Introduction
Much as the symbiotic evolutionary origins of human mitochondrial genes helps us understand their role in disease, so the symbiotic origins of HERVs helps us understand the pathogenic role of whole viruses, viral genes, viral regulatory sequences and other virus-related sequences in the human genome. Extrapolating from Part 2,1 we might anticipate some general principles. For example, viral elements may cause disease through virus-specific evolutionary mechanisms, such as recombination, or through replication and unwanted insertion. Disease may also result from the dysregulation of an established symbiotic viral gene, or genetic pathway, or through the cooption of such ‘normal’ viral roles in complex, multistep disease progressions. We shall look at some general examples before extrapolating these to the autoimmune diseases.
HERVs in miscellaneous disease
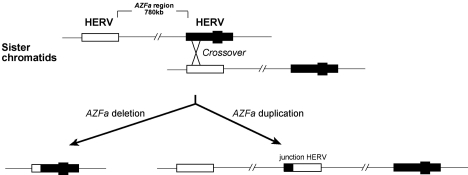
Pandemic flu viruses arise through the genomic recombination of different viruses within the same host, a symbiogenetic evolutionary mechanism.2 HERVs retain this essentially viral capacity and this can lead to recombinations across homologous, and even non-homologous, chromosomes. Since the Y chromosome is solitary (haploid), its vertebrate lineage cannot undergo homologous sexual recombination during meiosis, but its viral components can recombine, and when they do so they carry the associated fragments of the chromosome with them. For example, deletions of a genetic block known as ‘azoospermia factor a’ (AZFa) are brought about by recombination between HERV15 elements flanking this region, leading to the complete failure of development of germ cells. This is known as Sertoli-cell only syndrome (SCOS), a form of male infertility.3–5 This is illustrated in Figure 1, where we note that the same recombination can also cause duplication of region AZFa – in this case, associated with normal fertility. Foerster and colleagues have reported a possible link between a HERV-K insert and susceptibility to psoriasis.6 Kazazian and colleagues have reported two separate insertions of truncated LINE-1s into the factor VIII gene, resulting in haemophilia.7 LINE-1 insertions into the dystrophin gene have also been found to cause muscular dystrophy.8,9
Figure 1.
HERV-15 recombination between Y chromosomes during meiosis, leading to deletion or duplication of AZFa genetic domain
Kudaka and colleagues have shown significant decrease in expression of the fusogenic HERV protein, syncytin-1, in placentas of women with pregnancy-induced hypertension, concluding that this dysfunction may be linked to the pathogenesis of the hypertension.10 Other authors have raised the possibility that HERVs might contribute to organic disease of the brain as well as mental diseases. Schizophrenia is a complex disorder, with family and twin studies suggesting multiple environmental and genetic factors. Among the environmental factors, infectious agents and viruses in particular, have been considered as possible triggers. The discovery of retroviral transcripts in brain tissue, cerebrospinal fluid and plasma of recently diagnosed individuals suggested that HERVs might play some role in the pathogenesis. In 2005, Frank and colleagues performed a comprehensive micro-array-based analysis of HERV transcriptional activity in 215 human brain samples derived from normal controls and patients with schizophrenia and bipolar disorders.11 They reported a ‘brain-specific’ HERV activity profile in both test and control brain samples that included HERV-E, HERV-F, ERV9 and HERV-K, meanwhile a subgroup of HERV-K10 was weakly associated with schizophrenia and bipolar disorder. This tallies with earlier reports, which showed qualitative and quantitative differences in HERV expression in brain tissues from patients with schizophrenia and other neuro-psychiatric disorders.12–15 However, the pattern of these findings suggests more a physiological response to the disease rather than its causation. Earlier studies had raised the possibility that exogenous viral infection might trigger pathogenic HERV expression. When Nellåker and colleagues looked at the effects of influenza and herpes viruses on HERV expression profiles in human cell lines, they found that HERV-W elements, whose env genes code for syncytin, were expressed in cell-specific patterns that appeared to be modified by the exogenous viral influences.16 The role of such viral interactions in schizophrenia remains speculative. While it seems likely, given the widespread expression of HERVs and their products in the normal human brain, that these will play some role in neuro-psychiatric disorders, we need to unravel their roles in normal physiology before we can extrapolate this to any significant role in disease.
