Abstract
We introduce a homonuclear version of third spin assisted recoupling, a second-order mechanism that can be used for polarization transfer between 13C or 15N spins in magic angle spinning (MAS) NMR experiments, particularly at high spinning frequencies employed in contemporary high field MAS experiments. The resulting sequence, which we refer to as proton assisted recoupling (PAR), relies on a cross-term between 1H–13C (or 1H–15N) couplings to mediate zero quantum 13C–13C (or 15N–15N recoupling). In particular, using average Hamiltonian theory we derive an effective Hamiltonian for PAR and show that the transfer is mediated by trilinear terms of the form for 13C–13C recoupling experiments (or for 15N–15N). We use analytical and numerical simulations to explain the structure of the PAR optimization maps and to delineate the PAR matching conditions. We also detail the PAR polarization transfer dependence with respect to the local molecular geometry and explain the observed reduction in dipolar truncation. Finally, we demonstrate the utility of PAR in structural studies of proteins with 13C–13C spectra of uniformly 13C, 15N labeled microcrystalline Crh, a 85 amino acid model protein that forms a domain swapped dimer (MW=2×10.4 kDa). The spectra, which were acquired at high MAS frequencies (ωr2π>20 kHz) and magnetic fields (750–900 MHz 1H frequencies) using moderate rf fields, exhibit numerous cross peaks corresponding to long (up to 6–7 Å) 13C–13C distances which are particularly useful in protein structure determination. Using results from PAR spectra we calculate the structure of the Crh protein.
INTRODUCTION
Solid-state NMR (SSNMR) structural studies of proteins are generally performed under high resolution conditions obtained via magic angle spinning (MAS), an approach which averages second rank tensor interactions such as the chemical shift anisotropy and the homonuclear dipolar interaction.1 Thus, while MAS introduces high resolution by attenuating the shift anisotropy, it also suppresses the distance information that is the source of structural data in the spectra. This structural information can be reintroduced selectively in a manner consistent with the goal of high resolution by the application of a carefully chosen sequence of rotor synchronized rf pulses,2, 3, 4, 5, 6, 7, 8 an approach that was introduced approximately 20 years ago and is referred to as dipolar recoupling. Since the original experiments, homonuclear and heteronuclear recoupling sequences have been refined in many ways and now allow accurate measurement3, 9, 10, 11 of distances and torsion angles12 as well as distance estimates using spin diffusion based techniques.13, 14, 15, 16, 17
Among the repertoire of dipolar recoupling techniques, homonuclear experiments, which recouple 13C–13C and 15N–15N spins, play an especially important role in biomolecular structural studies. In particular, the favorable dispersion of 13C and 15N chemical shifts and the development of efficient methods for 1H–13C∕15N decoupling18, 19, 20 ensure high spectral resolution that is essential for spectral assignments. Moreover, 13C–13C spectra also provide structurally valuable restraints, and they are potentially more numerous than either 15N–15N or 15N–13C distance restraints. Thus, homonuclear 13C–13C recoupling sequences have been used extensively in SSNMR structural studies.14, 16, 21, 22, 23
Despite their importance for structure determination, the measurement of long 13C–13C distances with nonselective techniques in uniformly labeled samples is complicated by a phenomenon known as dipolar truncation. In particular, the magnetization transfer due to weak dipolar couplings, characteristic of long distances, is strongly attenuated by the presence of larger noncommuting dipolar interaction characteristic of short distances. While this effect is much maligned and is usually discussed as the major bottleneck for structure determination, it is actually important for performing efficient, error-free resonance assignments. In fact, efficient truncation of medium- and long-range transfers by strong one-bond couplings induces a one-bond relayed transfer along the 13C–13C chains. This is illustrated below with broadband double quantum (DQ) cosine modulated rotary resonance (CM5RR) 8, 24 spectra of [U–13C,15N]-Crh protein25 that contain exclusively one-bond cross peaks. At longer mixing times, additional cross peaks appear with alternating signs which is a signature of a DQ process that depends on the number of one-bond couplings involved in the transfer. While this spectral behavior is obviously a very attractive feature of the technique for performing resonance assignments, it, nevertheless, does not provide distance information that could be directly related to the fold of the protein, except for what is available through statistical analyses of secondary chemical shifts.26
In order to utilize 13C–13C recoupling for three-dimensional (3D) structure determination of proteins, we require methods that either attenuate or quench dipolar truncation. A number of different groups of techniques have been proposed to circumvent the problem,8, 10, 11, 27, 28, 29, 30, 31, 32 and three of them have lead to structures of model proteins: (1) second-order techniques including Proton Driven Spin Diffusion (PDSD) (Ref. 33) and Dipolar Assisted Rotational Resonance RF Assisted Diffusion (DARR∕RAD),13, 34 (2) techniques combining 1H–1H spin diffusion with indirect detection such as CHHC∕NHHC,22, 35, 36 and (3) rotational resonance (R2) based on selective first-order techniques.37
Both PDSD and DARR∕RAD rely on the 1H–13C×13C–13C and 13C–13C×13C–13C second-order cross terms, respectively, to promote 13C–13C polarization transfer on a time scale of milliseconds to seconds.38 To date, 3D structure determinations using PDSD and DARR have been demonstrated primarily on selectively labeled spin systems14, 15, 39 and very recently on uniformly labeled systems.16, 23 A second approach—CHHC and NHHC—employs 1H–1H contacts that are probed in the initial rate regime (τm∼102 μs) and detected indirectly via rare spins.35, 40, 41, 42 CHHC∕NHHC based approaches were successfully used for structural studies of uniformly 13C and 15N labeled peptides∕proteins36, 43 including the 3D structure determination of Crh dimer.42 Finally, R2-based frequency selective techniques are well established as methods for measuring accurate 13C–13C distances and were used extensively in the context of small peptide structure determination. Recently, it was demonstrated that combined restraints from homogenously broadened R2W and PDSD are sufficient to calculate the structure of a uniformly 13C and 15N model protein GB1.37
In this paper we introduce a homonuclear recoupling technique that exhibits substantially reduced dipolar truncation and concurrently functions well at the high spinning frequencies that are desirable for experiments performed at high magnetic fields. It is in this important regime (high B0 and ωr∕2π) that the performance of PDSD and DARR begins to degrade. This sequence, proton assisted recoupling (PAR), is based on the more general third spin assisted recoupling (TSAR) mechanism that was used recently to design experiments for 15N to 13C cross polarization44 and to understand the spin dynamics underlying the beneficial effect of a weak (<0.25 ωr) 1H irradiation on the DQ CMpRR 13C–13C transfer efficiency.8 More generally, the B-[A]-C TSAR mechanism serves to connect two spins B and C via a cross term involving dipolar couplings with a third spin A (B-A and C-A dipolar couplings, respectively). In particular, the TSAR effective Hamiltonian contains trilinear terms of the form B±C∓AZ, which induce zero quantum (ZQ) polarization transfer between spins B and C. In the specific cases of 13C–13C and 15N–15N recouplings, the terms that mediate polarization transfer are of the form or .
In proteins and nuclei acids, protons are ideal candidates to mediate second-order TSAR transfers. Thus, the homonuclear PAR experiment is used for 13C–[1H]–13C and 15N–[1H]–15N polarization transfers. In contrast to most recoupling sequences that require decoupling of 1H from the 15N–13C, 13C–13C, and 15N–15N spin dynamics, the TSAR effect described here uses the intrinsic properties of protons (high abundance, large γ) to facilitate the transfer process. As a consequence, and in spite of the fact that TSAR is a second-order effect, it significantly accelerates the polarization transfer between remote 13C’s or 15N’s when compared to mechanisms based on 13C–13C or 15N–15N dipolar coupling alone. Note that the homonuclear dipolar coupling is not involved in the TSAR mechanism, a fact that intrinsically differentiates it from the spin diffusion methods that involve 13C–13C and 15N–15N couplings. Moreover, even though the 1H’s are involved in the PAR mechanism, the polarization is not transferred through the proton network using 1H–1H couplings. This is an essential difference with respect to CHHC-type experiments where the polarization is transferred from 13C to 1H, then between 1H’s, and indirectly detected on 13C. The TSAR mechanism also leads to attenuated dipolar truncation as we demonstrate below both theoretically and experimentally, allowing the detection of cross peaks corresponding to distances up to ∼6–7 Å in the spectra of uniformly [U–13C,15N]-Crh protein.
The paper is organized into the following eight additional sections. In Sec. 2 we delineate the principles of PAR, the effective Hamiltonian [from average Hamiltonian theory (AHT)], and the associated ZQ-TSAR subspace. Section 3 compares analytical and numerical PAR simulations, describes the PAR optimization map, and details the dependence on local molecular geometry. Section 4 discusses the effect of the spin system topology on the efficiency of PAR transfers. Using a simple model system, Sec. 5 describes the reduced dipolar truncation observed with PAR. Section 6 highlights various aspects of the PAR polarization transfer in uniformly labeled proteins with a focus on dipolar truncation, the issue of relayed versus direct polarization transfer, as well as the effect of multiple protons mediating PAR. Section 7 discusses using the PAR experiment for estimating distances. Section 8 highlights an important phenomenon, namely, that additional ZQ 13C–[1H]–13C TSAR transfer may be present during most of the contemporary first-order recoupling sequences. Section 9 demonstrates that the PAR technique is a suitable approach for protein structure determination and compares its features to other alternative methods such as PDSD∕DARR and CHHC∕NHHC. Note that a detailed description of the materials and methods can be found in the Supporting Information (SI),69 including Sec. IIB that describes the PAR optimization protocol.
PRINCIPLES OF PAR
Figure 1a illustrates the pulse sequence used throughout this paper to record homonuclear two-dimensional (2D) correlation spectra [except for the spectra in Figs. 9a, 12a for which the pulse sequences can be found in De Paepe et al.8]. In what follows we cast the discussion in terms of 13C–13C recoupling, but all of the theory and discussion are equally applicable to the case of 15N–15N recoupling, which is specifically described in separate paper.45 The initial 1H to 13C cross polarization (CP) step is followed by 13C indirect t1 evolution, and then the PAR recoupling block, consisting of cw irradiation, is applied to both the 13C and 1H channels. The final step is 13C detection during t2 in the presence of TPPM (Ref. 18) decoupling. As we shall see below, the PAR mechanism relies on second-order recoupling involving 13C1–1H and 1H–13C2 dipolar interactions, or in other words the 13C–[1H]–13C TSAR mechanism.
Figure 1.
(a) PAR pulse sequence for obtaining 2D homonuclear correlation spectra. The PAR mixing consist of continuous wave (cw) irradiation on the 1H and 13C channels that induces a second-order cross term between 1H–13C dipolar couplings [terms 2 and 3 of Eq. 6] in order to transfer polarization from 13C1 to 13C2. (b) The PAR subspace can be seen as a coupled basis between a fictitious ZQ operator involving two 13C’s and a 1H spin. The red arrows indicate PAR recoupling axis and longitudinal tilting field resulting from autocross terms (see Sec. 4).
Figure 9.
2D 13C–13C correlation spectra of [U–13C,15N]-Crh protein comparing two advanced recoupling pulse sequences at ω0H∕2π=750 MHz and ωr∕2π=20 kHz: (a) Broadband CM5RR spectrum recorded with τm=0.8 ms ω1C∕2π∼100 kHz and displaying only one-bond dipolar 13C–13C cross peaks (see the gray monomer of the Crh dimer structure representation in the inset). Note that the spectrum was acquired in ∼15 h without 1H irradiation during the CMRR mixing time. (b) PAR spectrum corresponding to τm=14 ms with ω1C∕2π∼53 kHz and ω1H∕2π∼50 kHz cw fields displaying 13C–13C cross peaks corresponding to medium (4.3 Å) and long (∼5.4 Å) distances (acquired in ∼40 h). Several illustrative examples are shown on the green monomer of the Crh dimer structure representation. A detailed description of the PAR optimization protocol can be found in the SI (Ref. 69).
Figure 12.
