Abstract
SR proteins are essential pre-mRNA splicing factors that have been shown to bind a number of exonic splicing enhancers where they function to stimulate the splicing of adjacent introns. Members of the SR protein family contain one or two N-terminal RNA binding domains, as well as a C-terminal arginine–serine (RS) rich domain. The RS domains mediate protein–protein interactions with other RS domain containing proteins and are essential for many, but not all, SR protein functions. Hybrid proteins containing an RS domain fused to the bacteriophage MS2 coat protein are sufficient to activate enhancer-dependent splicing in HeLa cell nuclear extract when bound to the pre-mRNA. Here we report progress towards determining the protein sequence requirements for RS domain function. We show that the RS domains from non-SR proteins can also function as splicing activation domains when tethered to the pre-mRNA. Truncation experiments with the RS domain of the human SR protein 9G8 identified a 29 amino acid segment, containing 26 arginine or serine residues, that is sufficient to activate splicing when fused to MS2. We also show that synthetic domains composed solely of RS dipeptides are capable of activating splicing, although their potency is proportional to their size.
INTRODUCTION
In higher eukaryotes, the majority of genes encode pre-mRNAs that contain one or more introns that are removed by the process of splicing. Intron removal is facilitated by the splicesome—a multi-component RNA–protein machine (1). The spliceosome is composed of five small ribonucleoprotein particles (snRNPs) called U1, U2, U4, U5 and U6. Each snRNP contains a U small nuclear RNA (snRNA) and several proteins. In addition, the spliceosome contains many non-snRNP proteins. Spliceosome assembly is initiated by the formation of the early or E complex in which U1 snRNP, SF1/mBBP, and the 65 and 35 kDa subunits of U2 snRNP auxiliary factors (U2AF), are bound to the 5′ splice site, branchpoint, pyrimidine tract and AG dinucleotide, respectively. Subsequently, A complex is formed when U2AF recruits U2 snRNP to the branchpoint, which displaces SF1/mBBP. Next, B complex is formed when the U4/U6·U5 tri-snRNP is incorporated into the spliceosome. Finally, the spliceosome undergoes an extraordinary conformational rearrangement to form the catalytically active C complex.
Perhaps the best-characterized non-snRNP proteins are the SR proteins, which constitute a family of essential pre-mRNA splicing factors present in all metazoans (2). Members of the SR protein family contain one or two N-terminal RRM type RNA binding domains, as well as a C-terminal arginine–serine (RS) rich domain. The RS domains are thought to mediate protein–protein interactions with other RS domain containing proteins (3) and are essential for many, but not all, SR protein functions (4). In constitutive splicing, SR proteins have been proposed to promote both cross-intron and cross-exon interactions, and to participate in the recruitment of the tri-snRNP into the spliceosome. It is thought that SR proteins facilitate cross-intron and cross-exon interactions by simultaneously interacting with U2AF35 bound to the 3′ splice site and U1-70K bound to the 5′ splice site (3). These activities most likely involve interactions between the RS domains contained in each of these proteins.
SR proteins also have been shown to play a role in alternative splicing (5). In this context, SR proteins have been shown to bind a number of exonic splicing enhancers (ESEs), where they function to stimulate the splicing of adjacent introns. Presumably, the splicing stimulatory action of SR proteins is the result of recruitment of components of the general splicing machinery to the weak splice site via RS domain mediated protein interactions (3). For example, several studies have shown that ESE-bound SR proteins can activate splicing by recruiting the general splicing factor U2AF to upstream 3′ splice sites (6,7). ESEs not only play an important role in the regulation of alternative splicing but also in the correct splice site recognition of constitutively spliced pre-mRNAs (8,9).
It has been shown previously that artificially tethering an RS domain to the pre-mRNA, via fusion to the MS2 coat protein, is sufficient to activate splicing (10). Thus, RS domains function as splicing activation domains. These previous experiments also demonstrated that enhancer-dependent splicing requires an ESE-bound SR protein as well as an additional SR protein. This system, there fore, allows for the properties of RS domains in enhancer-dependent splicing to be examined independent of the other SR protein activities.
