Abstract
Purpose
To study the relationship between the 24-hour pattern of intraocular pressure (IOP) with optic disc appearance in primary open angle glaucoma (POAG) patients.
Design
Observational clinical study.
Participants
Seventy-five eyes of 45 POAG patients.
Methods
Patients underwent 24-hour IOP assessment in a sleep laboratory. Two observers classified the optic disc appearance for each eye into either concentric or non-concentric category. IOP measurements were taken in supine and sitting position during the diurnal period and in supine position during the nocturnal period. The mean, peak, trough and IOP range (peak – trough) were calculated for the office-hour period (9 AM to 4 PM), the diurnal period (7 AM-11PM), the nocturnal period (11 PM–7AM) and the 24-hour period. Further the difference in supine and sitting IOP during the diurnal periods were calculated and generalized estimating equations were used to compare IOP measurements in both groups.
Main Outcome Measures
Diurnal and nocturnal IOP measurements.
Results
Forty eyes were classified as having concentric optic disc appearance and 35 eyes as having non-concentric optic disc appearance. The mean nocturnal IOP was significantly greater in the concentric group (24.0 ± 3.8 mm Hg; mean ± standard deviation [SD]) compared to the non-concentric group (21.9 ± 1.9 mm Hg; mean ± SD) (P = 0.004). The majority of IOP peaks of patients with the concentric optic disc appearance occurred during the nocturnal period as opposed to the diurnal period of patients with the non-concentric optic disc appearance.
Conclusions
Concentric optic disc appearance may be associated with higher nocturnal IOP compared to non-concentric optic disc appearance.
Four distinct appearances of glaucomatous optic neuropathy have been characterized: focal ischemic (FI), myopic glaucomatous (MG), senile sclerotic (SS), and generalized cup enlargement (concentric).1, 2 Each of these appearances have been associated with specific clinical characteristics. 1–6 For an example, patients with focal ischemic damage tend to be predominantly female and have a greater prevalence of vasospastic disorders, such as migraine, whereas patients with senile sclerotic discs are usually older and have a higher prevalence of systemic vascular abnormalities.3 In contrast, patients with myopic glaucomatous optic disc appearance and concentric optic disc enlargement, on the other hand, tend to be younger than patients in the other groups and have less prevalence of systemic disorders.4
It also has been proposed that each of these optic disc appearances is associated with distinct pathogenic mechanisms that could be involved in their development of glaucomatous damage. Nicolela and Drance investigated the association between intraocular pressure (IOP) and patterns of damage and reported that patients with concentric enlargement of the cup had significantly higher IOP levels than patients in the other groups.4 Ninety-one (91%) percent of patients with concentric enlargement of the cup had maximum IOP levels of 30 mmHg or more, whereas 38% of patients with focal ischemic discs, 42% with myopic discs and 32% with senile sclerotic discs had maximum IOP levels below 21 mmHg. Although some differences in IOP levels were found among the different groups, the authors concluded that IOP levels could not be used to reliably distinguish patients among the different groups.
Previous investigations of the association between IOP and patterns of glaucomatous optic nerve damage have focused only on IOP measurements obtained during office hours. It is known that IOP may show large fluctuations during the 24h period and that office measurements may not be representative of the pattern of changes occurring in a particular patient throughout the whole day.7–12 Therefore, evaluation of 24-hour IOP measurements is needed for a better understanding of the association between IOP and patterns of optic nerve damage in primary open-angle glaucoma (POAG). The purpose of the present study was to evaluate the relationship between 24-hour IOP levels obtained in a sleep laboratory to patterns of optic nerve damage occurring in POAG.
Patients and Methods
This observational cohort study included POAG patients who had undergone 24-hour IOP measurements in a sleep laboratory, as part of other investigations.9, 10 All patients had been followed at the Hamilton Glaucoma Center, University of California, San Diego. Informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization forms were obtained from all participants and the University of California San Diego Human Subjects Committee approved all protocols. The methods described adhere to the tenets of the Declaration of Helsinki.
