Abstract
Background
The objectives of this analysis were to compare various measures associated with lymph node (LN) dissection and to identify threshold values associated with disease-specific survival (DSS) outcomes in patients with melanoma.
Methods
Patients with node positive melanoma who underwent therapeutic LN dissection of the neck, axilla, and inguinal region were identified from the SEER database (1988–2005). We performed Cox multivariate analyses to determine the impact of the total number of LNs removed, number of negative LNs removed, and LN ratio on DSS. Multivariate cut-point analyses were conducted for each anatomic region to identify the threshold values associated with the largest improvement in DSS.
Results
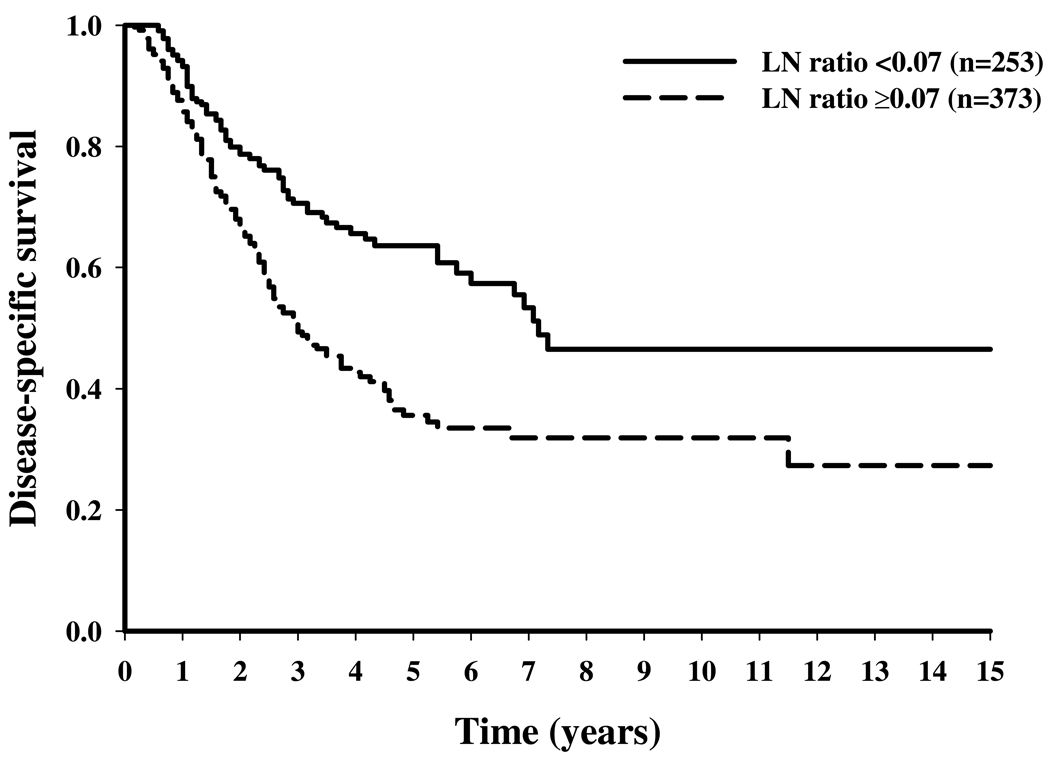
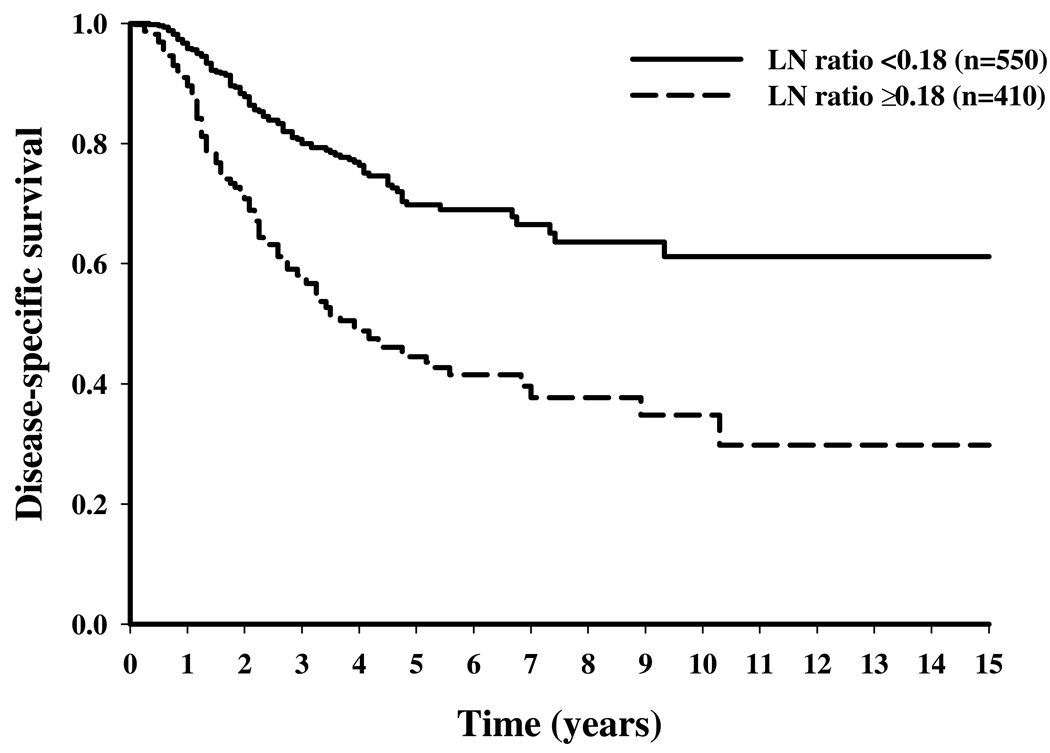
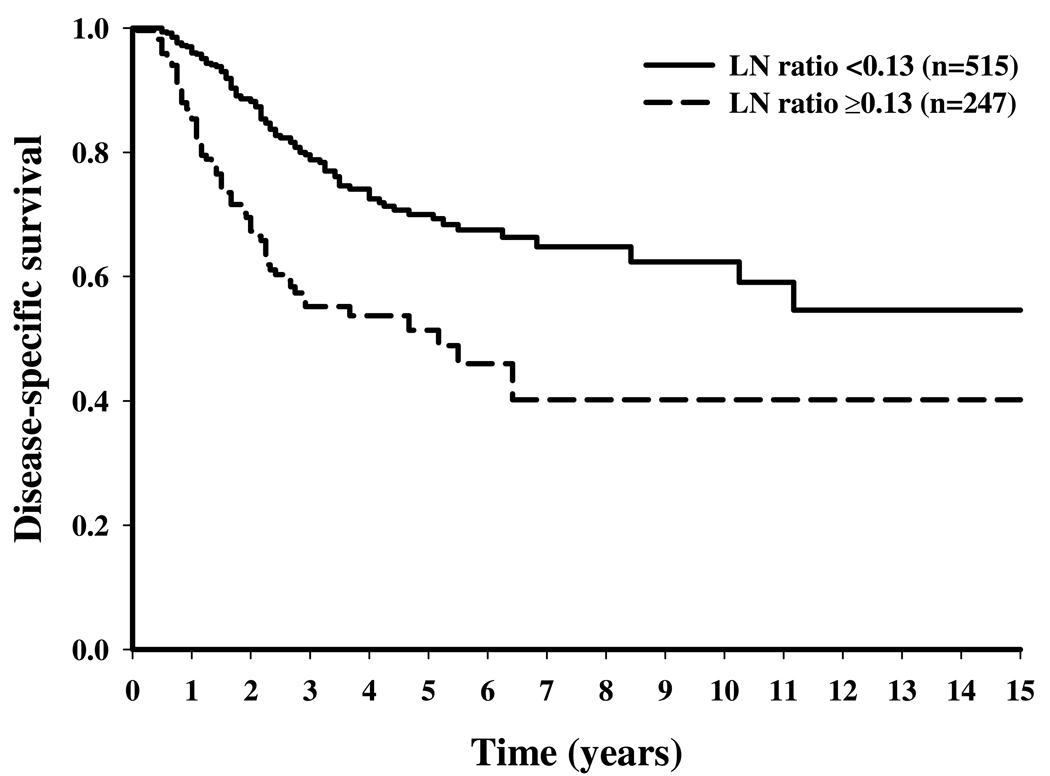
The LN ratio was significantly associated with DSS for all LN regions. The LN ratio thresholds resulting in the greatest difference in 5-year DSS were 0.07, 0.13, and 0.18 for neck, axillary, and inguinal regions, respectively, corresponding to 15, 8, and 6 LNs removed per positive node. After adjustment for other clinicopathologic factors, the hazard ratios (HRs) were 0.53 (95% confidence intervals (CIs), 0.40 to 0.71) in the neck, 0.52 (95% CI, 0.42 to 0.65) in the axillary, and 0.47 (95% CI, 0.36 to 0.61) in the inguinal regions for patients in whom the LN ratio threshold was met.
Conclusions
Among the prognostic factors examined, LN ratio was the best indicator of the extent of LN dissection, regardless of anatomic nodal region. These data provide evidence-based guidelines for defining adequate LN dissections in melanoma patients.
Keywords: melanoma, lymphadenectomy, lymph node ratio, disease-specific survival
INTRODUCTION
Lymph node (LN) metastasis is one of the most important prognostic factors in patients with melanoma. In addition, the number of involved LNs is a principal component of the American Joint Committee on Cancer (AJCC) staging system.1 Because systemic treatment options for melanoma patients are of limited effectiveness, precise pathologic staging,2 and surgical treatment can influence recurrence and survival rates.3, 4 Although most studies of the quality of surgical treatment of melanoma patients have focused on the width of excision margins for the primary tumor5, 6 and adherence to practice guidelines for pathologic staging,7 few studies8, 9 have examined the extent of LN dissection (LND) and its influence on outcomes.