The vast number of Alu inserts in the human genome creates abundant opportunities for unequal homologous recombination events, mostly intra-chromosomal, which result in deletion or duplication of exons within genes. Like HERVs, they also give rise to unequal homologous recombination of whole segments of chromosomes, resulting in major genetic abnormalities. These ‘Alu-induced mutations’ give rise to a formidable range of diseases, which includes familial hypercalciuric hypercalcaemia and neonatal severe hyperparathyroidism, neurofibromatosis, XSCID, haemophilia, Apert's syndrome, cholinesterase deficiency, hereditary desmoid disease, X-linked agammaglobilinaemia, complement deficiency, glycerol kinase deficiency, diabetes mellitus type II, together with a wide range of germ-line disorders.17,18 Although Alus produce diseases in a manner similar to classical mutations, their behaviour and genetic mechanisms are essentially viral. Villarreal classifies Alus separately from SINES, though both appear to function as ‘hyperparasites’ (genetic parasites of other genetic parasites), yet ‘all [such viral elements] appear to be acting in some concerted fashion, capable of both cooperating and interfering’ (personal communication).19
HERVs in autoimmune disease
Autoimmune diseases constitute an important group of conditions that affect approximately 4% of the population in industrialized countries.20 The pathology appears to involve an immune reaction to the body's own cells or tissues – or, to put it another way, the failure of the adaptive immune system to recognize self. Historically, we recognize that various genes within the human extended Major Histocompatibility Complex (xMHC) (similar but not identical to the Human Leukocyte Antigen Complex [HLA]), are intimately associated with auto-immunity. Indeed the human xMHC is a key genetic region with regard to the evolution, and expression, of our adaptive immunity, and 22% of its 421 genes have putative immunoregulatory function.21 Thus an understanding of the evolutionary origins of both the xMHC and the adaptive immune system should assist understanding of the processes involved in auto-immunity. Genetic analysis has revealed that the xMHC, which is still rapidly evolving, arose through block duplications of simpler ancestral patterns, followed by diversification, leading to five subregions, the extended class I, classical class I, classical class III, classical class II, and extended class II.22,23 We have seen how HERV recombination may create this pattern of block duplications, and, as Dawkins and colleagues demonstrate, the human MHC is densely colonized by HERVs and retroelements, which are likely to have played an important part in its evolution.24 Villarreal has probed the evolution of adaptive immunity, and the origin of self, to propose a comprehensive hypothesis in which viruses in general, and retroviruses in particular, have played a key role in the evolution of immunity and the recognition of self from the simpler non-adaptive systems of marine invertebrates, to the sudden, almost explosive, origins of true adaptive immunity in jawed fish, with its subsequent refinements, each accompanied by new expansions of retroviruses, with the origins of mammals, and, finally, primates.25 Such a system, evolving in important part by symbiogenetic interaction between retroviruses and vertebrate host, and still dense today with HERVs and their products, make it likely that viral elements will play a significant role in autoimmune diseases. Indeed, as Dawkins suggests, ‘if HERV sequences can be protective, there are exciting prospects for [therapeutic] manipulation’.
But before we can extrapolate this further, we require a clearer understanding of the genetic mechanisms that underlie autoimmune diseases, and further we need to disentangle the role of HERVs and their products within the complex whole.
Several hundred diseases have xMHC specific associations.23,24 For example, Lie and Thorsby accept the highly specific association of ankylosing spondylitis with HLA B27, type 1 diabetes with the primary risk genes DRB1, DQA1 and DQB1, and Coeliac Disease with HLA-DQ2 and HLA-DQ8, all of which are likely to be linked to pathogenesis.20 Meanwhile the original associations between a wide range of diseases, including systemic lupus erythematosis and specific HLA loci, are now seen to be inadequate, with problems arising from linkage disequilibrium and polymorphic gene variability, coupled with a more complex, multigenetic linkage. It seems increasingly likely that many autoimmune conditions will be linked not to a single locus or allele but to multiple predisposing genes within the extended MHC locus. This would fit with the tendency for diverse groups of autoimmune diseases to be associated with clusters of genetic loci known as ‘ancestral haplotypes’, such as the AH 8.1 haplotype, which gathers together the alleles HLA-A*01, −B*08, −DRB1*03, −DQB1*02 and −DQA1*05, and which is associated with more than 30 autoimmune diseases, including type 1 diabetes, Grave's disease, Addison's disease, SLE and myasthenia gravis. Researchers are extending such studies to the links between various autoimmune diseases and viruses, notably HERVs and HERV-related products.
Type 1 diabetes
Superantigens are toxins, usually produced by microbes, that elicit a massive immune over-reaction that is useless physiologically and damaging to the host. In 1997, Conrad and colleagues reported that the env gene of a newly reported HERV-K 10-like endogenous retrovirus appeared to encode a superantigen that was a candidate autoimmune gene in insulin-dependent diabetes.26 However, these findings were disputed by colleagues,27,28 who suggested that more refined experiments were needed. In 2002 Portis reviewed the prevailing perspectives on HERVs in autoimmune disease, acknowledging the difficulties.29 By now circulating antibodies to various HERV antigens or viral gene expression had been found in patients with SLE,30 rheumatoid arthritis,31 alopecia areata,32 Sjögren's syndrome,33 congenital heart block,34 type 1 diabetes, multiple sclerosis and primary biliary cirrhosis.35 Unfortunately, all such associations were confounded by the lack of understanding of the normal expression of HERV sequences at tissue or cellular level. This was further complicated by the fact that HERV promoter sequences, found in the viral LTRs, contained binding sites for a variety of transcription factors involved in inflammatory responses, suggesting that HERV expression might well be a response to, rather than the cause of, these diseases.