Although arginine and serines have been shown to be important in RS domain function in the context of an intact SR protein (11,12), the precise sequences required for RS domains to function as a splicing activation domain have yet to be determined. Krainer and colleagues have shown that 10 RS dipeptides can functionally substitute for the SF2/ASF RS domain (13), and more recently, that RS dipeptides, as well as RE and RD dipeptides can activate splicing in vitro when tethered to the pre-mRNA via a complementary oligonucleotide (14). In addition, it has been shown that the potency of a splicing activation domain is proportional to the number of RSRS tetrapeptide sequences it contains (15). Nonetheless, a more detailed understanding of the sequence requirements for splicing activation domain function will help elucidate the molecular mechanisms by which these protein interaction domains function. Here we report that the RS domains present in three non-SR proteins—U2AF65, U2AF35 and U1-70K, all potential targets of ESE-bound SR proteins—can all activate splicing when tethered to the pre-mRNA. We have also identified a 29 amino acid segment of the 9G8 RS domain, consisting almost exclusively of arginine and serine residues, that is sufficient to activate splicing when tethered to the pre-mRNA. Finally, we show that synthetic RS domains composed solely of RS dipeptides are sufficient to constitute a functional splicing activation domain, although they do so in a length-dependent manner. These studies enhance our understanding of the sequence requirements of RS domains in splicing activation.
MATERIALS AND METHODS
Proteins
The expression and purification of MS2, MS2-RS9G8, MS2-RSp55 and MS2-RSSF2/ASF have been described previously (15). To generate fusion proteins containing the RS domains of U1-70K, U2AF65 and U2AF35, oligonucleotides were designed to amplify each fragment such that the products would contain a BamHI site at the 5′ end and a stop codon and HindIII site at the 3′ end. PCR products encoding amino acids 211–438 of human U1-70K, 1–94 of human U2AF65 and 194–241 of human U2AF35 were amplified for these constructs. The PCR products were digested with BamHI and HindIII and ligated into pHIS-BIVT-MS2-RS9G8 (15) that was digested previously with BamHI and HindIII. To generate fusion proteins containing deletions of the 9G8 RS domain, PCR was performed with oligonucleotides designed to amplify each fragment. The oligonucleotides were designed such that a BamHI site would be added to the 5′ end, and a stop codon and HindIII site would be added to the 3′ end. The PCR products were digested with BamHI and HindIII and ligated into pHIS-BIVT-MS2-RS9G8 that was digested previously with BamHI and HindIII. To generate the hybrid proteins containing the synthetic RS domains, oligonucleotides encoding seven RS dipeptides were synthesized. First, a pair of RS7 oligonucleotides was synthesized such that when annealed, the 5′ end contained an overhang compatible with BamHI, and the 3′ end contained an in frame stop codon and an overhang compatible with HindIII. These oligonucleotides were ligated into pHIS-BIVT-MS2-RS9G8 digested with BamHI and HindIII. Subsequently, a second set of RS7 oligonucleotides was synthesized in which both ends were compatible with BamHI. However, the junction at the 5′ end could be re-digested with BamHI, but the 3′ junction destroyed the BamHI site. These oligonucleotides were inserted into pHIS-BIVT-MS2-RS7 digested with BamHI to generate pHIS-BIVT-MS2-RS14. Next, the same set of oligonucleotides was inserted into pHIS-BIVT-MS2-RS14 to generate pHIS-BIVT-MS2-RS21. All clones were sequenced to confirm their identity. The resulting plasmids were used to generate recombinant baculoviruses as described by the manufacturer (Invitrogen). Each protein was expressed in infected Sf9 cells for 3 days and purified under native conditions as described (10).
In vitro splicing
The plasmid encoding the dsx(70)M1 pre-mRNAs was described previously (10,15). Capped, 32P-labeled RNAs were transcribed with either T7 or SP6 RNA polymerase and gel-purified prior to use. Splicing assays were performed as described previously (10), for the times indicated.