An association between the extent of LND (measured by the total number of LNs removed, the number of uninvolved LNs removed, and the ratio of involved to total number of LNs (LN ratio)) and improved survival outcomes with more extensive LND has been demonstrated for colon,10–12 lung,13 gastric,14, 15 bladder,16 pancreatic,17 and esophageal18 cancers. Although these improved outcomes are likely due to multiple factors including patient-related,19, 20 process-related,21 and hospital-related characteristics,22 the National Quality Forum and other organizations including the American College of Surgeons and the College of American Pathologists have endorsed the evaluation of at least 12 LNs as a quality standard for patients with colorectal cancer.23 However, no quality guidelines for therapeutic LND for melanoma patients exist, and only a few single-institution studies have suggested that the extent of LND may be important for improved patient outcomes.8, 9 The objectives of this analysis were to compare clinically relevant measures associated with the extent of LND in melanoma patients, to identify threshold values for those measures, and to report associated disease-specific survival (DSS) outcomes.
PATIENTS AND METHODS
Data on node-positive melanoma patients who underwent therapeutic LND from 1988 to 2005 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database, a population-based cancer registry supported by the U.S. National Cancer Institute.24 The SEER database contains data from 18 population-based registries and includes patient demographics, tumor site and morphology, first course of treatment, and stage at diagnosis.
Patients
We first identified patients who had a confirmed diagnosis (other than death certificate) of invasive cutaneous malignant melanoma (n = 137,809). The population was then limited to patients (n = 10,677) who had had at least 1 LN examined, had undergone LND, and did not have stage IV disease at the time of LND. Patients who underwent sentinel LN biopsy alone were excluded. The resultant cohort comprised 3,917 stage III melanoma patients with at least 1 LN positive for metastatic disease. Because data on the specific anatomic sites of LND were not available from the SEER database, we selected patients with primary tumors of the head and neck region (n = 626), upper extremity (n = 762), and lower extremity (n = 960) with the assumption that the associated LND for each primary site was the neck, axillary, and inguinal region, respectively. Patients with primary melanoma of the truncal region (n = 1,569) were excluded because of the ambiguous lymphatic drainage patterns associated with this anatomic site. Similarly, patients with melanoma of unknown primary were not included. The final study cohort consisted of 2,348 patients.
Beginning in 1998, SEER recorded the sentinel node biopsy procedure for melanoma patients in the new field of scope of regional lymph node surgery.25 The coding scheme for this variable has been expanded since 2003 to collect more detailed information on the timing and number of LNs removed from sentinel node biopsy.26 Since 2003, the scope of the pathology is confirmed to be cumulative adding the number of all the lymph nodes removed during each surgical procedure.26 In order to account for the aforementioned changes in coding, the year of diagnosis was categorized into three time periods as follows, 1988 to 1997, 1998 to 2002, and 2003 to 2005. To account for potential geographic variation, the SEER registries were grouped into 4 regions: the West (San Francisco, Hawaii, Alaska, Seattle, San Jose/Monterey, Los Angeles, Utah, New Mexico, and Greater California), the North Central (Iowa and Detroit), the Northeast (Connecticut and New Jersey), and the South (Atlanta, rural Georgia, Louisiana, and Kentucky).
Statistical Analysis
Descriptive statistics were calculated for clinicopathologic factors, and differences among the distribution of factors among the 3 LND regions (neck, axillary, and inguinal) were compared using Chi-square or Fisher’s exact test where appropriate. We performed Cox multivariate analyses27 which adjusted for age, sex, year of diagnosis, SEER region, primary tumor characteristics (Clark level, thickness, histologic subtype, and ulceration) to determine whether the total number of LNs removed, the number of uninvolved LNs removed, and LN ratio (number of involved LNs divided by the total number of LNs removed) had any significant effect on DSS. Multivariate cut-point analyses28 were conducted to define LN ratio threshold values for each LND region. We used Kaplan-Meier methods29 to estimate 5-year DSS from the time of LND. Log-rank tests were used to compare DSS for each group (below the LN ratio threshold v above or equal to the LN ratio threshold).30 Associations between LN ratio and DSS were estimated with the Cox proportional hazards regression model for patients undergoing neck or inguinal LND.27 For patients who underwent axillary LND, we used a log-normal parametric model to identify the prognostic factors for DSS because the proportional hazard assumption was violated and the log-normal parametric model produced more precise estimates of the hazard ratios.31 All calculations were performed with S-PLUS software (version 6.2; Insightful Corporation, Seattle, WA). All P values were 2-sided. A P value of < .05 was considered significant.