Katsumata and colleagues confirmed this when they showed that HERV expression was increased in vascular endothelial cells by known products of the inflammatory responses, such as TNF-α, IL-1α, and IL-1β,36 and when Johnson and colleagues observed that the expression of HERV mRNAs increases on exposure of macrophages to stimulants, such as PMA, which also trigger the release of extracellular virus-like particles.37 Portis, in his review, re-visited the superantigen-in-diabetes controversy, drawing attention to the fact that the HERV-K related sequence found in the pancreatic islets of patients with insulin dependent diabetes mellitus (IDDMK1222) had now been identified as the env gene of HERV-K18, and this had been located to the first intron of the CD48 gene on chromosome 1. Conrad and colleagues had also shown that HERV-K18, which appears to be a solitary insert, coded for three alleles, one of which was IDDMK1222 and the other two were full-length env genes, all of which encoded superantigens. Expression of HERV-K18 superantigens could be induced by interferon-α (IFNα) and was associated with rapid expansion of Vβ7+ T cells, which can be associated with insulin-dependent diabetes.38 Since these interferons are key regulators of the adaptive immune response to exogenous virus infection, Portis suggested a way in which exogenous virus infection might trigger the highly specific HERV-K18 superantigen response, leading to the expansion of autoreactive T cells in an organ-specific fashion – in other words the organ specificity might be based on the tissue tropism of the exogenous virus. These findings merit further research and investigation.
Systemic lupus erythematosis
Systemic lupus erythematosis (SLE) is associated with dysregulated activation of both T and B lymphocytes and with the development of autoantibodies, notably against double-stranded DNA. Susceptibility to the disease has been linked to a number of MHC genes, but, despite extensive study, no primary risk genes have emerged. This has encouraged various groups to explore the possibility of endogenous viral involvement. HERV clone 4-1 is a member of the HERV-E family, which is widely distributed in the human genome. A complete endogenous retrovirus, with open reading frames in gag, env and pol, there are 85 copies of the virus at various integrations sites in the human chromosomes. Sekigawa and colleagues investigated this particular HERV for a potential role in SLE.39 They began by showing that messenger RNA, encoding HERV clone 4-1 gag sequences, was expressed in peripheral blood lymphocytes of SLE patients but not in normal controls. They also showed significantly higher expression of the gag domain in the lymphocytes of patients with SLE when compared with rheumatoid arthritis. Steroids and immunosuppressant therapy inhibited this HERV expression. Components of endogenous retroviruses, notably p15E, an env-encoded transmembrane protein, induce several immune abnormalities in vitro, for example inhibition of IL-2 production and suppression of the lymphocyte proliferative response. The same authors showed that synthetic clone 4-1-derived p15E peptides induced CD4+ T-cell activation and anergy in vitro, as well as inducing the production of several cytokines, such as IL-16, the latter strongly associated with disease activity in SLE. This led them to propose a mechanism for activation of CD4+ T-cells, involving viral components from HERV clone 4-1, that might contribute to the loss of self-tolerance and the induction of SLE-related autoimmune phenomena.
An additional, instructive, finding emerged from this study. In the standard human sequence of the clone 4-1 domain, the gag gene is switched off by four separate stop codons. Stop codons are mutations that permanently switch off vertebrate genes. But not so viral genes, where viral recombination is capable of removing them. In all three SLE patients tested, Sekigawa discovered unstopping of three of the four codons. This may have enabled an unusual expression of the gag sequences in these patients. The authors concluded that inappropriate expression of the gag sequences, perhaps coupled with the evidence for lower levels of demethylation of the viral sequences in patients with SLE, may play a part in the pathogenesis.
Another line of study has pointed to a role for HERVs in the pathogenesis of SLE. Mice deficient in the enzyme deoxyribonuclease 1 (DNase1), which helps to break down unwanted DNA and chromatin-protein complexes in cells, have clinical features and serological findings that closely resemble human SLE. Thus it seemed particularly relevant when Yasutomo and colleagues discovered a mutation in the gene, DNASE1, in two young female patients with SLE.40 In these patients, the level of antibodies to nucleosomal antigens was 7–8 times greater than in patients with SLE without the mutation, and 70–80 times greater than normal controls, and the level of antibodies to double-stranded DNA was also proportionately higher. The authors concluded that low activity of DNASE1 in these two patients led to the accumulation of unwanted chromatin and chromatin-protein complexes, which contributed to the pathogenesis. This encouraged researchers to widen the exploration of DNASE1 expression, and to include the potential of HERV interaction at the locus.
In a third line of study, Stetson and colleagues have discovered a cell-intrinsic mechanism that suggests a novel contribution of endogenous retroelements.41 Detection of potentially alien nucleic acids and the induction of type I interferons (IFNs) are key elements of the body's defence against exogenous viral invasion, but dysregulation of the same mechanisms can cause autoimmunity. Detection of DNA within the cytoplasm of cells (cytosolic detection) activates a potent cell-intrinsic antiviral response that, while poorly understood, is coordinated by type I interferons (IFNs), and these in turn direct a multifaceted programme of response that restricts viral replication within infected cells, alerting neighbouring cells to the presence of infection and expanding effector lymphocytes to provide specific protection against a potential viral invader. Two different nucleic acid detection systems – toll-like receptors within the membranes of sentinel immune cells and cytosolic sensors within individual infected body cells – account for all IFN-mediated antiviral immunity. However, the ability of these pathways to discriminate between viral and self nucleic acids is imperfect and defective clearance of self-derived nucleic acids can cause severe, IFN-associated autoimmunity. This, for example, was the pattern seen with low activity of DNASE1 seen in Yasumoto's two patients.
