Abstract
Objective
The mechanism by which non-nucleoside reverse transcriptase inhibitors (NNRTIs) increase HDL cholesterol (HDL-C) in HIV+ patients and the benefits of this with respect to cardiovascular risk are not known. Studies were conducted to test the hypothesis that NNRTIs have a beneficial effect on HDL-C and reverse cholesterol transport (RCT).
Methods
LDLr −/− and hA-I transgenic mice were fed a Western diet containing either nevirapine (20 mg/kg/day), efavirenz (10 mg/kg/day), or diet alone. hA-I transgenic mice underwent a study to measure RCT (measured by excretion of macrophage [3H]-cholesterol into HDL and feces) at 8 weeks.
Results
LDLr −/− and hA-I transgenic mice treated with nevirapine and efavirenz had a significant increase in HDL-C level (up to 23% in hA-I transgenic) at 4 weeks. However, there was no difference in HDL levels beyond 4 weeks of treatment. At 4 weeks, the FPLC profile of hA-I transgenic mice showed an increase in large HDL. hApoA-I transgenic mice treated with efavirenz for 4 weeks had increased expression of human apoA-I in liver and an increased human apoA-I production rate. Incubation of plasma from hA-I transgenic mice treated for 4 weeks with [3H]-cholesterol-labeled macrophages revealed increased cholesterol efflux to plasma from mice treated with efavirenz and nevirapine. Following injection of hA-I transgenic mice treated for 8 weeks with [3H]-cholesterol-labeled macrophages, RCT was increased in the efavirenz (p=.01) group and trended towards an increase in the nevirapine (p=.15) group.
Conclusion
Nevirapine and efavirenz transiently increased HDL-C in LDLr −/− and hA-I transgenic mice fed a Western diet that was associated with increased apoA-I production. An increase in RCT in hA-I transgenic mice at 8 weeks despite no difference in HDL levels indicates that these drugs affect additional factors in the RCT pathway that enhance cholesterol efflux from the macrophage and peripheral tissues to plasma and delivery to liver for excretion. These results suggest that treatment with NNRTIs has a beneficial effect on cholesterol efflux and RCT.
Keywords: NNRTIs, RCT, apoA-I, LDL receptor, cholesterol efflux, HIV
Introduction
Infection with HIV results in progressive impairment of immune function that, if left untreated, has a high rate of morbidity and mortality. Treatments for HIV infection, while effective in slowing disease progression, are commonly associated with undesirable side-effects. Combination antiretroviral therapy for HIV infection is known to be associated with several physiological or metabolic changes such as fat redistribution and dyslipidemia 1. Some nucleoside reverse transcriptase inhibitors and protease inhibitors are associated with a lipoprotein profile that is considered atherogenic 2 and that includes elevated levels of total cholesterol, triglyceride, low density lipoprotein (LDL) and reduced high density lipoprotein (HDL) cholesterol levels.
Nevirapine and efavirenz are non-nucleoside reverse transcriptase inhibitors (NNRTIs) that are used in the treatment of HIV. In contrast to the atherogenic lipoprotein profile observed in patients treated with other anti-HIV drugs 3 several studies have demonstrated that treatment with NNRTIs increase plasma levels of HDL cholesterol by up to 49% 4–11 through an unknown mechanism. In the non-HIV-infected population elevated HDL cholesterol levels have been associated with reduced risk of cardiovascular disease 12. The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) demonstrated that increasing HDL levels by 6% through treatment with gemfibrozil was associated with 22% reduction in coronary events 2. Additional epidemiological studies have suggested that there is a 2% decrease in cardiovascular risk with each 1% increase in HDL cholesterol level 12.
HDL and its major protein component, apolipoprotein A-I (apoA-I), are thought to reduce atherosclerosis by promoting efflux of excess cholesterol from peripheral tissue (including the macrophage), and return this excess cholesterol to the liver for excretion into bile in a process known as reverse cholesterol transport (RCT) 13. RCT is essential for preventing toxic effects of cholesterol accumulation in the macrophage which can lead to foam cell formation and atherosclerosis. RCT is complex and involves many gene products in addition to apoA-I including scavenger receptor B type 1 (SRB1), ATP binding cassette transporters A-1 (ABCA-1) and G-1 (ABCG-1) and several lipases13. These factors, which are regulated by several nuclear receptors 13,14, control the net flux of cholesterol from macrophages and peripheral tissues to HDL and from HDL to liver where it is excreted.