RESULTS
RS domains from non-SR proteins can function as splicing activation domains
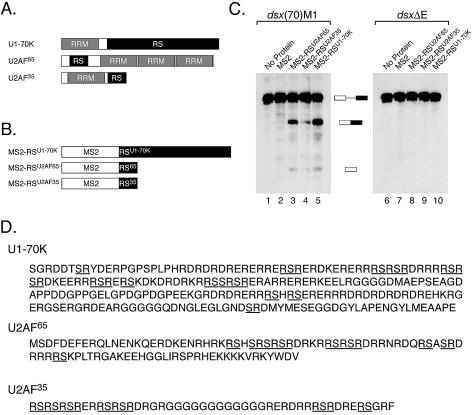
Many splicing factors other than SR proteins contain RS domains (16). We were therefore interested in testing whether any of these non-SR protein RS domains can function as a splicing activation domain while fused to MS2. For these experiments we have focused on the RS domains present in the general splicing factors U1-70K, U2AF65 and U2AF35. These proteins were selected because both U1-70K and U2AF are thought to be targets of ESE-bound SR proteins (3,17). Thus, these experiments also test whether the potential targets of ESE-bound SR proteins can function as splicing activators when placed in a different context. U1-70K, a component of U1 snRNP, contains a large bipartite RS domain (Fig. 1A and D) that is sufficient to mediate protein interactions with SR proteins (3,18). U2AF65 contains an N-terminal RS domain that is required for the recruitment of U2 snRNP to the branchpoint, and appears to function as a protein–RNA interaction domain rather than a protein–protein interaction domain (19). Finally, U2AF35 contains a C-terminal RS domain that is thought to mediate protein interactions with SR proteins (3). To test whether any of these RS domains could function as splicing activation domains, we fused each of these to MS2 (Fig. 1B), expressed the proteins in Sf9 cells, and purified them under native conditions.
Figure 1.
Non-SR protein RS domains can function as splicing activation domains. (A) The domain organization of three non-SR proteins that contain RS domains. Depicted are the domain organizations of the U1-70K, U2AF65 and U2AF35. (B) Hybrid proteins containing non-SR protein RS domains. The RS domains of U1-70K, U2AF65 and U2AF35 were fused to MS2. These proteins were expressed in Sf9 cells and purified. (C) The RS domains of non-SR proteins can function as splicing activation domains. The proteins depicted in (B) were tested for their ability to activate the splicing of the dsx(70)M1 or dsxΔE pre-mRNAs in HeLa nuclear extract. The reactions contained ∼200 nM of each protein and were incubated for 2 h. (D) The sequences of the RS domains of U1-70K, U2AF65 and and U2AF35 that were fused to MS2. Stretches of contiguous, alternating arginine and serine residues are underlined.
As done previously (15), all of these experiments were performed using protein concentrations at which the MS2 binding site on the pre-mRNA is fully occupied. This ensures that observed differences in activity are due to differences in the potency of the activation domain, rather than differences in RNA binding affinity. Therefore, initial splicing reactions were performed in HeLa cell nuclear extract with the dsx(70)M1 pre-mRNA in which each hybrid protein was titrated and the apparent Kd for each protein preparation was calculated as the amount of protein necessary to achieve half the maximal level of splicing [data not shown, see (15) as an example]. The dsx(70)M1 pre-mRNA contains the enhancer-dependent intron from the Drosophila doublesex pre-mRNA and a single MS2 binding site 70 nt downstream from the 3′ splice site (15). The Kdapp for these proteins ranged from 10 to 30 nM, in accordance with the affinity of the MS2–RNA interaction (20). Gel shift experiments demonstrated that all the proteins described here could bind RNA specifically (data not shown). For the experiments presented in this paper, protein concentrations approximately 10 times the Kdapp were used (∼200 nM).