[(a) and (b)] Examples of 13C–13C correlation spectra of [U–13C,15N]-Crh protein at ω0H∕2π=900 MHz and ωr∕2π=20 kHz. Expansion of the aliphatic region for (a) CM5RR (0.8 ms) and (b) PAR (15 ms). The PAR spectrum contains numerous cross peaks corresponding to medium to long distances that involve methyl groups. As a comparison, the CM5RR spectrum displays only one-bond cross peaks. A detailed description of the PAR optimization protocol can be found in the SI (Ref. 69). (c) Ensemble of structures of a Crh monomer (residues 12–85) calculated using a unique 2.5–6 Å distance class for all the unambiguous 13C–13C cross peaks identified using the x-ray structure (Ref. 56) as a homology model. (e) Numerical simulations of the polarization transfer between CH3 and CH illustrating the influence of the threefold methyl group hopping on the overall polarization transfer. The coordinates for spin system (d) used in the simulations were taken for A20Cα and I47Cδ1 from the x-ray structure (Ref. 56) of the Crh protein. Simulations do not include chemical shift.
The PAR experiment can be analyzed with AHT (Ref. 46) that permits visualization and understanding of the spin dynamics in the PAR subspace. Accordingly, we consider a three spin system consisting of two dipolar coupled 13C’s–13C1, 13C2–, and an assisting 1H spin subject to two cw rf fields of strengths ω1C and ω1H applied to the 13C and 1H spins, respectively. The internal Hamiltonian can therefore be written as
| (1) |
where ΔωC1, ΔωC2, and ΔωH denote the shift tensors and resonant offsets of the 13C (or 15N) and 1H nuclei, respectively, and ωC1C2, ωC1H, and ωHC2 the homonuclear and heteronuclear dipolar couplings. Note that rotation at the magic angle induces a time dependence of the spatial anisotropy of the interactions.
For computational convenience, the Hamiltonian 1 is rewritten using spherical tensor notation,
| (2) |
In order to appreciate the effect of the cw rf fields, it is convenient to further recast the Hamiltonian in the interaction frame defined by the two fields. Such a transformation can be decomposed into two rotations: R1(α1=0,β1=π∕2,γ1=0) which rotates the z-axis to the x-axis, and R2(α2=ω1C∕1Ht,β2=0,γ2=0), which rotates the spin system around the new z-axis by angles ω1Ct and ω1Ht. Each of the operators transforms as follows:
| (3) |
where is a reduced Wigner matrix element. We reduce the dependence on three different averaging frequencies (ωr∕2π, the rotor frequency, and ω1C∕2π and ω1H∕2π the strength of the 13C and 1H cw fields, respectively) to a single frequency dependence by assuming that these frequencies are commensurate, implying that we can find indices pC and pH, and integers , , , and , such that
| (4) |
| (5) |
where denote irreducible ratios. Assuming that the frequencies are commensurate is not a demanding constraint and results in a Hamiltonian that is periodic modulo nτr, to which AHT is applicable. Here , given that n is sufficiently small to ensure rapid convergence and τr is a rotor period. The expression for the Hamiltonian in the interaction frame can thus be obtained using Eqs. 2, 3, 4, 5),
 |
(6) |
where we use the following expressions:
| (7) |
and sgn(q) is the sign function of q.
We assume that the rf fields are chosen so that neither Hartmann–Hahn47 (HH) nor rotary resonance recoupling (R3) (Ref. 48) conditions are matched, i.e., X1≠0,X2≠0,XCi≠0,XH≠0, and that the 13C–13C dipolar coupling is not recoupled to first order (X3≠0). Under these conditions, the first-order average Hamiltonian vanishes.
In order to describe the PAR recoupling mechanism, we calculate the cross term between terms 1 and 2 in Eq. 6,
| (8) |
The above expression is nonzero if and only if
| (9) |
| (10) |
which implies that
| (11) |
Equation 11 has two solutions.
DQ solution: For q1C=q2C=±1 and pC∊±{0,0.5,1,1.5,2} with no restriction on pH, which results in DQ terms of the form .
ZQ solution: For q1C=−q2C and m1=−m2 with no restriction on pC and pH, which results in ZQ terms of the form . Note the change in sign of the subscript——yielding a ZQ Hamiltonian.
Thus, it appears that the ZQ solution, as opposed to the DQ solution, is much easier to fulfill, and ZQ TSAR recoupling occurs when the following conditions are satisfied:
| (12) |
and providing that we concurrently avoid the DQ conditions associated with pC={0,±0.5,±1,±1.5,±2}, HH conditions with pC=±pH±{1,2}, and R3 conditions with pC={1,2}.
Using the identity , we obtain the following expression for the effective PAR term:
| (13) |
and the PAR coupling
| (14) |
with
| (15) |
The above expressions permit us to visualize the subspace in which the TSAR spin dynamics evolve. The PAR subspace [Fig. 1b] can be viewed as a coupled basis described extensively in the context of solution NMR (Ref. 49) between a fictitious ZQ operator involving the two 13C’s and a 1H. The proton spin is essentially a bystander, i.e., no magnetization is sent to this proton in the spin dynamics described by Eq. 13. In this process, the dipolar couplings to the 1H are used to create an effective transverse PAR component composed of trilinear terms of the form that can invert the z-component in the PAR subspace ((C1Z−C2Z)∕2) and thus induce polarization transfer between the two 13C’s. The similar case that is encountered in solution NMR is described further in Supporting Information, Sec. 1.
The PAR recoupling term [Eq. 13] can be decomposed into two terms which we refer to as the m=1 and m=2 contributions, where m denotes for the spatial component index of the dipolar tensor during MAS. The dependence of the PAR term on the local geometry and the powder Euler angles is detailed in Sec. 4. Note that the PAR mechanism usually involves multiple protons: any proton is potentially a candidate to participate in such a process, but only the closest ones will contribute significantly to the polarization transfer. This issue is discussed further in Sec. 6.
The three spin term derived above drives the polarization transfer in a PAR experiment. However, the PAR term is not the only possible cross term present during double cw irradiation, and a complete picture of the spin dynamics of the PAR experiment requires that we evaluate the other second-order cross terms. For example, an important contribution, which we refer to as an autocross term, arises from a cross term of term 1 in Eq. 6 with itself (i.e., 1H–13C1 term crossed with 1H–13C1). These autocross terms yield nonzero contributions that can be expressed as a function of pC and pH, the rf field strengths in units of the MAS frequency.
The autocross term of term 1 in Eq. 6 (i.e., the term involving the 1H–13C1 dipolar coupling crossed with the 1H–13C1 dipolar coupling) can be written as follows:
| (16) |
with
| (17) |
Similarly, one can derive -the second-order contribution of term 2 in Eq. 9 with itself (i.e., the term involving 1H–13C2 dipolar coupling cross 1H–13C2 dipolar coupling).
In order to obtain a more complete expression of the longitudinal contribution, one should also evaluate the cross term of the chemical shift tensors of the 13C’s and 1H’s with themselves:
| (18) |
where
| (19) |
The autocross term arising from J coupling may also be present, but is generally negligible compared to the -type terms considered above. Finally, the cross terms yield longitudinal T10 operators [along the irradiation axis, see Fig. 1b] that directly interfere with the ZQ PAR polarization transfer described in Eq. 13. The relevant Hamiltonian in the ZQ-13C subspace can hence be described by the following equation:
| (20) |
where
| (21) |
To summarize, we have learned that the ZQ-PAR mechanism can potentially be active for all (pC, pH) combinations except when pH=±pC±{0,1,2,3,4} or pC={0.5,1,1.5,2}. Moreover, autocross terms from both the heteronuclear dipolar interactions and the chemical shift tensors yield contributions that are depicted in the PAR subspace introduced above as longitudinal contributions [see Fig. 1b]. The importance of these longitudinal terms is discussed in Sec. 4.
NUMERICAL AND ANALYTICAL SIMULATIONS OF THE PAR MECHANISM
Even though the expressions derived in Sec. 2 provide considerable insight into the spin dynamics in the PAR subspace, we believe it is instructive to examine numerical simulations that account for the influence of other interactions and higher-order terms on the PAR spin dynamics. As we will see the salient features of the numerical simulations are in excellent agreement with the analytical expressions derived above.
Figure 2 shows simulations illustrating the PAR recoupling mechanism for a simple spin system consisting of two directly bonded 13C’s (C1 and C2) and a single 1H directly bonded to C1. The magnetization is initially present on C1. The usual CH (1.12 Å) and CC (1.54 Å) bond lengths and a 109.5 H–C1–C2 angle lead to a C1–H–C2 angle of ∼42°. The three contour plots in Figs. 2a, 2b, 2c represent the magnetization on C1, C2, and 1H, respectively, after 3 ms of simultaneous 13C∕1H PAR irradiation. The x- and y-axes of the contour plots indicate the rf field strengths applied in units of spinning frequency ωr for the 13C (pC=ω1C∕ωr) and 1H (pH=ω1H∕ωr) channels, respectively. The black dashed lines indicate the 1H–13C HH matching conditions and its sidebands (i.e., pH=pC±n where n=0,1,2).
Figure 2.
PAR optimization map simulated using SPINEVOLUTION (Ref. 50). The initial magnetization is on the C1 spin (C2,X operator) and is detected after 3 ms PAR mixing on the three spins: (a) C1 spin, (b) C2 spin, and (c) H spin (C1,X, C2,X, HX operators, respectively). The spin system is composed of two directly bonded 13C’s and one 1H bonded to the 13C1 spin (see the inset of the figure). The distance between the 13C2 spin and the 1H is 2.15 Å. The angle between the two CH dipolar vectors is 42° and arises from the usual tetrahedral geometry. Simulations include typical chemical shift tensor values (see SI for details) (Ref. 69). The black dashed lines indicate the locations of the n=0 and ±1 and ±2 HH matching conditions.
As expected, we observe magnetization transfer from 13C1 to the 1H at the HH conditions. However, we transfer a substantial amount of magnetization to C2 for rf fields that depart from the HH condition and match the PAR conditions in two regions of the optimization map that are illustrated in Fig. 2b and denoted with red X’s. The first lies just below the line corresponding to the n=0 matching condition (ω1H<ω1C but with pC>2), and since this region uses high power cw irradiation, it corresponds to relative broadband recoupling. In contrast, the second area (pC<1, pH>3) employs lower power cw 13C irradiation and results in more selective recoupling.
As we will see below the TSAR term alone, [Eqs. 13, 14, 15], is not sufficient to fully explain the features of the PAR optimization map. Thus, in order to obtain a more complete understanding of the underlying spin dynamics, we performed analytical simulations of the magnetization exchange. The spin dynamics in the TSAR subspace are described by Eq. 20 [and depicted in Fig. 1b]. If the magnetization starts on the first carbon (C1z) and is detected on the second carbon (C2z), the polarization transfer efficiency for a given crystallite orientation can be written , where ωeff represents the recoupling frequency along the effective tilted axis and θeff is the angle between the transverse PAR component and the effective tilted component when longitudinal cross terms are considered. Both parameters can be expressed as a function of ωPAR (the PAR recoupling frequency) and ωauto (the autocross-term contribution) using the following expressions: tan(θeff)=ωauto∕ωPAR and . The scaling factor cos2(θeff) accounts for the fact that the effective recoupling axis is not perpendicular the z-axis of the PAR subspace (which stands as both the initial magnetization axis and detection axis). After averaging over the Euler angles, the polarization transfer efficiency can be thus be written as
| (22) |
Figure 3 compares analytical and numerical simulations performed on the same spin system as in Fig. 2. Note that these simulations do not include chemical shift tensors in order to isolate the effect of the 1H–13C autocross terms on the TSAR spin dynamics. Shift tensor effects can be incorporated into the discussion using Eq. 21. The resulting analytical simulations are in excellent agreement with numerical simulations shown in Fig. 2 (data not shown).
Figure 3.
PAR optimization maps where the polarization transfer between the 13C is monitored as a function of 13C and 1H irradiation strengths (in units of ωr) and a 1.5 ms mixing period. The spin system is identical to that in Fig. 2. No chemical shift interactions were included in these simulations. The three panels represent (a) analytical simulations of the 13C polarization transfer from 13C1 to 13C2 arising from only the TSAR term, (b) 13C signal intensity showing the analytical simulation obtained with the TSAR term and the longitudinal autocross-term contributions, and (c) 13C signal intensity depicting the numerical simulations performed with SPINEVOLUTION. The two white lines displayed on the contour plots in panels (b) and (c) represent points where χ(1,pC,pH)=0 and χ(2,pC,pH)=0 (i.e., autocross terms for spatial components m=1 and m=2 are equal to 0), described by equations and , respectively.