Since increased HDL levels are associated with reduced risk of CHD, the increase in HDL cholesterol levels in response to NNRTIs might be expected to be beneficial. However, while the majority of data support that increased HDL levels are associated with reduced atherosclerotic risk, elevated HDL levels have been observed in patients with atherosclerosis 15 16. Furthermore, SRB1 knockout mice have elevated HDL-C levels, but develop atherosclerosis due to the absence of this functional element of the RCT pathway 17 . Recently, raising HDL through CETP inhibition has been associated with increased atherosclerotic risk 18. The benefits of an HDL increase in response to NNRTIs in relation to RCT and atherosclerotic risk has not been demonstrated. In this paper, we focus on the effects of nevirapine and efavirenz on HDL levels in LDL receptor −/− and human apoA-I transgenic mice. In the latter model we determine the effects of these agents on cholesterol efflux and RCT.
Methods
Animals
Female human apoA-I transgenic mice (n=8 per group) aged 6–8 weeks were placed on a Western diet (Research Diets #D12079B; 21% fat by weight) containing either nevirapine (Viramune®, 20 mg/kg per day), efavirenz (Sustiva®, 10 mg/kg per day), or no additive as control. Mice were bled via the retro-orbital plexus using heparinized capillary tubes using isoflurane anesthesia. Blood samples were collected at baseline and at weeks 4 and 8. At week 8 the human apoA-I transgenic mice, which are resistant to atherosclerosis, underwent a metabolic study which measured reverse cholesterol transport 19. Plasma samples were analyzed for plasma lipid levels and lipoprotein profile by fast protein liquid chromatography (FPLC).
In a separate experiment, female LDL receptor −/− mice (n=12 per group) aged 6–8 weeks were placed on the same Western diets as above containing either nevirapine, efavirenz or no additive. Mice were bled at baseline and at weeks 4, 8 and 12. Plasma samples were analyzed for plasma lipid levels.
Mice were euthanized by intraperitoneal injection of ketamine/xylazine prior to dissection. All procedures involving mice were performed under protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Lipid analyses
Plasma was immediately separated from whole blood by centrifugation at 4°C and stored at −80°C before use. Plasma total cholesterol, HDL and triglyceride concentrations were determined using enzymatic reagents (Wako Chemicals USA, Richmond, VA).
Fast Protein Liquid Chromatography (FPLC)
Pooled plasma samples from mice of the same experimental group were subjected to FPLC gel filtration by using 2 Superose 6 columns (Pharmacia LKB Biotechnology) as previously described 13, 20. Individual fractions were assayed for cholesterol concentrations by using commercially available assay kits (Wako Pure Chemical Industries, Ltd). Large and small HDL peaks were measured following peak deconvolution as previously described 21.
Real time polymerase chain reaction (PCR)
Liver tissue (10 mg) was homogenized and RNA isolated using the EZ1 RNA tissue mini kit (Qiagen). Real-time polymerase chain reaction (PCR) assays were performed with an Applied Biosystems 7300 sequence detector using SYBR green reagent and the following primers: for human apoA-I (APOA1), forward, 5'-agcttgctgaaggtggaggt-3' and reverse, 5'-atcgagtgaaggacctggc-3'; for Cytochrome P450 3A11 (CYP3A11), forward, 5'- gacccacagcactggtcaga-3' and reverse, 5'-aggatcaatgctgcccttgt-3'; for 36B4, forward, 5'-tcatccagcaggtgtttgaca-3' and reverse, 5'-ggcaccgaggcaacagtt-3'. Expression was normalized to the housekeeping gene 36B4 and expressed relative to control.