All patients had a complete review of medical history and underwent an eye examination including slit-lamp biomicroscopy, gonioscopy, Goldmann applanation tonometry, pachymetry, dilated fundoscopy, optic disc stereophotography and visual field (VF) examination with standard automated perimetry (SAP) performed using 24-2 program (Carl Zeiss Meditec, Dublin, CA), full threshold or Swedish Interactive Threshold Algorithm strategy. Central corneal thickness (CCT) was calculated as the average of 3 measurements obtained using ultrasound pachymetry (Pachette DGH 500, DGH technology, Inc., Philadelphia, PA). Inclusion criteria were diagnosis of POAG with either no prior treatment or a 4-week IOP lowering medication washout period. POAG was diagnosed by characteristic glaucomatous optic disc changes with or without reliable and repeatable VF loss with open angles on gonioscopy. An abnormal VF was defined as a pattern standard deviation with P<5%, glaucoma hemifield test outside normal limits or both. Fellow eyes of a glaucoma patient with a normal optic disc appearance were excluded. Patients with prior laser treatment or surgery for IOP lowering, ocular inflammation or trauma, a narrow iridocorneal angle, advanced glaucomatous changes that needed continued treatment or with optic disc stereoscopic photograph of inadequate quality were excluded. Individuals with irregular sleeping habits or who smoked were also excluded.
All patients had completed 24-hour IOP measurements in a laboratory specifically designed to study human circadian rhythms. The details of 24-hour IOP measurement method have been previously published.7, 8 In brief, patients were asked to maintain a daily 8-hour sleep schedule for 7 days, which was verified using a wrist monitor (Actiwatch, Mini Mitter: Sunriver,Oregon) before laboratory recording. Participants had to abstain from caffeine and alcohol for 3 days and to discontinue contact lens wear for 24 hours. Subjects reported to laboratory at approximately 2 PM, and were housed there for entire 24-hour study period. The 8-hour sleep period was adjusted to correspond to individual’s sleep/wake cycle. The light-dark conditions in the laboratory were strictly controlled with light intensity during light-diurnal period held constant at 500 lux to 1000 lux and absolute darkness during the dark-nocturnal period. For purposes of data analysis and presentation, clock times were normalized as if each subject had a sleep period from 11 PM to 7 AM. IOP, blood pressure and heart rate were measured every 2 hours over the 24-hour period by experienced ophthalmic technicians. IOP was measured using a calibrated pneumotonometer (Model 30 Classic, Mentor O & O, Norwell, MA). Topical 0.5% proparacaine was instilled prior to each IOP measurement. Supine measurements were performed after the subjects were instructed to lay down for 5 minutes. The subject then sat up for 5 minutes and the sitting IOP was taken. Initial diurnal IOP measurements were performed at 3:30 PM, 5:30 PM, 7:30 PM and 9:30PM. Lights were turned off at 11 PM and nocturnal IOP measurements were performed at 11:30 PM, 1:30 AM, 3:30 AM and 5:30 AM. To minimize the disturbance of nocturnal physiology, IOP measurements at night-time were performed in supine position immediately after waking up the participant under a dim red room light (< 10 lux).9 The lights were turned on at 7 AM and measurements were then performed at 7:30 AM, 9:30 AM, 11:30 AM and 1:30 PM. The statistical analysis was performed on habitual IOP readings (sitting IOP readings during the diurnal hours and supine IOP readings during the nocturnal period), and supine IOP readings throughout 24 hours separately.
For this study two experienced optic disc stereophotograph graders independently classified optic disc morphology into one of the following groups according to Nicolela at al’s description:1
Focal ischemic: optic discs with localized neuroretinal rim loss (< 2 clock hours) either at the superior and/or inferior pole with the remaining neuroretinal rim relatively well preserved.
Myopic glaucomatous: tilted optic discs with temporal crescent with additional glaucomatous damage characterized by neuroretinal rim thinning superiorly, inferiorly and/or temporally in absence of degenerative myopia.
Senile sclerotic: optic discs with a saucerized and shallow cup with a relatively pale, moth-eaten neuroretinal rim, parapapillary atrophy and choroidal sclerosis.
Concentric cup enlargement: optic disk with enlarged round cups with no localized neuroretinal rim loss or pallor and well preserved parapapillary retina.
Any optic disc with a mixed appearance was further classified into one of the 4 groups depending on the predominant feature. In case of disagreement a third experienced grader adjudicated. Normal appearing, non-classifiable optic discs and advanced glaucomatous optic discs were excluded from the study. All graders had been trained and certified using a set of standard reference photographs used in the Optic Disc Reading Center at Hamilton Glaucoma Center at the University of California, San Diego. The optic discs were evaluated with simultaneous stereoscopic optic disc photographs (TRC-SS, Topcon Instrument Corp. of America, Parasmus, NJ) using a stereoscopic viewer (Pentax, Asahi, Japan). For the purpose of analysis the optic discs were grouped into concentric group and non-concentric group (FI, MG, SS). This broad classification was adopted as underlying pathogenesis is thought to be distinctly different in concentric and non-concentric optic disc appearance.1–4 Both observers agreed in their classification in 71 of 75 eyes (95.5%) and adjudication from a third experienced observer was sought for the remaining 4 eyes. The kappa statistic between the two original graders was 0.91. All observers were masked to the IOP measurements and classified the discs independently. Stereo-photographs of the study eye were taken within a 12-month period of 24-hour IOP measurements.