While screening for proteins that might be controlling this cytosolic receptor response, Stetson and colleagues identified a key enzyme, known as Trex 1. Mutations in the human Trex 1 gene cause Aicardi-Goutieres syndrome, which presents in infancy with severe encephalitis, lymphocyte infiltrations of the brain and elevated type I IFN levels in cerebrospinal fluid, accompanied by demyelination of motor neurones and psychomotor retardation. Other Trex 1 mutations cause monogenic chilblain lupus, and these same mutations can be associated with SLE. But up to now little was known about the specific mechanisms linking Trex 1 to autoimmunity. In mice, these authors found that Trex 1 is an essential negative regulator of the cytosolic sensor response and they went on to show how Trex 1 deficiency gave rise to systemic autoimmunity in the animals with lethal myocarditis in early life. Their data suggested that Trex 1 deficiency resulted in the accumulation of DNA substrates within the affected cardiac cells. When they analysed the DNA within affected cells, they discovered that the bulk of the fragments were derived from the LINE-1s, LTRs and SINEs of endogenous retroviruses. They went on to demonstrate that Trex 1 specifically targets retroviral DNA sequences. Presumably this retroviral DNA turnover was part of the normal cell metabolism and would have been degraded and removed from the cell through the action of Trex 1. In the lack of Trex 1, this DNA waste accumulated, triggering the dysregulated interferon-mediated response, which appears to have led to the observed autoimmunity.
The authors proposed that, given the ubiquity of endogenous retroviruses and their sequences in normal metabolism, Trex 1 may have evolved as a defence mechanism against autoimmunity – and if so, this may have more general implications for autoimmune diseases.
Multiple sclerosis
In 1997 Perron and colleagues reported a novel retrovirus (MS-associated retrovirus, or MSRV), which was later recognized as belonging to the HERV-W family, and which was repeatedly isolated from patients with multiple sclerosis.42 In 2001, the same authors produced evidence that the env gene of this retrovirus induced a T-lymphocyte response that might play a significant role in the immunopathology of MS, with a pattern similar to that of a superantigen.43 A year later, they developed a hybrid animal model in which severe combined immunodeficiency mice were grafted with human lymphocytes and injected intraperitoneally either with MSRV virion or a control, after which the MSRV-injected mice developed fatal brain haemorrhages leading to death.44 However, that same year Nowak and colleagues reported MSRV pol sequences in the blood of patients with other neurological conditions as well as controls, albeit the incidence was significantly higher in untreated MS patients.45 The situation was further complicated when Perron's group reported that gag and env proteins encoded by HERV-W are expressed by normal cells in the central nervous system, a finding confirmed by other reserchers.46 Nevertheless they felt that the quantitative expression was significantly raised in MS lesions.
Much the same conclusion was drawn by Dolei and colleagues,47,48 who not only confirmed the presence of HERV-W env and pol RNA transcripts in normal brain, but also found considerable difference in the quantitative expression of the viral transcripts, which showed a 20- to 25-fold increase in brain samples from MS patients. Employing a monoclonal anti-HERV-W antibody, 6A2B2, they also showed striking immunoreactivity for MSRV/HERV-W in specific MS lesions when compared to controls, with intense staining for MSRV/HERV-W env expression in chronically active MS lesions, where it was localized to cells that resembled microglia and astrocytes. Intense staining for the viral env expression was also found within astrocytes at the plaque core.
In 2004, Antony and colleagues demonstrated increased expression of the HERV-W env gene, syncytin-1, in glial cells within acute demyelinating lesions.49 The syncytin-1 induced astrocytes to produce redox reactants that were lethally cytotoxic to oligodendocytes, the cells responsible for myelin. These authors also showed that expression of syncytin-1 in murine models resulted in demyelination in vivo. In 2007, Mameli's group extended this line of study with the discovery that the same cytokines involved in the pathogenesis of MS played an important role in the regulation of syncytin in the astrocytes.50 Antony and colleagues looked further at the role of syncytin-1 in its mediation of neuroimmune activation and oligodendroycyte damage, discovering that a key receptor for syncytin-1, known as ASCT1, was selectively suppressed in the astrocytes of brain tissue obtained from patients with MS.51 They also showed that syncytin-1 induced the expression of the endoplasmic reticulum stress sensor, known as old astrocyte specifically induced substance, or OASIS, in cultured astrocytes, again seen in MS brains. When they reduced the expression of syncytin-1, using RNAi, this blocked the suppression of ASCT1, which in turn prevented the release of the oligodendrocyte-directed toxins from the astrocytes. They also showed that syncytin-1 regulated neuroinflammation and its receptor expression in MS, suggesting a role for the endoplasmic reticulum stress sensor in the pathogenesis.