Reverse cholesterol transport
RCT was measured as previously described 13. Briefly, J774 cells were grown in suspension in RPMI 1640 supplemented with 10% FBS. Cells were radiolabeled with 5 μCi/ml [3H]-cholesterol, washed twice, equilibrated in RPMI plus 0.2% BSA for 6 hours, spun down, and resuspended in RPMI medium immediately before use. The cell suspension contained 17.7 × 106 cells/ml at 20.6 × 106 cpm/ml. [3H]-cholesterol-labeled J774 cells (0.5 ml) were injected intraperitoneally into individually caged mice. Plasma was collected at 24 hours and 48 hours and was used for liquid scintillation counting and lipoprotein analysis. Plasma radioactivity is expressed as percent of total injected [3H]-cholesterol per milliliter plasma. Feces were collected over 48 hours and stored at −20°C prior to lipid extraction. At 48 hours, mice were anesthetized, their livers perfused with cold PBS, then harvested flash frozen and stored at −80°C until lipid extraction. Fecal cholesterol and bile acid were extracted as described by Batta et al 22. Values are expressed as a percent of total injected [3H]-cholesterol. Liver lipids were extracted by the Bligh-Dyer method 23 and expressed as a percent of total [3H]-cholesterol-injected/whole organ.
HDL apoA-I kinetics
Female apoA-I transgenic mice maintained on a Western diet were gavaged with either efavirenz (10 mg/kg) (n=4) or vehicle (n=3) once daily for 4 weeks. There was no nevirapine treatment group in this experiment. At the end of 4 weeks mice were injected with radioiodinated human HDL (d=1.063–1.21 g/ml) isolated from pooled plasma. Blood was collected over a 48 hour period and radioactivity in plasma counted using a gamma counter. Fractional catabolic rates were calculated by fitting data to a single pool model containing an extravascular exchange using data normalized to the 2 minute timepoint. Production rates were calculated by multiplying the FCR by the estimated apoA-I pool size.
Cholesterol efflux
Untreated donor wild-type (C57Bl/6) mice were euthanized and bone marrow was flushed from femur and tibia of each leg using PBS-heparin (100ug/ml). Cells were washed with PBS and resuspended in DMEM containing 30% L-929 cells conditioned medium and 10% FBS (bone marrow growth medium) . Conditioned medium from L-929 mouse fibroblast-like cells favors differentiation of marrow cells into macrophages. Isolated bone marrow cells were seeded in 12-well plates (for in vitro experiments) or in 100mm Petri dishes (for RCT studies) and cultured at 37°C and 5% CO2. 4 days after plating, medium containing nonadherent cells was removed and the adherent cells were refed with fresh bone marrow growth medium and cultured for additional 3 days.
Statistics
Values are presented as mean ± SD. Data were analyzed by Student's t test for independent samples. Statistical significance for comparisons was assigned at p < 0.05.
Results
Studies in LDL receptor −/− mice
Treatment of LDL receptor −/− mice with nevirapine or efavirenz in a Western diet for 12 weeks resulted in a significant increase in the HDL cholesterol level in the nevirapine (+11%, p=0.001) and efavirenz (+8%, p=0.005) groups as compared to control group at week 4. However, this difference was transient and all groups had similar HDL cholesterol levels at the 8 and 12 week timepoints (table 1). Plasma non-HDL (containing VLDL and LDL) cholesterol levels remained unchanged throughout the study period (table 1).
Table 1.
Plasma lipid levels in LDL receptor −/− mice following treatment with nevirapine or efavirenz during the course of the study. All values, in mg/dl, are mean (SD).