Figure 3a shows the polarization transfer from the 13C1 to 13C2 after 1.5 ms of simultaneous cw 13C∕1H irradiation when only the PAR term is included. As expected from Eq. 13, we observe an efficient polarization transfer for a wide range of pC and pH values. The influence of the inclusion of the autocross term [see Eq. 20] on the overall spin dynamics is illustrated in the analytical simulation in Fig. 3b. The structure of the resulting optimization map is very different than in Fig. 3a, but very similar to the numerical SPINEVOLUTION simulation in Fig. 3c.
Note that the PAR transfer is maximized for points close to the diagonal of the map (pC=pH, pC>2.25) and for points where pC<1 and pH>3. The optimal rf settings yield a minimal tilt angle θeff. (i.e., corresponding to points where the PAR recoupling term dominates the longitudinal T10 component). This can easily be seen for the central recoupling condition (near the n=0 matching condition). The two white lines displayed on the contour plots in Figs. 3b, 3c represent points where χ(1,pC,pH)=0 and χ(2,pC,pH)=0 (i.e., autocross terms for spatial components m=1 and m=2 are equal to 0), described by equations and , respectively. If only one of the two spatial components is taken into account, we clearly observe that either the first or the second conditions maximize the polarization transfer [see Fig. SI 3 (Ref. 69)]. In the case where both m=1 and m=2 components are used, the maximized polarization transfer appears closer to the m=1 matching condition, which is consistent with the fact that the PAR scaling factor is higher for the m=1 component. Practically, this means that the optimal transfer will utilize a 1H rf field slightly smaller than the 13C rf field (with 13C rf >2.25ωr).
The remarkable agreement between Figs. 3b, 3c demonstrates that second-order AHT provides considerable insight into the TSAR process. The TSAR transfer is active as long as the longitudinal off-resonance contribution (autocross terms) is sufficiently small, and first-order recoupling (HH, R3) is avoided. The agreement between the numerical and analytical plots could be further improved by including higher-order corrections to the AHT expansion. This does not, however, appear necessary to understand the fundamentals of the TSAR mechanism.
From the discussion and results above, it is clear that a knowledge of the longitudinal cross terms is of primary importance for implementing the TSAR mechanism and that the maximum TSAR polarization transfer occurs when this longitudinal component is minimized. The next step is to understand the influence of the various other interactions present in the spin Hamiltonian during a TSAR polarization transfer. Figure 4 illustrates polarization buildup curves corresponding to pC=2.6 and pH=2.35 (the area indicated with a red X on the C2 optimization map in Fig. 2). Figure 4a shows that there is a substantial polarization transfer from C1 to C2 with all couplings included in the simulation. Figure 4b depicts the same simulation as in (a) except that the C1–C2 coupling is absent from the spin system. We note that identical transfer curves are observed Figs. 4a, 4b, illustrating that the polarization transfer does not rely on the C1C2 coupling. Since the TSAR mechanism is based on the cross term involving the C1H and C2H couplings, we would therefore predict, based on Eqs. 13, 14, 15, that removing one or both of the CH couplings would either attenuate or quench polarization transfer from 13C1 to 13C2. This point is illustrated in Figs. 4c, 4d, 4f. The small polarization transfer to the H [Figs. 4d, 4e] is due to the cross terms involving the C1H interactions and through higher-order AHT analysis. The proton polarization buildup is indeed almost unchanged when the C2H coupling and both the C2H∕C1C2 couplings are removed [Figs. 4d, 4e]. However, the 1H buildup collapses when the C1H coupling is removed [Fig. 4c]. The same explanation should also hold for the C2H interactions via symmetry arguments, however, its effect is not appreciable since the C2H interaction is much smaller than the C1H for the particular spin geometry. Thus, only the cross term involving the C1H dipolar interaction is able to induce polarization transfer from C1 to 1H on a timescale under 20 ms. Finally, a comparison of Figs. 4d, 4e shows that the residual slowly rising C2 polarization transfer present in Fig. 4d does rely on the 13C–13C couplings. In this case the C2 polarization is driven by the cross terms involving terms 1 and 3 from Eq. 6. Note, however, that simulation, Fig. 4b, indicates that this contribution is negligible compared to the TSAR term.
Figure 4.
Simulations of polarization transfer in the PAR experiment for a HC1C2 spin system. The chemical shift is not included in the simulations. The PAR 13C and 1H cw rf field strengths correspond to pC=2.6 and pH=2.35, respectively [see Fig. 3b]. The panels illustrate simulations that include the following couplings: (a) all dipolar couplings; (b) couplings C1H and C2H but not C1C2; (c) couplings C2H and C1C2, but not C1H (note that the polarization remains on C1); (d) couplings C1H and C1C2 but not C2H; (e) coupling C1H but not C2H or C1C2; (f) C1C2 but not C1H and C2H.
SPIN SYSTEM GEOMETRY AND PAR TRANSFER EFFICIENCY
In the discussion above we assumed a model spin system with a particular geometry to analyze the TSAR transfer mechanism. Although this is a useful point of departure, it is clear that the details of the spin system geometry and the averages over the powder Euler angles will influence the TSAR polarization transfer process. We now discuss these two factors in more detail.
As demonstrated above, the TSAR recoupling term is described by Eq. 13, and the effective TSAR recoupling frequency contains terms of the form representing the product of the dipolar coupling components. The m-component of the 13Ci–1H dipolar coupling interaction can be expressed by the following equation:
| (23) |
where (0,bCiH,cCiH) represents the Euler angles between the principal axis system (PAS) of the dipolar 13Ci–1H interaction and the crystal frame (CF) and (α,β,χ) represent the Euler angles between the CF and the rotor frame. Here the angle between the rotor axis and the laboratory magnetic field B0 is set to the magic angle, i.e., θM=54.7°. dCiH, the dipolar coupling constant between the 13Ci and 1H spins, and is related to the internuclear distance by
| (24) |
where γ is the gyromagnetic ratio of the spins and rCiH the distance between the two spins.
In order to simplify the derivation of the expression for , one can arbitrarily choose the CF to match the PAS of the 13Ci–1H dipolar interaction which implies (0,bCiH,cCiH)=(0,0,0). We can also rewrite (0,bHCj,cHCj)=(0,θ,ϕ), where θ and ϕ represent the spherical coordinates of a system centered on the 1H spin with the CiH vector aligned with the z-axis, as shown in Fig. 5a.
Figure 5.
Dependence of PAR polarization transfer on the local geometry of the spin system. (a) The three-spin system geometry used in the simulations: the first 13C and the 1H are fixed in space, whereas the position of the second 13C is defined by θ and ϕ, the spherical coordinates with the origin at the 1H. The distances between the 13C’s and the 1H are constant and equal to 1.1 and 2.6 Å, respectively. The spherical map represents the 13C–13C polarization transfer efficiency for a PAR mixing time of 4.2 ms using pC=2.75 and pH=2.5. Polarization transfer for θ=0 orientation (aligned geometry) as a function of time is presented in panels (b) and (c). The buildup curves labeled (1)–(5) represent analytical simulations performed with the following contributions: (1) both m=1 and m=2 components without autocross terms, (2) both m=1 and m=2 with autocross terms, [(3) and (4)] only m=1 without and with autocross terms, [(5) and (6)] only m=2 without and with autocross terms.
With the simplifications above,
| (25) |
and
| (26) |
we finally obtain the following two equations for the m=1 and m=2 components, respectively:
| (27) |
| (28) |
Equations 27, 28 show the angular dependence of the PAR process with respect to the two powder Euler angles α, β and the two polar angles θ, ϕ. Note that the Euler angle γ is absent from the expression for the PAR recoupling frequency. Thus, the PAR mechanism appears as a γ -compensated process (note that it is distinct from a γ-encoded mechanisms where γ-angle appears as a phase factor).
Figure 5a illustrates the PAR polarization transfer for a three spin system similar to the one in Fig. 2. More specifically, the system is composed of a directly bonded 13C1–H spin pair and a 13C2 located on a sphere of constant radius centered on the proton spin [see Fig. 5a]. The polarization transfer from C1 to C2 after 4.2 ms is illustrated on the spherical map shown in Fig. 5a. A substantial polarization transfer is present over the entire sphere with maxima occurring around the poles and the equator. Note that the polarization transfer efficiency does not depend on the ϕ-angle.
The angular dependence of the PAR recoupling sequence mechanism is given in Eq. 14, and the recoupling frequency is composed of four terms that can be rewritten as
 |
(29) |
The first and second terms, denoted and , of Eq. 29 correspond to the m=1 and m=2 components, respectively. We note that all of the analytical simulations shown in Fig. 5 were performed assuming pC=2.75 and pH=2.5, and that these values where chosen based on numerical simulations of the same system. In particular, they maximize the polarization transfer after 4.2 ms, but maintain the rf strengths within experimentally reasonable limits (≤55 kHz in this case). The rf field strengths (in units of the MAS frequency) almost fulfill the matching condition that corresponds to points when the m=1 component of the autocross term is canceled. This reduction in the longitudinal contribution results, for example, in about 50% maximum polarization transfer in 4.2 ms for the geometry corresponding to the spins close to the poles of the sphere (see Fig. 5).
In order to gain additional insight into the TSAR process (or more specifically to elucidate the maximum theoretical efficiency of the TSAR process), we can choose θ=0 and simulate the TSAR polarization transfer with the three spins aligned in a linear array. In this case, Eqs. 27, 28 simplify yielding a sin2(2β) and sin4(β) dependence for the m=1 and m=2 component, respectively. Figure 5b shows the corresponding analytical magnetization buildup curves [(1)–(6)] where, in the case without the autocross terms, the contributions of both the m=1 and m=2 terms reach a theoretical maximum of ∼58% [(3) and (4)]. The difference in buildup times, i.e., slower for the m=2 component, is also consistent with the sin2(2β) and sin4(β) dependences. Interestingly, when both components simultaneously contribute to the transfer, the maximum polarization is enhanced by constructive interference of the two-component process. Note that in the case of first-order γ-encoded recoupling sequences, the Euler angle dependences are sin(2β) and sin2(β), yielding a theoretical maximum of ∼73%.6, 8, 51 Including longitudinal autocross terms [see Eq. 21] leads to an attenuation of the magnetization transfer in the three spin case mentioned above. The attenuation is more significant for the m=2 component due largely to the choice of rf power levels and consequently the scaling factor of the spin part. Indeed, as mentioned above, pC and pH were chosen (in this simulation) in order to minimize the m=1 component of the autocross terms. When both the m=1 and m=2 components are included, the maximum transfer efficiency can still approach 50%.
The PAR transfer should thus be seen as a superposition of two simultaneous recoupling pathways involving the m=1 and m=2 components, and we can decompose the contributions of the different components arising from the two cross terms and the autocross terms. Figure 6 shows maps of polarization transfer efficiency as a function of the θ-angle and the mixing time (with ϕ=0 since as we have shown above ϕ does not influence the transfer). The m=1 pathway [Figs. 6a, 6b] has a better scaling factor than the m=2 component [Figs. 6c, 6d]. This can easily be understood by examining Eqs. 27, 28. The comparison between Fig. 6a and Fig. 6b shows the influence of the autocross terms for the m=1 component. The overall features of Fig. 6a are preserved with some attenuation, except around θ=0° and θ=180°, where the autocross terms quench the TSAR polarization transfer. This effect can be explained by noting that the orientation dependence of the m=1 PAR frequency and the m=1 autocross terms are identical for θ=0° or 180° and limited to the β-angle. Even if the choice of pC and pH minimizes the importance of this longitudinal contribution, its effect is further enhanced when the orientation dependence is identical: i.e., both the m=1 PAR and autocross term are minimized for β=0, π∕2 and maximized for β=π∕4, 3π∕4. For the m=2 component [Figs. 6c, 6d], the effect of the autocross terms is larger. Again this is related to the choice of pC and pH that strongly attenuates the m=1 autocross terms compared to the m=2 condition. For similar reasons as with m=1, the area around θ=0° and θ=180° is the most affected. The last two panels—Figs. 6e, 6f—show similar simulations as the previous panels but with both the m=1 and m=2 components included. In this case, the two components constructively interfere yielding 50%–70% transfer with and without inclusion of the autocross terms.