One hundred and twelve eyes of 64 glaucoma subjects met the inclusion criteria of the study. Thirty-seven eyes (25%) were excluded as the optic disc was considered to be non-classifiable either due to poor quality of photographs (19), mixed appearance with lack of a predominant feature (8) or advanced optic disc damage (10). Seventy-five eyes of 45 POAG subjects consisting of 17 males and 28 females with the mean age of 65 years (range, 45–82 years) were enrolled in the data analysis and included 32 Caucasians, 6 African Americans, 6 Asians and one Hispanic. The study group included both eyes from 30 subjects. Forty eyes were classified as having concentric optic disc appearance and 35 eyes as having non-concentric optic disc appearance (FI 8 eyes, MG 12 eyes, SS 15 eyes). There was no significant difference in age, gender, CCT, PSD, blood pressure parameters and heart rate between groups (Table 1). Systemic medication usage included anti-hypertensive medications (15), cholesterol lowering medications (8), low-dose aspirin (10) and other systemic medications (6). Nineteen subjects were on no systemic medications.
Table 1.
Clinical characteristics of patients in the concentric and non-concentric groups
| Concentric Mean (SD) |
Non-concentric Mean (SD) |
P value | |
|---|---|---|---|
| Age (years) | 64.6 (11.7) | 65.9 (10.7) | 0.63 |
| Male:Female * | 10:17 | 11:14 | 0.73 |
| CCT (µm) | 549 (51) | 562 (35) | 0.22 |
| PSD (dB) | 2.14 (1.35) | 2.46 (2.01) | 0.42 |
| SBP (mm Hg) | 130.0 (13.1) | 125.9 (13.2) | 0.22 |
| DBP (mmHg) | 75.4 (8.7) | 73.0 (8.7) | 0.26 |
| Heart Rate | 69.0 (9.0) | 69.3 (7.9) | 0.45 |
7 subjects had one eye with concentric and one eye with non-concentric optic disc appearance. Fisher’s exact test
SD = Standard Deviation, CCT = central corneal thickness, PSD = pattern standard deviation, SBP = systolic blood pressure, DBP = diastolic blood pressure
Statistical analysis
The mean, peak, trough and range in IOP (peak – trough) were calculated for the office-hour period (9 AM to 4 PM), the diurnal period (7 AM–11PM), the nocturnal period (11 PM–7AM) and the 24-hour period. The range of the 24-hour IOP was used as a surrogate for IOP fluctuation. Further, the difference between the nocturnal and diurnal values for each subject was calculated.
Generalized estimating equations (GEE) were used to evaluate differences in IOP values between the two groups (concentric vs non-concentric) while adjusting for CCT, age and pattern standard deviation (PSD). GEEs were also used to adjust for the correlation between both eyes of the same individual. An overlay plot was constructed to present the habitual and supine 24-hour IOP data in graphical form.
Specific analyses were performed to evaluate 24-hour rhythm of IOP. The best fitting 24-hour cosine was estimated for 12 mean IOP readings. The circadian amplitude (height of the rhythm) and acrophase (timing of the fitted peak) were estimated13 and compared between the experimental groups using Mann Whitney-U test. Area under curve (AUC) for 24 hour IOP, diurnal IOP and nocturnal IOP was calculated using trapezoidal technique on Matlab software and compared between the groups. Statistical analyses were performed using JMP software version 6.0.2 (SAS institute, Cary, North Carolina, USA) and SPSS software version 13.0 (SPSS inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
IOP readings were significantly greater in the concentric group compared to the non-concentric group at all nocturnal time points. The mean nocturnal IOP was greater in the concentric group (24.0 ± 3.8 mm Hg; mean ± standard deviation [SD]) compared to the non-concentric group (21.9 ± 1.9 mm Hg; mean ± SD), P = 0.004 (Table 2). The mean nocturnal IOP was significantly greater than the mean diurnal IOP in both groups ([concentric group: 24.0 mm Hg vs 20.4 mm Hg, P < 0.001], [non-concentric group: 21.9 mm Hg vs 19.8 mm Hg, P < 0.001]). However, the difference between the mean nocturnal IOP and the habitual mean diurnal IOP was significantly greater in the concentric group compared to the non-concentric group (Table 2). There were no significant differences between the groups for mean, peak, trough and range of habitual IOP during the office period, the diurnal period and the whole 24-hour period (Table 2). Differences in the mean nocturnal IOP between the groups remained significant after adjusting for age, CCT and PSD (Table 3).