In 2008, Mameli's group showed that the presence, and viral load, of MSRV in the blood and cerebrospinal fluid of MS patients was positively associated with the clinical stage and progression of MS, and blood levels fell below detection limits in the majority of a group of patients after three months of beta-interferon therapy, suggesting that not only is the virus playing a role in the pathogenesis but also that evaluation of plasma MSRV might offer a prognostic marker for therapy outcome in individual patients.52
By now the weight of evidence was suggesting that the env genetic domain of a HERV-W virus was expressing a syncytin-like protein that contributed, to an important degree, to the pathogenesis of MS. Power's group went so far as to propose that these findings may offer a ‘target for therapeutic intervention’. But key questions remained. What is the source of the syncytin-like protein expressed in the astrocytes? Is it ERVWE1, the env gene on chromosome 7 that codes for placental syncytin-1? Is it the putative MSRV/HERV-W? If MSRV/HERV-W, is it possible, for example, that this alternative source of a syncytin-like env protein might be dysregulating a physiological role of syncytin-1 in the astrocytes?
In 2009 a study by Laufer and colleagues analysed the transcribed loci of HERV-W env sequences within the human genome, with the express aim of clarifying the MS-related retrovirus env contribution.53 This produced several surprises. In a previous study, Pavlicek and colleagues had shown that the HERV-W family has inserted into roughly 650 positions dispersed throughout the human chromosomes.54 Many of these have been reduced to isolated LTRs, leaving some 280 elements with internal genetic sequences, most of which had been rendered defective through the acquisition of stop codons, frameshift mutations and deletions. The only known completely intact and functional HERV-W env locus was the established ERVWE1, located on chromosome 7 (7q21.2), which codes for the syncytin-1 complete envelope protein important to placentation. But in 2006 Rolland and colleagues had reported that the env sequence (AF331500) from the purported MSRV virus was significantly different from that of ERVWE1, showing 87% sequence homology.55 Messenger RNA can be transcribed in vitro using reverse transcriptase, to produce its complementary DNA sequence, which is known as cDNA. In the genomes of peripheral blood mononuclear cells of MS patients and controls, the Ruprecht group now focused on these complementary DNA sequences to show that almost 30% of the HERV-W env cDNAs extracted by this technique were the result of recombinations of HERV-W env elements from different chromosomal integrations sites, most likely generated in vitro. In other words, the MSRV env and gag sequences published in previous studies could be explained as sequences originating either from HERV-W loci on the chromosomes or from recombinations of the products of these viral loci in vitro. If so, this might have given rise to confusion in previous studies. By clarifying the origin of MSRV sequences, they attempted to resolve the longstanding confusion and debate surrounding this putative virus and its potential role in the pathogenesis of multiple sclerosis.
In all they identified seven transcribed HERV-W env loci in the chromosomes of human peripheral blood mononuclear cells. They confirmed the HERV-W env locus, ERVWE1, coding for the placental syncytin-1, and located on chromosome 7 (7q21.2), is transcribed in the mononuclear cells of normal controls and patients with MS. The other six transcribed HERV-W loci had various deletions and truncations of their genetic domains and LTRs. But among these they identified a second transcriptionally active HERV-W env element, located on the X chromosome (Xq22.3), which contained an almost complete env gene, interrupted by a single premature stop codon in its 5′ region at amino acid codon position 39. The longest possible transcribable sequence from this locus would give rise to a truncated syncytin-like sequence of 475 amino acids. The env sequence (AF331500) reported by Rolland could be explained as an in vitro recombination of this sequence and another defective HERV-W env sequences, on chromosome 5. In their analysis, the env clone AF127228 and the region coding for the surface domain of the env clone AF331500 corresponded to the HERV-W env element on chromosome X. As the authors express it, ‘the amino acid sequence of a recombinant MSRV env SU protein, which has been shown by Rolland to have proinflammatory effects in various assays, and which was generated using the AF331500 MSRV env clone, is identical to the amino acid sequence of the HERV-W env protein putatively encoded by Xq22.3 HERV-W env’.
There is an additional, important, inference. The stop codons in the two env sequences, AF331500 and Xq22.3, differ in a single nucleotide. The elimination of the stop codon at position 39 of the HERV-W env Xq22.3 would result in an uninterrupted full-length HERV-W env open reading frame capable of encoding a complete env protein that contained a signal peptide. This stop codon of HERV-W env Xq22.3 might readily be unstopped by recombination with other HERV-W env elements, which were shown to contain the necessary triplet at the right place. They also showed that the monoclonal antibody, 6A2B2, which reacts with a HERV-W env antigen in MS lesions, was actually generated against a fragment of the Xq22.3 HERV-W env sequence. Although the 6A2B2 antibody may cross-react with syncytin-1, these findings raised the possibility that the antigen detected in MS lesion could be encoded by the Xq22.3 HERV-W env locus.