Figure 6.
Analytical contour plots of the PAR polarization transfer arising from the m=1 and m=2 components as a function of θ angle for a three spin system described in Fig. 5a with the mixing time and irradiation settings used in Fig. 5 with ϕ=0. (a) m=1 PAR component, (b) m=1 PAR term plus m=1 autocross-term components, (c) m=2 PAR component included, (d) m=2 PAR term and m=2 autocross-term components, (e) m=1 and m=2 PAR components, and (f) m=1 and m=2 PAR plus m=1 and m=2 autocross-term components.
Note that the rf settings that optimize the transfer for a particular spin system can be understood as a compromise between minimizing the autocross terms and maximizing the constructive interference of the two TSAR pathways (m=1 and m=2), given that they do have a different dependence on θ and the Euler angles, and thus different scaling factors.
The spin system that we have studied thus far is composed of a directly bonded 13C1–1H spin pair and a third 13C2. As we have seen, the optimal rf fields correspond to points that minimize the size of the autocross terms. From Eq. 21, we also surmise that the TSAR autocross terms disappear when the assisting spin is equidistant from 13C1 and 13C2. This situation is encountered for the spin system in Fig. SI5 (Ref. 69) where the three spins are aligned and the 1H is at 3 Å from each of the 13C’s (rCC=6 Å). The analytical simulation in Fig. SI5 (Ref. 69) shows that efficient polarization transfers can be achieved over a wider range of rf levels. Since the heteronuclear autocross term is absent in Fig. SI5a,69 the optimal rf fields correspond only to the maximum interference between the m=1 and m=2 components of the TSAR recoupling frequency. Note that in this case over 85% of the polarization is transferred over a 6 Å distance in 60 ms.
Other examples illustrating various levels of autocross-term compensation with different spin system geometries can be found in the Supporting Information. Figure SI4 (Ref. 69) shows the PAR polarization transfer map in the case of a nonaligned three spin system where the 1H spin is equidistant from the two 13C’s. In such a situation, the off-resonance contribution from the autocross terms vanishes in the ZQ PAR subspace. Figure SI6 (Ref. 69) illustrates that in the case of a nonsymmetric spin system (i.e., two different couplings corresponding to a case where the proton is directly bonded to C1 and at 4.9 Å from C2), the polarization transfer occurs only along the white line corresponding to the cancellation of the m=1 off-resonance autocross terms. In this case the influence of the autocross terms is significant because of the very different 1H–13C interactions involved.
The autocross-term compensation can be generalized as soon as each of the two 13C’s is coupled equally to the 1H. This is illustrated in Fig. SI7 (Ref. 69) with the example of a four spin system consisting of two 13C’s, each with a directly bonded 1H. In Fig. SI7a (Ref. 69) the symmetry of the spin system ensures that the ZQ-TSAR off-resonant contributions vanish [see Eq. 24]. The effect of the geometrical autocross-term compensation can be seen in the map shown in Fig. SI7a (Ref. 69) since the TSAR mechanism is active over a large part of the map and does not need to be close to the two lines defined by χ(1,pC,pH)=0 and χ(2,pC,pH)=0. If the spin system symmetry is broken (as in Fig. SI7b),69 the ZQ off-resonance contribution is no longer zero, but still sufficiently small so that Fig. SI7b (Ref. 69) looks closer to Fig. SI7a (Ref. 69) compared to the case where only one proton is considered (Fig. SI7c) (Ref. 69).
In practice, this means that the ZQ off-resonant autocross term is intrinsically reduced when considering a TSAR transfer between 13C’s arising from the same types of groups (e.g., CH and CH, CH2 and CH2, and CH3 and CH3) and that the TSAR effect is much easier to fulfill in this case (which is manifest as broader range of rf powers leading to appreciable TSAR effect).
DIPOLAR TRUNCATION AND LONG DISTANCE TRANSFER
Dipolar truncation is frequently alluded to in the literature and refers to the difficulty of detecting cross peaks corresponding to long distances arising from weak couplings in the presence of strong dipolar couplings.52, 53 This phenomenon is illustrated in Fig. 7a with the example of a first-order broadband CM5RR where, in the absence of a directly bonded Cβ spin, we achieve significant polarization transfer from the Cα spin to the Cremote spin (dashed black line) with a ∼25 ms buildup time for a 4.5 Å distance. However, in the presence of Cβ spin, the polarization transferred from Cα to the Cremote (dash-dot red line) is severely attenuated. Note that in the latter case most of the magnetization is directed to the directly bonded Cβ spin. Thus, for example, γ-encoded dipolar recoupling sequences6, 7, 8, 51, 54 exhibit spectra that are dominated by a one-bond relayed transfer mechanism and thus provide excellent tools for assigning 13C–13C spectra of proteins.55
Figure 7.
Illustration of dipolar truncation in the CM5RR and PAR homonuclear recoupling schemes. The spin system 1 is composed of a directly bonded CαHα pair and Cremote spins of 4.5 and 3.56 Å distant from the Cα and Hα spins. In the spin system 2, a Cβ spin, directly bonded Cα, is also present. (a) The black dashed line depicts the polarization transfer (∼30%) from Cα to Cremote (rC−C=4.5 Å) using the broadband DQ CM5RR in the three spin system 1. When a directly bonded Cβ spin is added to the spin system (rC−C=1.5 Å), the polarization transfer to the Cremote (red dash-dot line) is quenched for CM5RR with most of the polarization being transferred to the directly bonded Cβ (blue solid line), thus demonstrating the phenomenon of dipolar truncation. (b) In the PAR simulation the presence of a third strongly coupled spin leads to a partial decrease in polarization transfer to Cremote (red dash-dot line) showing that dipolar truncation is attenuated in the TSAR transfer mechanism. Simulations were performed with SPINEVOLUTION (Ref. 50) ωr∕2π=20 kHz, ω0H∕2π=750 MHz 1H frequency and do not include chemical shifts.
However, in order to determine 3D structures, it is necessary to measure weak couplings corresponding to structurally important long distances in the presence of the strong couplings from directly bonded neighbors. Thus, the design of new polarization transfer methods that suppress 13C–13C dipolar truncation in uniformly 13C labeled compounds is an area of active research. The initial solutions proposed for this problem were based on frequency selective 13C–13C recoupling methods such as rotational resonance (R2) and its variants—R2 tickling,27R2 width,10, 28 and R2 in the tilted rotating frame (R2TR).11, 29 More recently, two additional experimental approaches were introduced and tested on model compounds with the aim of circumventing truncation and providing more accurate 13C–13C distance restraints.
The first category includes the SEASHORE and COMICS experiments, which use the chemical shift interactions to dephase unwanted DQ coherences. In the SEASHORE approach DQ excitation periods are alternated with delay windows where DQ coherences are dephased except if the carrier frequency matches the mean offset of the two carbons.30 COMICS is based on the generalization of the second averaging principle and consists of the application of two successive averaging fields equal to 0.25ωr that simultaneously reintroduce the isotropic chemical shift interaction and the DQ dipolar terms leading to an extremely narrowband sequence.8
The second category relies on the active truncation of a ZQ dipolar Hamiltonian by chemical shift tensors,31 an intellectually elegant approach recently introduced by Marin-Montesinos and employed in the triple oscillating field technique rotor assisted dipolar refocusing (TOFU-RADAR) experiment.32 Whereas COMICS relies on a two step averaging at 0.25ωr, TOFU-RADAR adds a third averaging step that produces the recoupling of the ZQ dipolar interaction and the chemical shift tensor. To date these sequences have been demonstrated on uniformly labeled model systems and allow measurement of 13C–13C distances from sites selected using a Gaussian inversion pulse.
The PAR experiment differs significantly from the methods presented above since the spin dynamics do not depend on the 13C–13C couplings, but involve couplings to the surrounding protons. An important consequence of this mechanism is the attenuation of dipolar truncation when compared to first-order 13C–13C recoupling sequences. This is illustrated in Fig. 7b with numerical simulations where in the presence of the Cβ spin, and in contrast to broadband DQ recoupling, we can still transfer a significant amount of polarization between the Cα and the Cremote spin. This prediction is confirmed experimentally with 2D spectra obtained with DQ CM5RR where at a short mixing time (0.8 ms) only one-bond 13C–13C dipolar transfer is observed (vide infra).
POLARIZATION TRANSFER IN UNIFORMLY LABELED SYSTEMS
With the simulations in Sec. 3 we theoretically demonstrated the PAR mechanism for 13C–13C polarization transfer. To confirm these predictions with experimental data we have studied two systems—the tripeptide [U–13C,15N]N-f-MLF-OH and [U–13C,15N]-Crh protein—and 13C–13C spectra of these two molecules are shown in Figs. 89, respectively. The spectra permit us to illustrate the features of the PAR experiment and importantly permits us to measure distances >4 Å at high spinning frequencies (>20 kHz) and subsequently, in the case of the Crh protein, to calculate the structure of a monomer.
Figure 8.
(a) 2D 13C–13C correlation spectrum of [U–13C,15N]N-f-MLF-OH diluted to 10% in a natural abundance lattice. The spectrum was recorded at ω0H∕2π=750 MHz and ωr∕2π=20 kHz with τmix=7.5 ms, ω1C∕2π≈50 kHz, ω1H∕2π≈47 kHz, and the 13C offset at 101 ppm. The circled cross peaks in (a) correspond to ≥4 Å 13C–13C distances. (b) Numerical simulations of PAR polarization transfer between MCβ and LCα (highlighted 4.3 Å distance) using rf power levels specified in (a). The spin system includes nearby protons (2xMHβ, MCα, LHα, LH) (back solid line) and nearby protons plus MCα and MC’ (red dash line). The dotted blue line represents simulation on a spin system including nearby protons plus MCα and MC′ with the 1H–1H couplings removed from the calculation. Simulations include typical chemical shift tensor values (see SI for details) (Ref. 69). The plot illustrates that the contribution of the polarization relayed through MCα and MC′ to the polarization transfer between MCβ and LCα is negligible compared to the direct polarization transfer.
In Fig. 8a we display a 2D 13C–13C PAR correlation spectrum obtained from the [U–13C,15N]-N-f-MLF-OH diluted to 10% in a natural abundance lattice which insures that the observed contacts are due to the intramolecular interactions. In this 2D spectrum the circled cross peaks correspond to 13C–13C distances ≥4 Å. Thus, this simple spectrum clearly demonstrates polarization transfer between 13C’s separated by medium to long distances. Concurrently, the presence of these cross peaks raises important questions about the mechanism of the polarization transfer: (1) how can we extend the description of the PAR mechanism from a model three spin process involving a single 1H to a real lattice where multiple assisting 1H’s spins can contribute; (2) what is the nature of the polarization transfer between distant spins, i.e., is the polarization transfer directly through space or is it relayed via a chain of 13C’s and which 1H’s are involved in the TSAR mechanism? As shown below the answer to these questions depends strongly on the topology of the spin system together with the 13C–13C distances. Nevertheless, it is possible to predict some qualitative general trends that are useful for evaluating the reliability of the distance estimates for various cases.
As mentioned above, only the 1H’s in close proximity to the 13C’s contribute significantly to PAR polarization transfer. With simulations we have considered PAR polarization transfer as a function of a 1H–13C distance for several different three spin topologies [Fig. SI8 (Ref. 69)]. From these simulations it is apparent that the main contribution to the PAR polarization transfer involves directly bonded 1H–13C spin pairs (since they result in the stronger TSAR couplings). At the same time, it is also clear that, in order to accurately simulate the polarization transfer, 1H’s other than those that are directly bonded to 13C’s also have to be considered. Indeed the simulations suggest that, in general, the inclusion of the 1H’s within 2.5 Å of the 13C’s should lead to an adequate description of the spin dynamics. The contributions from more remote protons have TSAR couplings that are small and do not significantly alter the spin dynamics on the timescale of ∼20–30 ms. For example, even in the very favorable case where the remote proton is at an equal distance of 3 Å from the 13C’s, the PAR polarization transfer requires more than 100 ms to achieve a maximum [see Fig. SI5 (Ref. 69)].