| Total Cholesterol | Non-HDL Cholesterol | HDL Cholesterol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Week 12 | Baseline | Week 4 | Week 8 | Week 12 | Baseline | Week 4 | Week 8 | Week 12 | |
| Control | 197 (15) | 1012 (73) | 1093 (89) | 1170 (185) | 114 (11) | 811 (68) | 876 (82) | 969 (170) | 83 (5) | 200 (11) | 217 (10) | 218 (28) |
| Efavirenz | 198 (21) | 975 (65) | 1040 (82) | 1082 (96) | 117 (15) | 760 (55) | 823 (76) | 870 (85) | 81 (7) | 215* (14) | 217 (15) | 212 (20) |
| Nevirapine | 210 (36) | 1024 (103) | 1156 (109) | 1167 (162) | 123 (30) | 803 (97) | 936 (97) | 950 (140) | 87 (8) | 221* (20) | 220 (22) | 217 (30) |
P < 0.05 vs. corresponding to control
Studies in human apoA-I transgenic mice
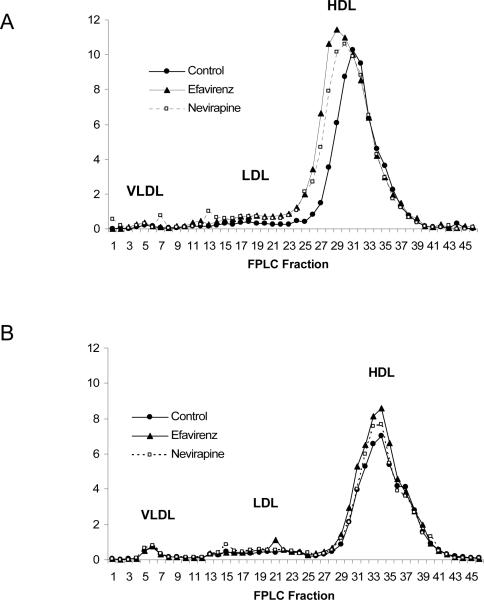
Treatment of human apoA-I transgenic mice with nevirapine or efavirenz was associated with a significant increase in plasma total and HDL cholesterol levels at 4 weeks in the nevirapine (+15%, p=0.04 and +23%, p=0.01, total and HDL cholesterol respectively) and efavirenz (+23%, p=0.01 and +23%, p=0.01, total and HDL cholesterol respectively) groups as compared to controls (table 2). However, by 8 weeks the HDL level of the control group rose to a level that was similar to the nevirapine and efavirenz groups (table 2). There was also a significant increase in the non-HDL (containing VLDL and LDL) cholesterol levels in the efavirenz, but not nevirapine group at week 4 with no differences observed at 8 weeks (table 2). The plasma lipoprotein profile by FPLC shows the differences in HDL cholesterol levels at week 4 (figure 1A) that were not apparent at week 8 (figure 1B). Consistent with the transient difference observed for HDL cholesterol, the major protein on HDL in these mice, human apoA-I, was significantly increased at 4 weeks in the animals treated with nevirapine and efavirenz (table 2). However, at 8 weeks there were no differences detected for human apoA-I among groups (table 2).
Table 2.
Plasma lipid levels in human apoA-I transgenic mice following treatment with nevirapine or efavirenz during the course of the study. All values, in mg/dl, are mean (SD).
| Total Cholesterol | Non-HDL Cholesterol | HDL Cholesterol | ApoA-I | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | |
| Control | 169 (21) | 245 (27) | 275 (49) | 34 (9) | 64 (68) | 76 (18) | 135 (17) | 180 (22) | 200 (32) | 294 (21) | 408 (23) | 364 (24) |
| Efavirenz | 163 (14) | 302* (51) | 304 (22) | 38 (6) | 79* (55) | 80 (12) | 125 (14) | 223* (51) | 224 (18) | 269 (20) | 440 (53) | 361 (22) |
| Nevirapine | 165 (13) | 281* (38) | 258 (32) | 41 (14) | 58 (97) | 66 (14) | 124 (23) | 224* (38) | 192 (20) | 285 (25) | 441 (40) | 351 (18) |
P < 0.05 vs. corresponding to control
Figure 1.
FPLC profile showing cholesterol content (mg/dl)of plasma lipoprotein fractions from human apoA-I transgenic mice fed a Western diet (Control) or a Western diet containing nevirapine or efavirenz at (A) week 4 and (B) week 8.
The HDL portion of the FPLC profile of human apoA-I transgenic mice run on plasma collected at 4 weeks was deconvoluted to differentiate its two major components, one large HDL (peak at fraction 31) and one small HDL component (peak at fraction 35). There was a change towards an increased size of the larger HDL component in plasma of nevirapine and efavirenz treated mice as compared to control while the size of the smaller peak was unchanged. We estimate that there was an increase of amount of the 21–27% in the larger HDL component in response to nevirapine and efavirenz while the amount of the smaller component was unchanged.