RESULTS
Patient and clinicopathologic characteristics stratified by LND region at presentation are presented in Table 1. The median follow-up for all 2,348 patients was 3.3 years. There were a larger proportion of men in the neck LND cohort than in the axillary and inguinal LND cohorts (78.9% v 62.7% and 45.1%, respectively, P < .001). There were fewer Caucasian (93.9%) patients in the inguinal LND group than in the neck and axillary LND groups. Most patients were diagnosed between 1998 and 2005 and were from the West. Nodular melanoma was the most common histologic subtype and acral lentiginous melanomas were more prevalent in patients who underwent inguinal LND than in patients who underwent axillary LND (9.1% vs 3.4%, respectively, P < .001). More patients who underwent axillary LND had N1 disease (68.8%) than patients who underwent LND of the neck (52.3%) or inguinal regions (51.7%) (P < .001). Fewer patients who underwent inguinal LND were treated with radiation therapy (2.5%) compared to those with neck (20%) and axillary (3.2%) LND (P < .001).
Table 1.
Demographics and Clinical Factors of Patient Cohort Stratified by Anatomic Region of Lymph Node Dissection (LND)
| Neck (n = 626) | Axillary (n = 762) | Inguinal (n = 960) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | P | ||||||
| No. of patients | (%) | No. of patients | (%) | No. of patients | (%) | ||
| Age | < .0001 | ||||||
| <50 years | 205 | (32.7) | 261 | (34.3) | 414 | (43.1) | |
| ≥50 years | 421 | (67.3) | 501 | (65.7) | 546 | (56.9) | |
| Sex | < .0001 | ||||||
| Male | 494 | (78.9) | 478 | (62.7) | 433 | (45.1) | |
| Female | 132 | (21.1) | 284 | (37.3) | 527 | (54.9) | |
| Race | < .0001 | ||||||
| Caucasian | 621 | (99.2) | 743 | (97.5) | 902 | (93.9) | |
| African American | 2 | (0.3) | 4 | (0.5) | 19 | (2.0) | |
| Other | 3 | (0.5) | 15 | (2.0) | 39 | (4.1) | |
| Marital status | NS | ||||||
| Married | 407 | (65.0) | 474 | (62.2) | 573 | (59.7) | |
| Unmarried | 197 | (31.5) | 266 | (34.9) | 360 | (37.5) | |
| Unknown | 22 | (3.5) | 22 | (2.9) | 27 | (2.8) | |
| Year of diagnosis | NS | ||||||
| 1988–1997 | 89 | (14.2) | 90 | (11.8) | 128 | (13.3) | |
| 1998–2002 | 283 | (45.2) | 310 | (40.7) | 424 | (44.2) | |
| 2003–2005 | 254 | (40.6) | 362 | (47.5) | 408 | (42.5) | |
| Region | NS | ||||||
| West | 372 | (59.4) | 485 | (63.6) | 588 | (61.2) | |
| North Central | 90 | (14.4) | 84 | (11.0) | 120 | (12.5) | |
| Northeast | 87 | (13.9) | 111 | (14.6) | 143 | (14.9) | |
| South | 77 | (12.3) | 82 | (10.8) | 109 | (11.4) | |
| Histology | < .0001 | ||||||
| Superficial spreading | 116 | (18.5) | 147 | (19.3) | 192 | (20.0) | |
| Nodular | 159 | (25.4) | 214 | (28.1) | 238 | (24.8) | |
| Acral lentiginous | 2 | (0.3) | 26 | (3.4) | 87 | (9.1) | |
| Other/unknown | 349 | (55.8) | 375 | (49.2) | 443 | (46.1) | |
| Thickness | NS | ||||||
| T1 (≤ 1 mm) | 137 | (21.9) | 204 | (26.8) | 233 | (24.3) | |
| T2 (1.01 – 2.0 mm) | 95 | (15.2) | 125 | (16.4) | 157 | (16.4) | |
| T3 (2.01 – 4.0 mm) | 121 | (19.3) | 143 | (18.7) | 202 | (21.0) | |
| T4 (> 4.0 mm) | 106 | (16.9) | 105 | (13.8) | 146 | (15.2) | |
| Unknown | 167 | (26.7) | 185 | (24.3) | 222 | (23.1) | |
| Clark level | .005 | ||||||
| II–III | 79 | (12.6) | 118 | (15.5) | 131 | (13.6) | |
| IV–V | 409 | (65.3) | 509 | (66.8) | 685 | (71.4) | |
| Unknown | 138 | (22.1) | 135 | (17.7) | 144 | (15.0) | |
| Primary tumor ulceration | NS | ||||||
| Yes | 99 | (15.8) | 112 | (14.7) | 168 | (17.5) | |
| No/unknown | 527 | (84.2) | 650 | (85.3) | 792 | (82.5) | |
| N stage | <.0001 | ||||||
| N1 (1 positive LN) | 327 | (52.3) | 524 | (68.8) | 496 | (51.7) | |
| N2 (2–3 positive LN) | 198 | (31.6) | 165 | (21.6) | 326 | (33.9) | |
| N3 (≥ 4 positive LN) | 101 | (16.1) | 73 | (9.6) | 138 | (14.4) | |
Abbreviation: NS, not significant; No., number, LN, lymph node
Table 2 summarizes the patients’ surgical and pathologic characteristics and LN ratio thresholds by LND site. A median of 1 positive LN was removed from patients who underwent neck, axillary and inguinal LND (P < .001). Fewer negative LNs (median, 9; interquartile range [IQR], 5 to 14) and total LNs (median, 11; IQR, 7 to 16) were removed from patients who underwent inguinal LND than from patients who underwent neck and axillary LND. Consequently, the median LN ratio was higher in inguinal LND patients than in neck and axillary LND patients (0.15 v 0.09 and 0.08, respectively; P < .001).