The relative contribution of 13C relayed versus direct polarization transfer is illustrated below for three typical spin systems encountered in proteins. In all cases, the PAR polarization transfers correspond to structurally valuable 13C–13C distances (>4 Å). Let us first examine the case of a sequential inter-residue polarization transfer between Met-Cβ and Leu-Cα (rC−C=4.3 Å) in N-f-MLF-OH [one of the cross peaks highlighted in Fig. 8a]. The SPINEVOLUTION simulations of the polarization transfer are displayed in Fig. 8b and highlight the influence of other nearby spins. The black solid line corresponds to the case with only neighboring 1H’s included in the simulation, the red dashed line includes adjacent 1H’s, Met-Cα, and Met-C′. The fact that the simulations from these three cases overlap indicates that the Met-Cα and Met-C′ (i.e., 13C’s in between Met-Cβ and Leu-Cα) do not really alter the polarization transfer between Met-Cβ and Leu-Cα. The polarization transfer is dominated by the Met-Cβ-[1H]-Leu-Cα TSAR coupling, and thus the 13C relayed magnetization is a negligible fraction of the overall magnetization transferred.
It is, however, possible to find spin systems where the situation is more complicated and where nearby 13C’s do influence a particular magnetization transfer. This is illustrated in Fig. SI9 (Ref. 69) for an intraresidue polarization transfer along the leucine sidechain. Here we consider two cases: from Leu-Cα to Leu-Cδ and to Leu-Cδ′ in [U–13C,15N]-N-f-MLF-OH. These very interesting cases illustrate that the nearby carbons (here Cβ and Cγ) can influence the polarization transfer in different ways. In the case of the Leu-Cα to Leu-Cδ transfer, the polarization transfer maximum is significantly decreased, but the buildup time is very similar with Cβ and Cγ included and without Cβ and Cγ. In this case, the decrease in overall efficiency is explained by the fact that the initial magnetization present on the Leu-Cα is now distributed over three spins. However, because the buildup time is essentially constant, the polarization transfer is mainly direct. In the case the Leu-Cα to Leu Cδ′ transfer, the buildup time is changed when we include Cβ and Cγ in the simulation, accounting for the fact that a substantial part of the polarization transfer in this case is relayed by the Cβ and Cγ carbons. This is consistent with the fact that the Cβ and Cγ carbons are located in the path between the Cα and Cδ2 spins. This is illustrated in Fig. SI9 (Ref. 69).
Note that the three-bond PAR polarization transfers from LCα to LCδ and LCδ′ with Cβ and Cγ included in the simulation are almost identical even though the 13C–13C distances differ by almost 1 Å. This means that intraresidue contacts generally lead to less accurate 13C–13C distance estimates.
In summary, the 13C relayed TSAR mechanism between two distant 13C spins is likely to be significant for spin topologies where additional 13C’s are located in between them. Cases with larger TSAR relayed couplings than direct TSAR coupling will be encountered primarily for intraresidue transfers and sequential contacts (except for Cα,i–Cα,i+1 and Cα,i–Cβ,i+1). For the rest of the inter-residue transfer, as demonstrated in Sec. 7, it is possible to relate the PAR experimental data to 13C–13C distance estimates and to use them for protein structure determination in an approach analogous to Nuclear Overhauser Effect (NOE)-based methods in solution NMR.
13C–13C Crh DISTANCE ESTIMATES FROM 13C–[1H]–13C PAR DATA
In Sec. 3 we discussed the TSAR mechanism for the case of three spins, involving a single proton, and have seen that the PAR polarization transfer depends on the local geometry in this case. It is important to determine how this translates to situations where multiple 1H’s are involved in the mechanism and if the polarization transfer can be used to estimate 1H–13C or 13C–13C distances in spin topologies where the contribution of relayed transfers is negligible.
Figure 9 shows 13C–13C PAR spectra obtained from [U–13C,15N]-Crh protein, and Figs. 10a, 10b, 10c, 10d experimental polarization buildup curves for a number of isolated unambiguously assigned 13C–13C pairs obtained using the PAR technique at ωr∕2π=20 kHz. The polarization transfer curves are sorted into different classes depending on the corresponding 13C–13C distance extracted from the x-ray structure [1MU4 (Ref. 56)]: Fig. 10a corresponds to one-bond distances, (b) to two-bond distances, (c) to medium (4–5.5 Å), and (d) to long (5.5–7 Å) distance classes. Thus, the first two panels (a) and (b) represent intraresidue contacts, whereas the last two panels (c) and (d) show inter-residue distance ranging from 4 to 7 Å.
Figure 10.
PAR polarization transfer as a function of the 13C–13C distance. [(a)–(d)] A sampling of experimental 13C–13C PAR polarization transfer curves obtained on [U–13C,15N]-Crh protein with ωr∕2π=20 kHz, ω0H∕2π=900 MHz and a carrier frequency set to 38.9 ppm: (a) one-bond distance class; (b) 2.5–3.5 Å distance class; (c) 3.5–5 Å distance class; (d) >5 Å distance class. (e) Spin system used in the PAR polarization transfer simulations [(f) and (g)]. Atom coordinates and chemical shift tensors used in the simulations can be found in the SI (Table SI3).
Buildup curves corresponding to one-bond distances are easily distinguishable from other polarization transfers: the maximum signal occurs at around 2 ms, and the intensity is larger than any other distance transfer. In this case, directly bonded 1H’s are primarily responsible for the transfer since their TSAR couplings are dominant. Figure 10b shows PAR polarization transfer corresponding to two-bond distances and the optimal buildup time is about 5 ms. Figure 10c shows medium distance inter-residue transfer corresponding to the 4–5.5 Å distances, and the buildup time is around 5–10 ms with the maximum efficiency is comparable to the two-bond distance case. Note that it does not seem possible to differentiate between the distance classes in Figs. 10b, 10c based on the polarization profiles alone. For longer nonsequential inter-residue distances (>5.5 Å), the PAR buildups are quite distinguishable with the optimal polarization transfer occurring around τmix=15 ms. Note that in this case, the polarization transfer efficiency is set to zero for PAR mixing times equal to 2 and 5 ms because the cross peak intensities were below the noise level.
Finally, we illustrate in Figs. 10e, 10f, 10g the detailed data for the case of polarization transfer between G67Cα and S31Cα. The spin system is composed of ten spins [see Fig. 10e and Table SI3] and does not involve relaxation. The magnetization is initially on the G67Cα spin and the simulation use similar settings as for the experimental data shown in Fig. 9b. The first striking observation is that the buildup curves corresponding to the G67Cα−S31Cα and G67Cα−S31Cβ cross peaks have similar characteristic times. This is a very satisfying result since the two distances are close to 4 Å. Moreover, the transfer to the carbonyls is small (even for the directly bonded G67C′). This behavior is expected since the carrier frequency was set in the middle of the aliphatic 13C region. The PAR 13C cw rf field is not large enough to compensate for the carbonyl chemical shift autocross term which induces a significant off-resonance component in the corresponding TSAR subspace. Figure 10g shows the G67Cα−S31Cα transfer in the presence or in the absence of the S31Cβ and S31C′ spins. The maximum polarization transfer is higher in the latter case but the overall buildup time is preserved ensuring that the TSAR transfer is direct in this case.
Finally, we should mention that the influence of the network of 1H–1H couplings on the TSAR mechanism is a complex question. As shown in Figs. 8b, 10f, 10g, the presence of 1H–1H couplings can have different effects on the 13C–[1H]–13C PAR polarization transfer. In the case of the sequential transfer from LCα to MCβ simulated in Fig. 8b, the polarization transfer is only slightly affected by the removal of the 1H–1H couplings. Concurrently, the long-range PAR transfer between S31Cα and G67Cα spins simulated in Fig. 10g is dependent on the presence and the absence of 1H–1H couplings. The influence of 1H–1H couplings on the 13C–[1H]–13C TSAR spin dynamics depends on the spin system topology and the rf settings chosen. Moreover, the influence of the 1H–1H couplings appears through second-order effects (i.e., pH≠0.5,1). However, in examples highlighted here, the presence or the absence of 1H–1H couplings does not significantly change the 13C–[1H]–13C buildup profiles, i.e., the maximum buildup time is preserved and the optimal polarization transfer is similar within 30%.
In summary, Figs. 10a, 10b, 10c, 10d indicate that the homonuclear TSAR mechanism yields buildup profiles that can be used to estimate 13C–13C distances in fully protonated protein samples (with the exception of intraresidue >2-bond and sequential contacts different from iCα–i+1Cα, iCα–i+1Cβ). Furthermore, the effect of the 1H–1H couplings does not appear as a limiting factor for using the PAR experiments to estimate 13C–13C distances.
13C–[1H]–13C TSAR RECOUPLING DURING FIRST-ORDER 13C–13C HOMONUCLEAR RECOUPLING
In Sec. 2 we demonstrated that 13C–[1H]–13C TSAR transfers utilize two cw rf fields and that one of the matching conditions implies similar rf fields on the 1H and 13C channels. That is, appreciable PAR transfer is achieved when the mismatch between 1H and 13C rf field strengths is not sufficient to decouple the 1H and 13C baths. This in turn suggests the possibility of concurrent 13C–[1H]–13C TSAR transfers during other homonuclear recoupling experiments, particularly those where the 1H decoupling is nonideal. Potentially, this situation arises in first-order 13C–13C dipolar recoupling experiments, where insufficient 1H decoupling is known to interfere with the 13C–13C recoupling spin dynamics and produces intensity losses in DQ filtered experiments5, 57 or 13C–13C J-based transfer experiments. This interference becomes acute at high ωr∕2π and has stimulated the design of DQ recoupling sequences where no additional 1H irradiation is required.7, 53
In the Supporting Information (Figs. SI10–SI12) (Ref. 69) we investigate with simulations the influence of the ZQ 13C–[1H]–13C TSAR mechanism during the application of four 13C–13C dipolar recoupling sequences [HORROR,6 CMpRR,8 RFDR,4, 58 and SR62 (Refs. 6, 59)] as well as P912 (Ref. 1) TOBSY (Ref. 60) through bond polarization transfer experiments. We show that the TSAR mechanism may become significant or even the dominant homonuclear polarization transfer mechanism for 1H irradiation conditions considered as adequate 1H decoupling fields (especially in applications on proteins). Note that this is in contrast to previous studies that have only considered the effects of inadequate 1H decoupling fields in the context of 13C–13C polarization transfer losses and not in terms of potential additional polarization transfer.
This is an important result as it shows that we should exercise caution when interpreting experimental data obtained on protonated samples on the basis of a 13C–13C recoupling mechanism. For instance, if the goal is to assign the spectrum of an unknown molecule, it is crucial to be able to distinguish cross peaks due to covalently bonded nuclei from the cross peaks due to noncovalently bonded nuclei that are in close spatial proximity. In theory this can be achieved either using a broadband dipolar recoupling technique, in which dipolar truncation ensures one-bond relayed transfer, or using J coupling based through-bond methods.20, 60, 61 In practice, the presence of additional efficient through-space TSAR transfer is likely to jeopardize, or at least greatly complicate, the correct interpretation of the assignment data by producing additional cross peaks that are not distinguishable from the cross peaks resulting from the intended dipolar or J-based mechanism. We note that for techniques that recouple and decouple with 13C irradiation alone (CMpRR,8, 55 CMAR,7 radio-frequency-driven dipolar recoupling (RFDR),62 and some R and C sequences63) that TSAR is suppressed.