The expression of human apoA-I in mice treated with nevirapine and efavirenz was measured using real time PCR. Expression of human apoA-I in human apoA-I transgenic mice treated with efavirenz for 4 weeks was significantly increased (1.44 ± 0.17 fold change from control, p=0.02). Expression of human apoA-I in human apoA-I transgenic mice treated with nevirapine and efavirenz for 8 weeks was similar to control animals (fold changes from control 0.94 ± 0.28, p=0.58 and 1.10 ± 0.39, p=0.39, nevirapine and efavirenz respectively).
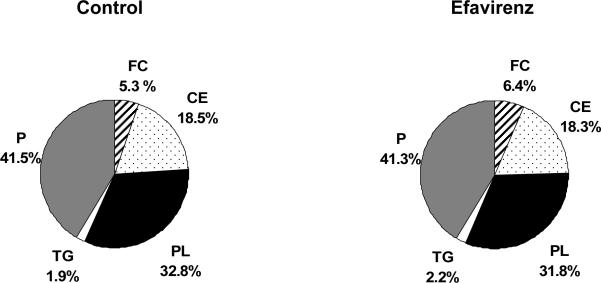
We performed labeled HDL in vivo kinetic study in human apoA-I transgenic mice fed a Western diet treated with efavirenz for 4 weeks. In the efavirenz treated group, we saw significant increase in the plasma apoA-I concentration (630 ± 9 vs. 798 ± 85 mg/dl). There was significant increase in the apoA-I production rate as compared to the control group (14.2 ± 7.0 vs. 27.4 ± 2.9 mg/kg/hr, p=0.01). There was no significant difference in the human apoA-I fractional catabolic rate between the two groups (1.55 vs. 2.38 pools/day, p=0.11). In this study, we also performed composition analysis with HDL isolated by ultracentrifugation. The composition of HDL did not change between control and efavirenz treated mice (table 2
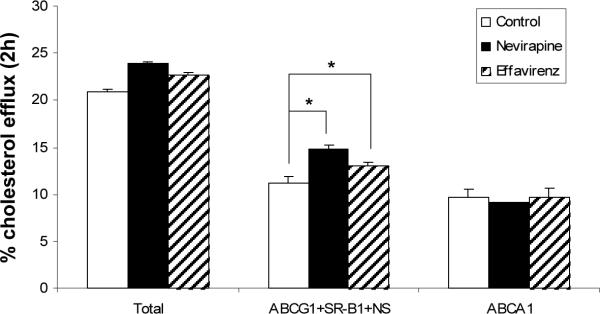
We conducted in vitro assay of cholesterol efflux from bone marrow-derived macrophages from wild-type mice to lipoprotein acceptors in control, nevirapine and efavirenz treated human apoA-I transgenic mouse plasma from the 4 week timepoint. We saw significantly increased ABCA1-independent efflux capacity of plasma from nevirapine (32%, p<0.01, figure 3) and efavirenz (16%, p<0.01, figure 3) treated mice. There was no significant difference in the ABCA1-specific efflux capacity between treatment groups.
Figure 3.
Results from in vitro cholesterol efflux study. Cholesterol efflux capacity of plasma from human apoA-I transgenic mice treated with nevirapine and efavirenz. Bone marrow-derived macrophages were isolated from wild-type mice and differentiated with DMEM/ FBS/10% L929 cells for 8 days. Cells were labeled with [3H]-cholesterol for 24 hours and loaded with 25ug/ml of acetylated LDL at the same time. After the incubation, efflux was initiated on the addition of 2.5% control mouse plasma, nevirapine-treated plasma and efavirenz-treated plasma. (* p=0.01).
