000.0196. [DOI] [PubMed] [Google Scholar]
- 7.Combet E, Paterson S, Iijima K, Winter J, Mullen W, Crozier A, Preston T, McColl KE. Fat transforms ascorbic acid from inhibiting to promoting acid catalyzed N-nitrosation. Gut. 2007;56:1678–84. doi: 10.1136/gut.2007.128587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirsch M, Korth HG, Sustmann R, de Groot H. H. Carbon dioxide but not bicarbonate inhibits N-nitrosation of secondary amines. Evidence for amine carbamates as protecting entities. Chem Res Toxicol. 2000;13:451–61. doi: 10.1021/tx990138z. [DOI] [PubMed] [Google Scholar]
- 9.Gladwin MT. Haldane, hot dogs, halitosis, and hypoxic vasodilation: the emerging biology of nitrite anion. J Clin Invest. 2004;113:19–21. doi: 10.1172/JCI200420664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milner JA. Mechanisms by which garlic and allyl sulfur compounds suppress carcinogen bioactivation, Garlic and carcinogenesis. Adv Exp Med Biol. 2001;492:69–81. doi: 10.1007/978-1-4615-1283-7_7. [DOI] [PubMed] [Google Scholar]
- 11.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–13. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohshima H, Bartsch H. Quantitative estimation of endogenous N-nitrosation in humans by monitoring N-nitrosoproline excreted in the urine. Cancer Res. 1981;41:3658–62. [PubMed] [Google Scholar]
- 13.Brunnemann KD, Enzmann HG, Perrone CE, Iatropoulos MJ, G MJ, Williams M. In ovo carcinogenicity assay (IOCA): evaluation of mannitol, caprolactam and nitrosoproline. Arch Toxicol. 2002;76:606–12. doi: 10.1007/s00204-002-0380-4. G. M. [DOI] [PubMed] [Google Scholar]
- 14.Mirvish SS, Bulay O, Runge RG, Patil K. Study of the carcinogenicity of large doses of dimethylnitramine, N-nitroso-L-proline, and sodium nitrite administered in drinking water to rats. J Natl Cancer Inst. 1980;64:1435–42. doi: 10.1093/jnci/64.6.1435. [DOI] [PubMed] [Google Scholar]
- 15.Garland WA, Kuenzig W, Rubio F, Kornychuk H, Norkus EP, Conney A. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effects of administered ascorbic acid and alpha-tocopherol. Cancer Res. 1986;46:5392–400. [PubMed] [Google Scholar]
- 16.Krause RJ, Glocke SC, Elfarra AA. Sulfoxides as qrinary metabolites of S-allyl-L-cysteine in rats: evidence for the involvement of flavin-containing monooxygenases. Drug Metab Dispos. 2002;30:1137–42. doi: 10.1124/dmd.30.10.1137. [DOI] [PubMed] [Google Scholar]
- 17.Mirvish SS, Grandjean AC, Moller H, Fike S, Maynard T, Jones L, Rosinsky S, Nie G. N-Nitrosoproline excretion by rural Nebraskans drinking water of varied nitrate content. Cancer Epidemiol Biomarkers Prev. 1992;1:455–61. [PubMed] [Google Scholar]
- 18.Jandke J, Spiteller G. Unusual conjugates in biological profiles originating from consumption of onions and garlic. J Chromatogr. 1987;421:1–8. doi: 10.1016/0378-4347(87)80373-0. [DOI] [PubMed] [Google Scholar]
- 19.Stillwell WG, Glogowski J, Xu HX, Wishnok JS, Zavala D, Montes G, Correa P, Tannenbaum SR. Urinary excretion of nitrate, N-nitrosoproline, 3-methyladenine and 7-methylguanine in a Colombian population at high risk for stomach cancer. Cancer Res. 1991;51:190–4. [PubMed] [Google Scholar]
- 20.Chung MJ, Lee SH, Sung NJ. Inhibitoryeffect of whole strawberries, garlic juice or kale juice on endogenous formation of N-nitrosodimethylamine in humans. Cancer Lett. 2002;182:1–10. doi: 10.1016/s0304-3835(02)00076-9. [DOI] [PubMed] [Google Scholar]
- 21.Bartsch H, Ohshima H, Pignatelli B. Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutat Res. 1988;202:307–24. doi: 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- 22.Leaf CD, Vecchio AJ, Roe DA, Hotchkiss JH. Influence of ascorbic acid dose on N-nitrosoproline formation in humans. Carcinogenesis. 1987;8:791–5. doi: 10.1093/carcin/8.6.791. [DOI] [PubMed] [Google Scholar]
- 23.Dion ME, Agler M, Milner JA. S-Allyl cysteine inhibits nitrosoproline formation and bioactivation. Nutr Cancer. 1997;28:1–6. doi: 10.1080/01635589709514545. [DOI] [PubMed] [Google Scholar]
- 24.Pedraza-Chaverri J, Barrera D, Maldonado P, Chirino Y, Macias-Ruvalcaba N, Medina-Campos O, Castro L, Salcedo M, Hernandez-Pando R. S-Allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clinical Pharmacology. 2004;4:5. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina-Campos ON, Barrera D, Segoviano-Murillo S, Rocha D, Maldonado PD, Mendoza-Patino N, Pedraza-Chaverri J. S-Allylcysteine scavenges singlet oxygen and hypochlorous acid and protects LLC-PK(1) cells of potassium dichromate-induced toxicity. Food Chem Toxicol. 2007;45:2030–2039. doi: 10.1016/j.fct.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 26.de Rooij BM, Boogaard PJ, Rijksen DA, Commandeur JN, Vermeulen NP. Urinary excretion of N-acetyl-S-allyl-L-cysteine upon garlic consumption by human volunteers. Arch Toxicol. 1996;70:635–9. doi: 10.1007/s002040050322. [DOI] [PubMed] [Google Scholar]
- 27.Verhagen H, Hageman GJ, Rauma AL, Versluis-de Haan G, van Herwijnen MH, de Groot J, Torronen R, Mykkanen H. H. Biomonitoring the intake of garlic via urinary excretion of allylmercapturic acid. Br J Nutr. 2001;86(Suppl 1):S111–4. doi: 10.1079/bjn2001343. [DOI] [PubMed] [Google Scholar]
- 28.de Rooij BM, Boogaard PJ, Commandeur JN, van Sittert NJ, Vermeulen NP. Allylmercapturic acid as a urinary biomarker of human exposure to allylchloride. Occup Environ Med. 1997;54:653–61. doi: 10.1136/oem.54.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]