Abstract
Introduction
Observational studies show reduced incidence of Alzheimer’s dementia (AD) in users of non-steroidal anti-inflammatory drugs (NSAIDs). One hypothesis holds that the subset of NSAIDs known as “selective Aβ42-lowering agents” (SALAs) is responsible for this apparent reduction in AD risk.
Methods
We pooled individual-level data from six prospective studies to obtain a sufficient sample to examine AD risk in users of SALA vs. non-SALA NSAIDs.
Results
Of 13,499 initially dementia-free participants (70,863 person-years), 820 developed incident AD. Users of NSAIDs (29.6%) showed reduced risk of AD (adjusted hazard ratio or aHR 0.77, 95% confidence interval or CI 0.65–0.91). The point estimates were similar for SALAs (aHR 0.87, CI 0.72–1.04) and non-SALAs (aHR 0.75, CI 0.56–1.01). Because 573 NSAID users (14.5%) reported taking both a SALA and non-SALA, we examined their use alone and in combination. Resulting aHRs were 0.82 (CI 0.67–0.99) for SALA only, 0.60 (CI 0.40–0.90) for non-SALA only, and 0.87 (CI 0.57–1.33) for both NSAIDs (Wald test for differences, p=0.32). The 40.7% of participants who used aspirin also showed reduced risk of AD, even when they used no other NSAIDs (aHR 0.78, CI 0.66–0.92). By contrast, there was no association with use of acetaminophen (aHR 0.93, CI 0.76–1.13).
Conclusions
In this pooled dataset, NSAID use reduced the risk of AD. However, there was no apparent advantage in AD risk reduction for the subset of NSAIDs shown to selectively lower Aβ42, suggesting that all conventional NSAIDs including aspirin have a similar protective effect in humans.
Search terms: Epidemiology - cohort studies (54), Epidemiology - risk factors (59), Alzheimer’s disease (26)
INTRODUCTION
Alzheimer’s disease (AD) currently causes dementia in some 3 million North Americans and 26 million people worldwide.(1) Many observational studies,(2) including five with prospective design,(3–7) suggest that non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) may delay or prevent the onset of AD, especially with prolonged use.(3,4,7) In contrast, results from randomized trials of NSAIDs for prevention or treatment of AD or mild cognitive impairment have been discouraging.(8–10)
Laboratory experiments suggest that NSAIDs can reduce Alzheimer pathology by suppressing microglial activation (11,12) or deposition of amyloid-beta peptide (Aβ),(11,13) possibly through inhibition of cyclooxygenases (COX).(14) Alternatively, a subset of NSAIDs appear to modify γ-secretase cleavage away from the more fibrillogenic Aβ42 species toward peptides such as Aβ40 and Aβ38 (15,16) These findings have provoked speculation that this γ-secretase effect is key to NSAIDs’ apparent ability to protect against AD, with the subset of NSAIDs known as “selective Aβ42-lowering agents” (SALAs) being responsible for the reduced AD risk with NSAIDs overall. Accordingly, some have proposed that the lack of benefit in NSAID clinical trials reflects their choice of the “wrong” non-SALA compounds (17) - this has motivated the initiation of new trials of the non-NSAID SALA tarenflurbil.(18)
Only two epidemiological studies have investigated the differential association of SALAs vs. non-SALAs and AD. The Rotterdam study found that SALAs were associated with a greater reduction in AD incidence than non-SALAs (19) while the Cardiovascular Health Study found no such difference.(20) To investigate these conflicting findings and to overcome the limitation of small sample size in individual studies, we pooled individual-level data from six cohort studies to clarify whether SALA NSAIDs appear to confer preferential protection against AD.
METHODS
Settings, subjects, and design
We contacted investigators of prospective cohort studies with specified study inclusion criteria of 1) diagnoses of incident AD made using clinical research criteria,(21) 2) systematic data on individual over-the-counter (OTC) and prescription NSAIDs, and 3) exposure measurement(s) collected prior to dementia diagnosis. The six participating studies were: the Baltimore Longitudinal Study of Aging (BLSA), the Cache County Study (CCS), the Canadian Study of Health and Aging (CSHA), the Cardiovascular Health Study (CHS), the Framingham Heart Study (FHS), and the Monongahela Valley Independent Elders Study (MoVIES).(22–28) Each study was approved by appropriate Institutional Review Boards (IRB) / Research Ethics Board, and the present pooled analysis was approved by the IRB at the Johns Hopkins Bloomberg School of Public Health. Of the seven other study investigators who were contacted but did not participate, one stated that their design was not prospective, two did not systematically collect data on OTC NSAIDs, and four declined participation.
Outcome and exposure measurement
All studies used a primary outcome measure of incident Probable or Possible AD, diagnosed using NINCDS-ADRDA criteria (21) applied by consensus conferences of expert clinicians after review of extensive data that typically included neurocognitive assessments, detailed clinical evaluations, neuroimaging, and laboratory tests. Use of aspirin, acetaminophen, and NSAIDs was assessed by self-report. We analyzed acetaminophen as a “control” medication because it is often used for indications similar to those for NSAIDs (e.g., pain management) but has a different mechanism of action. NSAIDs were categorized as SALAs if found to lower Aβ42 compared to Aβ40 using in vitro or in vivo models by Eriksen and colleagues,(16) or non-SALAs if they did not show selective Aβ42 lowering.(16) SALAs included diclofenac, diflunisal, fenoprofen, flurbiprofen, ibuprofen, indomethacin, meclofenamate, piroxicam, and sulindac. Non-SALAs included celecoxib, etodolac, ketoprofen, ketorolac, mefanamic acid, nabumetone, naproxen, and phenylbutazone. A few participants had reported use of NSAIDs not characterized by Eriksen et al., including oxaprozin, rofecoxib, tiaprofen, and tolmetin. We considered these agents unclassifiable. Dose information was not consistently available and could not be investigated.
Statistical analyses
We compared baseline demographic characteristics of NSAID user groups using χ2 tests. The relationship between NSAIDs and AD was analyzed using two approaches, a Pooled Participant analysis and a Pooled Study analysis, to test the robustness of the findings. For the Pooled Participant analysis we pooled individual-level study data and used extended Cox hazards regression (29) to obtain crude and adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for the association between incident AD and three medications groups – non-aspirin NSAIDs, aspirin, and acetaminophen. In turn, we examined separate models with SALA or non-SALA NSAIDs. All models used chronological age at observation as the time axis (to provide tight control of potential confounding by age), and medication use was modeled as ever-used vs. never-used as a time-dependent covariate. Thus, a participant who entered the analysis as a “never user” could later switch to an “ever user” if NSAID use was initiated during follow-up. To account for potential variability in baseline hazards between studies we stratified all analyses by study. We further adjusted by sex, education, and age at first visit (to additionally control for possible cohort effects). In addition, we used self-report of arthritis as a covariate in the model looking at any NSAID use. Analyses were performed using SAS 8.2.(30)
The Pooled Study analysis obtained separate aHRs for each study which were then pooled using standard inverse-variance weighted fixed- and random-effects meta-analytic models. Because the fixed- and random-effects estimates did not differ substantively, we report only results from fixed-effects analyses. The Pooled Study analysis allowed for further examination of heterogeneity and degree of influence of each study. We used the Q-statistic to assess heterogeneity and conducted influence analyses by removing one study at a time and re-calculating the aHRs and Q-statistics without that study. Analyses were performed using the meta program in Stata 8.0.(31)
RESULTS
Qualitative analysis
Five studies had been carried out in the United States and one in Canada (Table 1). Their baseline samples of cognitively normal participants numbered between 1,001 and 3,244, contributing 70,863 total person-years of follow-up. Of 13,499 individual participants, 820 developed AD. The age distribution across studies was relatively heterogeneous, with BLSA participants being somewhat younger and FHS participants older. Education level appeared homogeneous across the studies except for the CSHA where it was lower. While all studies used self-report of medication, three validated this by viewing medication bottle labels. Five used structured interviews and one used a mailed questionnaire. The average time between study visits ranged from 1.0 to 4.7 years. Two studies (CCS and CHS) specifically excluded those participants with AD plus vascular dementia from the AD group.
Table 1.
Qualitative analysis of studies
| Study | BLSA | CCS | CSHA | CHS | FHS | MoVIES |
|---|---|---|---|---|---|---|
| Country | USA | USA | Canada | USA | USA | USA |
| Base cohort | Community volunteers, predominantly white | Geographic cohort, predominantly white | Provincial healthcare system registrants or electoral rolls, predominantly white | Geographic cohort from 4 counties; African Americans recruited from 3 of the counties | Geographic cohort | Geographic cohort; originally designed as population-based dementia registry |
| Published* | yes | yes | yes | no | no | no |
| Men, % | 58.4 | 41.8 | 35.8 | 39.8 | 34.9 | 40.7 |
| ≥ High school, % | 98.3 | 84.9 | 29.4 | 76.5 | 68.7 | 59.6 |
| Age range† | 17 to 95 | 64 to 100 | 65 to 101 | 66 to 95 | 72 to 99 | 65 to 106 |
| Calendar years of data collection | 1980–1995 | 1994–1999 | 1991–1997 | 1992–1998 | 1990–2001 | 1987–2001 |
| Average years between visits | 2.3 | 3.2 | 4.7 | 1.0 | 2.0 | 2.2 |
| Maximum number of visits‡ | 9 | 2 | 2 | 6 | 5 | 6 |
| Exposure measurement | Self-report (in-person structured interview); current use or any time between previous and current interview; no criteria for use per week | Self-report (in-person structured interview); corroborated by viewing pill bottles; current or former use of NSAIDs 4 or more times per week for 1 month or longer | Self-report (self-administered questionnaire); current use; no criteria for use per week | Self-report (in-person structured interview); corroborated by viewing pill bottles; current or use within 2 weeks; no criteria for use per week | Self-report (in-person structured interview); current use; no criteria for use per week | Self-report (in-person structured interview); corroborated by viewing pill bottles; current use; no criteria for use per week |
| Medication use, %¶ | ||||||
| NSAID | 15.4 | 17.5 | 16.4 | 19.4 | 14.9 | 7.4 |
| SALA | 12.5 | 14.7 | 10.5 | 14.1 | 10.8 | 6.5 |
| non-SALA | 4.3 | 6.0 | 5.0 | 5.2 | 2.9 | 0.6 |
| aspirin | 41.7 | 30.2 | 24.9 | 33.9 | 45.3 | 29.5 |
| acetaminophen | 15.2 | 10.4 | 8.2 | 16.3 | 12.9 | 13.8 |
| Outcome | probable and possible AD; DSM-III-R and NINCDS-ADRDA; diagnostic conference | probable and possible AD (excluded those with AD plus VaD); DSM-III-R and NINCDS-ADRDA; diagnostic conference | probable and possible AD; DSM-III-R and NINCDS-ADRDA; diagnostic conference | probable and possible AD (excluded those with AD plus VaD); DSM-IV and NINCDS-ADRDA; diagnostic conference | probable and possible AD; DSM-IV and NINCDS-ADRDA; diagnostic conference | probable and possible AD; DSM-III-R and NINCDS-ADRDA; diagnostic conference |
| Sample size, N | 1,686 | 3,188 | 3,244 | 3,008 | 1,001 | 1,372 |
| Person-years | 13,659 | 10,075 | 15,376 | 13,366 | 6,620 | 11,767 |
| Incident AD, N | 81 | 101 | 152 | 231 | 78 | 177 |
| Number of AD / PY × 100 | ||||||
| age <75 | 10/10,593 = 0.09 | 9/5,432 = 0.17 | 12/6,453 = 0.19 | 27/4,169 = 0.65 | 0/205 | 19/4,376=0.43% |
| age 75 to 80 | 11/1,364 = 0.81 | 17/2,349 = 0.72 | 14/4,036 = 0.35 | 65/5,407 = 1.20 | 9/1,933 = 0.47% | 37/3,877=0.95% |
| age >80 | 60/1,701 = 3.53 | 75/2,294 = 3.27 | 126/4,887 = 2.58 | 139/3,790 = 3.67 | 69/4,482 = 1.54% | 121/3,515 = 3.44% |
published NSAID-AD results at the time of data collection for the current project;
age at first visit;
these numbers also represent the number of visits at which medication data were collected. For CCS and CSHA data were used from the baseline visit only to be consistent with published results.
Medication use at first visit; percent of SALA plus non-SALA users may not add exactly to percent of NSAID users because some participants took both a SALA and a non-SALA, some participants took unclassifiable NSAIDs, and data were missing on NSAID type for some participants.
Quantitative analysis
Baseline characteristics by NSAID use
In the pooled dataset the frequency of NSAID use at any time in the follow-up interval was 29.6%; aspirin was used by 47.0%, and acetaminophen by 25.3%. These frequencies were fairly consistent among the six studies (data not shown). NSAID users tended more often to be women, highly educated, and younger at baseline (Table 2). Ibuprofen was the most commonly used SALA and accounted for 52.9% of NSAID use. Naproxen was the most commonly used non-SALA, accounting for 20.4% of NSAID use. Less than 1% of participants reported use of selective COX-2 inhibitors (E-Table 1). We found no sex differences between SALA and non-SALA users (34.7% vs.35.3% men; χ2(1) 0.09, p=0.79). However, SALA users were slightly more educated (48.7% vs 43.8% educated beyond high school; χ2(2) 6.1, p=0.05) and tended to be younger at the first visit (12.2% vs 7.8% <65 years of age; χ2(4) 13.1, p=0.01).
Table 2.
Baseline characteristics by NSAID use
| Non-users n (%) | Users† n (%) | |
|---|---|---|
| Sex* | ||
| Men | 4,207 (44.3) | 1,376 (34.4) |
| Women | 5,294 (55.7) | 2,622 (65.6) |
| Education* | ||
| no high school diploma | 3,338 (35.1) | 1,019 (25.5) |
| high school diploma | 2,123 (22.3) | 1,031 (25.8) |
| post high school | 4,013 (42.2) | 1,939 (48.5) |
| missing | 27 (0.3) | 9 (0.2) |
| Age at first visit* | ||
| < 65 | 626 (6.6) | 454 (11.4) |
| 65 – 69.99 | 1,847 (19.4) | 722 (18.1) |
| 70 – 74.99 | 2,722 (28.6) | 1,228 (30.7) |
| 75 – 79.99 | 2,351 (24.7) | 960 (24.0) |
| ≥ 80 | 1,955 (20.6) | 634 (15.9) |
| Total | 9,501 (100) | 3,998 (100) |
NSAID=non-steroidal anti-inflammatory drug
χ2 p-values all <0.001, results for sex and education did not change when adjusted for age in logistic regression models
for these counts, participants were considered an NSAID user if they reported exposure at any time during follow-up
Association between AD and NSAID, aspirin, or acetaminophen use
Table 3-Model 1 shows the results from the Pooled Participant analysis with any NSAID use. Risk of AD was reduced among those who reported use of any NSAIDs (HR 0.75, CI 0.64–0.89; aHR 0.77, CI 0.65–0.91). Results were similar for the three previously published investigations (aHR 0.69, CI 0.52–0.91) and the three unpublished studies (aHR 0.83, CI 0.67–1.03). Controlling for arthritis mitigated the observed association between NSAIDs and AD slightly (aHR 0.83, CI 0.69–0.99) but did not change the overall conclusion. As shown in Table 3-Model 2, there was an association between aspirin use and AD, even in those who used aspirin but no NSAIDs (aHR 0.78, CI 0.66–0.92). Table 3-Model 3 showed no significant association between acetaminophen use and AD (aHR 0.93, CI 0.76–1.13).
Table 3.
Adjusted HRs for NSAID, aspirin, or acetaminophen use and AD
| N | PY | AD | aHR (95% CI) | |
|---|---|---|---|---|
| Model 1 | ||||
| No NSAID | 9,501 | 47,293 | 642 | 1.0 |
| Any NSAID | 3,998 | 23,569 | 178 | 0.77 (0.65 – 0.91) |
| Model 2* | ||||
| No NSAID, no aspirin | 5,301 | 23,673 | 385 | 1.0 |
| No NSAID, yes aspirin | 4,193 | 23,600 | 256 | 0.78 (0.66 – 0.92) |
| Yes NSAID, +/− aspirin | 3,992 | 23,554 | 178 | 0.68 (0.57 – 0.82) |
| Model 3† | ||||
| No NSAID, no acetaminophen | 7,610 | 35,618 | 502 | 1.0 |
| No NSAID, yes acetaminophen | 1,885 | 11,656 | 140 | 0.93 (0.76 – 1.13) |
| Yes NSAID, +/− acetaminophen | 3,993 | 23,555 | 178 | 0.75 (0.63 – 0.90) |
aHR=adjusted hazard ratio, N=number, PY=person years, AD=incident Alzheimer’s dementia, CI=confidence interval, NSAID=non-steroidal anti-inflammatory drug
Each model shows the relationship between a medication classification and the risk of incident AD. All models are stratified by study and adjusted for age, sex, and education. The variables shown within each model are mutually exclusive categorizations of the medication exposure of interest.
Data on aspirin use missing for 13 participants
Data on acetaminophen use missing for 13 participants
Data on NSAID type missing for 42 participants
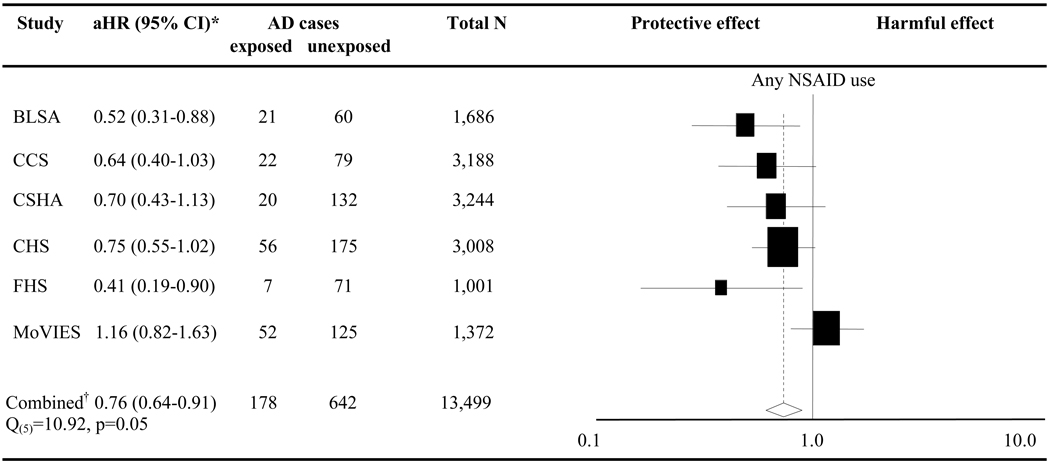
Figure 1 depicts the results from the Pooled Study analysis. There was an inverse association between AD and NSAID use in five of the studies (BLSA, CCS, CSHA, CHS, FHS) but not in one other (MoVIES). Although the analysis gave a combined aHR of 0.76 (CI 0.64–0.91), the Q-statistic indicated heterogeneity among the study results (Q(5) 10.92, p 0.05). As expected, influence analysis suggested that the heterogeneity was attributable to results from MoVIES (E-Table 2). Removing MoVIES changed the aHR to 0.66 (CI 0.54–0.80), with the Q-statistic no longer significant.
Figure 1. Forest plot of NSAID use and AD.
NSAID=non-steroidal anti-inflammatory drug, aHR=adjusted hazard ratio, CI=confidence interval, AD=incident Alzheimer’s dementia, N=number; Black squares and horizontal lines represent each study’s risk estimate and 95% CI. The size of each square is indicative of the weight each study contributed to the meta-analysis; *aHRs result from a model adjusted for age, sex, and education; †Data analyzed using pooled study fixed-effects meta-analysis
Association between AD and NSAID categorized by Aβ42-lowering capability
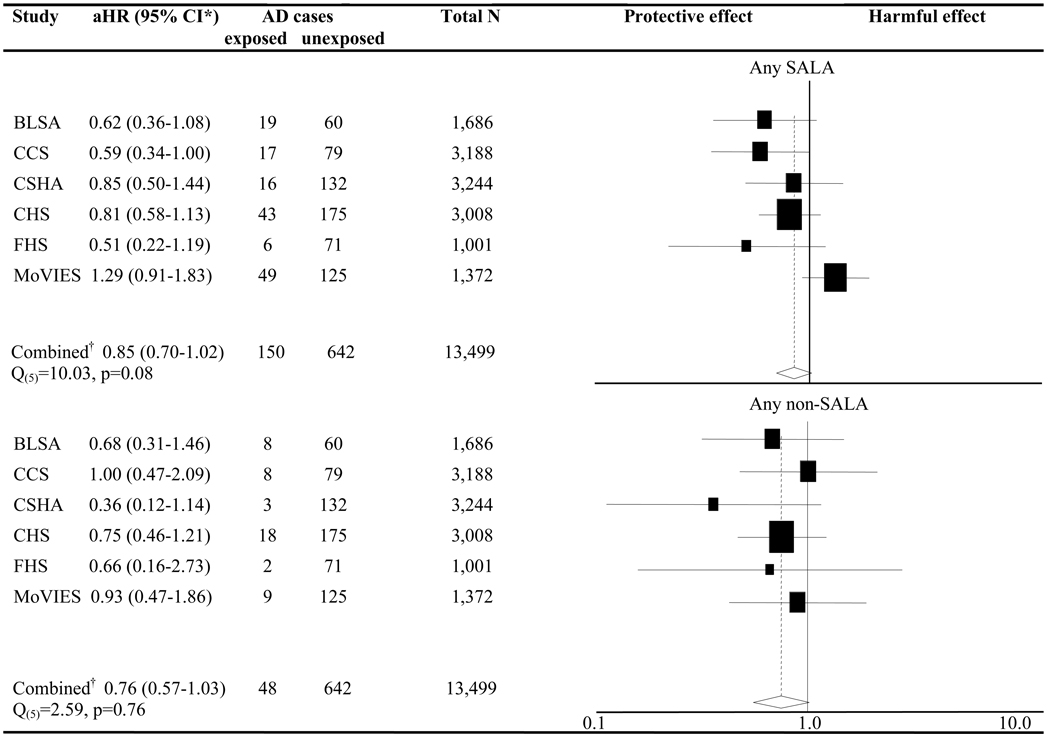
The results from the Pooled Participant analysis with NSAIDs categorized as SALAs and non-SALAs showed similar aHRs for the two NSAID groups (SALA aHR 0.87, CI 0.72–1.04; non-SALA aHR 0.75, CI 0.56–1.01). These analyses included terms in the model to control simultaneously for the use of the other NSAID type. Figure 2 depicts the results for a corresponding model in the Pooled Study analysis. The combined aHR for use of any SALA (0.85, CI 0.70–1.02) was again similar to that for any non-SALA (0.76, CI 0.57–1.03). Heterogeneity and influence analyses suggested that MoVIES contributed to the statistical heterogeneity among the studies in the SALA group (Q(5) 10.03, p 0.08) but not in the non-SALA group (Q(5) 2.59, p 0.76). Without MoVIES, the aHR for SALAs changed to 0.72 (CI 0.58–0.90; Q(4) p 0.68) while the non-SALA aHR remained almost unchanged at 0.73 (CI 0.52–1.01; Q(4) p 0.70) (E-Table 3).
Figure 2. Forest plot of NSAID use and AD by Aβ42-lowering capability.
NSAID=non-steroidal anti-inflammatory drug, aHR=adjusted hazard ratio, CI=confidence interval, AD=incident Alzheimer’s dementia, N=number, SALA=selective Aβ42-lowering agent; Black squares and horizontal lines represent each study’s risk estimate and 95% CI. The size of each square is indicative of the weight each study contributed to the meta-analysis; *The aHRs result from one model including variables for any SALA (comparing risk among participants who took a SALA during follow-up vs. those who did not) and any non-SALA (comparing risk among participants who took a non-SALA during follow-up vs. those who did not), as well as adjusting for age, sex, and education; †Data analyzed using pooled study fixed-effects meta-analysis
Because 573 NSAID users (14.5%) reported taking both a SALA and another NSAID, we considered the effects with each NSAID type alone and in combination. The results from the Pooled Participant analysis are shown in Table 4-Model 1. There was no meaningful difference between the aHR for use of SALAs alone (0.82, CI 0.67–0.99), non-SALAs alone (0.60, CI 0.40–0.90), or both (0.87, CI 0.57–1.33) (Wald p=0.32). What appears in Table 4 Models 2 and 3 to be a somewhat stronger inverse association in the Pooled Participant analyses between AD and use of the most common non-SALA, naproxen, and the most common SALA, ibuprofen, was partially mitigated when we removed MoVIES. Doing so resulted in an aHR of 0.75 (CI 0.58 – 0.98) for ibuprofen and 0.52 (CI 0.32 – 0.83) for naproxen.
Table 4.
Adjusted HRs for various categorizations of NSAID use
| N | PY | AD | aHR (95% CI) | |
|---|---|---|---|---|
| Model 1* | ||||
| No NSAID | 9,501 | 47,293 | 642 | 1.0 |
| SALA alone | 2,544 | 14,828 | 127 | 0.82 (0.67 – 0.99) |
| Non-SALA alone | 743 | 4,162 | 25 | 0.60 (0.40 – 0.90) |
| Both SALA & non-SALA | 573 | 3,704 | 23 | 0.87 (0.57 – 1.33) |
| Unclassifiable alone | 96 | 556 | 3 | 0.61 (0.19 – 1.89) |
| Model 2* | ||||
| No NSAID | 9,501 | 47,293 | 642 | 1.0 |
| Any ibuprofen | 2,093 | 12,731 | 103 | 0.88 (0.71 – 1.09) |
| Other than ibuprofen | 1,863 | 10,518 | 75 | 0.67 (0.53 – 0.86) |
| Model 3* | ||||
| No NSAID | 9,501 | 47,293 | 642 | 1.0 |
| Any naproxen | 806 | 4,887 | 22 | 0.55 (0.36 – 0.85) |
| Other than naproxen | 3,150 | 18,363 | 156 | 0.82 (0.69 – 0.99) |
aHR=adjusted hazard ratio, N=number, PY=person years, AD=incident Alzheimer’s dementia, CI=confidence interval, NSAID=non-steroidal anti-inflammatory drug, SALA=selective Aβ42-lowering agent
Each model shows the relationship between a medication classification and the risk of incident AD. All models are stratified by study and adjusted for age, sex, and education. The variables shown within each model are mutually exclusive categorizations of the medication exposure of interest.
Data on NSAID type missing for 42 participants
DISCUSSION
Results from this pooled analysis of six prospective studies were consistent with published data as they suggested a 23% reduction in AD incidence with any NSAID use. Three of the six studies (BLSA, CMS, and CSHA) had previously published on the association between NSAIDs and AD and had noted a reduction in risk.(3,4,6) Two of the seven contacted studies that declined to participate had also published similarly on the NSAID-AD relationship.(5,7) Although the results from the CHS, FHS, and MoVIES had not been published prior to our analyses, we found that two of these also supported the notion that NSAIDs reduce the risk of AD. Thus, we saw little evidence of differences between those studies that had been published and other unpublished work.
Our pooled analyses showed no suggestion of greater risk reduction for those NSAIDs shown in laboratory experiments to lower Aβ42 (SALAs) vs. others that do not. In addition, use of aspirin (which does not reduce production of Aβ42), but not acetaminophen, was associated with a reduced risk of AD. Aspirin is a non-SALA but, unlike the other agents, it inhibits COX by irreversibly acetylating the enzyme’s binding site. As a result, any new COX activity must be mediated by newly synthesized enzyme.(32) Five prospective studies (3,4,6,33) have reported on aspirin use and all but one (7) have shown a modest reduction in risk of AD. Our results were consistent with previous findings of a reduced risk in aspirin users, and the large sample size enabled us to show that the risk reduction was apparent in those who used aspirin alone, without other NSAIDs.
Two individual studies have previously reported on the association of SALAs and non-SALAs with the risk of AD. Data from one of these, the CHS,(20) were included here. Those results were, as expected, consistent with the present findings indicating no advantage in AD risk reduction with SALAs. By contrast, results reported at a scientific meeting from the Rotterdam study suggested stronger protection with SALAs used for two years or more, as compared with non-SALAs.(19) Interestingly, when we included the risk estimates from the Rotterdam group (reported in their abstract) in our Pooled Study analysis, we saw no change in the overall results, nor did this addition introduce any suggestion of statistical heterogeneity (data not shown).
Our findings appear to have some implications for the mechanism by which NSAIDs may reduce risk of AD. A current hypothesis holds that this effect is mediated by a subset of agents that modify γ-secretase activity and reduce production and deposition of Aβ42. (34,35) If that hypothesis were correct, then only SALA NSAIDs should reduce risk of AD. Our results are not in accord with this prediction of the hypothesis.
A few randomized trials of NSAIDs in AD have shown a weak suggestion of benefit,(18,36) but others have been null or negative.(8,9,37) These trials have tested both SALAs (18,36,38) and non-SALAs.(8,39) The only primary prevention trial, the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), tested naproxen and celecoxib, both non-SALAs, and failed to show efficacy of either compound, at least within the first years after initiation of treatments.(10) The present results suggest that this discrepancy between the observational and randomized studies does not reflect the trials’ choice of NSAIDs, as has been suggested,(17) but may instead be the result of other factors such as timing and duration of exposure, or in other systematic differences in participants of epidemiologic studies versus clinical trials. (40)
As with all observational studies, this study faces methodologic limitations. These include differential recall error by those who may be in the prodromal stages of AD, and confounding by factors that have been inadequately controlled such as socioeconomic class or medical conditions such as arthritis that may be “indications” for NSAID use but might also be associated with development of AD. Our null findings with acetaminophen may offer some reassurance on these concerns; however, this question is best tested in randomized prevention trials.
There were differences in the number of SALA and non-SALA users, and we may not have had adequate power to detect differences in AD risk reduction between the two. These analyses may also have been vulnerable to confounding by “indication” or other systematic differences among individuals who used SALAs vs non-SALAs. We did observe some differences in the age and education between the two groups, but we controlled for these in the analyses. We also note that this is the largest sample used to investigate the SALA vs. non-SALA issue.
To safeguard against the vagaries of meta-analyses, we used two methods to pool data with results that were reassuringly similar. The pooling of individual-level data provides a powerful analytic design, but combining individual data from studies with substantive design differences and participant characteristics can produce misleading results.(41) In this instance, a qualitative assessment of the study characteristics suggested that the design features were homogeneous enough to allow pooling of the data. The main source of heterogeneity among the studies was in detection of exposure assessment: three studies assessed current use,(6,28,42) one assessed current use plus use over the previous two weeks(20), one assessed use over the prior two years,(3) and one defined use as current or former use of four or more doses per week for one month or longer.(4) In each of these studies it is possible, but not certain, that recent or current use also serves to indicate prior use. Predictably, the different criteria for “use” resulted in varying baseline rates of NSAID use, but they did not appear to affect the relationship between exposure and outcome, which was itself measured with quite consistent results. One study, the MoVIES, seemed to produce somewhat divergent results, especially for SALAs. We note that this is one of the older studies included, the participants were drawn from a somewhat lower socioeconomic region, and most of the reported exposures were to ibuprofen (a SALA). One may conjecture that individuals with lesser education and financial resources were less likely to purchase and use their NSAIDs consistently, thus reducing their overall exposure, but we lacked data on adherence or frequency of use needed to test this idea.
Because of concerns about potential cardiotoxicity (43) and other side effects of NSAIDs (44) as well as discouraging results from ADAPT, enthusiasm for NSAIDs as a potential preventative for AD has diminished. Nevertheless, it is notable that our analysis of three previously unpublished studies do suggest in two instances that NSAIDs or other agents with similar activities may protect against AD. A better understanding of these effects will be important, even if the current generation of drugs has limitations. Given current hypotheses about AD pathogenesis, the Aβ-lowering hypothesis has provided an attractive alternate interpretation of the accumulated observational data on NSAIDs and AD, and this hypothesis will be further tested by a recently completed pivotal trial of tarenflurbil (the r-enantiomer of flurbiprofen), ostensibly having no COX-inhibiting activity but still modifying γ-secretase activity similarly to SALAs. There is much still to learn about the role of NSAIDs in the pathogenesis of AD, including whether the putative neuroprotective effects of NSAIDs depend upon the timing, amount, or duration of their use or on particular characteristics of the subgroups of people who take them.
Supplementary Material
Acknowledgements
The research reported in this article was supported by the Intramural Research Program of the NIH, the NIA and NIA R01-AG08325 (BLSA); NIA R01-AG88930, R01-AG85477, R01-AG11380 and administrative supplement to R01-AG11380 (CCS); CIHR MOP-42530 (CSHA); NHLBI N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 to N01-HC-85086, N01 HC-15103, N01 HC-55222, U01-HL080295 and NIA R01-AG15928, R01-AG-09556 (CHS); NHLBI N01-HC25195, NIA R01-AG08122, R01-AG16495, and BU ADC P30 AG13846 (FHS); K01-AG023014, K24-AG022035, NIA R01-AG07562 (MoVIES); NHGRI R01-HG02213, NIA K24-AG027841 and R01-AG09029. The authors thank Curtis Meinert and Jim Tonascia for their valuable input. We thank additional study investigators for their involvement including Sudha Seshadri and Rhoda Au (FHS). Particular thanks are due to the participants of each of the individual studies for their cooperation.
Footnotes
Disclosure: Dr. Welsh-Bohmer has a royalty interest in several patents on use of NSAIDs as a prevention/treatment for AD. No other conflicts of interest.
REFERENCES
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's and Dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Szekely CA, Thorne JE, Zandi PP, et al. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 4.Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002 Sep 24;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 5.Cornelius C, Fastbom J, Winblad B, Viitanen M. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology. 2004 May;23:135–143. doi: 10.1159/000075957. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002 Sep 1;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 7.in 't Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 8.Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003 Jun 4;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 9.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005 Jun;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 10.ADAPT Research Group. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007 Apr 25;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 11.Lim GP, Yang F, Chu T, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000 Aug 1;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netland EE, Newton JL, Majocha RE, Tate BA. Indomethacin reverses the microglial response to amyloid beta-protein. Neurobiol Aging. 1998 May;19:201–204. doi: 10.1016/s0197-4580(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 13.Yan Q, Zhang J, Liu H, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003 Aug 20;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vane JR. Introduction: mechanism of action of NSAIDs. Br J Rheumatol. 1996 Apr;35 Suppl 1:1–3. doi: 10.1093/rheumatology/35.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 15.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001 Nov 8;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen JL, Sagi SA, Smith TE, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003 Aug;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbehenn E, Lurie P, Wolfe SM. Letter to HHS Secretary Tommy Thompson that raises ethical concerns about the "Alzheimer's Disease Anti-inflammatory Prevention Trial"(ADAPT) HRG Publication #1637. 2002 [Google Scholar]
- 18.Black SE, Wilcock G, Haworth J, et al. Washington, DC: Society for Neuroscience; 2005. A placebo-controlled, double-blind trial of the selective Abeta42-lowering agent Flurizan in patients with mild to moderate Alzheimer's disease: Efficacy, safety, and follow-on results: Program No. 585.6. [Google Scholar]
- 19.Haag MD, van Oijen M, de Jong FJ, et al. Amyloid beta-42-level lowering non-steroidal anti-inflammatory drugs and the risk of Alzheimer's disease. Madrid, Spain. Presented at the 10th International Conference on Alzheimer's Disease and Related Disorders.2006. [Google Scholar]
- 20.Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID Use and Dementia Risk in the Cardiovascular Health Study: Role of APOE and NSAID Type. Neurology. 2008 Jan 1;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Shock NW, Greulich RC, Andres R, et al. Normal human aging: The Baltimore Longitudinal Study of Aging. Washington DC: USA: Government Printing Office; 1984. [Google Scholar]
- 23.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000 Jun 13;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 24.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002 Jan 22;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 25.Canadian study of health and aging: study methods and prevalence of dementia. CMAJ. 1994 Mar 15;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991 Feb;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 27.Beiser A, D'Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000 Jun 15;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000 Mar 14;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 29.Cox DR. Regression Models and Life Tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 30.The SAS System for Windows V8.2. Cary, NC: SAS Institute; 2001. [Google Scholar]
- 31.Stata Statistical Software: Release 8.0. College Station, Texas: Stata Corporation; 2003. [Google Scholar]
- 32.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson SE, Johansson B, Takkinen S, et al. Does aspirin protect against Alzheimer's dementia? A study in a Swedish population-based sample aged > or =80 years. Eur J Clin Pharmacol. 2003 Aug;59:313–319. doi: 10.1007/s00228-003-0618-y. [DOI] [PubMed] [Google Scholar]
- 34.Weggen S, Eriksen JL, Sagi SA, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003 Aug 22;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- 35.Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer's disease: old and new mechanisms of action. J Neurochem. 2004 Nov;91:521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [DOI] [PubMed] [Google Scholar]
- 36.Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993 Aug;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 37.Reines SA, Block GA, Morris JC, et al. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004 Jan 13;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- 38.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999 Jul 13;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 39.Sainati S, Ingram D, Talwalker S, Geis G. Results of a double-blind, randomized, placebo-controlled study of celecoxib in the treatment of progression of Alzheimer's Disease. Stockholm, Sweden: 6th International Stockholm-Springfield Symposium of Advances in Alzheimer's Therapy; 2000. [Google Scholar]
- 40.Breitner JC, Zandi PP. Do nonsteroidal antiinflammatory drugs reduce the risk of Alzheimer's disease? N Engl J Med. 2001;345:1567–1568. doi: 10.1056/NEJM200111223452110. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Schneider M, Davey SG. Spurious precision? Meta-analysis of observational studies. BMJ. 1998 Jan 10;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992 Jan;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 43.Drazen JM. COX-2 inhibitors--a lesson in unexpected problems. N Engl J Med. 2005 Mar 17;352:1131–1132. doi: 10.1056/NEJMe058038. [DOI] [PubMed] [Google Scholar]
- 44.Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994 Mar 26;343:769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.