Table 2.
Pathologic Characteristics and LN Ratio Thresholds with Associated DSS Stratified by Lymph Node Dissection Region
| Neck (n = 626) | Axilla (n = 762) | Inguinal (n = 960) | |
|---|---|---|---|
| Univariate analyses | |||
| Median no. of positive LNs removed (IQR) | 1 (1–3) | 1 (1–2) | 1 (1–2) |
| Median no. of negative LNs removed (IQR) | 19 (7–35) | 15 (9–22) | 9 (5–14) |
| Median no. of LNs removed (IQR) | 22 (10–38) | 16.5 (11–23) | 11 (7–16) |
| Median LN ratio (IQR) | 0.09 (0.04–0.24) | 0.08 (0.05–0.17) | 0.15 (0.09–0.33) |
| Multivariate cut-point analyses | |||
| LN ratio threshold | 0.07 | 0.13 | 0.18 |
| No. of LNs per positive node | 15 | 8 | 6 |
| 5-year DSS (< threshold v ≥ threshold) | 64% v 36% | 70% v 51% | 70% v 45% |
Abbreviations: no., number; LN, lymph node; DSS, disease-specific survival; IQR, interquartile range.
LN ratio thresholds and DSS
Cox multivariate analyses demonstrated that increasing LN ratio was an adverse prognostic factor for DSS for all LND regions (data not shown). The number of total LNs removed and the number of negative LNs removed were not significantly associated with DSS, even after adjusting for the number of positive LNs and other clinicopathological characteristics.
Table 2 shows the results of multivariate cut-point analyses to determine the LN ratio thresholds. The LN ratio thresholds were 0.07, 0.13, and 0.18 for the neck, axillary, and inguinal regions, respectively, corresponding to 15, 8, and 6 LNs removed per positive node. Stratifying patients according to their status above or below the LN ratio threshold resulted in significant differences in 5-year DSS rates: 64% versus 36% for neck LND, 70% versus 51% for axillary LND, and 70% versus 45% for inguinal LND (P < .001) (Figure 1 to Figure 3).
Figure 1.
Disease-specific survival stratified by lymph node (LN) ratio (below v at or above the LN ratio threshold) for melanoma patients who underwent neck LN dissection.
Figure 3.
Disease-specific survival stratified by lymph node (LN) ratio (below v at or above the LN ratio threshold) for melanoma patients who underwent inguinal LN dissection.
Multivariate survival analyses
Hazard ratios (HRs) and 95% confidence intervals (CIs) for each prognostic factor for DSS stratified by LND region are summarized in Table 3. After adjustment for age, sex, year of diagnosis, SEER region, Clark level, primary tumor (T) stage, histologic subtype, and the presence/absence of primary tumor ulceration, patients who had a LN ratio less than the threshold had a 50% reduction in the risk of disease-specific death compared with patients who had a LN ratio at or above the threshold (P <.001). Specifically, the HR was 0.53 (95% CI, 0.40 to 0.71) in the neck, 0.52 (95% CI, 0.42 to 0.65) in the axillary, and 0.47 (95% CI, 0.36 to 0.61) in the inguinal LND groups for patients who met LN ratio threshold. In the axillary LND group, nodular melanoma (HR, 1.40; 95% CI, 1.02 to 1.94; P = .04) was a poor prognostic factor for DSS; while Clark levels IV and V (HR, 1.56; 95% CI, 1.0 to 2.43; P = .05) and primary tumor ulceration (HR, 1.44; 95% CI, 1.07 to 1.94; P = .016) were adverse prognostic factor for DSS in the inguinal LND group.
Table 3.