To test if non-SR protein RS domains could activate splicing, the proteins shown in Figure 1B were added to splicing reactions containing HeLa cell nuclear extract and 32P-labeled dsx(70)M1 pre-mRNA. As shown in Figure 1C, each of these proteins activates splicing of the dsx(70)M1 pre-mRNA (lanes 1–5), but not of dsxΔE, a similar RNA lacking an MS2 binding site (lanes 6–10). Thus, the ability of each of these domains to activate splicing requires that they be tethered to the pre-mRNA. MS2-RSU1-70K, which contains the largest of the three RS domain, is one of the most potent splicing activators we have tested to date (lane 5). Interestingly, the U1-70K RS domain only contains 14 RS dipeptides (Fig. 1D). However, the U1-70K RS domain also contains several other dipeptides of alternating charge (RE, RD, KE, KD). The U2AF65 and U2AF35 RS domains are also both capable of activating splicing, although the U2AF35 RS domain does so weakly. This is interesting because the U2AF65 and U2AF35 RS domains contain nine and seven RS dipeptides, respectively (Fig. 1D). As with the U1-70K RS domain, the U2AF RS domains also contain several dipeptides of alternating charge. Although we do not know the precise sequences responsible for their activity, these results demonstrate that the RS domains of non-SR proteins can function as splicing activation domains when tethered to the pre-mRNA. Moreover, they demonstrate that the potential targets of ESE-bound SR proteins can function as splicing activators when placed in a different context.
Identification of a minimal RS domain
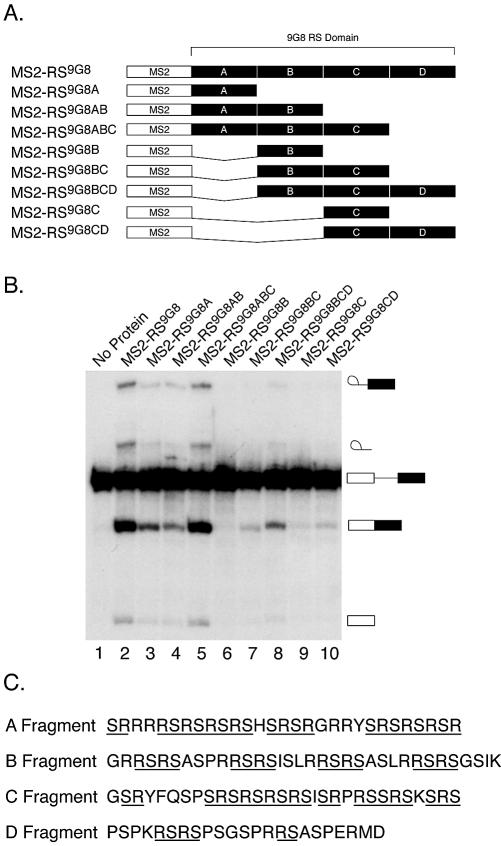
To examine the relationship between RS content and splicing activation domain strength in more detail we have tested the ability of several deletion mutants of the 9G8 RS domain to function as a splicing activation domain when fused to the MS2 protein. The 9G8 RS domain is one of the most potent natural splicing activation domains (15). The 9G8 RS domain was arbitrarily divided into four segments of roughly equal size called A, B, C and D (Fig. 2A and C). We generated eight hybrid proteins containing different portions of the 9G8 RS domain fused to MS2. These proteins were expressed in Sf9 cells and purified under native conditions. We then assayed the ability of saturating concentrations of each of these proteins to activate the splicing of the dsx(70)M1 pre-mRNA. The majority of the hybrid proteins tested retained the ability to activate splicing (Fig. 2B). The A fragment is the smallest fragment tested that is able to activate splicing when fused to MS2 (lane 3). However, despite the presence of several RS dipeptides, the B and C fragments alone (lanes 6 and 9) were unable to support efficient splicing. We were unable to purify a hybrid protein containing only the D fragment in soluble form (data not shown). However, since the RS domain containing only the A, B and C fragments, but lacking the D fragment, was as potent an activator as the full-length RS domain (lanes 2 and 5), the D fragment does not appear to contribute significantly to the overall activity of the 9G8 RS domain. Consistent with this, the D fragment contains 12 arginine or serine residues, and only three RS dipeptides.