Table 2.
Comparison of 24-hour intraocular pressure (IOP) measurements based on optic disc appearance (habitual position)
| Period | IOP parameter | Concentric Mean (SD) mm Hg |
Non-concentric Mean (SD) mm Hg |
P value |
|---|---|---|---|---|
| Diurnal | Mean | 20. (3.6) | 19.8 (2.6) | 0.40 |
| Peak | 23.7 (4.4) | 23.4 (3.3) | 0.69 | |
| Trough | 17.4 (3.5) | 16.8 (2.6) | 0.29 | |
| Range | 6.2 (2.0) | 6.1 (2.7) | 0.55 | |
| Nocturnal | Mean | 24.0 (3.7) | 21.9 (1.9) | 0.004 * |
| Peak | 26.0 (4.2) | 23.7 (2.5) | 0.008 * | |
| Trough | 22.0 (3.4) | 20.1 (1.8) | 0.02 * | |
| Range | 4.1 (2.4) | 3.6 (1.7) | 0.20 | |
| Office | Mean | 20.9 (3.9) | 20.3 (2.5) | 0.50 |
| Peak | 22.2 (4.0) | 22.3 (3.2) | 0.90 | |
| Trough | 18.9 (3.9) | 18.7 (2.4) | 0.79 | |
| Range | 3.6 (1.7) | 3.5 (1.8) | 0.85 | |
| 24-hour | Mean | 21.6 (3.5) | 20.5 (2.2) | 0.11 |
| Peak | 26.6 (4.5) | 24.9 (2.3) | 0.050 | |
| Trough | 17.5 (3.5) | 16.6 (2.4) | 0.21 | |
| Range | 6.2 (2.0) | 6.6 (2.7) | 0.55 | |
| Nocturnal-Diurnal | Mean | 3.5 (3.1) | 2.1 (2.1) | 0.021* |
P < 0.05
SD = Standard Deviation
Table 3.
Multivariate model for the relationship between mean nocturnal intraocular pressure and optic disc appearance adjusting for age, central corneal thickness and pattern standard deviation.
| Covariates | Mean Nocturnal Intraocular Pressure | |
|---|---|---|
| Standardized β Coefficient (SE) |
P value | |
| Disc Appearance * | 2.73 (0.81) | 0.001 |
| Age | −0.029(0.03) | 0.374 |
| CCT | 0.023 (0.01) | 0.014 |
| PSD | 0.062 (0.28) | 0.825 |
Non-concentric optic disc appearance used as a reference category
SE = standard error, CCT = central corneal thickness, PSD = pattern standard deviation
Separate data analysis for three non-concentric disc appearances, grouped together for the purpose of this study, did not show any significant differences in the IOP readings. The mean nocturnal IOP readings for senile sclerotic, myopic glaucomatous and focal ischemic optic disc appearances were 22.0 ± 1.8 mm Hg, 20.9 ± 1.5 mm Hg and 19.5 ± 2.5 mm Hg respectively (P = 0.09). Though mean nocturnal IOP was greater than mean diurnal IOP in all three non-concentric optic disc appearances, the difference did not reach statistical significance.