Results of Regression Analyses of Prognostic Factors for Disease-Specific Survival by Lymph Node Dissection Region
| Neck (n = 626) | Axillary (n = 762) | Inguinal (n = 960) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Minimal LN ratio threshold | < .001 | < .001 | < .001 | ||||||
| < threshold v > threshold | 0.53 | 0.40–0.71 | 0.52 | 0.42–0.65 | 0.47 | 0.36–0.61 | |||
| Clark Level | NS | NS | .056 | ||||||
| IV – V v II – III | 1.46 | 0.91–2.36 | 1.20 | 0.86–1.66 | 1.56 | 1.28–3.75 | |||
| Unknown v II – III | 1.66 | 0.98–2.82 | 1.17 | 0.79–1.73 | 1.79 | 1.08–2.98 | |||
| Histologic type | NS | 0.041 | NS | ||||||
| Nodular v superficial spreading | 1.32 | 0.88–1.97 | 1.40 | 1.02–1.94 | 1.29 | 0.88–1.90 | |||
| Other/unknown v superficial spreading | 1.03 | 0.71–1.50 | 1.06 | 0.78–1.44 | 1.15 | 0.80–1.66 | |||
| Primary tumor ulceration | |||||||||
| Yes v no | 1.32 | 0.94–1.84 | NS | 1.26 | 0.97–1.64 | NS | 1.44 | 1.07–1.94 | .016 |
Abbreviations: CI, confidence interval; LN, lymph node; NS, not significant.
Note: All hazard ratios were adjusted for age (≥ 50 v < 50 years), sex, year of diagnosis (2003–2005 v 1988–1997, 1998–2002 v 1988–1997), geographic region, and T stage.
Disease-specific survival (DSS) for the neck and inguinal regions were calculated using Cox proportional hazard models, while DSS for the axilla region was calculated using a log-normal parametric model.
DISCUSSION
Our multivariate analysis demonstrates that LN ratio is a significant predictor of DSS in stage III melanoma patients undergoing neck, axillary, or inguinal LND. Given that the AJCC staging system identifies the number of positive LNs as the strongest independent prognostic factor for patients with stage III melanoma, it is not surprising that the LN ratio, which incorporates the number of positive LNs [numerator] and total number of LNs removed [denominator], would also be an important prognostic factor. For patients with only 1 positive node, we determined that the number of LNs that should be removed is 15, 8, and 6 in the neck, axillary, and inguinal regions, respectively. This threshold was associated with 5-year DSS differences of approximately 20% for patients in each LND group. These results suggest that anatomic LNDs should be performed for patients with melanoma to ensure that a sufficient number of nodes are removed.
Studies have shown that improved survival outcomes are associated with a greater number of LNs removed for various tumor histologies. Similar to the current report, the majority of these analyses used population-based data from the SEER database to examine the association of LND and survival outcomes in lung cancer,13 esophageal cancer,18, 32 gastric cancer,14 pancreatic cancer,17 colon cancer,10, 12, 22, 33 and bladder cancer16 patients. The studies identified thresholds that ranged from 10 to 16 total LNs removed for lung,13 stomach,14 and pancreatic17 tumors. Other studies have reported that LN ratio, ranging from 0.1 for esophageal cancer,32 gastric cancer,34 and melanoma8 to 0.125 to 0.2 for bladder cancer35, 36 is a significant prognostic factor. A limitation of those reports is that unlike the current analysis, LN ratio thresholds were assigned according to expert opinion or median and quartile cut-points determined with unadjusted or univariate analysis, and survival differences were reported according to these thresholds. The current study utilized multivariate cut-point analyses adjusting for known melanoma-specific prognostic factors to determine LN ratio thresholds thereby providing more accurate estimates.
Two single-center studies have examined the prognostic significance of the extent of LND reported as total LNs removed,9 or LN ratio8 for melanoma patients. Galliot-Repkat et al studied 67 melanoma patients (26 [39%] trunk, 35 [52%] extremity, and 6 [9%] unknown) and found that overall survival, but not DSS, significantly improved when more than 10 total LNs were removed.9 Rossi et al, in a study of 213 melanoma patients (124 [56%] trunk, 80 [38%] limb, 13 [6%] head and neck), found that LN ratio (≤ 0.10, 0.11 to 0.25, or > 0.25) was a significant predictor of overall survival.8
The current analysis has several strengths which distinguish if from previous reports. First, outcomes were examined from a large, population-based cohort stratified by anatomic LN region to account for potential anatomic variation in the number of LN. Second, three potential indicators of extent of LND which have previously been reported were examined: total number of LNs removed, number of negative nodes removed, and LN ratio. In addition, we also used multivariate cut-point analyses to account for the effects of other known prognostic factors which were included in SEER.28 The primary limitation of this and other observational analyses is that a direct cause-and-effect relationship between LN ratio and DSS cannot be assumed. There are additional limitations to using SEER data in our analysis. These include: (1) the relatively short median follow-up time of 3.3 years for the cohort, (2) the lack of data pertaining to more contemporary melanoma-specific prognostic factors including mitotic rate, and (3) the evolution of pathologic assessment techniques over the study period to identify microscopic nodal disease.