Figure 2.
Identification of a minimal, natural RS domain. (A) 9G8 RS domain deletion mutants. The 9G8 RS domain was arbitrarily divided into four equal pieces (A–D). Deletions of the 9G8 RS domain were generated and fused to the MS2 protein. Each protein was expressed in Sf9 cells using recombinant baculoviruses and purified under native conditions by Ni-NTA chromatography. (B) Splicing activity of the 9G8 RS domain deletion mutants. Approximately 200 nM of each hybrid protein was added to a splicing reaction containing the dsx(70)M1 pre-mRNA and the reactions incubated for 2 h at 30°C. The reactions were resolved on a denaturing polyacrylamide gel. (C) The sequences of each fragment of the 9G8 RS domain are depicted. Stretches of contiguous, alternating arginine and serine residues are underlined.
Some of these results are consistent with our previous finding that the potency of an activation domain is proportional to its RS content (15). For example, the potency of the hybrid proteins increases with the size of the activation domain (see for example, lanes 6–8). The exception to this is that the activity of the hybrid protein containing both the A and B fragments is lower than that of the protein containing the A fragment alone (lanes 3 and 4). This result has been reproduced in at least five experiments using three independent protein preparations (data not shown). Although, RS content is clearly one determinant, these results suggest that there are additional properties of activation domain potency. This may be attributable to the formation of unfavorable tertiary structures of the RS domain containing the A and B fragments. Nonetheless, these results support the conclusion that one determinant of activation domain potency is RS content.
Due to the fact that the A fragment of the 9G8 RS domain is 29 amino acids in length, and is composed almost entirely of arginine and serine residues and the majority of these are alternating (Fig. 2C), we were interested in testing whether RS repeats are sufficient to constitute a functional splicing activation domain. We therefore generated hybrid proteins containing 7, 14 or 21 RS dipeptides fused to MS2 (Fig. 3A). These proteins were expressed in Sf9 cells and purified under native conditions. Western blotting using mAb104 (2), which recognizes a phosphoepitope in RS domains, indicates that each of these fusion proteins is phosphorylated (data not shown). We next tested whether the synthetic RS domains could activate splicing of the dsx(70)M1 pre-mRNA. As shown in Figure 3B, MS2-RS7 is unable to activate splicing to levels significantly above background (lanes 1–4, data not shown). In contrast, MS2-RS14 (lanes 5–8) and MS2-RS21 (lanes 9–12) activate splicing of the dsx(70)M1 pre-mRNA (lane 5). Thus, RS repeats are sufficient to constitute a splicing activation domain.
Figure 3.
RS Repeats are sufficient to function as a splicing activation domain. (A) Schematic diagram of the MS2 fusion proteins containing 7, 14 or 21 RS dipeptides. The proteins were expressed in Sf9 cells and purified. (B) The dsx(70)M1 pre-mRNA was incubated in HeLa nuclear extract in the presence of saturating concentrations (∼200 nM) of MS2-RS7 (lanes 1–4), MS2-RS14 (lanes 5–8), MS2-RS21 (lanes 9–12), MS2-RSSF2/ASF (lanes 13–16), MS2-RSp55 (lanes 17–20) or MS2-RS9G8A (lanes 21–24) for 3 h. Time points were taken every hour. Quantitation of the data is shown in (C). (D) The rate of splicing promoted by each hybrid protein was calculated as described in Graveley et al. (6), based on the data in (B) and (C). These values were plotted versus the number of RS dipeptides contained within each RS domain.