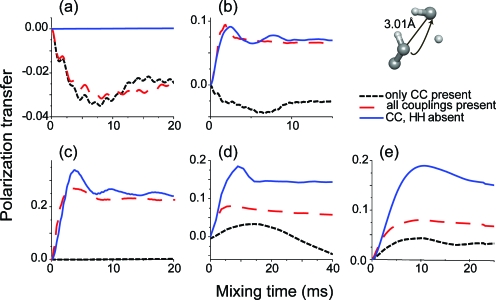
In Fig. 11 we delineate these ideas with simulations using five spins (three 13C’s and two 1H’s) that illustrate the effect of a directly bonded spin (C3) on a long distance transfer from C1 to C2 (3.01 Å). As we will see the presence of the additional 13C–[1H]–13C TSAR mechanism during first-order 13C–13C recoupling pulse sequences may lead to an overall increase in long distance polarization transfer (C1-C2) which may be incorrectly interpreted as a reduction in dipolar truncation in the 13C–13C recoupling mechanism.
Figure 11.
Modification of the long distance polarization transfer in uniformly labeled systems when both first-order 13C–13C recoupling and second-order TSAR mechanism are simultaneously present. Note the different behavior for the simulations with only 13C–13C couplings included and the simulations also including 1H’s. The chosen 1H irradiations yield substantial TSAR mechanism contribution to overall polarization transfer (except CM5RR where no 1H irradiation was used in order to illustrate a case of pure second-order 13C–13C spin dynamics without TSAR contribution). (a) DQ CM5RR with 100 kHz 13C rf and no 1H rf. (b) DQ HORROR with 15 kHz 13C rf and 80 kHz 1H rf. (c) ZQ PAR with 56 kHz 13C rf and 54 kHz 1H rf. (d) ZQ SR62 (Ref. 6) with 20 kHz 13C rf and 82 kHz 1H rf. (e) ZQ RFDR with 12.5 kHz pulses and 69 kHz 1H rf. The simulations were performed at [(a), (c), (d), and (e)] ωr∕2π=20 kHz or (b) ωr∕2π=30 kHz and ω0∕2π=700 MHz and include isotropic chemical shift and CSA typical for the aliphatic sites (see SI) (Ref. 69). The spin system is based on the leucine sidechain in the structure of N-f-MLF-OH (Ref. 64). Note that all the pulse sequences except PAR [in (d)] are designed to reintroduce the 13C–13C dipolar coupling to the first order.
If we consider the first set of simulations (dashed black lines) in Fig. 11 where only the 13C spins are included, we confirm the general observation that most first-order 13C–13C recoupling sequences lead to efficient dipolar truncation. The two other sets of curves (dashed red and blue lines) in Fig. 11 represent the same simulations with a larger spin system that includes three additional protons. More specifically, the second set of lines (dashed red) represents simulations where all the couplings are present, whereas the third set of lines (blue) accounts only for the TSAR transfer as the 13C–13C and 1H–1H couplings are removed.
In the case of CMpRR, no 1H irradiation is present since the sequence was designed to efficiently recouple and decouple using only a 13C irradiation field.7, 8 In this case the spin dynamics is free from any TSAR contribution and dominated by first-order 13C–13C recoupling yielding a small polarization transfer to the remote spins (about 3% when all couplings are included). On the other hand, for PAR recoupling, the polarization transfer does not rely on 13C–13C couplings, and long-range polarization transfer can reach about 25% efficiency (all couplings included) solely based on 13C–[1H]–13C TSAR mechanism. Note that the polarization transfer is only slightly affected by the 1H–1H couplings.
For the other examples, the long distance polarization transfers are substantially different between simulation performed with 13C’s only (black dashed) and simulations in the presence of protons under 1H decoupling (second set) and can even be larger in the latter case. This can be explained by the presence of a concurrent 13C–[1H]–13C TSAR mechanism and is clearly demonstrated by comparing simulations without 13C–13C and 1H–1H couplings and simulations with all couplings included. The 1H “decoupling” field in each case was chosen to obtain an appreciable (although not necessarily optimal) TSAR effect [see Fig. SI10 (Ref. 69)]. The 1H field strengths are 80, 82, and 69 kHz for HORROR, SR62, and RFDR, respectively. Note that these 1H fields correspond to values often used in SSNMR protein studies. This is a very interesting observation as it explains why some first-order recoupling sequences under some conditions are able to provide medium to long-range contacts. In all the cases studied here, the reason behind involves a second-order 13C–[1H]–13C TSAR effect. This is a very satisfying observation as the dipolar truncation efficiency should not vary much between first-order recoupling sequences (either DQ or ZQ). Indeed the truncation efficiency primarily relies on the ratio between weak versus strong couplings and is thus independent of the pulse sequence scaling factor. For a given spin system geometry, the powder Euler angle dependence of the recoupling pulse sequence should be able to mainly account for the small variation in dipolar truncation efficiency.
Finally, it is important to highlight that the simultaneous reintroduction of first-order 13C–13C terms and second-order 13C–[1H]–13C TSAR terms reduces the polarization transfer to the remote spin compared to the PAR case. As a consequence, if the objective is to record medium to long distance contacts in uniformly or extensively labeled systems, the obvious choice is to use a pulse sequence inducing a pure TSAR spin dynamics and not a mixture of first-order 13C–13C and TSAR spin dynamics.
To summarize, when a sufficiently large second-order 13C–[1H]–13C TSAR effect is concurrently present during first-order 13C–13C recoupling experiments, then it manifests itself as an apparent reduction in dipolar truncation. Thus, in the case of first-order ZQ sequences such as RFDR, it is possible to observe polarization transfer over distances that are long compared to the situation where only 13C–13C couplings are present. This significant effect should be taken into account when interpreting experimental data both for assignments and structural studies.
PROTEIN STRUCTURE CALCULATION
Finally, we illustrate the potential of the TSAR based techniques for 3D structure determination of proteins. More precisely, we show that the PAR data presented here contain information on a sufficient number of distances, including medium range (from contacts between sites that are between two to four residues apart) and long-range restraints (from contacts between the sites that are equal or more than five residues apart) to successfully calculate the structure of the Crh protein.
The 13C–13C correlation spectra presented in Fig. 9 were recorded at 900 MHz and ωr∕2π=20 kHz from a sample of microcrystalline [U–13C,15N]-Crh protein with mixing times of (a) 0.8 ms (CM5RR) and (b) 15 ms (PAR). To reduce the experimental acquisition period, compared to the N-f-MLF-OH PAR spectra presented above, we focused on the aliphatic-aliphatic correlations in which case we need to sample only a ∼14 kHz spectral width in the indirect dimension rather than ∼40 kHz for the full bandwidth at 900 MHz 1H frequency. Moreover, this approach allows us to work with rf fields of ∼50 kHz without having to address bandwidth issues and concentrates the magnetization on the aliphatic sites, without distributing polarization to the carbonyl and aromatic nuclei.
The 13C–13C PAR data shown in Fig. 9b contain ∼800 cross peaks and assigning these unambiguously is a nontrivial task. Thus, we used the x-ray structure56 as a homology model to facilitate in the assignment of ambiguous cross peaks present in the PAR data set. More precisely, we combined the chemical shift assignment data25 and 13C–13C distances extracted from the x-ray structure to simulate a 13C–13C spectrum with a 13C–13C distance cutoff of 7 Å. By allowing the chemical shifts to vary within ±0.25 ppm and comparing this simulated spectrum with the experimental data, we were able to unambiguously assign 163 structurally meaningful 13C–13C cross peaks. 92 of these correspond to long-range 13C–13C contacts.
Using the unambiguously assigned cross peaks with a unique distance class of 2.2–6 Å, we then performed molecular dynamics simulated annealing in torsion angle space [CNS (Ref. 65)]. The backbone precision for the 10 best structures from the 100 calculated conformers was found to be 2.06 Å, and the accuracy (defined here as the backbone rmsd of the 10 best conformers with respect to the x-ray structure) is 2.31 Å. The calculated structure resulting from this simple procedure is presented in Fig. 12c. Using only one 13C–13C PAR data set and one distance class for all the contacts, we obtain the correct fold of the Crh monomer. Thus, this demonstrates that the PAR data presented here contain information on a sufficient number of distances to calculate a protein structure. It is worth mentioning that we did not use dihedral angle predictions from chemical shifts.26
Note, that we have used only a single class of distances in the above calculation. However, as we have demonstrated in Sec. 7 with polarization transfer curves, we can establish several distance classes that should enhance the precision and accuracy of the structure calculations using data from the TSAR based techniques. A de novo structure calculation utilizing 13C–13C PAR and 15N–13C PAIN-CP (Ref. 44) (i.e., 15N–[1H]–13C TSAR) restraints, that we have performed in a separate study will be reported elsewhere.
In order to complete our discussion of the PAR experiment, we briefly contrast it with other techniques used for SSNMR 3D structure determination of proteins, primarily PDSD33 and DARR.13, 34 Using these two approaches, structures of model proteins were determined using U–13C,15N and alternately 13C labeled spin systems.14, 16, 23, 66 The use of alternate labeling schemes67 is motivated by excessive spectral crowding obtained at long mixing times on uniformly labeled systems, which complicates cross peak assignments. The extent of crowding in DARR spectra is dependent on several variables, such as the protein size, the magnetic field, and the MAS frequency. However, because the DARR mechanism relies on 13C–13C couplings, it efficiently relays magnetization through the 13C chain and thus leads to efficient polarization transfer for intraresidue (and also sequential) contacts, and this interferes with the observation of cross peaks arising from medium and long-range contacts.68 Since the TSAR mechanism does not involve 13C–13C couplings, the same is not true for PAR. Indeed, if we compare cross peak intensities at τmix=20 ms, we find that the ratio of cross peaks corresponding to long (>5 Å) as compared to short distances is between 1:1 and 1:2.
In contrast to PDSD and DARR and similarly to the PAR method, CHHC∕NHHC35, 36 yields spectra with reduced spectral crowding. These experiments are used to extract structurally valuable cross peaks and therefore appear more suitable for application to uniformly labeled systems. This was recently illustrated with a structure determination of the [U–13C,15N]-Crh dimer based on CHHC∕NHHC data,42 and similar work is currently under progress using the PAR data.
Even though CHHC∕NHHC and TSAR-based techniques appear as complementary approaches to extract relevant structural restraints, it is worth pointing out that they differ in terms of sensitivity. In particular, the CHHC∕NHHC techniques utilize three polarization transfer steps, and as a consequence their sensitivity is low. In contrast, the PAR method uses a single, efficient transfer step and thus yields improved signal to noise. This point is illustrated in Fig. SI13 (Ref. 69) which compares a CHHC spectrum of Crh recorded with ∼20 mg of sample at ω0H∕2π=500 MHz in ∼46 h with a 13C–13C PAR spectrum obtained with ∼6 mg of sample at ω0H∕2π=750 MHz in ∼21.4 h. In both spectra, an intermediate mixing time was chosen and the lowest contour level was set at five times the noise level. These spectra illustrate the improved signal to noise of the PAR data obtained at high magnetic fields on small samples.
Another interesting observation is the significant attenuation or even the absence of cross peaks involving methyl groups in the CHHC spectra. This effect can be explained by the intrinsically inefficient polarization step from the 13C spin to methyl 1H’s and a reduction in 1H–1H polarization transfer efficiency due to threefold methyl group hopping.40 In contrast, cross peaks involving methyl groups are featured prominently in the PAR spectra. Figure 12b illustrates this point with an expansion of the aliphatic region of the spectrum focusing on the cross peaks involving methyl groups.
The data suggest that the motion of the −CH3’s has a positive effect on the polarization transfer in the PAR experiment, providing a sensitive tool to probe (long-range) contacts involving methyl groups. This is of primarily importance for methyl-methyl side-chain contacts which are often buried in the hydrophobic core of the protein and thus particularly useful in structure calculations. This point is corroborated by the simulations shown in Fig. 12d that compare the 4.3 Å distance transfer between A20Cα and I47Cδ1 sites of Crh protein with and without methyl group rotation. The polarization transfer is greatly enhanced when the methyl protons are simulated under fast exchange conditions. It is worth noting that methyl groups have been recognized as very valuable probes of structure and dynamics in a wide range of biomolecular systems including membrane and amyloid proteins, and they were recently used to probe protein-ligand interaction in SSNMR.66
The last feature of PAR that should be pointed out, especially in contrast to PDSD and DARR sequences, is its flexibility with respect to the MAS regime. The PAR sequence was used in this work at ωr∕2π=20 kHz, but its application at higher, as well as lower frequencies is also possible. This is depicted in Fig. SI14 (Ref. 69) with a numerical simulation of a 13C–13C PAR experiment at the lower and higher spinning frequencies, ωr∕2π=10 and 70 kHz, respectively. In PDSD the optimal buildup time for a fixed distance increases with ωr∕2π, which limits the applicability of the technique to low and moderate spinning frequencies (<15 kHz). The DARR experiment, which relies on the same type of mechanism as PDSD with an improved scaling factor, enables access to higher MAS frequencies. However, it is not practical to use DARR for obtaining long distance contacts ≥30 kHz MAS for two reasons. First, as in the case of PDSD, the spin part of the DARR scaling factor is fixed, so the mixing time required to observe long distance contacts increases with ωr∕2π; and second, the application of DARR for ∼100 ms to observe long distances at >30 kHz MAS frequencies may induce a considerable amount of rf heating, which is not desirable for biological samples.