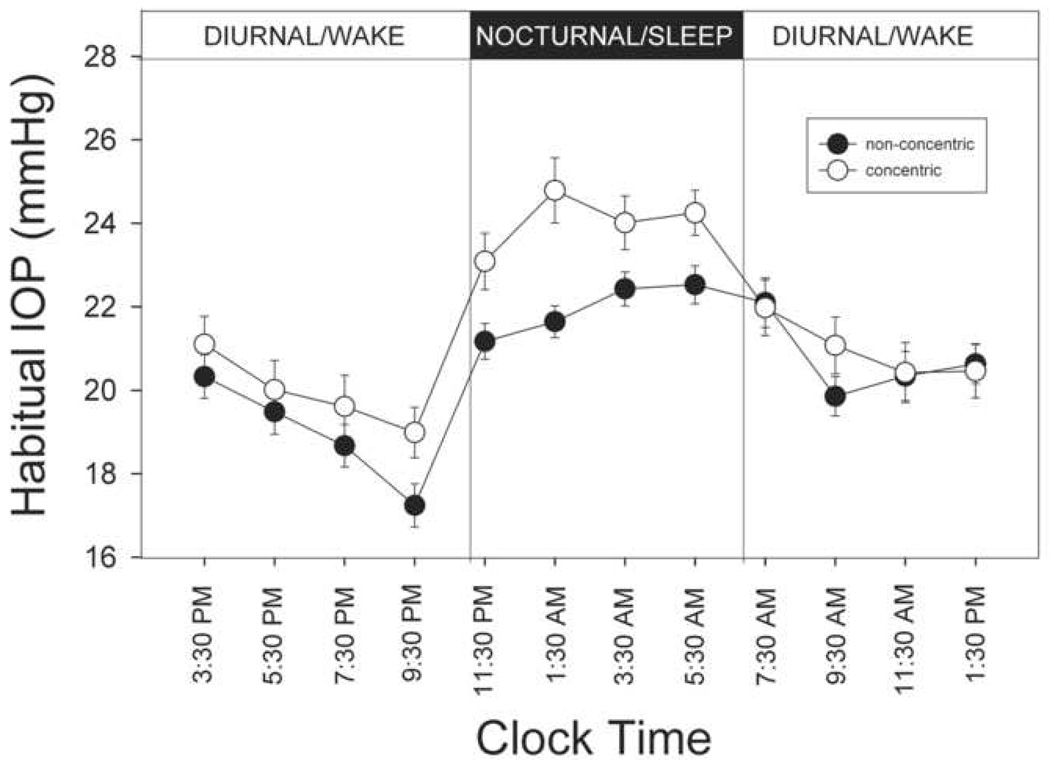
Figure 1 presents the habitual 24-hour IOP profile in eyes with the concentric and the non-concentric optic disc appearance. Mean IOP reached trough at 9:30 PM and peaked at 1:30 AM and then remained elevated through the nocturnal period in the concentric group. In the non-concentric phenotype, the trough IOP was reached at 9:30 PM followed by progressive rise in IOP in the nocturnal period with IOP peaking at 5:30 AM. Cosine fits of the 24-hour habitual IOP data showed that the mean acrophase occurred at different times in the concentric (05:00 hour) and the non-concentric group (06:30 hours) (P = 0.049). Similarly the mean amplitude of the circadian rhythm in the concentric group (2.7±1.5 mm Hg) was higher than the mean amplitude in the non-concentric group (2.1±0.9) (P value = 0.044).
FIGURE 1.
Profiles of habitual 24-hour intraocular pressure (IOP) in the concentric and non-concentric optic disc phenotype. Measurements were taken in sitting during the diurnal period and supine during the nocturnal period. Solid symbols represent non-concentric disc phenotypes (n=35) and open symbols represent concentric optic disc phenotype (n=40). Error bars represent standard error of measurement. Significant difference in IOP readings was found for all measurements taken in the nocturnal period.
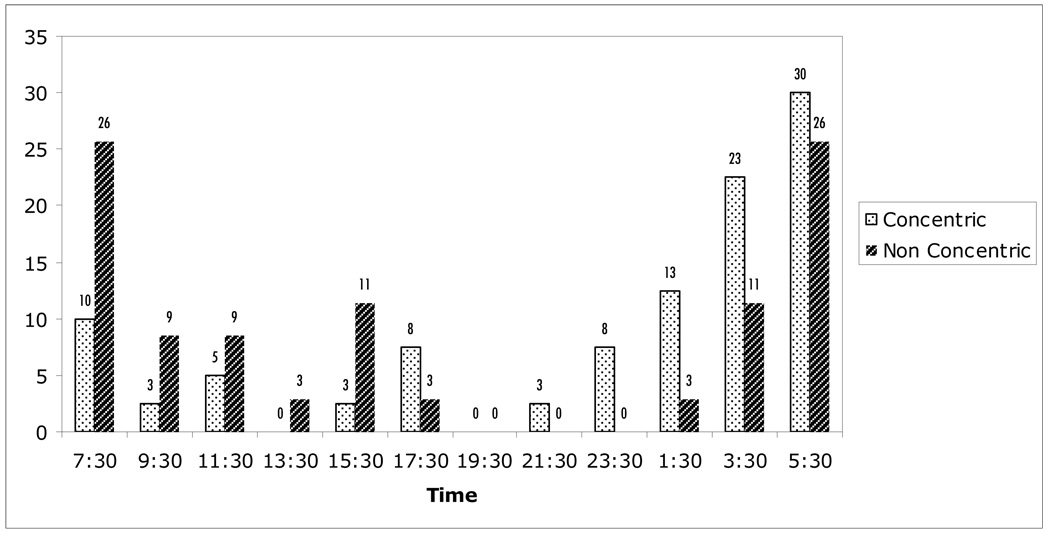
Figure 2 shows the frequency of habitual peak IOP by optic disc appearance at each of the 12 time points. The distribution of habitual peak IOP during the 24-hour period was significantly different between the groups, with peak IOP in the concentric group more prevalent during the nocturnal period compared to the non-concentric group (67.5% vs 48.5%, P = 0.040). This remained significant even after including one additional time point on either side of the nocturnal time period to compensate for the variations in circadian rhythms of different individuals (P= 0.05). Similarly, the area under the curve for nocturnal IOP was significantly higher for the concentric group compared to the non-concentric group (Table 4).