While LND has been shown to enhance regional disease control in melanoma patients,37 the relationship between the extent of LND and DSS remains unclear. Furthermore, whether total LN count alone or as a component of LN ratio can serve as a reliable proxy for the extent of LND is controversial, as there are many factors beyond the extent of surgical dissection which contribute to the total LN count. LN count has also been shown to be related to variation in pathologic processing of surgical specimens both within and between institutions as well as biological variation among individuals.19 Studies have suggested that patients with higher numbers of LNs may in fact have enhanced immunological responses which may account for improved survival outcomes.20, 38 We accounted for some of this variation by stratifying and independently assessing three separate anatomic nodal regions.
Of interest is a recent study by Wong et al which found no significant relationship between the number of LNs examined and survival in node-positive colon cancer patients after adjustment for hospital characteristics.22 Such findings question whether patient selection bias— rather than LN counts—contributed to disparities in outcome. Although the hospital-adjusted analysis employed by Wong et al raises questions about the value of LN count alone as a hospital-level quality indicator for node-positive colon cancer surgery, it confirms previous studies that suggested a better prognosis for node-negative patients in whom more LNs are examined.22 However, it is difficult to interpret these findings given that the investigators defined hospital quartiles based on aggregate data from SEER-Medicare data in which there was wide variation. For example, outcomes were compared according to the percentage of patients who achieved a dissection in which 12 LN were removed, which varied from 16% of patients in the lowest quartile hospitals to only 61% of patients in the highest quartile hospitals. Given that the median age at melanoma diagnosis is approximately 55 years, similar hospital-adjusted analyses based on Medicare-linked data would not be appropriate, since conclusions drawn from such a small subset of Medicare patients ages 65 and above would not be generalizable.
In some other cancer types, studies of the extent of LND have been used to propose guidelines to define adequate LN sampling for proper pathologic staging for patients without nodal metastasis39 and to define adequate LND for patients with confirmed nodal metastasis. Specifically, the National Quality Forum has endorsed a minimum 12-node evaluation in the setting of colon cancer as a measure of hospital performance.23 This guideline was introduced to avoid inadequate classification or understaging10, 40 at the time of colon cancer resection. However, the rationale for such guidelines is not entirely applicable to melanoma patients who undergo surgery because elective LND for pathologic nodal staging has been abandoned in favor of sentinel LN biopsy39 with selective LND for patients with confirmed nodal metastasis. We would argue that the LN ratio is the most effective prognostic factor that can be used to assess the extent of therapeutic LND for patients with nodal metastasis. While the benefits of immediate, complete LND in patients with microscopic metastases in sentinel LNs continue to be debated, current practice guideline recommend therapeutic LND for patients with node-positive disease, regardless of tumor burden.41 Although it is not possible to discriminate between microscopic versus macroscopic nodal metastasis in our data, the improved DSS outcomes observed when 15, 10, and 8 LNs were removed in the neck, axillary, and inguinal regions provide support for the concept of completion LND in patients with a single positive node.
Although we agree with Simunovic et al,40 who advocate caution when using quality indicators supported only with evidence from observational studies, we would submit that discouraging research of thresholds for the extent of LND could be detrimental to patient care and negatively affect patient outcomes. However crude LN ratio may be as a patient-level quality benchmark, some indicator of surgical quality is needed for the standardization of clinical practice.42 Our intent is that the current analysis, which defines the LN ratios for several anatomic regions in patients with stage III melanoma, is to (1) provide evidence to support anatomic operations to ensure that adequate numbers of LN are removed and (2) to provide a foundation for further inquiry that may lead to improved quality measures.
Figure 2.
Disease-specific survival stratified by lymph node (LN) ratio (below v at or above the LN ratio threshold) for melanoma patients who underwent axillary LN dissection.
Acknowledgements
We would like to acknowledge Beth Notzen for editorial assistance and Debbie Dunaway for manuscript preparation.
Acknowledgement of research support: This project was supported by Grant Number 1 R01 CA127328-01 (Cormier, PI) from National Cancer Institute, National Institutes of Health. The contents of this manuscripts are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
Footnotes
Presented at the 3rd Annual Academic Surgical Congress, Huntington Beach, CA, February, 2008.