CONCLUSIONS
We have introduced and characterized the homonuclear version of the TSAR mechanism applicable to 13C–13C and 15N–15N polarization transfer in biomolecules at moderate (10–20 kHz) to high (70 kHz) spinning frequencies and high magnetic fields with moderate power requirements. The PAR sequence relies on a three spin process involving second-order cross terms between 1H–13C1 (1H–15N1) and 1H–13C2 (1H–15N2) that promotes polarization transfer between 13C1 (15N1) and 13C2 (15N2) via trilinear operators such as . The analytical expressions derived from AHT permit visualization of the subspace in which the TSAR spin dynamics evolves and indicates that the processes are influenced by the presence of an autocross term with major contributions from cross terms of the 1H–13C coupling with itself. Moreover, in the context of analytical and numerical simulations, we discussed the dependence of PAR polarization transfer with respect to the local geometry of the spin system. We show that the autocross term may be compensated to a large extent for specific combinations of 13C and 1H rf fields, as well as for some particular spin topologies. In addition, we demonstrate that dipolar truncation is significantly reduced in the TSAR mechanism, and that we can use the 13C–13C PAR experiment to estimate 13C–13C distances. With this concept in mind, we applied 13C–13C PAR to the uniformly labeled Crh protein, which allowed us to obtain high quality spectra used to derive the structure of a Crh monomeric subunit. Moreover, we have discussed the possibility of inducing significant 13C–[1H]–13C TSAR polarization transfer during the application of other 13C–13C dipolar recoupling and J-based experiments that should be taken into account when interpreting the data. Interestingly, 13C–[1H]–13C TSAR contributions are present during the first-order 13C–13C recoupling experiments and may lead to the apparent reduction in dipolar truncation. In summary, TSAR based techniques—including 13C–13C PAR, 15N–15N PAR, and the previously introduced 15N–13C PAIN-CP—constitute a new family of techniques for high resolution structural studies of large biomolecules at high magnetic fields and high spinning frequencies.
ACKNOWLEDGMENTS
We would like to thank Dr. Patrick van der Wel for his active support and useful discussions and Dr. Carole Gardiennet for recording the CHHC Crh data. We are very grateful to Dr. Mikhail Veshtort for providing the SPINEVOLUTION software that was essential for this research. We also would like to thank Dr. David Ruben, Dr. Christopher Turner, Ajay Thakkar, and Dr. Anthony Bielecki for technical support, and Galia Debelouchina, Matt Eddy, Alexander Barnes, Marvin Bayro, Dr. Thorsten Maly, Marc Caporini, Andrew Casey, and Professor Anne-Frances Miller for many helpful discussions. This work was supported by National Institutes of Health (Grant Nos. EB-001960, EB-003151, and EB-002026) and the French ANR (Grant No. JC05_44957).
References
- Andrew E. R., Bradbury A., and Eades R. G., Nature (London) 10.1038/1821659a0 182, 1659 (1958) [DOI] [Google Scholar]; Andrew E. R., Bradbury A., and Eades R. G., Nature (London) 10.1038/1831802a0 183, 1802 (1959). [DOI] [Google Scholar]
- Munowitz M. G., Griffin R. G., Bodenhausen G., and Huang T. H., J. Am. Chem. Soc. 10.1021/ja00400a007 103, 2529 (1981) [DOI] [Google Scholar]; Munowitz M. G. and Griffin R. G., J. Chem. Phys. 10.1063/1.443386 76, 2848 (1982) [DOI] [Google Scholar]; Raleigh D. P., Levitt M. H., and Griffin R. G., Chem. Phys. Lett. 10.1016/0009-2614(88)85051-6 146, 71 (1988) [DOI] [Google Scholar]; Raleigh D. P., Creuzet F., Gupta S. K. D., Levitt M. H., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja00194a057 111, 4502 (1989) [DOI] [Google Scholar]; Tycko R. and Dabbagh G., Chem. Phys. Lett. 10.1016/0009-2614(90)87235-J 173, 461 (1990) [DOI] [Google Scholar]; Tycko R. and Dabbagh G., J. Am. Chem. Soc. 10.1021/ja00025a003 113, 9444 (1991) [DOI] [Google Scholar]; Bayro M. J., Ramachandran R., Caporini M. A., Eddy M. T., and Griffin R. G., J. Chem. Phys. 128, (2008) [DOI] [PubMed] [Google Scholar]; Brinkmann A., Eden M., and Levitt M. H., J. Chem. Phys. 10.1063/1.481458 112, 8539 (2000) [DOI] [Google Scholar]; Brinkmann A., Eden M., and Levitt M. H., J. Chem. Phys. 10.1063/1.1377031 115, 357 (2001). [DOI] [Google Scholar]
- Gullion T. and Schaefer J., J. Magn. Reson. 81, 196 (1989). [Google Scholar]
- Bennett A. E., Ok J. H., Griffin R. G., and Vega S., J. Chem. Phys. 10.1063/1.462267 96, 8624 (1992). [DOI] [Google Scholar]
- Bennett A. E., Rienstra C. M., Griffiths J. M., Zhen W. G., Lansbury P. T., and Griffin R. G., J. Chem. Phys. 10.1063/1.476420 108, 9463 (1998). [DOI] [Google Scholar]
- Nielsen N. C., Bildsoe H., Jakobsen H. J., and Levitt M. H., J. Chem. Phys. 10.1063/1.467759 101, 1805 (1994). [DOI] [Google Scholar]
- De Paepe G., Bayro M. J., Lewandowski J., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja0550430 128, 1776 (2006). [DOI] [PubMed] [Google Scholar]
- De Paepe G., Lewandowski J. R., and Griffin R. G., J. Chem. Phys. 10.1063/1.2834732 128, 124503 (2008). [DOI] [PubMed] [Google Scholar]
- Creuzet F., McDermott A., Gebhard R., Vanderhoef K., Spijkerassink M. B., Herzfeld J., Lugtenburg J., Levitt M. H., and Griffin R. G., Science 10.1126/science.1990439 251, 783 (1991) [DOI] [PubMed] [Google Scholar]; Carravetta M., Eden M., Johannessen O. G., Luthman H., Verdegem P. J. E., Lugtenburg J., Sebald A., and Levitt M. H., J. Am. Chem. Soc. 10.1021/ja016027f 123, 10628 (2001) [DOI] [PubMed] [Google Scholar]; Jaroniec C. P., Filip C., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja026385y 124, 10728 (2002) [DOI] [PubMed] [Google Scholar]; Jaroniec C. P., Tounge B. A., Herzfeld J., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja003266e 123, 3507 (2001) [DOI] [PubMed] [Google Scholar]; Jaroniec C. P., Tounge B. A., Herzfeld J., and Griffin R. G., Biophys. J. 80, 368A (2001) [Google Scholar]; Jaroniec C. P., Tounge B. A., Rienstra C. M., Herzfeld J., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja9921569 121, 10237 (1999) [DOI] [Google Scholar]; Carravetta M., Zhao X., Johannessen O. G., Lai W. C., Verhoeven M. A., Bovee-Geurts P. H. M., Verdegem P. J. E., Kiihne S., Luthman H., de Groot H. J. M., deGrip W. J., Lugtenburg J., and Levitt M. H., J. Am. Chem. Soc. 10.1021/ja039390q 126, 3948 (2004). [DOI] [PubMed] [Google Scholar]
- Ramachandran R., Ladizhansky V., Bajaj V. S., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja037761x 125, 15623 (2003) [DOI] [PubMed] [Google Scholar]; Ramachandran R., Lewandowski J. R., van der Wel P. C. A., and Griffin R. G., J. Chem. Phys. 10.1063/1.2194905 124, 214107 (2006). [DOI] [PubMed] [Google Scholar]
- Ladizhansky V. and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja037138c 126, 948 (2004). [DOI] [PubMed] [Google Scholar]
- Ladizhansky V., Jaroniec C. P., Diehl A., Oschkinat H., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja029082c 125, 6827 (2003) [DOI] [PubMed] [Google Scholar]; Feng X., Lee Y. K., Sandstrom D., Eden M., Maisel H., Sebald A., and Levitt M. H., Chem. Phys. Lett. 10.1016/0009-2614(96)00558-1 257, 314 (1996) [DOI] [Google Scholar]; Feng X., Verdegem P. J. E., Eden M., Sandstrom D., Lee Y. K., Bovee-Geurts P. H. M., de Grip W. J., Lugtenburg J., de Groot H. J. M., and Levitt M. H., J. Biomol. NMR 10.1023/A:1008377231625 16, 1 (2000) [DOI] [PubMed] [Google Scholar]; Ladizhansky V., Veshtort M., and Griffin R. G., J. Magn. Reson. 10.1006/jmre.2001.2488 154, 317 (2002) [DOI] [PubMed] [Google Scholar]; Feng X., Eden M., Brinkmann A., Luthman H., Eriksson L., Graslund A., Antzutkin O. N., and Levitt M. H., J. Am. Chem. Soc. 10.1021/ja972252e 119, 12006 (1997) [DOI] [Google Scholar]; Feng X., Verdegem P. J. E., Lee Y. K., Sandstrom D., and Eden M., BoveeGeurts P., deGrip W. J., Lugtenburg J., deGroot H. J. M., and Levitt M. H., J. Am. Chem. Soc. 10.1021/ja970710d 119, 6853 (1997). [DOI] [Google Scholar]
- Takegoshi K., Nakamura S., and Terao T., Chem. Phys. Lett. 10.1016/S0009-2614(01)00791-6 344, 631 (2001) [DOI] [Google Scholar]; Takegoshi K., Nakamura S., and Terao T., J. Chem. Phys. 10.1063/1.1534105 118, 2325 (2003). [DOI] [Google Scholar]
- Castellani F., van Rossum B., Diehl A., Schubert M., Rehbein K., and Oschkinat H., Nature (London) 10.1038/nature01070 420, 98 (2002). [DOI] [PubMed] [Google Scholar]
- Zech S. G., Wand A. J., and McDermott A. E., J. Am. Chem. Soc. 10.1021/ja0503128 127, 8618 (2005). [DOI] [PubMed] [Google Scholar]
- Manolikas T., Herrmann T., and Meier B. H., J. Am. Chem. Soc. 10.1021/ja078039s 130, 3959 (2008). [DOI] [PubMed] [Google Scholar]
- Scholz I., Meier B. H., and Ernst M., J. Chem. Phys. 127, (2007). [DOI] [PubMed] [Google Scholar]
- Bennett A. E., Rienstra C. M., Auger M., Lakshmi K. V., and Griffin R. G., J. Chem. Phys. 10.1063/1.470372 103, 6951 (1995). [DOI] [Google Scholar]
- Detken A., Hardy E. H., Ernst M., and Meier B. H., Chem. Phys. Lett. 10.1016/S0009-2614(02)00335-4 356, 298 (2002) [DOI] [Google Scholar]; De Paepe G., Elena B., and Emsley L., J. Chem. Phys. 10.1063/1.1773155 121, 3165 (2004) [DOI] [PubMed] [Google Scholar]; De Paepe G., Hodgkinson P., and Emsley L., Chem. Phys. Lett. 10.1016/S0009-2614(03)00966-7 376, 259 (2003) [DOI] [Google Scholar]; De Paepe G., Lesage A., and Emsley L., J. Chem. Phys. 10.1063/1.1595088 119, 4833 (2003) [DOI] [Google Scholar]; Ernst M., J. Magn. Reson. 10.1016/S1090-7807(03)00074-0 162, 1 (2003) [DOI] [PubMed] [Google Scholar]; Hodgkinson P., Prog. Nucl. Magn. Reson. Spectrosc. 46, 197 (2005). [DOI] [PubMed] [Google Scholar]
- De Paepe G., Giraud N., Lesage A., Hodgkinson P., Bockmann A., and Emsley L., J. Am. Chem. Soc. 10.1021/ja037213j 125, 13938 (2003). [DOI] [PubMed] [Google Scholar]
- van der Wel P. C. A., Lewandowski J. R., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja068633m 129, 5117 (2007) [DOI] [PubMed] [Google Scholar]; Jaroniec C. P., MacPhee C. E., Astrof N. S., Dobson C. M., and Griffin R. G., Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.252625999 99, 16748 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko R. and Ishii Y., J. Am. Chem. Soc. 10.1021/ja0342042 125, 6606 (2003). [DOI] [PubMed] [Google Scholar]
- Wasmer C., Lange A., Van Melckebeke H., Siemer A. B., Riek R., and Meier B. H., Science 10.1126/science.1151839 319, 1523 (2008). [DOI] [PubMed] [Google Scholar]
- De Paepe G., Lewandowski J., Bayro M. J., and Griffin R. G., 47th Experimental Nuclear Magnetic Resonance Conference, Asilomar (SF), 2006 (unpublished).