FIGURE 2.
Distribution of habitual intraocular pressure peaks (percentage of eyes). Measurements were taken in sitting during the diurnal period and supine during the nocturnal period.
Table 4.
Comparison of area under the intraocular pressure curve between optic disc appearances
| Period | Concentric mm Hg.h |
Non-concentric mm Hg.h |
P Value |
|---|---|---|---|
| Nocturnal | 143.02 (22.26) | 101.94 (13.24) | 0.0036* |
| Diurnal | 340.52 (13.94) | 328.334 (49.42) | 0.31 |
| 24-hour | 488.56 (70.34) | 460.30 (60.30) | 0.06 |
Statistically significant using student’s t-test
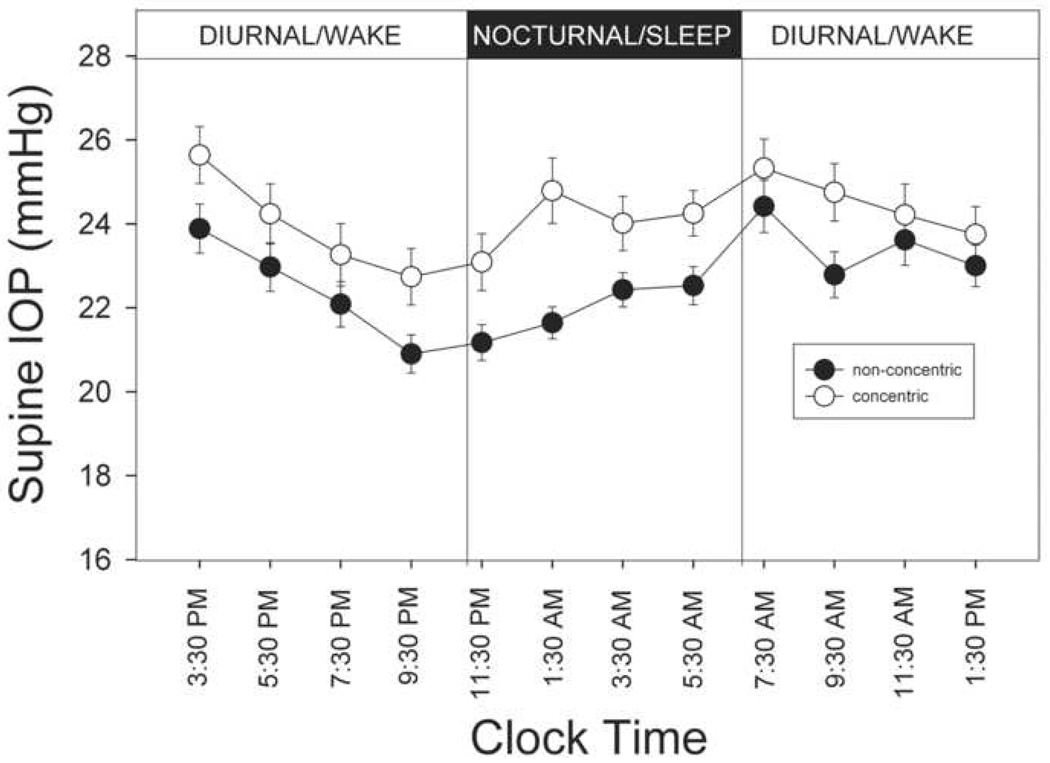
To account for the postural influence, the 24-hour IOP profile was examined again using only 24-hour supine IOP readings (Figure 3). During the diurnal period, the mean supine IOP was 23.6 ± 3.4 mm Hg (n=75). It was significantly higher than mean sitting IOP of 20.16 ± 3.30 mm Hg, obtained at comparable times. The difference between the supine IOP and sitting IOP was greater for the patients with the concentric optic disc appearance (3.8 ± 1.5 mm Hg) compared to those with the non-concentric optic disc appearance (3.1 ± 1.3 mm Hg), but it was not statistically significant (P = 0.054).