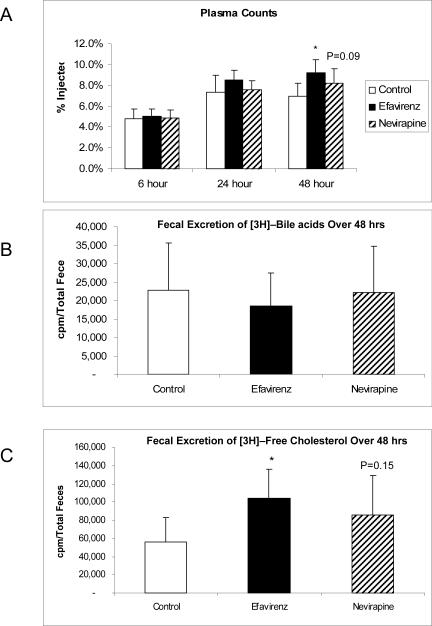
The results of the reverse cholesterol transport study conducted at the end of 8 weeks of treatment in human apoA-I transgenic mice showed that the mice in the efavirenz group had significantly higher plasma [3H]-cholesterol counts (p=.003 vs. control) at 48 hours after labeled macrophage injection, followed by the nevirapine group (p= 0.09 vs. control) with the control group being lowest (figure 2A). The pattern for cumulative [3H]-cholesterol excretion into feces as free cholesterol over 48 hours following labeled macrophage injection was significantly increased in the efavirenz group with a non-significant increase observed in the nevirapine group as compared to control (figure 2B). The cumulative [3H]-cholesterol excretion into bile as bile acid over 48 hours following labeled macrophage injection was similar among the study groups (figure 2C).
Figure 2.
Results from in vivo RCT study. [3H]-cholesterol counts in (A) plasma and (B) feces following injection of human apoA-I transgenic mice fed a Western diet (Control) or a Western diet containing nevirapine or efavirenz for 8 weeks with J774 macrophages labeled with [3H]-cholesterol.
Discussion
The use of combination antiretroviral therapy for HIV infection is often associated with an atherogenic lipoprotein profile that includes elevated levels of total cholesterol, triglyceride, LDL and reduced high density lipoprotein HDL cholesterol levels. Nevirapine and efavirenz are two non-nucleoside reverse transcriptase inhibitors used to treat HIV that have been reported to raise HDL cholesterol levels in humans 8. While an HDL-raising effect of these drugs would be expected to reduce the risk of atherosclerosis based on epidemiological studies 24, recent evidence suggests that raising HDL may not always be of benefit 15–17. The effects of the HDL increase in response to nevirapine and efavirenz on atherosclerotic risk are currently unknown. We sought to determine if nevirapine and efavirenz have a beneficial effect on HDL and enhance RCT in mice.
Human apoA-I transgenic mice treated with nevirapine or efavirenz displayed a significant increase in apoA-I and HDL cholesterol compared to control mice at 4 weeks while there was no difference from controls at 8 weeks. Similarly, LDL receptor −/− mice treated with nevirapine or efavirenz displayed a significant but transient increase in HDL cholesterol compared to control mice at 4 weeks that was no longer different from controls at 8 weeks. This contrasts to what has been reported in human adults treated with nevirapine or efavirenz where HDL increases have been maintained beyond 8 weeks of treatment 9. Interestingly, Sankatsing, et al reported a similar transient increase in HDL in newborns treated with nevirapine 25. It is possible that the transient nature of the observed HDL increase in the current study and that in newborns is due to enhanced metabolism of the drug over time since efavirenz has been reported to induce its own metabolism (Sustiva© prescribing information, January 2002 revision). Another contributing factor in the current study may be the Western diet used in these studies which may have increased the HDL levels in the control group over time and masked the effects of these drugs on HDL metabolism.
One mechanism that is likely responsible for the increase in HDL cholesterol levels in the apoA-I transgenic mice in response to NNRTIs is an increase in apoA-I production. We observed an increase in apoA-I production due to a significant increase in human apoA-I expression in human apoA-I transgenic mice treated for 4 weeks with efavirenz. This result is consistent with a recent report that nevirapine increases apoA-I production in humans 26. Haripasad et al 27 reported that efavirenz is an agonist for the bile-acid responsive nuclear receptor PXR (pregnane X receptor), the mouse homolog of the human nuclear receptor SXR (steroid and xenobiotic receptor). PXR has also been reported to regulate the expression of apoA-I in rodents 14. The constitutive androstane receptor (CAR) has also been reported to be activated by nevirapine and efavirenz 28. Phenytoin, which is known to increase HDL levels, has been shown to activate CAR 29. It is possible that the Western diet used in these studies led to an increase hepatic sterols which could activate PXR and CAR 30 and affect gene expression in a way similar to nevirapine and efavirenz. Thus a more pronounced and prolonged increase in HDL may be observed in mice fed a chow, rather than Western, diet.