We also compared the activity of the synthetic RS domain fusion proteins with fusion proteins containing the RS domains of SF2/ASF and SRp55, which are the weakest and strongest naturally occurring RS domains we have characterized previously (15). These results demonstrate that the activity of MS2-RS14 is comparable with that of MS2-RSSF2/ASF (Fig. 3B, lanes 13–16 and C), and that the activity of MS2-RS21 is intermediate to that of MS2-RSSF2/ASF and MS2-RSp55 (Fig. 3B, lanes 17–20 and C). In addition, we also compared the activity of the synthetic RS domains with the 9G8 A fragment (Fig. 3B, lanes 21–24 and C). This was clearly more potent than MS2-RS14 even though each RS domain has a similar RS content. Moreover, MS2-RS9G8A was even more potent than MS2-RS21. We used the data in Figure 3B and C to calculate the rate of splicing promoted by each hybrid protein and plotted these values versus the number of RS tetrapeptides contained within each RS domain. This analysis clearly shows that, with one exception (MS2-RS9G8A), there is a proportional relationship between RS content and activation domain potency (Fig. 3D). Together, these results demonstrate that RS repeats are sufficient to function as a splicing activation domain when tethered to the pre-mRNA. In addition, consistent with our previous finding of the relationship of RS domain strength and RS content with natural RS domains (15), the activity of the hybrid proteins containing the synthetic RS domains increases with increasing RS content. However, the results obtained comparing MS2-RS9G8A with the synthetic RS domains suggest that RS content is not the sole determinant of RS domain potency.
DISCUSSION
SR proteins play several key roles in pre-mRNA splicing (4,5). First, they serve as general splicing factors where they promote cross-intron and cross-exon interactions and participate in the recruitment of the U4/U6·U5 tri-snRNP into the spliceosome. In addition to these general activities that are thought to be required for the splicing of most, if not all introns, SR proteins function as splicing regulators. In this capacity, SR proteins act by binding to ESEs where they interact with and recruit components of the general splicing machinery to adjacent introns. As this activity appears to require the RS domain, determining how RS domains function will be important to understanding how SR proteins act to regulate alternative splicing. Here, we show that RS domains from non-SR proteins can function as splicing activation domains when tethered to the pre-mRNA. In addition, we have identified a 29 amino acid fragment within the 9G8 RS domain that is sufficient to activate splicing when tethered to the pre-mRNA. Finally, we show that RS repeats are sufficient to constitute a functional splicing activation domain and that the potency of these synthetic RS domains is proportional to their RS content. Together these results enhance our understanding of the sequence requirements of splicing activation domains.
Context-dependent activities of RS domains
One of the most interesting results we obtained was that the RS domains of non-SR proteins function as a splicing activation domain when tethered to the pre-mRNA. The three RS domains we examined were from U1-70K, U2AF65 and U2AF35. The RS domains of each of these proteins are thought to have specific functions in pre-mRNA splicing. The RS domains of both U1-70K and U2AF35 participate in protein interactions with SR proteins. These interactions are thought to mediate cross-intron interactions. In addition, ESE-bound SR proteins are thought to regulate alternative splicing and promote exon inclusion by interacting with the RS domains in U1-70K and U2AF35. Thus, the RS domains in U1-70K and U2AF35 normally function as targets of SR proteins. However, we find that when these RS domains are tethered to the pre-mRNA, they can be converted from a target to an activator. This suggests that the activity of RS domains is context dependent.
Further support for the context-dependent activity of RS domains comes from our observation that the U2AF65 RS domain functions as a splicing activation domain when fused to MS2. As mentioned, all evidence to date suggests that ESE-bound SR proteins activate splicing by interacting with components of the general splicing machinery via protein–protein interactions involving their RS domains. In contrast, the RS domain of U2AF65 is somewhat unique in that it has been shown to directly interact with the RNA at the branch point when U2AF is bound to the downstream pyrimidine tract (19). Interestingly, the RS domains from both U2AF35 and SF2/ASF—which normally participate in protein–protein interactions—can functionally replace the U2AF65 RS domain (19). Moreover, Valcárcel et al. also demonstrated that RS7, KS2, RA7 and RT7 can substitute for the U2AF65 RS domain, while AS7 and RD7 cannot (19). Presumably, when fused to U2AF65, these other RS domains participate in protein–RNA interactions as does the U2AF65 RS domain. Here we have performed a reciprocal experiment and found that the U2AF65 RS domain can function as a splicing activation domain when bound to the RNA in place of an ESE. Most likely, in this context, the U2AF65 RS domain is participating in protein–protein interactions. Similarly, Dauwalder and Mattox have found that the RS domain of the large U2AF subunit in Drosophila (dU2AF50) can replace the essential RS domain of the splicing regulator TRA2 in transgenic flies (21). Together, these experiments suggest that whether an RS domain will function as a protein–protein or a protein–RNA interaction domain is dictated by its context. When bound to an exon, an RS domain will participate in protein–protein interactions, while when present at the N-terminus of U2AF65 RS domains function as protein–RNA interaction domains.