FIGURE 3.
Profiles of 24-hour supine intraocular pressure (IOP) in the concentric and non-concentric optic disc phenotype. Solid symbols represent non-concentric disc phenotypes (n=35) and open symbols represent concentric optic disc phenotype (n=40). Error bars represent standard error of measurement. Significant difference in IOP readings was found for measurements taken at 21:30 PM, 9:30 AM and whole of the nocturnal period.
Discussion
This is the first study to evaluate the optic disc appearance in relation to 24-hour IOP. Our results show that although the nocturnal IOP was higher than diurnal IOP for both groups, eyes with the concentric optic disc appearance had a significantly higher IOP rise during the nocturnal period compared to the eyes with the non-concentric optic disc appearance. This difference was noted for overall nocturnal mean IOP and at all individual nocturnal time points. IOP measurements were obtained in habitual (sitting in daytime and supine at night time) positions in the sleep laboratory. Therefore, they are more likely to represent the IOP variations actually experienced by subjects during their daily routine.
The concentric optic disc enlargement has been reported to correlate with raised IOP.1, 2 In a study by Broadway et al the mean IOP was 32.2±3.6 mm Hg with the concentric optic disc appearance and ranged from 21mm Hg to 24 mm Hg with the non-concentric optic disc appearance.2 In contrast, a similar study by Rahman et al failed to show a significant difference in the IOP levels among various disc appearances.14 However, since the office-hour IOP levels alone were analyzed in their study, their findings cannot be extrapolated to a 24-hour period. Figure 1 demonstrates how the IOP pattern was similar between the groups over the diurnal and office hour period while in the habitual position. This suggests that by taking in consideration only the office hour IOP measurements, a clinician is likely to underestimate peak levels of IOP regardless of optic disc morphology. In fact, in the present study, 85% of IOP peaks occurred outside usual office hours, an observation that may lead to modification of management in a significant proportion of patients.15
Ability to detect elevated IOP is crucial in any type of glaucoma management and currently IOP lowering is the only effective treatment for glaucoma. A nocturnal rise in IOP that is undetected may therefore result in suboptimal treatment of glaucoma. Any feature that may suggest a likelihood of elevated IOP at times other than office hours can be clinically useful and may allow clinician to predict and manage the disease course better. The finding of greater rise in nocturnal IOP in patients with concentric optic disc appearance in our study suggests that the underestimation of the peak IOP will be even larger in the concentric group compared to the non-concentric group if only office hour IOP measurements are evaluated.
The biologic basis for the association of concentric optic disc enlargement with higher nocturnal IOP is unclear. It is possible that the concentric optic disc enlargement is not merely an association, but the consequence of raised nocturnal IOP. The level of IOP that an eye maintains depends on the interplay of factors including aqueous production, outflow facility and episcleral venous pressure. It is possible that the influence of any one factor on IOP differs between the disc appearances. Compared with eyes with the non-concentric optic disc appearance, it is possible that the higher nocturnal IOP of eyes with the concentric enlargement may be related to a greater reduction in the trabecular outflow facility than the reduction in the aqueous production.
A supine position leads to multiple hydrostatic changes in the body, including elevation of episcleral venous pressure, with consequent rise in IOP.16 In the present study, an analysis of 24-hour patterns demonstrated consistently greater mean supine IOP compared to mean sitting IOP for both the concentric and the non-concentric optic disc appearance. However, no significant difference was observed in IOP increase when moving from sitting to supine position between the concentric and the non-concentric groups. This indicates that the greater increase in pressure that occurs at night in the concentric group cannot be attributed entirely to postural changes.
The inter-observer agreement in the recognition of disc appearance was very high in the present study. This is likely due to the rigorous training observers had to undergo before being certified at our optic disc reading center and that both observers were from the same institution and have worked on a similar project in the past. Nicolela et al reported moderate agreement among 3 clinicians in the optic disc pattern recognition. However 2 out of 3 clinicians agreed 83.8% of the times, which is similar to the present study.17
Our study has some limitations. The disc stereo-photographs selected for the study were obtained within 12 months of the 24-hour IOP assessment. It is possible that the 24-hour IOP profile may have been different at the time stereo-photograph was evaluated in this study. However, as glaucoma is a slowly progressive disease it is unlikely that disc appearance would have changed sufficiently during the period between stereo-photograph and sleep laboratory assessment.