In addition to an increase in HDL we observed an increased HDL size in the FPLC profile of human apoA-I transgenic mice treated with efavirenz and nevirapine for 4 weeks. The lipid composition of HDL following treatment was unchanged and because of this it is likely that the large HDL produced maintain normal function. An in vitro cellular efflux study showed that these larger HDL particles promoted ABCA1-independent efflux of cholesterol from macrophages while ABCA1-dependent efflux was unchanged. ABCA1-independent efflux by larger HDL particles is mediated via mechanisms that include ABCG1, SR-BI and aqueous diffusion of cholesterol 31.
Despite having HDL cholesterol levels similar to control animals at 8 weeks of treatment, mice in the efavirenz group displayed a significant increase in RCT as measured by the movement of labeled cholesterol from macrophages to feces. The nevirapine group showed a trend towards an increase in RCT but was not significantly different. These changes are consistent with an enhanced uptake of cholesterol from macrophages to HDL and transfer to liver for excretion in mice treated with nevirapine and efavirenz. The lack of a difference among groups for bile acid excretion may indicate that there is no change in the expression of enzymes responsible for bile acid synthesis, the excess cholesterol delivered to liver being excreted in bile as free cholesterol. The increase in total sterol excretion suggests that either free cholesterol excretion via sterol transporter ABCG5/ABCG8 is increased or there is enhanced delivery of cholesterol to liver. The increase in plasma [3H]-cholesterol counts during the in vivo RCT study supports the latter mechanism and indicates that a process upstream of cholesterol unloading from HDL is the responsible for the increase in total sterol excretion, consistent with the results of the in vitro cholesterol efflux studies.
In summary, we observed a transient increase in HDL in both LDL receptor −/− and human apoA-I transgenic mice fed a Western diet in response to nevirapine and efavirenz treatment. This transient HDL increase was associated with an increase in cholesterol efflux from the macrophage to plasma and with enhanced fecal cholesterol excretion. These results suggest that treatment with NNRTIs has a beneficial effect on cholesterol efflux and RCT.
Figure 4.
Composition of HDL isolated by ultracentrifugation from plasma of mice treated with efavirenz for 4 weeks. P=protein, FC=free cholesterol, CE=cholesteryl ester, PL=phospholipid, TG=triglyceride. There was insufficient plasma available for analysis of HDL composition from mice treated with nevirapine.
Acknowledgements
This research was supported by a grant from Boehringer-Ingelheim. We would like to thank Dawn Marchadier for helpful comments and Aisha Wilson for assisting with the animal studies.
Source of Support: Research supported by a grant from Boehringer-Ingelheim.
References
- 1.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 3.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, Lo JC, Schambelan M. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Tebas P, Yarasheski K, Henry K, Claxton S, Kane E, Bordenave B, Klebert M, Powderly WG. Evaluation of the virological and metabolic effects of switching protease inhibitor combination antiretroviral therapy to nevirapine-based therapy for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20:589–594. doi: 10.1089/0889222041217374. [DOI] [PubMed] [Google Scholar]
- 5.Clotet B, van der Valk M, Negredo E, Reiss P. Impact of nevirapine on lipid metabolism. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S79–84. doi: 10.1097/00126334-200309011-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bonjoch A, Paredes R, Domingo P, Cervantes M, Pedrol E, Ribera E, Force L, Llibre JM, Vilaro J, Dalmau D, Cucurull J, Mascaro J, Masabeu A, Perez-Alvarez N, Puig J, Cinquegrana D, Clotet B. Long-term safety and efficacy of nevirapine-based approaches in HIV type 1-infected patients. AIDS Res Hum Retroviruses. 2006;22:321–329. doi: 10.1089/aid.2006.22.321. [DOI] [PubMed] [Google Scholar]