REFERENCES
- 1.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging handbook: from the AJCC cancer staging manual. ed. 6th. New York: Springer; 2002. Melanoma of the Skin; p. 239. [Google Scholar]
- 2.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 3.Riker AI, Kirksey L, Thompson L, Morris A, Cruse CW. Current surgical management of melanoma. Expert Rev Anticancer Ther. 2006;6:1569–1583. doi: 10.1586/14737140.6.11.1569. [DOI] [PubMed] [Google Scholar]
- 4.O'Day S, Boasberg P. Management of metastatic melanoma 2005. Surg Oncol Clin N Am. 2006;15:419–437. doi: 10.1016/j.soc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JM, Newton-Bishop J, A'Hern R, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350:757–766. doi: 10.1056/NEJMoa030681. [DOI] [PubMed] [Google Scholar]
- 6.Krown SE, Chapman PB. Defining adequate surgery for primary melanoma. N Engl J Med. 2004;350:823–825. doi: 10.1056/NEJMe038235. [DOI] [PubMed] [Google Scholar]
- 7.Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol. 2005;23:6054–6062. doi: 10.1200/JCO.2005.21.360. [DOI] [PubMed] [Google Scholar]
- 8.Rossi CR, Mocellin S, Pasquali S, Pilati P, Nitti D. N-ratio: a novel independent prognostic factor for patients with stage-III cutaneous melanoma. Ann Surg Oncol. 2008;15:310–315. doi: 10.1245/s10434-007-9641-z. [DOI] [PubMed] [Google Scholar]
- 9.Galliot-Repkat C, Cailliod R, Trost O, et al. The prognostic impact of the extent of lymph node dissection in patients with stage III melanoma. Eur J Surg Oncol. 2006;32:790–794. doi: 10.1016/j.ejso.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 11.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570–3575. doi: 10.1200/JCO.2006.06.8866. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 14.Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz RE, Smith DD. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2005;23:5404–5405. doi: 10.1200/JCO.2005.05.189. author reply 05. [DOI] [PubMed] [Google Scholar]
- 16.Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006;107:2368–2374. doi: 10.1002/cncr.22250. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 18.Bollschweiler E, Baldus SE, Schroder W, Schneider PM, Holscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 19.Canessa CE, Badia F, Fierro S, Fiol V, Hayek G. Anatomic study of the lymph nodes of the mesorectum. Dis Colon Rectum. 2001;44:1333–1336. doi: 10.1007/BF02234794. [DOI] [PubMed] [Google Scholar]
- 20.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 21.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21:1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149–2154. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 23.National Quality Forum. [Accessed July 23, 2008];Specifications of the national voluntary consensus standards for breast and colon cancer. Available at http://www.qualityforum.org/pdf/cancer/txbreastcolonAppA-Specsvoting01-18-07clean.pdf.
- 24.About SEER. [Accessed November 3, 2005];Surveillance, Epidemiology, and End Results. Available at http://seer.cancer.gov/about/
- 25. [Accessed July 8, 2008];SEER Program Code Manual. Available at http://seer.cancer.gov/manuals/AppendC.pdf.
- 26.National Cancer Institute. [Accessed July 8, 2008];The SEER Program Coding and Staging Manual 2004, Revision 1. Available at http://seer.cancer.gov/manuals/2004Revision%201/SPM_2004_maindoc.r1.pdf.
- 27.Cox DR. Regression models and life tables. J R Statistical Soc. 1972;B34:187–220. [Google Scholar]
- 28.Tableman M, Sung J, Portnoy S. Survival Analysis using S. Boca Raton: Chapman & Hall/CRC; 2003. [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–481. [Google Scholar]
- 30.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 31.Klein J, Moeschberger M. Survival Analysis. Berlin: Springer; 2003. [Google Scholar]
- 32.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–1608. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 34.Kunisaki C, Makino H, Akiyama H, et al. Clinical significance of the metastatic lymph-node ratio in early gastric cancer. J Gastrointest Surg. 2008;12:542–549. doi: 10.1007/s11605-007-0239-3. [DOI] [PubMed] [Google Scholar]
- 35.Hollenbeck BK, Ye Z, Wong SL, Montie JE, Birkmeyer JD. Hospital lymph node counts and survival after radical cystectomy. Cancer. 2008;112:806–812. doi: 10.1002/cncr.23234. [DOI] [PubMed] [Google Scholar]
- 36.Wright JL, Lin DW, Porter MP. The association between extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Cancer. 2008;112:2401–2408. doi: 10.1002/cncr.23474. [DOI] [PubMed] [Google Scholar]
- 37.Morton DL, Wanek L, Nizze JA, Elashoff RM, Wong JH. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes.Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214:491–499. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26:535–541. doi: 10.1200/JCO.2007.14.0285. [DOI] [PubMed] [Google Scholar]
- 39.Amersi F, Morton DL. The role of sentinel lymph node biopsy in the management of melanoma. Adv Surg. 2007;41:241–256. doi: 10.1016/j.yasu.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simunovic M, Baxter NN. Lymph node counts in colon cancer surgery: lessons for users of quality indicators. JAMA. 2007;298:2194–2195. doi: 10.1001/jama.298.18.2194. [DOI] [PubMed] [Google Scholar]
- 41.Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–796. doi: 10.1016/s0140-6736(97)08260-3. [DOI] [PubMed] [Google Scholar]
- 42.Bilimoria KY, Stewart AK, Edge SB, Ko CY. Lymph node examination rate, survival rate, and quality of care in colon cancer. JAMA. 2008;299:896. doi: 10.1001/jama.299.8.896-b. [DOI] [PubMed] [Google Scholar]