In a forthcoming paper, Mameli and colleagues searched for a reliable means of differentiating MSRVenv from syncytin-1 sequences.56 They included twelve variants of MSRV env and eight variants of syncytin-1 variant sequences, comprising all those deemed suitable from GenBank as well as those detected experimentally in their own cohort under study. From this extensive group, they discovered a 12-nucleotide insertion in the trans-membrane moiety of the MSRV env, which was present in all twelve MSRV env variants they tested, yet was not present in any of the eight syncytin-1 variants. They also noticed that the syncytin-1 sequences were highly conserved when compared with the MRSV env sequences, confirming the known operation of selection on the ERVWE1 locus at holobiontic level. Based on this newly-discovered insertion, they now developed discriminatory real time PCR assays that could selectively amplify either MRSV env or syncytin-1. Previous data had shown that both MSRV and ERVWE1 were expressed in the brains of MS patients, while only MSRV was found in peripheral blood, and was expressed by cultures of peripheral blood monocytes (PBMCs) in blood-positive individuals. While syncytin-1 had been found intracellularly and on the plasma membrane, it had not been detected extracellularly and its sequences had not been expressed in the MSRV virus-like particles, which were visible on electron microscopy, and contained demonstrable reverse transcriptase activity and all three HERV genetic domains. Now, using their newly constructed PCR assay, Mameli and colleagues were able to compare and contrast the expressions of HERV-W generic env, MRSV env and ERVWE1 env (syncytin-1) expression in the plasma, in cultured PBMCs and in the supernatant of the cultures cells, in four MS patients who had not yet been treated, in four MS patients who had been treated and in six healthy blood donors acting as controls. The results were striking.
The controls showed no expression of any of the three env sequences. The untreated MS patients showed high levels of expression of MSRV env in all three test situations, with markedly reduced titre of expression in the treated MS patients. A similar, but much lower level of expression of generic HERV-W env sequences was seen in all of the MS patients. Meanwhile there was no measurable expression of ERVWE1 env (syncytin-1) in the plasma or culture supernatant of any of the MS patients, and it was only seen to be expressed in very low titre in a single untreated MS patient within the PBMCs. The authors concluded that these patterns of env expression confirmed the link between MS and the putative MSRV.
These latest findings, complementing earlier studies, appear to provide important confirmation of the role of a specific HERV-W env gene in the pathogenesis of MS, suggesting that this is less likely to be related to the syncytin-1 coded by ERVWE1, located on chromosome 7 (7q21.2), and more likely corresponds to the unstopped or truncated HERV-W env on chromosome Xq22.3, or to a low-grade HERV-W-like exogenous retrovirus, MSRV. Mameli acknowledges, that ‘theoretically the (Laufer) findings are not in contrast with the data of the present study,’ while not being persuaded by the chromosome Xq22.3 findings. The remaining differences would appear to be resolvable through further study of larger numbers of untreated MS patients with active disease, and through the application of the new findings to the specific pathology in the central nervous system.
In summary
It seems likely that HERV-W env expression of a syncytin-like protein is important in the pathogenesis of MS. Meanwhile HERVs and their related sequences may play a part in the pathogenesis of other autoimmune diseases, such as type-1 diabetes and systemic lupus erythematosis. The growing evidence of these studies, coupled with the likely role of endogenous retroviruses in the evolutionary origins and prevailing structure of the MHC, suggests that the endogenous retroviral contribution should form an integral part of a coordinated research approach to the autoimmune disorders.
Part 4 of this series will examine the role of HERVs and related sequences in cancer.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor FPR
Contributorship FPR is the sole contributor
Acknowledgements
The author would like to thank Klemens Ruprecht, Guiseppe Mameli, Christopher Power and Erik Larsson for their assistance and advice with this paper. Figure 1 was kindly provided by Mark Jobling
References
- 1.Ryan FP. An alternative approach to medical genetics based on modern evolutionary biology. Part 2: retroviral symbiosis. J R Soc Med 2009;102:324–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roossinck MJ. Symbiosis versus competition in plant virus evolution. Nat Rev Microbiol 2005;3:917–24 [DOI] [PubMed] [Google Scholar]
- 3.Sun C, Skaletsky H, Rozen S, et al. Deletion of azoospermia factor a (AZFa) region of human Y chromosome caused by recombination between HERV15 proviruses. Hum Mol Genet 2000;9:2291–6 [DOI] [PubMed] [Google Scholar]