- Bockmann A., Lange A., Galinier A., Luca S., Giraud N., Juy M., Heise H., Montserret R., Penin F., and Baldus M., J. Biomol. NMR 10.1023/A:1025820611009 27, 323 (2003). [DOI] [PubMed] [Google Scholar]
- Cornilescu G., Delaglio F., and Bax A., J. Biomol. NMR 10.1023/A:1008392405740 13, 289 (1999). [DOI] [PubMed] [Google Scholar]
- Costa P. R., Sun B. Q., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja964313z 119, 10821 (1997). [DOI] [Google Scholar]
- Costa P. R., Sun B. Q., and Griffin R. G., J. Magn. Reson. 10.1016/S1090-7807(03)00083-1 164, 92 (2003). [DOI] [PubMed] [Google Scholar]
- Takegoshi K., Nomura K., and Terao T., Chem. Phys. Lett. 10.1016/0009-2614(94)01399-G 232, 424 (1995) [DOI] [Google Scholar]; Takegoshi K., Nomura K., and Terao T., J. Magn. Reson. 10.1006/jmre.1997.1191 127, 206 (1997). [DOI] [PubMed] [Google Scholar]
- Paravastu A. K. and Tycko R., J. Chem. Phys. 124, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Montesinos I., Mollica G., Carravetta M., Gansmuller A., Pilelo G., Bechmann M., Sebald A., and Levitt M. H., Chem. Phys. Lett. 10.1016/j.cplett.2006.10.101 432, 572 (2006). [DOI] [Google Scholar]
- Khaneja N. and Niels Chr N., J. Chem. Phys. 10.1063/1.2816140 128, 015103 (2008). [DOI] [PubMed] [Google Scholar]
- Szeverenyi N. M., Sullivan M. J., and Maciel G. E., J. Magn. Reson. 47, 462 (1982). [Google Scholar]
- Morcombe C. R., Gaponenko V., Byrd R. A., and Zilm K. W., J. Am. Chem. Soc. 10.1021/ja047919t 126, 7196 (2004). [DOI] [PubMed] [Google Scholar]
- Lange A., Seidel K., Verdier L., Luca S., and Baldus M., J. Am. Chem. Soc. 10.1021/ja034555g 125, 12640 (2003) [DOI] [PubMed] [Google Scholar]; Lange A., Luca S., and Baldus M., J. Am. Chem. Soc. 10.1021/ja026691b 124, 9704 (2002). [DOI] [PubMed] [Google Scholar]
- Heise H., Seidel K., Etzkorn M., Becker S., and Baldus M., J. Magn. Reson. 10.1016/j.jmr.2004.11.020 173, 64 (2005). [DOI] [PubMed] [Google Scholar]
- Peng X., Libich D., Janik R., Harauz G., and Ladizhansky V., J. Am. Chem. Soc. 10.1021/ja076658v 130, 359 (2008). [DOI] [PubMed] [Google Scholar]
- Grommek A., Meier B. H., and Ernst M., Chem. Phys. Lett. 10.1016/j.cplett.2006.07.005 427, 404 (2006). [DOI] [Google Scholar]
- Franks W. T., Wylie B. J., Schmidt H. L. F., Nieuwkoop A. J., Mayrhofer R. M., Shah G. J., Graesser D. T., and Rienstra C. M., Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0712393105 105, 4621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K., Etzkorn M., Sonnenberg L., Griesinger C., Sebald A., and Baldus M., J. Phys. Chem. A 10.1021/jp045605m 109, 2436 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou D. H., Shea J. J., Nieuwkoop A. J., Franks W. T., Wylie B. J., Mullen C., Sandoz D., and Rienstra C. M., Angew. Chem., Int. Ed. Engl. 10.1002/anie.200702905 46, 8380 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loquet A., Bardiaux B., Gardiennet C., Blanchet C., Baldus M., Nilges M., Malliavin T., and Boeckmann A., J. Am. Chem. Soc. 10.1021/ja078014t 130, 3579 (2008). [DOI] [PubMed] [Google Scholar]
- Lange A., Becker S., Seidel K., Giller K., Pongs O., and Baldus M., Angew. Chem., Int. Ed. 10.1002/anie.200462516 44, 2089 (2005). [DOI] [PubMed] [Google Scholar]
- Lewandowski J. R., De Paepe G., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja0650394 129, 728 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski J. R., De Paepe G., Eddy M., and Griffin R. G. (unpublished).
- Haeberlen U. and Waugh J. S., Phys. Rev. 10.1103/PhysRev.175.453 175, 453 (1968). [DOI] [Google Scholar]
- Hartmann S. R. and Hahn E. L., Phys. Rev. 10.1103/PhysRev.128.2042 128, 2042 (1962). [DOI] [Google Scholar]
- Levitt M. H., Oas T. G., and Griffin R. G., Isr. J. Chem. 28, 271 (1988) [Google Scholar]; Levitt M. H., Oas T. G., and Griffin R. G., J. Chem. Phys. 10.1063/1.455191 89, 692 (1988). [DOI] [Google Scholar]
- Sorensen O. W., Eich G. W., Levitt M. H., Bodenhausen G., and Ernst R. R., Prog. Nucl. Magn. Reson. Spectrosc. 10.1016/0079-6565(84)80005-9 16, 163 (1983). [DOI] [Google Scholar]
- Veshtort M. and Griffin R. G., J. Magn. Reson. 10.1016/j.jmr.2005.07.018 178, 248 (2006). [DOI] [PubMed] [Google Scholar]
- Lee Y. K., Kurur N. D., Helmle M., Johannessen O. G., Nielsen N. C., and Levitt M. H., Chem. Phys. Lett. 10.1016/0009-2614(95)00741-L 242, 304 (1995). [DOI] [Google Scholar]
- Costa P. R., Verel R., Ernst M., and Meier B. H., J. Magn. Reson. 10.1006/jmre.2001.2310 150, 81 (2001). [DOI] [PubMed] [Google Scholar]
- Hohwy M., Rienstra C. M., and Griffin R. G., J. Chem. Phys. 10.1063/1.1488136 117, 4973 (2002). [DOI] [Google Scholar]
- Hohwy M., Rienstra C. M., Jaroniec C. P., and Griffin R. G., J. Chem. Phys. 10.1063/1.478702 110, 7983 (1999). [DOI] [Google Scholar]
- Lewandowski J. R., De Paepe G., van der Wel P. C. A., Birkett N. R., Belenky M., Maly T., Bayro M. J., Sivertsen A. C., Dobson C. M., Herzfeld J., and Griffin R. G. (unpublished).
- Juy M., Penin F., Favier A., Galinier A., Montserret R., Haser R., Deutscher J., and Bockmann A., J. Mol. Biol. 332, 767 (2003). [DOI] [PubMed] [Google Scholar]
- Ishii Y., Ashida J., and Terao T., Chem. Phys. Lett. 10.1016/0009-2614(95)01136-5 246, 439 (1995) [DOI] [Google Scholar]; Rienstra C. M., Hatcher M. E., Mueller L. J., Sun B. Q., Fesik S. W., and Griffin R. G., J. Am. Chem. Soc. 10.1021/ja9810181 120, 10602 (1998). [DOI] [Google Scholar]
- Ishii Y., J. Chem. Phys. 10.1063/1.1359445 114, 8473 (2001). [DOI] [Google Scholar]
- Brinkmann A., Gunne J. S. A. D., and Levitt M. H., J. Magn. Reson. 10.1006/jmre.2002.2525 156, 79 (2002). [DOI] [PubMed] [Google Scholar]
- Hardy E. H., Verel R., and Meier B. H., J. Magn. Reson. 10.1006/jmre.2000.2258 148, 459 (2001). [DOI] [PubMed] [Google Scholar]
- Lesage A., Auger C., Caldarelli S., and Emsley L., J. Am. Chem. Soc. 10.1021/ja971089k 119, 7867 (1997) [DOI] [Google Scholar]; Lesage A., Bardet M., and Emsley L., J. Am. Chem. Soc. 10.1021/ja992272b 121, 10987 (1999) [DOI] [Google Scholar]; Lesage A., Sakellariou D., Steuernagel S., and Emsley L., J. Am. Chem. Soc. 10.1021/ja983048+ 120, 13194 (1998) [DOI] [Google Scholar]; De Paepe G., Lesage A., Steuernagel S., and Emsley L., ChemPhysChem 10.1002/cphc.200301062 5, 869 (2004) [DOI] [PubMed] [Google Scholar]; Mueller L. J., Elliott D. W., Leskowitz G. M., Struppe J., Olsen R. A., Kim K. C., and Reed C. A., J. Magn. Reson. 10.1016/j.jmr.2004.03.017 168, 327 (2004). [DOI] [PubMed] [Google Scholar]
- Bayro M. J., Ramachandran R., Caporini M. A., Eddy M. T., and Griffin R. G., J. Chem. Phys. 128, (2008). [DOI] [PubMed] [Google Scholar]
- Marin-Montesinos I., Brouwer D. H., Antonioli G., Lai W. C., Brinkmann A., and Levitt M. H., J. Magn. Reson. 10.1016/j.jmr.2005.07.020 177, 307 (2005) [DOI] [PubMed] [Google Scholar]; Hughes C. E., Luca S., and Baldus M., Chem. Phys. Lett. 10.1016/j.cplett.2004.01.009 385, 435 (2004). [DOI] [Google Scholar]
- Rienstra C. M., Tucker-Kellogg L., Jaroniec C. P., Hohwy M., Reif B., McMahon M. T., Tidor B., Lozano-Perez T., and Griffin R. G., Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.152346599 99, 10260 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., and Warren G. L., Acta Crystallogr., Sect. D: Biol. Crystallogr. 10.1107/S0907444998003254 54, 905 (1998). [DOI] [PubMed] [Google Scholar]
- Zech S. G., Olejniczak E., Hajduk P., Mack J., and McDermot A. E., J. Am. Chem. Soc. 10.1021/ja040086m 126, 13948 (2004). [DOI] [PubMed] [Google Scholar]
- Lemaster D. M. and Cronan J. E., J. Biol. Chem. 257, 1224 (1982). [PubMed] [Google Scholar]
- Marulanda D., Tasayco M. L., Cataldi M., Arriaran V., and Polenova T., J. Phys. Chem. B 10.1021/jp052774d 109, 18135 (2005). [DOI] [PubMed] [Google Scholar]
- See EPAPS Document No. E-JCPSA6-129-020846 for additional simulations and experimental results (including the PAR optimization procedure) as well as detailed discussions in order to fully support some important points of the manuscript. For more information on EPAPS, see http://www.aip.org/pubservs/epaps.html.