In the present study the forced choice of disc appearance resulted in some mixed appearances being included in non-concentric groups. Ideally, only pure examples of disc appearance should be selected. However, our aim was to replicate clinical practice, as most patients present with a mixed disc appearance in the clinic. Further, all non-concentric subgroups were grouped together. We elected this strategy based on literature that suggests that vascular abnormalities play a predominant role in glaucoma pathogenesis in all non-concentric optic disc appearance in contrast to IOP in concentric optic disc appearance.1–6 It is important to bear in mind that patients included in this study were either untreated or had undergone 4-week washout period and thus findings of this study will be applicable only to that group. Glaucoma patients with concentric optic disc enlargement who are on IOP lowering treatment may have a different 24-hour IOP profile.10, 18
In conclusion, untreated glaucoma patients with a concentric optic disc enlargement demonstrate higher mean IOP and greater number of IOP peaks in the nocturnal period compared to those with non-concentric optic disc appearance. This has clinical implications as measuring IOP only during office hours may lead to underestimation of IOP, particularly in the patients with concentric optic disc enlargement.
Acknowledgments
Financial Disclosure: Supported in part by the National Institutes of Health, Bethesda, Maryland (grant no. EY-07544). The funding organization had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: None
Conflict of Interest: No authors have any financial/conflicting interests to declare
References
- 1.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology. 1996;103:640–649. doi: 10.1016/s0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- 2.Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Surv Ophthalmol. 1999;43(suppl):S223–S243. doi: 10.1016/s0039-6257(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 3.Geijssen HC, Greve EL. The spectrum of primary open angle glaucoma I: Senile sclerotic glaucoma versus high-tension glaucoma. Ophthalmic Surg. 1987;18(3):207–213. [PubMed] [Google Scholar]
- 4.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 1998;82:862–870. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolela MT, McCormick TA, Drance SM, et al. Visual field and optic disc progression in patients with different types of optic disc damage: a longitudinal prospective study. Ophthalmology. 2003;110:2178–2184. doi: 10.1016/S0161-6420(03)00801-7. [DOI] [PubMed] [Google Scholar]
- 6.Nicolela MT, Walman BE, Buckley AR, Drance SM. Various glaucomatous optic nerve appearances: a color Doppler imaging study of retrobulbar circulation. Ophthalmology. 1996;103:1670–1679. doi: 10.1016/s0161-6420(96)30448-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 8.Liu JH, Kripke DF, Twa MD, et al. Twenty-four hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 9.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 10.Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of oncedaily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004;138:389–395. doi: 10.1016/j.ajo.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Phelps CD, Woolson RF, Kolker AE, Becker B. Diurnal variation in intraocular pressure. Am J Ophthalmol. 1974;77(3):367–377. doi: 10.1016/0002-9394(74)90743-0. [DOI] [PubMed] [Google Scholar]
- 12.Noël C, Kabo AM, Romanet JP, et al. Twenty-four hour time course of intraocular pressure in healthy and glaucomatous Africans: relation to sleep pattern. Ophthalmology. 2001;108:139–144. doi: 10.1016/s0161-6420(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 13.Zeimer RC. Circadian variations in intraocular pressure. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2nd ed. Vol 1. St. Louis, MO: Mosby; 1996. pp. 429–445. [Google Scholar]
- 14.Rahman R, Casson RJ, Gouveia SM, Salmon JF. Optic disc morphology on presentation of chronic glaucoma [letter] Eye. 2002;16:665–667. doi: 10.1038/sj.eye.6700153. [DOI] [PubMed] [Google Scholar]
- 15.Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12:232–236. doi: 10.1097/00061198-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kothe AC. The effect of posture on intraocular pressure and pulsatile ocular blood flow in normal and glaucomatous eyes. Surv Ophthalmol. 1994;38(suppl):S191–S197. doi: 10.1016/0039-6257(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 17.Nicolela MT, Drance SM, Broadway DC, et al. Agreement among clinicians in the recognition of patterns of optic disc damage in glaucoma. Am J Ophthalmol. 2001;132:836–844. doi: 10.1016/s0002-9394(01)01254-5. [DOI] [PubMed] [Google Scholar]
- 18.Orzalesi N, Rossetti L, Invernizzi T, et al. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2000;41:2566–2573. [PubMed] [Google Scholar]