Sequence requirements for RS domain function
All SR proteins contain variable length C-terminal domains containing alternating arginine and serine residues (2). Several studies have been conducted to determine the sequences required for RS domain function. Several years ago, several variants of SF2/ASF that contained mutated RS domains were tested for their ability to function in constitutive splicing and to promote splice site switching in vitro. SF2/ASF mutants in which all of the RS dipeptides were substituted with either RT, RG, GS or KS were unable to complement S100 extracts, indicating that RS dipeptides are critical for the function of SF2/ASF in constitutive splicing (11). In contrast, all of these mutants, as well as a variant lacking the entire RS domain, could all promote splice site switching (11).
More recently, using the MS2-RS hybrid protein system, it was shown that RS domains from six human SR proteins were sufficient to activate splicing when tethered to the pre-mRNA (10,15). A quantitive analysis of the activity of these hybrid proteins revealed that the potency of a natural RS domain is proportional to its RS content. More specifically, these results fit best to a model in which the functional unit within an RS domain is an RS tetrapeptide (RSRS or SRSR) (15). Here, we have extended these findings by showing that the RS domains from three non-SR proteins, U1-70K, U2AF65 and U2AF35, as well as simple RS repeats can all function as splicing activation domains. Moreover, consistent with our previous study, we find that the potency of the synthetic RS domains is proportional to their RS content. We have attempted to extend these studies by examining the activity of mutant versions of synthetic RS domains. We have generated baculoviruses that express hybrid proteins containing either 7, 14 or 21 consecutive RE, RD, RA, KE, KD, KS, KA, AS, AE, AD or VA dipeptides. Consistent with the negligible activity of the hybrid protein containing seven RS dipeptides (Fig. 3), none of the mutant proteins containing seven dipeptides are capable of activating splicing (data not shown). Unfortunately, to date, we have been unable to purify any of the mutant proteins containing 14 or 21 dipeptides in either soluble form, or in a form that can bind RNA (data not shown). This may be due to the unusual charges of these synthetic domains. Thus, we have been unable to further elucidate the amino acid requirements for splicing activation domain function. However, despite the rather low content of RS dipeptides, the U1-70K RS domain is very potent (Fig. 1). This indirectly suggests that sequences other than RS dipeptides may be capable of functioning as a splicing activation domain. Nonetheless, our results clearly demonstrate that RS dipeptides are sufficient to constitute a functional splicing activation domain.
Although our previous work and work described here show that RS content is a major contributor to activation domain potency, some of our results indicate that RS content is not the sole determinant. First, whereas the A fragment of the 9G8 RS domain, which contains 11 RS repeats or 5 RSRS tetrapeptides, activates splicing more strongly than either the AB fragment (19 RS dipeptides/9 tetrapeptides) or the BC fragment (17 RS dipeptides/6 RSRS tetrapeptides). Secondly, the 9G8 A fragment is also a more potent activator than fusion proteins containing either 14 or 21 RS dipeptides. Dauwalder and Mattox have shown that RS domains differ in their ability to replace the essential RS domain from Tra2. Interestingly, of the four RS domains tested—Transformer, dU2AF50, dSRp55/B52 and SC35—the dU2AF50 RS domain provided the strongest rescue yet has the lowest RS content (21). Together, these results indicate that properties in addition to RS content play a role in determining the potency of a splicing activation domain. One of these additional properties could be the three-dimensional structure of the splicing activation domain. It is possible that the non-RS sequences within natural RS domains function to place these residues in a favorable context. It is also possible, however, that the phosphorylation status of the RS domains plays an important role in their potency. Although we know that all of the RS domains we have tested are phosphorylated, given the sequence of the RS domains, it is difficult to determine which and how many of the serine residues are phosphorylated.
Recently, Krainer and colleagues have described two different assays in which RS repeats were found to functionally substitute for an SR protein RS domain. First, Zhu and Krainer demonstrated that a variant of SF2/ASF containing 10 RS dipeptides in place of the SF2/ASF RS domain was capable of performing the RS domain-dependent SR protein activities (13). Additionally, Cazalla et al. have shown that 10 RS dipeptides can functionally substitute for the SF2/ASF RS domain in vivo (22). However, because the RS domain of SF2/ASF can be removed without affecting its ability to carry out several SR proteins activities (13), these studies did not demonstrate conclusively that RS repeats alone are sufficient to activate splicing. Cartegni and Krainer extended these studies by demonstrating that chimeric molecules containing a peptide nucleic acid (PNA) complementary to an alternative exon and a peptide of 5, 10 or 15 RS dipeptides could enhance the inclusion of the targeted exon in vitro (14). In addition, these authors showed that similar chimeric molecules containing 10 RE and RD dipeptides, but not 10 RA dipeptides, could activate splicing (14). These results, therefore, show that peptides containing alternating positive (arginine) and negative (phosphoserine, aspartic acid or glutamic acid) charges are sufficient to activate splicing when tethered to the pre-mRNA via a PNA. Although performed in a completely different context, our results are consistent with those of Krainer and colleagues.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Kristen Lynch, Tom Maniatis and members of the Graveley lab for discussions and/or comments on the manuscript. This work was supported by an NIH grant (GM062516) to B.R.G.
REFERENCES
- 1.Staley J.P. and Guthrie,C. (1998) Mechanical devices of the spliceosome: motors, clocks, springs and things. Cell, 92, 315–326. [DOI] [PubMed] [Google Scholar]
- 2.Zahler A.M., Lane,W.S., Stolk,J.A. and Roth,M.B. (1992) SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev., 6, 837–847. [DOI] [PubMed] [Google Scholar]
- 3.Wu J.Y. and Maniatis,T. (1993) Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell, 75, 1061–1070. [DOI] [PubMed] [Google Scholar]
- 4.Hastings M.L. and Krainer,A.R. (2001) Pre-mRNA splicing in the new millennium. Curr. Opin. Cell Biol., 13, 302–309. [DOI] [PubMed] [Google Scholar]
- 5.Graveley B.R. (2000) Sorting out the complexity of SR protein functions. RNA, 6, 1197–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graveley B.R., Hertel,K.J. and Maniatis,T. (2001) The role of U2AF35 and U2AF65 in enhancer-dependent splicing. RNA, 7, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo P. and Maniatis,T. (1996) The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev., 10, 1356–1368. [DOI] [PubMed] [Google Scholar]
- 8.Schaal T.D. and Maniatis,T. (1999) Multiple distinct splicing enhancers in the protein-coding sequences of a constitutively spliced pre-mRNA. Mol. Cell. Biol., 19, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayeda A., Screaton,G.R., Chandler,S.D., Fu,X.D. and Krainer,A.R. (1999) Substrate specificities of SR proteins in constitutive splicing are determined by their RNA recognition motifs and composite pre-mRNA exonic elements. Mol. Cell. Biol., 19, 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graveley B.R. and Maniatis,T. (1998) Arginine/serine-rich domains of SR proteins can function as activators of pre-mRNA splicing. Mol. Cell, 1, 765–771. [DOI] [PubMed] [Google Scholar]
- 11.Cáceres J.F. and Krainer,A.R. (1993) Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J., 12, 4715–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo P. and Manley,J.L. (1993) