Abstract
It is still difficult to successfully cryopreserve in vitro-produced (IVP) swine embryos, as they are sensitive to chilling due to the abundance of intracellular lipids. Mechanical delipation through micromanipulation is successful, but this method increases the potential of pathogen transmission because of the damage inflicted upon the zona pellucida during micromanipulation, and it is labor intensive. Reported here is a method to remove the lipid of IVP porcine embryos, without significantly compromising the zona pellucida, by trypsin treating the embryos or exposing the embryo to a high-osmolality solution to enlarge the perivitelline space so that the lipid could be polarized and separated completely after subsequent centrifugation without micromanipulation. The procedures work both for nuclear transfer-derived embryos and in vitro-fertilized embryos. Both methods provide a high-throughput process that leaves the zona pellucida intact (or relatively intact for the trypsin treatment) to aid in preventing disease transmission. It is also demonstrated that this procedure results in viable piglets, a claim that could not be made in many previous reports. Although the efficiencies of cryopreservation have not been dramatically improved, these procedures allow a single person to process very large numbers of embryos without the necessity of manipulating each individual embryo on a micromanipulator. Such high-throughput processing overcomes the lack of high efficiency (i.e., the system can be overloaded with embryos for transfer to surrogates).
Keywords: cryopreservation, embryo, in vitro fertilization, osmotic stress
Centrifugation in a high osmolality solution without micromanipulation can efficiently separate the lipids in swine embryos to facilitate cryopreservation.
INTRODUCTION
Successful cryopreservation of early mammalian embryos provides opportunities for the preservation of germplasm, and the movement of genetics domestically and internationally. Unfortunately, the pig embryo has been more difficult to cryopreserve than many mammalian embryos. Significant advances have been made toward the successful cryopreservation of pig embryos based on the observation that pig embryos are very sensitive to hypothermic conditions, and that removal of intracellular lipids (delipation) appears to alleviate this sensitivity [1–4]. Most studies have focused on in vivo-produced embryos, as they are considered to be more developmentally competent than in vitro-produced (IVP) embryos. Alternatives to mechanical delipation include destabilizing the cytoskeleton [5] and altering the vitrification conditions [6–9]. Most recently, a solid-surface cryopreservation procedure was developed for the pig that shows great promise for early embryos [9], and has been shown to work on IVP embryos.
IVP embryos are even more difficult to cryopreserve [10]. There are three reports of the cryopreservation of IVP embryos that resulted in piglets: two used mechanical delipation through centrifugation and micromanipulation [10, 11], and the other used solid-surface vitrification [9]. However, mechanical delipation increases the potential of pathogen transmission, because of the damage inflicted upon the zona pellucida during micromanipulation. It is also labor intensive and time consuming. Development of a practical, noninvasive means of lipid removal for high-throughput cryopreservation of IVP porcine embryos is still necessary for research and commercial purposes.
After centrifugation of the pig oocyte or embryo with an intact zona pellucida, the polarized lipid droplets tend to remain connected with the cytoplasm of the oocyte or blastomere of the embryo via a bridge-like structure [12]. The polarized lipid droplets can redistribute into the oocyte or blastomere during subsequent culture or cryopreservation procedures. Thus, in vivo-derived embryos need to be cryopreserved immediately after centrifugation in order to prevent lipid redistribution prior to cryopreservation [13]. If the perivitelline space is enlarged, the bridge-like structure will break after centrifugation, and the lipid droplets will not redistribute into the cytoplasm of the oocyte or the blastomere of the embryo, but will stay within the intact zona pellucida. At least two methods might make the perivitelline space larger. One is to swell the zona pellucida through partial enzymatic digestion (such as trypsin, pronase, etc.). Another option is to condense the volume of the oocyte or embryo by high-osmolality treatment. Two groups [14, 15] demonstrated that partial enzymatic digestion and subsequent centrifugation improved the cryopreservation survival of pig parthenogenetic embryos and hand-made cloned embryos. But neither reported any piglets produced by using a combination of enzymatic digestion and centrifugation.
The lipid droplets are abundant and large in the early-stage porcine embryo, and gradually decline in size and abundance as the embryo advances to and beyond the blastocyst stage [16, 17]. Coincident with reduced lipid content is an increased freezability of late-stage embryos. Late-stage embryos (blastocyst) with low lipid content and smaller-sized lipid droplets survive cryopreservation better than early-stage embryos (two, four, and eight cell) [18–20]. Interestingly, the large lipid droplets in the early-stage embryos are easier to remove by centrifugation than the smaller droplets in the later-stage embryos.
Based on the above observations, we treated IVP (in vitro-matured, in vitro-fertilized) pig embryos with trypsin to swell the zona pellucida, or with high osmolality to shrink the embryo, followed by centrifugation to separate the lipids. These embryos were cultured to the blastocyst stage and subjected to cryopreservation. The high-osmolality treatment was also used for nuclear transfer-derived (NT) transgenic embryos. Live piglets resulted from both trypsin- and high osmolality-treated IVP and NT embryos. The high osmolality treatment, which doesn't compromise the zona pellucida, and high-throughput nature of the procedure make it attractive for possible commercial application. It is anticipated that, with further refinements, swine embryo cryopreservation will no longer be a limitation for commercialization of embryo transfer.
MATERIALS AND METHODS
General Experimental Design
The studies reported here include three sets of experiments. The first set of experiments evaluated cryopreserved IVP blastocysts that were earlier delipated at the two- to four-cell stage using a combination of trypsin and centrifugation treatments. In order to obtain a high percentage of lipid separation, in vitro-matured oocytes were treated with trypsin, so that oocytes rather than embryos could be cryopreserved. Unfortunately, these oocytes parthenogenetically activated after trypsin treatment, and 12.5% (8/64) developed to the blastocyst stage in vitro. Next, we treated cleaved embryos at 28–30 h after insemination to avoid any parthenogenetic activation. IVP embryos were treated with trypsin and trypsin plus centrifugation. The untreated embryos served as control. The first experiment investigated the sensitivity of different stages of embryos (two-, three- to four-, and ≥ four-cell-stage embryos) to the trypsin treatment. The number of nuclei and the percentage that hatched were also determined. Although the trypsin-treated zona pellucida was thinner, these embryos did not hatch at an increased percentage compared to the untreated embryos. We therefore attempted to provide additional assistance: the zona pellucida was either punctured with a micropipette, or completely removed by using pronase after vitrification and warming. Based on the results of the above experiments, the combination of trypsin treatment and pronase treatment was used to cryopreserve IVP embryos prior to embryo transfer.
The second set of experiments evaluated cryopreservation of lipid-separated IVP blastocysts derived from high-osmolality treatment and centrifugation of zygotes. Lipid separation, embryo development, and hatching were measured after exposure of zygotes to different osmolalities (300, 350, 400, 450, and 500 mOsm by adding NaCl) and different centrifugation durations (6, 12, and 20 min). Next, sucrose was used to make media with even higher osmolalities (600 and 800 mOsm), and then lipid separation, embryo development, and hatching were determined. The hatching ability of fresh embryos was not adversely affected, as with trypsin treatment. However, the hatching ability of cryopreserved embryos was obviously compromised by the cryopreservation process. The combination treatments of 350 mOsm plus 6-min centrifugation (350 + 6), 400 + 6, 450 + 12, and 450 + 20 were chosen for cryopreservation prior to embryo transfer. The zona pellucida was removed with pronase before transfer.
The third set of experiments evaluated cryopreservation of lipid-separated NT blastocysts derived from high-osmolality treatment and centrifugation at the one-cell stage. Based on the results of the second set of experiments (described above), and to avoid potentially negative effects of excessively high osmolality (e.g., 450∼500 mOsm) on development of the NT embryo, 350 and 400 mOsm were chosen for the NT embryo treatment. High-osmolality media at 350 or 400 mOsm were made by adding NaCl or sucrose. The NT embryos were treated with these media and centrifuged. In some cases, the lipid separation was not as complete as with the IVP embryos, so the lipid-unseparated embryos were treated and centrifuged a second time. The blastocysts derived from lipid separation were cryopreserved and transferred into surrogates. Before transfer, the zona pellucida was softened or removed with pronase.
Collection of Porcine Oocytes, In Vitro Maturation, and Fertilization
All chemicals were purchased from Sigma (St. Louis, MO), unless otherwise indicated. Ovaries were collected from prepubertal gilts at a local abattoir and transported to the laboratory in 0.9% NaCl at 30–35°C. Cumulus oocyte complexes were aspirated from antral follicles (3–6 mm in diameter) with a syringe and an 18-gauge needle. Approximately 50 oocytes were cultured in 500 μl maturation medium, TCM199 (no. 31100035; Gibco, Grand Island, NY) with 0.1% polyvinyl alcohol (PVA), 3.05 mmol/L glucose, 0.91 mmol/L sodium pyruvate, 0.57 mmol/L cysteine, 0.5 μg/ml luteinizing hormone (LH), 0.5 μg/ml follicle-stimulating hormone (FSH), 10 ng/ml epidermal growth factor (EGF), 75 μg/ml penicillin, and 50 μg/ml streptomycin for 40–44 h at 38.5°C, 5% CO2, in humidified air. After maturation the cumulus cells were removed from the oocytes by vortexing for 4 min in Tyrode lactate (TL)-Hepes [21] supplemented with 0.1% PVA and 0.1% hyaluronidase. Denuded oocytes with a visible polar body were selected in oocyte manipulation medium (stock solution: TCM199, with 0.6 mmol/L NaHCO3, 2.9 mmol/L Hepes, 50 μg/ml penicillin, 60 μg/ml streptomycin, 30 mmol/L NaCl, and 3 mg/ml bovine serum albumin [BSA]). For IVF, 30–40 oocytes with a polar body were transferred to 50-μl microdrops of IVF medium (a modified Tris-buffered medium, with 113.1 mmol/L NaCl, 3 mmol/L KCl, 7.5 mmol/L CaCl2, 5 mmol/L sodium pyruvate, 11 mmol/L glucose, 20 mmol/L Tris, 2 mmol/L caffeine, and 2 mg/ml BSA). One 0.1-ml frozen semen pellet was thawed into 3-ml sperm wash (PBS [Gibco], with 1 mg/ml BSA, 75 μg/ml penicillin, and 50 μg/ml streptomycin) at 39°C, and then layered onto 60% percoll in PBS for centrifugation (600 × g, 10 min). After centrifugation, the pellet was transferred into 10-ml sperm wash and centrifuged (600 × g, 5 min) for a second wash. The supernatant was removed and the sperm pellet was suspended at 0.5 × 106 sperm/ml in IVF medium. The sperm suspension (50 μl) was added to a 50-μl IVF drop with oocytes and cultured at 38.5°C, 5% CO2, in humidified air for 4 h.
Nuclear Transfer
Sow-derived oocytes were purchased from BoMed, Inc. (Madison, WI), and shipped overnight in maturation medium (TCM199, with 2.9 mmol/L Hepes, 10% porcine follicular fluid, 0.5 μg/ml p-FSH, 5 μg/ml insulin, 10 ng/ml EGF, 25 mg/L gentamycin, 0.91 mmol/L pyruvate, 0.57 mmol/L cysteine), and moved to fresh medium after 24 h. After a total 42∼44 h of maturation, the cumulus cells were removed from the oocytes by vortexing for 4 min in TL-Hepes supplemented with 0.1% PVA and 0.1% hyaluronidase. The first polar body and the adjacent cytoplasm from these oocytes were aspirated while in manipulation medium with 7.0 μg/ml cytochalasin B. A donor cell was then transferred into the perivitelline space. Fusion and activation were accomplished simultaneously with two 30-μsec pulses of 1.2 kV/cm in fusion/activation medium (0.3 M mannitol, 1.0 mM CaCl2, 0.1 mM MgCl2, 0.5 mM Hepes) or, alternatively, in a fusion-only medium with a lower concentration of calcium (0.3 M mannitol, 0.1 mM CaCl2, 0.1 mM MgCl2, and 0.5 mM Hepes was used for fusion, then the fused oocytes were exposed to 200 μM thimerosal for 10 min in the dark, and then 8 mM dithiothreitol for 30 min to accomplish activation [22]).
Culture of IVP Embryos
The IVP and NT embryos were cultured in PZM3 (108.0 mmol/L NaCl, 10.0 mmol/L KCl, 0.35 mmol/L KH2PO4, 0.4 mmol/L MgSO4 · 7H2O, 25.07 mmol/L NaHCO3, 0.2 mmol/L Na-pyruvate, 2.0 mmol/L Ca(lactate)2 · 5H2O, 1.0 mmol/L glutamine, 5.0 mmol/L hypotaurine, 20 ml/L Eagle basal medium amino acid solution, 10 ml/L modified Eagle medium amino acid solution, 0.05 mg/ml gentamicin, 3 mg/ml BSA, 288 ± 2 mOsm, pH 7.3 ± 0.2) at 38.5°C, 5% CO2 in humidified air.
Trypsin and High-Osmolality Treatment of IVP and NT Embryos
To make high-osmolality medium, the oocyte manipulation medium stock was used as the basic medium. The osmolality of this stock was 300–310 mOsm. Adding 0.016 g NaCl to 10 ml of this stock increased the osmolality 50 mOsm. When sucrose was used to increase osmolality, 1 M sucrose (made with the oocyte manipulation stock as solvent) was diluted to make 350-, 400-, 500-, 600-, and 800- to 850-mOsm solutions. Osmolality was verified by using a vapor pressure osmometer (Model 5520; Wescor, Logan, UT).
The IVP embryos were treated with trypsin or high osmolality. For the trypsin treatment, cleaved embryos were selected at 28–30 h after insemination and treated with 2%–4% trypsin (T1426; Sigma) in PBS for 2–15 min. After trypsin treatment, the embryos were centrifuged at 13 400 × g for 5 min in oocyte manipulation medium with 7.0 μg/ml cytochalasin B and then cultured in PZM3. To determine the number of nuclei in Day 6 (D6) blastocysts, 4 μg/ml Hoechst 33342 was used to stain the nuclei, which were examined by epifluorescent microscopy. As an additional indicator of embryo viability, D5 and D6 blastocysts were moved to fresh PZM3 or Buffalo rat liver (BRL) cell-conditioned medium (TCM199, with 26.2 mmol/L NaHCO3, 0.2 mmol/L Na-Pyruvate, and 10% fetal bovine serum [FBS]) to investigate the ability of embryos to hatch and attach to the bottom of the dish. For the high osmolality treatment, at 18–20 h after insemination, the embryos were equilibrated in 2 ml manipulation medium with different osmolalities (300, 350, 400, 450, and 500 mOsm), 7.0 μg/ml cytochalasin B, and 0.1 mg/ml BSA for 6 min, and then centrifuged in 0.35 ml of the same medium in 1.5 ml eppendorf tubes (30–50 embryos were put in each tube) for 6, 12, or 20 min at 13 400 × g. A 6-min equilibration period was chosen because it has been shown previously to be sufficient for sperm [23]. After centrifugation, the embryos were transferred into 2 ml oocyte manipulation medium, washed in PZM3 three times, and then cultured in PZM3. After 12 h, lipid separation was checked, and embryos in which the lipids had not separated were removed, while the lipid-separated embryos were cultured in PZM3 to check the blastocyst and hatching percentages.
The NT embryos were treated with 350 or 400 mOsm by adding NaCl or sucrose. PVA at 0.1 mg/ml concentration was added instead of BSA when sucrose was used. The NT embryos were treated the same as the IVP embryos 14–18 h after fusion/activation, and then cultured in PZM3 medium. After 12-h culture, the lipid separation was checked, and the embryos in which the lipids had not separated were removed and treated again with medium at the same osmolality and checked again for lipid separation 12 h after treatment. The lipid-separated embryos from both the first and second high-osmolality treatments were cultured in PZM3. Interestingly, there appeared to be no leakage through the small hole made in the zona pellucida by the transfer pipette.
Vitrification and Embryo Transfer
Vitrification of embryos was carried out by using the open pulled straw (OPS) method. The OPS straws were purchased from LEC Instruments P/L. All solutions used during vitrification were prepared with holding medium (oocyte manipulation medium with 20% FCS instead of 3 mg/ml BSA). D5 and D6 IVP and NT blastocysts (day of NT or insemination was considered D0) were placed in equilibration solution (10% ethylene glycol, 10% dimethyl sulfoxide [DMSO]) for 2 min, followed by exposure to vitrification solution (20% ethylene glycol, 20% DMSO). Embryos were loaded into an OPS and immediately plunged into liquid nitrogen. All processes before plunging into nitrogen were conducted on a warm stage at 38.58°C. The time from exposure to the vitrification solution to plunging was 25–30 sec.
For embryo warming, both cryopreserved IVP and NT embryos were warmed by immersing the end of the OPS into 0.3 M sucrose for 5 min at 38.5°C, then transferring the thawed embryos to 0.2 M sucrose for 5 min, and then to holding medium for 5 min. The warmed embryos were cultured in BRL cell-conditioned medium to check reexpansion or used for the assisted hatching experiment or embryo transfer after softening or removing zona pellucida with 0.5% pronase.
For assisted hatching, embryos were either punctured by manipulation by using a 20-μm outer diameter pipette, or stripped of their zona pellucida by exposure to 0.5% pronase for 20–30 sec and then cultured in BRL cell-conditioned TCM199. To soften or remove the zona pellucida before transfer, the embryos were treated in 0.5% pronase (in PBS) for 10–15 sec (to soften) or 20–30 sec (to remove), and then transferred into holding medium immediately before the zona pellucida completely disappeared. The blastocysts with the zona pellucida softened or removed were surgically transferred into the ampullary-isthmic junction of the oviduct of a surrogate 4 or 5 days after observed estrus. All experiments with animals were approved by the Institutional Animal Care and Use Committee.
Statistic Analysis
All data were subjected to ANOVA by using the PROC GLM commands in SAS (Sas Institute, Cary, NC). The main effects of trypsin treatment, centrifugation time, zona pellucida removal or puncture, osmolality, and solute on percent lipid separation, percent blastocyst, percent hatching, the percentage of embryo attachment on the bottom of dish after hatching, and the number of nuclei in the blastocysts were tested as appropriate in each experiment. Percentage data were arcsine transformed prior to analysis. Differences between treatment groups were assessed via post hoc, pairwise comparisons of least squares mean values. Significance was assigned to P values less than 0.05.
RESULTS
Cryopreservation of Lipid-Separated IVP Blastocysts Derived from Trypsin-Treated and Centrifuged Cleavage-Stage Embryos
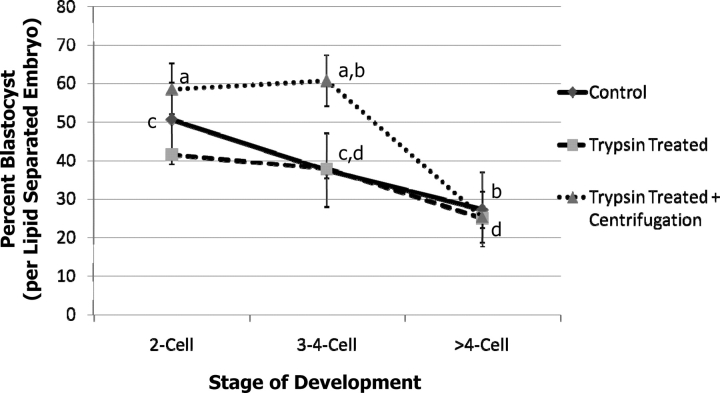
Overall, while neither trypsin treatment nor trypsin treatment plus centrifugation affected the percentage of embryos that developed to the blastocyst stage or the number of nuclei in the blastocysts (34.2 ± 2.7 vs. 37.7 ± 2.7, trypsin + centrifugation and control, respectively) there was a significant difference in development to the blastocyst stage from two-cell versus more than four-cell stage embryos for two of the treatments (Fig. 1; Supplemental Table S1 available at www.biolreprod.org). This may be because those embryos that are already beyond the four-cell stage at 28–30 h postinsemination may be a result of fragmentation, and are thus of lower quality. In addition, the percent blastocyst from the trypsin plus centrifugation treatment for the three- to four-cell-stage embryos was higher than the controls or trypsin-only treatments. While the trypsin-treated zona pellucida was thinner, these embryos did not hatch when cultured in PZM3. BRL cell-conditioned TCM199 with 10% FBS improved the hatching percentage of trypsin-treated and centrifuged embryos to 15.4%, which was still lower than the controls (61.3%), but higher than PZM3 alone (0%). The only outgrowth and attachment that was observed was with conditioned TCM199 in the controls (8% of those that hatched) (Supplemental Table S2).
FIG. 1.
Development of IVP embryos after trypsin treatment or trypsin treatment plus centrifugation. Different superscripts within a treatment are different: a,b and c,d (P < 0.01). Summary of four replications (n = 701). Values shown are means ± SEM. There was no difference in the trypsin-treated embryos.
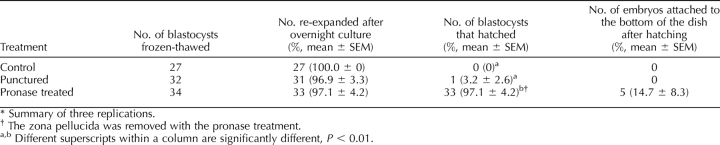
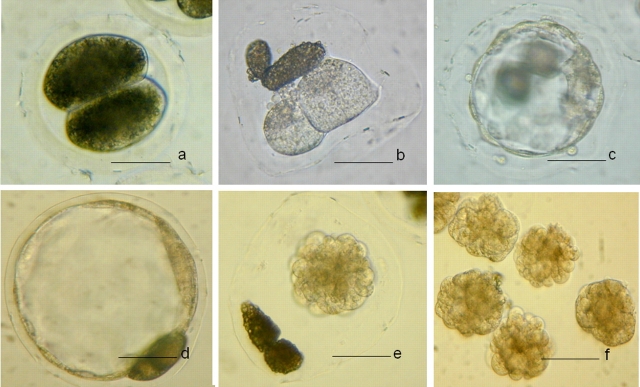
After vitrification and warming, good-quality embryos (embryos that appeared to be morphologically normal) were selected for an assisted hatching experiment. The embryos had their zona pellucida punctured, were treated with pronase, or were used as controls. The percentage that reexpanded was not different for the different treatments (96%–100%). Only those embryos that had their zona pellucida removed with pronase attached to the dish, and only one embryo hatched of the 32 that had their zona pellucida punctured (Table 1). After zona removal, 14.7% of the vitrified-warmed embryos attached on the bottom of the dish and continued to grow in BRL cell-conditioned medium. Developmental competence was determined by transfer to four surrogates; two surrogates received 50 vitrified-warmed and zona-removed embryos, and another two received 25 or 24 vitrified-warmed and zona-removed embryos. One surrogate from each group maintained the pregnancy and delivered nine piglets (eight males and one female) and five piglets (all of them were male), respectively (Supplemental Table S3). Photos of embryos before and after trypsin treatment or vitrification are shown in Figure 2.
TABLE 1.
Hatching ability of trypsin- and centrifugation-treated blastocysts after vitrification-warming and culture in BRL cell conditioned TCM199.*
FIG. 2.
Trypsin treated embryos. a) Before trypsin treatment. b) After trypsin treatment and centrifugation. c and d) Fresh blastocysts. e) Collapsed blastocyst after vitrification and warming. f) Collapsed blastocysts after zona removal. Bar = 50 μm.
Cryopreservation of Lipid-Separated IVP Blastocysts Derived from Zygotes Treated with High-Osmolality Media and Centrifugation
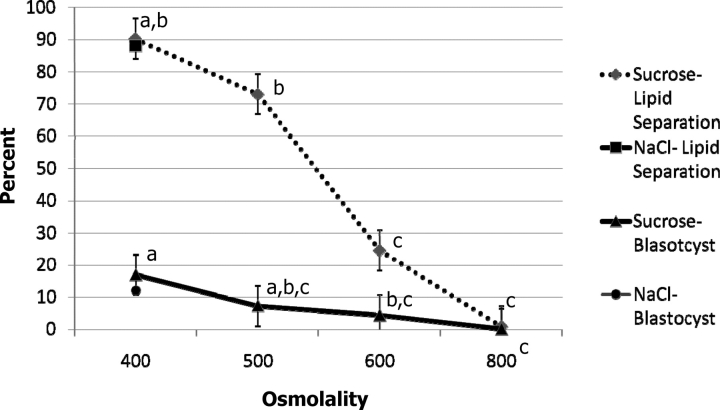
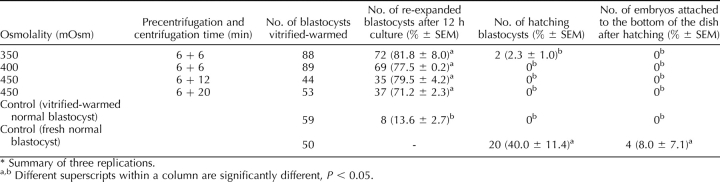
Both increasing osmolality and extending the centrifugation time enhanced the percentage of lipid separation (Fig. 3; Supplemental Table S4). For 6-, 12-, and 20-min centrifugation, the percentage of delipated embryos was the highest, at 450, 500, and 400 mOsm, respectively. In general, the lipid separation was more complete if the centrifugation time was longer (e.g., 20 min was more effective than 6 min when the osmolality was 400 or lower). These embryos hatched from 0% to 4.2% per treatment. Even higher osmolality (600 mOsm), achieved by using sucrose in place of NaCl, could not improve the lipid separation. In fact, the opposite was true, as the percentage of embryos in which lipid separation occurred decreased as the osmolality was increased, and there was almost no separation when the osmolality reached 800 mOsm (Fig. 4; Supplemental Table S5). The ability of lipid-separated embryos to develop to the blastocyst stage was adversely affected by 600 mOsm or higher osmolality. The general efficiencies (how many lipid-separated blastocysts could be produced from the initial oocytes) were lower when 300 mOsm and 6 min centrifugation, 500 mOsm and 20 min centrifugation (Supplemental Table S4) and higher than 500 mOsm treatments (Supplemental Table S5) were applied. The other treatments had similar efficiencies of blastocyst production compared with the controls. Although the blastocysts derived from different osmolality and centrifugation treatment survived cryopreservation, the hatching ability was compromised by the cryopreservation process. Only 2.3% of the blastocysts derived from the 350 + 6 treatment hatched after vitrification and warming, and none of the blastocysts derived from 400–450 mOsm treatment hatched, while 40% of the controls hatched, and 8% attached to the dish (Table 2).
FIG. 3.
Lipid separation of IVP embryos after treatment with different osmolalities and different centrifugation times at 18–20 h after insemination. Error bars represent SEM. Details of significant differences can be found in Supplemental Table S4.
FIG. 4.
Lipid separation and development of IVP embryos after treatment with different osmolalities and centrifugation for 6 min. In this experiment, 400 mOsm was used for the NaCl lipid separation and development to the blastocyst stage (n = 721, three replications). Details of significant differences can be found in Supplemental Table S5. Different footnote symbols (a, b, c) within a treatment are different P < 0.05.
TABLE 2.
Re-expansion and hatching of high osmolality-treated embryos after vitrification-warming and culture in BRL cell conditioned medium.*
One hundred vitrified-warmed zona-intact embryos were transferred to two surrogates (50 embryos/surrogate), in which none established a pregnancy. Subsequently, 175 vitrified-warmed embryos with zona removed after warming were transferred to seven surrogates (25 embryos/surrogate). Three surrogates established pregnancies and farrowed, producing normal offspring (Table 3). The conclusions from this section are that zona removal is prerequisite for establishing pregnancy with cryopreserved-warmed porcine IVP embryos. In addition, centrifugation from 6 to 20 min and exposure to osmolalities ranging from 350 to 450 mOsm (Fig. 5) is compatible with term development. Thus, a combination of osmolarity and centrifugation in this range will work for producing pig embryos for cryopreservation, as shown in Figure 6.
TABLE 3.
Transfer of IVP embryos derived from high osmolality treatment and centrifugation after vitrification and warming.*
FIG. 5.
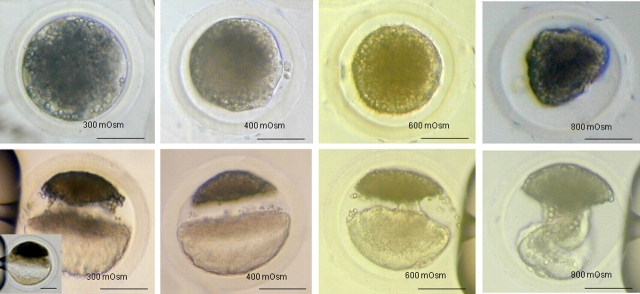
Zygotes treated with different osmolarities. Row 1: immediately after high-osmolality treatment. Row 2: immediately after centrifugation. The bridge-like structure could be seen in the 300-mOsm group (and insert). Complete lipid separation was observed in oocytes treated with 400 mOsm. Large bridge-like structures were present in oocytes treated with 600 or 800 mOsm and centrifuged (600 and 800 mOsm achieved with sucrose). Bar = 50 μm.
FIG. 6.
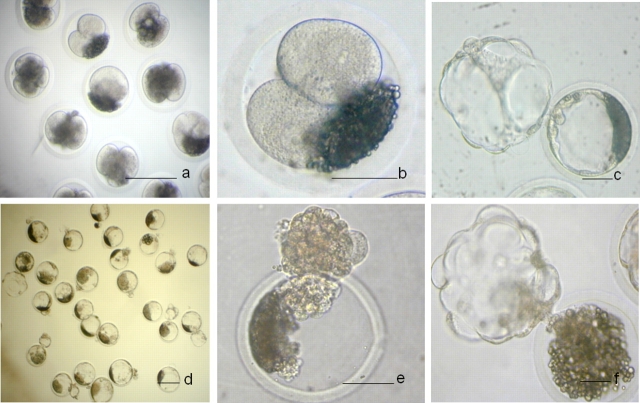
IVP embryos after high-osmolality treatment and centrifugation. a) After high-osmolality treatment and several-hour culture. b and c) After high-osmolality treatment and culture to blastocyst. d) After warming. e) After removal of the zona pellucida. f) Reexpansion after in vitro culture in BRL cell-conditioned medium. Bar = 50 μm (a, b, d, and e) and 150 μm (c and f).
Cryopreservation of Lipid-Separated NT Blastocysts Derived from High-Osmolality Treatment and Centrifugation
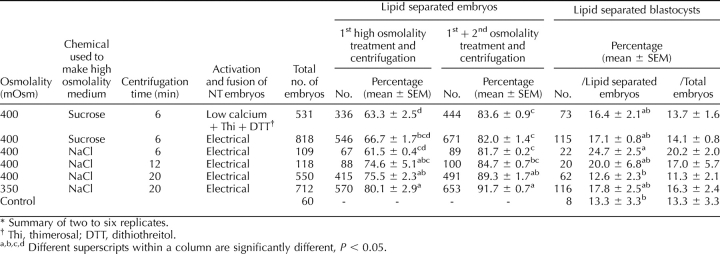
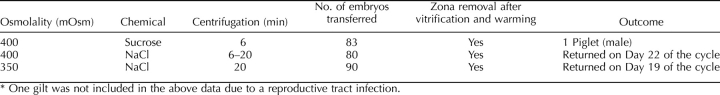
The percentage of NT embryos with complete lipid separation was numerically lower than that of the IVP embryos. Extending the centrifugation time enhanced the lipid separation, but increasing the osmolality did not. By treating the lipid-unseparated embryos with high osmolality for a second time, the total lipid separation from both first and second treatments tended to be similar between the different osmolality and centrifugation treatments (Table 4). The highest percentage of lipid separation was obtained from 350 mOsm and 20-min centrifugation. Sucrose and NaCl were not different in regard to lipid separation when they were used to make high-osmolality medium (Table 4). Three embryo transfers were performed; for each transfer, 80∼90 embryos with the zona pellucida softened or removed by pronase treatment were transferred into the surrogate. One of the three established a pregnancy, and one delivered a single male piglet (Table 5). The photos of embryos after high-osmolality treatment, and before and after vitrification, are shown in Figure 7.
TABLE 4.
Lipid separation of NT embryos after treatment with different osmolalities and centrifugation times at 14–18 h after fusion.*
TABLE 5.
Transfer of NT embryos after vitrification and warming.*
FIG. 7.
Nuclear transfer embryos after high-osmolality treatment and centrifugation. a and b) After high-osmolality treatment and several-hour culture. c and d) After high-osmolality treatment and culture to blastocyst stage. e) After warming. f) Reexpanded after in vitro culture in BRL cell-conditioned medium. Bar = 50 μm (b, c, e, and f) and 150 μm (a and d).
DISCUSSION
Pigs are an increasingly important model for studying and understanding human medicine, in addition to their extensive role in agriculture. In vitro production of pig embryos or by nuclear transfer has been used to create disease models or potential organ donors for xenotransplantation [24–26]. As a result, the demand for effective cryopreservation of IVP embryos has dramatically increased. However, a practical and efficient protocol for this purpose is currently unavailable. Here, we report two practical protocols to produce pig embryos in vitro, which can be successfully cryopreserved. They are trypsin or high-osmolality treatment of IVP embryos and subsequent high-speed centrifugation. Both methods can enlarge the periviteline space by partial zona pellucida digestion (trypsin) or oocyte cytoplasmic condensation (high osmolality). With centrifugation, the lipids could be separated completely from the cytoplasm. Both methods provide a high-throughput process that leaves the zona pellucida intact (or relatively intact, as is the case for the trypsin treatment) to aid in preventing disease transmission. It is also demonstrated that this procedure results in viable piglets, a claim that could not be made in many previous reports. Although the efficiencies of cryopreservation have not been dramatically improved, these procedures allow a single person to process very large numbers of embryos without the necessity of manipulating each individual embryo on a micromanipulator. A summary of the data from the first and second sets of experiments (except for those embryos in which the zona pellucida was not removed prior to embryo transfer) shows 324 embryos transferred to 11 surrogates, resulting in 5 pregnancies and 26 offspring. The time required to process 324 embryos is 30–40 min. If the previous procedures that require micromanipulation were used, it would require not only the expensive equipment and the specialized training, but also about 5 additional hours of labor. Such high-throughput processing overcomes the lack of high efficiency (i.e., the system can be overloaded with embryos for transfer to surrogates).
Lipids could be separated without compromising development to the blastocyst stage with an osmolality of 450 mOsm and 6-min centrifugation. By increasing the centrifugation time to 20 min, 350 mOsm resulted in more than 90% of the embryos having lipid separation. A number of the different combinations of osmolality and duration of centrifugation resulted in not only blastocyst-stage embryos, but also normal offspring. For practical applications, we suggest using the high-osmolality treatment in place of trypsin because of the variability of the trypsin, as described below.
Interestingly, for the IVP embryos, 400 mOsm appeared to be an optimal osmolality for lipid separation, but it did not work as well on NT embryos. There were many differences between IVP and NT embryos. The first was that the NT embryos had a smaller volume of cytoplasm, since some cytoplasm was removed during enucleation. The osmolality, therefore, did not need to go to as high as 400 mOsm to provide extra space in the zona pellucida. However, the 350 mOsm and 20-min centrifugation resulted in a numerically lower percentage of lipid separation in the NT group as compared with the IVP group. Interestingly when we tried 300 mOsm (unpublished data), the lipid separation was similar to that for 350 mOsm. A second round of treatments for those embryos that did not achieve complete lipid separation 12 h after the first treatment improved the overall percentage of NT embryos that had complete separation. A second difference between the IVP and NT embryos was that they were derived from gilt and sow oocytes, respectively, and cultured in different maturation media. The different ovary sources and different maturation culture conditions could result in different cytoplasmic properties, and thus affect the results. A third difference is that the physical characteristics of the cytoplasm or plasma membrane may be different in the NT embryo, because the parthenogenetic activation may not exactly replicate the events that normally occur at fertilization.
It cannot be categorically stated that a larger perivitelline space will necessarily result in a higher percentage of lipid separation. The lower cytoplasmic volume and larger perivitelline space after 600 mOsm treatment had only 24.5% lipid separation. When the osmolality reached 800 mOsm, almost none of the lipids could be separated. During the centrifugation, the lipid fraction will rise to the top, due to its lower density, while the nonlipid fraction of the cytoplasm will stay at the bottom, due its higher density. The high osmolality (600 or 800 mOsm) caused an extreme change in the properties of the oocyte cytoplasm, so that the cytoplasm did not separate.
There may be a positive effect of higher osmolality on early embryo development [27], as the embryos appear to have a reduced level of apoptosis through regulation of Bax-α/Bcl-xl gene expression [28]. In our study, the embryos were exposed not only to high osmolality, but also to high-speed centrifugation. While development was not improved above that of controls when the IVP embryos were treated with high-osmolality medium followed by centrifugation, there was an improvement for the NT embryos (400 mOsm [by adding NaCl] significantly improved the NT embryo development). However, development to term in high-osmolality treatment was not as high as anticipated.
Two groups [29, 30] found that the percent hatching of in vivo-derived pig morula/early blastocysts that had been centrifuged, vitrified-warmed, and cultured in vitro was very low. Initially, they wondered if the polarized material left in contact with the blastomere during vitrification may have had some inhibitory effect following warming and recovery. Subsequent experiments indicated that the presence of the lipid material in the perivitelline space was not the reason for difficulty in hatching. Either the zona pellucida was altered by the conditions, or the embryo was compromised such that it could not hatch. Removal or thinning of the zona pellucida after warming improved the survival rate of cyopreserved embryo, and resulted in live piglets [30]. Our results indicate that both trypsin treatment and cryopreservation itself can harden the zona pellucida, so the removal of the zona pellucida after warming and before transfer is necessary for both trypsin and high-osmolarity treatments.
The enzyme digestion method used a partial digestion of the zona pellucida with trypsin [14] or pronase [15]. Pronase has been shown to not harm porcine embryos if used appropriately [31, 32], and has been used for assisted hatching of embryos, but we found that it was very harsh, and resulted in rapid zona pellucida digestion. Previous work [14] in addition to our studies indicates that trypsin is also compatible with pig embryo development, as we were able to obtain blastocyst-stage embryos that resulted in piglets. However, trypsin treatment has some disadvantages when it is used for lipid separation. We found that trypsin treatment can elicit parthenogenetic activation of oocytes; thus, to avoid parthenogenetic activation, treatment of oocytes with trypsin cannot be done before or immediately after fertilization. In addition, the trypsin treatment did not work consistently. The effect of the trypsin treatment depended on the batch of trypsin powder. As such, the embryos had to be observed when they were being exposed to the trypsin solution, and the treatment worked best if the oocytes were treated in small groups so that they could be observed and the digestion monitored. However, even with these caveats, pregnancies and offspring can be produced with these procedures.
The procedures described here require that the zona pellucida be removed prior to embryo transfer. An intact zona pellucida is beneficial for preventing disease transmission. The high-osmolality treatments result in an intact zona pellucida throughout the cryopreservation process; thus, after cryopreservation, it can be confirmed that the zona pellucida is intact. Trypsin treatment results in a swelling of the zona pellucida; additional experiments are required to determine if the zona pellucida is compromised to such an extent as to allow pathogen entry. In either case—high-osmolality or trypsin treatment—the integrity of the zona pellucida can be determined just after or prior to transfer.
In preliminary experiments, BSA was added at 3 mg/ml to the media with high osmolalities. However, after several days of storage at 4°C, the media did not work well for lipid separation (i.e., the percent lipid separation was decreased). A possible reason could be that the highly charged proteins from the BSA chelate ions in the solution alter the osmolality [33]. Next, we decreased the BSA concentration to 0.1 mg/ml or replaced BSA with 0.1 mg/ml PVA. Both PVA and a lower concentration of BSA worked stably for separating the lipids. The embryos could be treated in large groups, several hundred at a time.
In 2006, we reported two litters of transgenic piglets produced from cryopreserved NT embryos [10]. Subsequently, Nagashima et al. [11] reported piglets produced from cryopreserved IVP embryos. Here, we report more practical noninvasive delipation methods (e.g., no micromanipulation is required), thus leaving the zona pellucida intact by high-osmolality treatment plus centrifugation. The results reported here establish a procedure that may have important implications for the cryopreservation of porcine IVP embryos on a commercial scale.
Acknowledgments
We would like to acknowledge Lonnie Dowell for helping to manage the pigs at the farm, Jianguo Zhao for helping with the nuclear transfer, and the people who drove to the slaughterhouse to retrieve ovaries.
Footnotes
1Supported in part by National Institutes of Health, National Center for Research Resources grants R01 RR13438 and U42 RR018877, and by Food for the 21st Century.
REFERENCES
- Polge C, Wilmut I, Rowson LEA.The low temperature preservation of cow, sheep, and pig embryos. Cryobiology 1974; 11: 560 [Google Scholar]
- Wilmut I.The low temperature preservation of mammalina embryos. J Reprod Fertil 1972; 31: 513–514. [DOI] [PubMed] [Google Scholar]
- Dobrinsky JR.Cellular approach to cryopreservation of embryos. Theriogenology 1996; 45: 17–26. [Google Scholar]
- Nagashima H, Kashiwazaki N, Ashman RJ, Grupen CG, Seamark RF, Nottle MB.Removal of cytoplasmic lipid enhances the tolerance of porcine embryos to chilling. Biol Reprod 1994; 51: 618–622. [DOI] [PubMed] [Google Scholar]
- Dobrinsky JR, Pursel VG, Long CR, Johnson LA.Birth of piglets after transfer of embryos cryopreserved by cytoskeletal stabilization and vitrification. Biol Reprod 2000; 62: 564–570. [DOI] [PubMed] [Google Scholar]
- Berthelot F, Martinat-Botte F, Perreau C, Terqui M.Birth of piglets after OPS vitrification and transfer of compacted morula stage embryos with intact zona pellucida. Reprod Nutr Dev 2001; 41: 267–272. [DOI] [PubMed] [Google Scholar]
- Beebe LFS, Cameron RDA, Blackshaw AW, Higgins A, Nottle MB.Piglets born from centrifuged and vitrified early and peri-hatching blastocysts. Theriogenology 2002; 57: 2155–2165. [DOI] [PubMed] [Google Scholar]
- Misumi K, Suzuki M, Sato S, Saito N.Successful production of piglets derived from vitrified morulae and early blastocysts using a microdroplet method. Theriogenology 2003; 60: 253–260. [DOI] [PubMed] [Google Scholar]
- Somfai T, Ozawa M, Noguchi J, Kaneko H, Nakai M, Maedomari N, Ito J, Kashiwazaki N, Nagai T, Kikuchi K.Live piglets derived from in vitro-produced zygotes vitrified at the pronuclear stage. Biol Reprod 2009; 80: 42–49. [DOI] [PubMed] [Google Scholar]
- Li R, Lai L, Wax D, Hao Y, Murphy CN, Rieke A, Samuel M, Linville ML, Korte SW, Evans RW, Turk JR, Kang JX, et al. Cloned transgenic swine via in vitro production and cryopreservation. Biol Reprod 2006; 75: 226–230. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Hiruma K, Saito H, Tomii R, Ueno S, Nakayama N, Matsunari H, Kurome M.Production of live piglets following cryopreservation of embryos derived from in vitro-matured oocytes. Biol Reprod 2007; 76: 900–905. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Cameron RD, Kuwayama M, Young M, Beebe L, Blackshaw AW.Survival of porcine delipated oocytes and embryos after cryopreservation by freezing or vitrification. J Reprod Dev 1999; 45: 167–176. [Google Scholar]
- Cameron RDA, Beebe LFS, Blackshaw AW, Keates HL.Farrowing rates and litter size following transfer of vitrified porcine embryos into a commercial swine herd. Theriogenology 2004; 61: 1533–1543. [DOI] [PubMed] [Google Scholar]
- Esaki R, Ueda H, Kurome M, Hirakawa K, Tomii R, Yoshioka H, Ushijima H, Kuwayama M, Nagashima H.Cryopreservation of porcine embryos derived from in vitro-matured oocytes. Biol Reprod 2004; 71: 432–437. [DOI] [PubMed] [Google Scholar]
- Du Y, Zhang Y, Li J, Kragh PM, Kuwayama M, Ieda S, Zhang X, Schmidt M, Bogh IB, Purup S, Pedersen AM, Villemoes K, et al. Simplified cryopreservation of porcine cloned blastocysts. Cryobiology 2007; 54: 181–187. [DOI] [PubMed] [Google Scholar]
- Norberg HS.Ultrastructural aspects of the preattached pig embryo: cleavage and early blastocyst stage. Z Anat Entwicklungsgesch 1973; 143: 95–114. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Ekwall H, Tienthai P, Kawai Y, Noguchi J, Kaneko H, Rodriguez-Martinez H.Morphological features of lipid droplet transition during porcine oocyte fertilisation and early embryonic development to blastocyst in vivo and in vitro. Zygote 2002; 10: 355–366. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Kato Y, Yamakawa H, Matsumoto T, Ogwa S.Changes in freezing tolerance of pig blastocysts in peri-hatching stages. Jpn J Anim Reprod 1989; 35: 130–134. [Google Scholar]
- Nagashima H, Yamakawa H, Niemann H.Freezability of porcine blastocysts at different peri-hatching stages. Theriogenology 1992; 37: 839–850. [DOI] [PubMed] [Google Scholar]
- Li R, Hosoe M, Shioya Y, Bou S.The preliminary research on freezing viability of bovine in vitro fertilized embryos. Chinese J Scientia Agricultura Sinica 2002; 35: 1125–1129. [Google Scholar]
- Tao T, Machaty Z, Boquest AC, Day BN, Prather RS.Development of pig embryos reconstructed by microinjection of cultured fetal fibroblast cells into in vitro matured oocytes. Anim Reprod Sci 1999; 56: 133–141. [DOI] [PubMed] [Google Scholar]
- Machaty Z, Wang WH, Day BN, Prather RS.Complete activation of porcine oocytes induced by the sulfhydryl reagent, thimerosal. Biol Reprod 1997; 57: 1123–1127. [DOI] [PubMed] [Google Scholar]
- Walters EM, Men H, Agca Y, Mullen SF, Critser ES, Critser JK.Osmotic tolerance of mouse spermatozoa from various genetic backgrounds: acrosome integrity, membrane integrity, and maintenance of motility. Cryobiology 2005; 50: 193–205. [DOI] [PubMed] [Google Scholar]
- Niemann H, Rath D, Wrenzycki C.Advances in biotechnology: new tools in future pig production for agriculture and biomedicine. Reprod Domest Anim 2003; 38: 82–89. [DOI] [PubMed] [Google Scholar]
- Prather RS, Hawley RJ, Carter DB, Lai L, Greenstein JL.Transgenic swine for biomedicine and agriculture. Theriogenology 2003; 59: 115–123. [DOI] [PubMed] [Google Scholar]
- Lai LX, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295: 1089–1092. [DOI] [PubMed] [Google Scholar]
- Li RF, Whitworth K, Lai LX, Wax D, Spate L, Murphy CN, Rieke A, Isom C, Hao YH, Zhong ZS, Katayama M, Schatten H, et al. Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol Reprod Dev 2007; 74: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IS, Park MR, Moon HJ, Shim JH, Kim DH, Yang BC, Ko YG, Yang BS, Cheong HT, Im GS.Osmolarity at early culture stage affects development and expression of apoptosis related genes (Bax-a and Bcl-xl) in preimplantation porcine NT embryos. Mol Reprod Dev 2008; 75: 464–471. [DOI] [PubMed] [Google Scholar]
- Dobrinsky JR.Advancements in cryopreservation of domestic animal embryos. Theriogenology 2002; 57: 285–302. [DOI] [PubMed] [Google Scholar]
- Beebe LFS, Cameron RDA, Blackshaw AW, Keates HL, Nottle MB.Assisted hatching improves post-warming in vitro viability of vitrified porcine embryos. Reprod Fertil Dev 2004; 16: 164 [Google Scholar]
- Du Y, Kragh PM, Zhang X, Purup S, Yang H, Bolund L, Vajta G.High overall efficiency of porcine handmade cloning (HMC) combining partial zona digestion and oocyte trisection with sequential culture. Cloning Stem Cells 2005; 7: 199–205. [DOI] [PubMed] [Google Scholar]
- Kragh PM, Du Y, Corydon TJ, Purup S, Bolund L, Vajta G.Efficient in vitro production of porcine blastocysts by handmade cloning with a combined electrical and chemical activation. Theriogenology 2005; 64: 1536–1545. [DOI] [PubMed] [Google Scholar]
- Collins JL, Baltz JM.Estimates of mouse oviductal fluid tonicity based on osmotic responses of embryos. Biol Reprod 1999; 60: 1188–1193. [DOI] [PubMed] [Google Scholar]