- 7.Young J, Weber R, Rickenbach M, Furrer H, Bernasconi E, Hirschel B, Tarr PE, Vernazza P, Battegay M, Bucher HC. Lipid profiles for antiretroviral-naive patients starting PI- and NNRTI-based therapy in the Swiss HIV cohort study. Antivir Ther. 2005;10:585–591. [PubMed] [Google Scholar]
- 8.Fisac C, Fumero E, Crespo M, Roson B, Ferrer E, Virgili N, Ribera E, Gatell JM, Podzamczer D. Metabolic benefits 24 months after replacing a protease inhibitor with abacavir, efavirenz or nevirapine. AIDS. 2005;19:917–925. doi: 10.1097/01.aids.0000171405.46113.bf. [DOI] [PubMed] [Google Scholar]
- 9.van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, Wood R, Bloch M, Katlama C, Kastelein JJ, Schechter M, Murphy RL, Horban A, Hall DB, Lange JM, Reiss P. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1:e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negredo E, Ribalta J, Paredes R, Ferre R, Sirera G, Ruiz L, Salazar J, Reiss P, Masana L, Clotet B. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. AIDS. 2002;16:1383–1389. doi: 10.1097/00002030-200207050-00010. [DOI] [PubMed] [Google Scholar]
- 11.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, Glesby M, Behrens G, Clotet B, Stellato RK, Molhuizen HO, Reiss P. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8:737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 13.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sviridov D, Chin-Dusting J, Nestel P, Kingwell B, Hoang A, Olchawa B, Starr J, Dart A. Elevated HDL cholesterol is functionally ineffective in cardiac transplant recipients: evidence for impaired reverse cholesterol transport. Transplantation. 2006;81:361–366. doi: 10.1097/01.tp.0000197556.83675.a6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanne JH. Pfizer stops clinical trials of heart drug. BMJ. 2006;333:1237. doi: 10.1136/bmj.39059.438044.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 20.Gerdes LU, Gerdes C, Klausen IC, Faergeman O. Generation of analytic plasma lipoprotein profiles using two prepacked superose 6B columns. Clin Chim Acta. 1992;205:1–9. doi: 10.1016/0009-8981(92)90348-t. [DOI] [PubMed] [Google Scholar]
- 21.Moore RE, Navab M, Millar JS, Zimetti F, Hama S, Rothblat GH, Rader DJ. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- 22.Batta AK, Salen G, Batta P, Tint GS, Alberts DS, Earnest DL. Simultaneous quantitation of fatty acids, sterols and bile acids in human stool by capillary gas-liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;775:153–161. doi: 10.1016/s1570-0232(02)00289-1. [DOI] [PubMed] [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer EJ. Lipoproteins, nutrition, and heart disease. Am J Clin Nutr. 2002;75:191–212. doi: 10.1093/ajcn/75.2.191. [DOI] [PubMed] [Google Scholar]
- 25.Sankatsing RR, Wit FW, Pakker N, Vyankandondera J, Mmiro F, Okong P, Kastelein JJ, Lange JM, Stroes ES, Reiss P. Effects of nevirapine, compared with lamivudine, on lipids and lipoproteins in HIV-1-uninfected newborns: the stopping infection from mother-to-child via breast-feeding in Africa lipid substudy. J Infect Dis. 2007;196:15–22. doi: 10.1086/518248. [DOI] [PubMed] [Google Scholar]
- 26.Sankatsing R, Franssen R, Hassink E, Sauerwein H, Brinkman K, Oesterholt R, Arenas-Pinto A, Storffer S, Kastelein J, Reiss P, Stroes E. Nevirapine Increases High Density Lipoprotein-cholesterol by Stimulation of Apolipoprotein A-I Synthesis. Antiviral Therapy. 2007;12:L5. doi: 10.1161/ATVBAHA.109.192088. abstract. [DOI] [PubMed] [Google Scholar]
- 27.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and Phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 28.Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson JP, Ferguson SS, Negishi M, Goldstein JA. Phenytoin induction of the cyp2c37 gene is mediated by the constitutive androstane receptor. Drug Metab Dispos. 2006;34:2003–2010. doi: 10.1124/dmd.106.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handschin C, Meyer UA. Regulatory network of lipid-sensing nuclear receptors: roles for CAR, PXR, LXR, and FXR. Arch Biochem Biophys. 2005;433:387–396. doi: 10.1016/j.abb.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]