- 4.Bosch E, Jobling MA. Duplications of the AZFa region of the human Y chromosome are mediated by homologous recombination between HERVs and are compatible with male fertility. Hum Mol Genet 2003;12:341–7 [DOI] [PubMed] [Google Scholar]
- 5.Kamp C, Hirschmann P, Voss H, et al. Two long homologous retroviral sequence blocks in proximal Yq11 cause AZFa microdeletions as a result of intrachromosomal recombination events. Hum Mol Genet 2000;9:2563–72 [DOI] [PubMed] [Google Scholar]
- 6.Foerster J, Nolte I, Junge J, et al. Haplotyupe sharing analysis identifies a retroviral dUTPase as candidate susceptibility gene for psoriasis. J Invest Derm 2005;124:99–102 [DOI] [PubMed] [Google Scholar]
- 7.Kazazian HH, Wong C, Youssoufian H, et al. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature 1988;332:164–6 [DOI] [PubMed] [Google Scholar]
- 8.Narita N, Nishio H, Kitoh Y, et al. Insertion of a 5′ truncated L1 element into the 3′ end of exon 44 in the dystrophin gene resulted in skip of the exon during splicing in a case of Duchenne muscular dystrophy. J Clin Invest 1993;91:1862–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes SE, Dombroski BA, Krebs C, et al. A new retrotransposable human L1 element from the LRE2 locus on chromosome 1q produces a chimaeric insertion. Nat Genet 1994;7:143–8 [DOI] [PubMed] [Google Scholar]
- 10.Kudaka W, Oda T, Jinno Y, et al. Cellular localization of placenta-specific human endogenous retrovirus (HERV) transcripts and their possible implication in pregnancy-induced hypertension. Placenta 2008;29:282–9 [DOI] [PubMed] [Google Scholar]
- 11.Frank O, Giehl M, Zheng C, et al. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J Virol 2005;79:10890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yolken RH, Karlsson, Yee F, et al. Endogenous retroviruses and schizophrenia. Brain Res Rev 2000;31:193–9 [DOI] [PubMed] [Google Scholar]
- 13.Karlsson H, Bachmann S, Schroder J, et al. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. PNAS 2001;98:4634–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otowa T, Tochigi M, Rogers M, et al. Insertional polymorphisms of endogenous retrovirus HERV-K115 in schizophrenia. Neurosci Lett 2006;408:226–9 [DOI] [PubMed] [Google Scholar]
- 15.Kim H-S, Ahn K, Kim D-S. Quantitative expression of the HERV-W env gene in human tissues. Arch Virol 2008;153:1587–91 [DOI] [PubMed] [Google Scholar]
- 16.Nellåker C, Yuanrong Y, Jones-Brando L, et al. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology 2006;3:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet 2002;3:370–80 [DOI] [PubMed] [Google Scholar]
- 18.Sukarova E, Dimovski AJ, Tchacarova P, et al. An alu insert as the cause of a severe form of hemophilia A. Acta Haematologica 2001;106:126–9 [DOI] [PubMed] [Google Scholar]
- 19.Villarreal LP. Viruses and the Evolution of Life. Washington, DC: ASM Press; 2005 [Google Scholar]
- 20.Lie BA, Thorsby E. Several genes in the extended human MHC contribute to predisposition to autoimmune disease. Curr Opin Immunol 2005;17:526–31 [DOI] [PubMed] [Google Scholar]
- 21.Fernando MMA, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genetics 2008;4:e1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukami-Kobayashi K, Shiina T, Anzai T, et al. Genomic evolution of MHC class I region in primates. PNAS 2005;102:9230–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiina T, Inoko H, Kulski JK. An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens 2004;64:631–49 [DOI] [PubMed] [Google Scholar]
- 24.Dawkins R, Leelayuwat C, Gaudieri S, et al. Genomics of the major histocompatibility complex: haplotypes, duplication, retroviruses and disease. Immunol Rev 1999;167:275–304 [DOI] [PubMed] [Google Scholar]
- 25.Villarreal L. The source of self: genetic parasites and the origin of adaptive immunity. Ann New York Acad Sci (in press) [DOI] [PubMed] [Google Scholar]
- 26.Conrad B, Weissmahr RN, Böni J, et al. A human endogenous retroviral superantigen as candidate autoimmune gene in type 1 diabetes. Cell 1997;90:303–13 [DOI] [PubMed] [Google Scholar]
- 27.Murphy VJ, Harrison LC, Rudert WA, et al. Retroviral superantigens and type 1 diabetes mellitus. Cell 1998;95:9–11 [DOI] [PubMed] [Google Scholar]
- 28.Löwer R, Tönjes RR, Boller K, et al. Development of insulin-dependent diabetes mellitus does not depend on specific expression of the human endogenous retrovirus HERV-K. Cell 1998;95:11–16 [DOI] [PubMed] [Google Scholar]
- 29.Portis JL. Perspectives on the role of endogenous human retroviruses in autoimmune diseases. Virology 2002;296:1–5 [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson A, Blomberg J, Nived O, et al. Selective antibody reactivity to peptides from human endogenous retroviruses and nonviral poly(amino acids) in patients with systemic lupus erythematosus. Arthritis Rheum 1996;39:1654–63 [DOI] [PubMed] [Google Scholar]
- 31.Seidl C, Horst D, Petershofen E, et al. An endogenous retroviral long terminal repeat at the HLA-DQB1 gene locus confers susceptibility to rheumatoid arthritis. Hum Immunol 1999;60:63–8 [DOI] [PubMed] [Google Scholar]
- 32.La Placa M, Vitone F, Bianchi T, et al. Serum antibodies against human intracisternal A-type particle (HIAP)endogenous retrovirus in alopecia areata patients: a hallmark of autoimmune disease? J Invest Dermatology 2004;123:407–9 [DOI] [PubMed] [Google Scholar]
- 33.Yamano S, Renard JN, Mizuno F, et al. Retrovirus in salivary glands from patients with Sjögren's syndrome. J Clin Pathol 1997;50:223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JM, Fan WS, Horsfall AC, et al. The expression of human endogenous retrovirus-3 in fetal cardiac tissue and antibodies in congenital heart block. Clin Exp Immunol 1996;104:388–93 [PubMed] [Google Scholar]
- 35.Mason AL, Xu L, Guo L, et al. Retroviruses in autoimmune liver disease: genetic or environmental agents? Arch Immunol Ther Exp (Warsz) 1999;47:289–97 [PubMed] [Google Scholar]
- 36.Katsumata K, Ikeda H, Sato M, et al. Cytokine regulation of env gene expression of human endogenous retrovirus-R in human vascular endothelial cells. Clin Immunol 1999;93:75–80 [DOI] [PubMed] [Google Scholar]
- 37.Johnson JB, Silva C, Holden J, et al. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann Neurol 2001;50:434–42 [DOI] [PubMed] [Google Scholar]
- 38.Stauffer Y, Marguerat S, Meylan F, et al. Interferon-alpha-induced endogenous superantigen: a model linking environment and autoimmunity. Immunity 2001;15:591–601 [DOI] [PubMed] [Google Scholar]
- 39.Sekigawa I, Ogasawara H, Naito T, et al. Systemic lupus erythematosus and human endogenous retroviruses. Mod Rheumatol 2003;13:107–13 [DOI] [PubMed] [Google Scholar]
- 40.Yasutomo K, Horiuchi Y, Kagami S, et al. Mutation in DNASE1 in people with systemic erythematosus. Nat Genet 2001;28:313–14 [DOI] [PubMed] [Google Scholar]
- 41.Stetson DB, Ko JS, Heidmann T, et al. Trex 1 prevents cell-intrinsic initiation of autoimmunity. Cell 2008;134:587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perron H, Garson JA, Bedin F, et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. PNAS 1997;94:7583–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perron H, Jouvin-Marche E, Ounanian-Paraz A, et al. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vβ16-T-lymphocyte activation. Virology 2001;287:321–32 [DOI] [PubMed] [Google Scholar]
- 44.Firouzi R, Rolland A, Michel M, et al. Multiple sclerosis-associated retrovirus particles cause T Lymphocyte-dependent death with brain hemorrhage in humanized mice model. J Neurovirol 2003;9:79–93 [DOI] [PubMed] [Google Scholar]
- 45.Nowak J, Januszkiewicz D, Pernak M, et al. Multiple sclerosis-associated virus-related pol sequences found both in multiple sclerosis and healthy donors are more frequently expressed in multiple sclerosis patients. J Neurovirol 2003;9:112–17 [DOI] [PubMed] [Google Scholar]
- 46.Perron H, Lazarini F, Ruprecht K, et al. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J Neurovirol 2005;11:23–33 [DOI] [PubMed] [Google Scholar]
- 47.Dolei A, Serra C, Mameli G, et al. Multiple sclerosis-associated retrovirus (MSRV) in Sardinian MS patients. Neurology 2002;58:471–3 [DOI] [PubMed] [Google Scholar]
- 48.Mameli G, Astone V, Giannina A, et al. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J Gen Virol 2007;88:264–74 [DOI] [PubMed] [Google Scholar]
- 49.Anthony JM, van Marle G, Opii W, et al. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci 2004;7:1088–95 [DOI] [PubMed] [Google Scholar]
- 50.Mameli G, Astone V, Khalili K, et al. Regulation of the syncytin-1 promoter in human astrocytes by multiple sclerosis-related cytokines. Virology 2007;362:120–30 [DOI] [PubMed] [Google Scholar]
- 51.Antony JM, Ellestad KK, Hammond R, et al. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J Immunol 2007;179:1210–24 [DOI] [PubMed] [Google Scholar]
- 52.Mameli G, Serra C, Astone V, et al. Inhibition of multiple sclerosis-associated retrovirus as biomarker of interferon therapy. J Neurovirol 2008;14:73–7 [DOI] [PubMed] [Google Scholar]
- 53.Laufer G, Mayer J, Mueller BF, et al. Analysis of transcribed human endogenous retrovirus W env loci clarifies the origin of multiple sclerosis-associated retrovirus env sequences. Retrovirology 2009; doi 10.1186/1742-4690-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavlicek A, Paces J, Elleder D, Hejnar J. Processed pseudogenes of human endogenous retroviruses generated by LINEs: their integration, stability, and distribution. Genome Res 2002;12:391–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolland A, Jouvin-Marche E, Michel M, et al. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol 2006;176:7636–44 [DOI] [PubMed] [Google Scholar]
- 56.Mameli G, Poddighe L, Astone V, et al. Novel reliable real-time PCR for differential detection of MSRVenv and syncytin-1 in RNA and DNA from patients with multiple sclerosis. J Virol Methods 2009;161:98–106 [DOI] [PubMed] [Google Scholar]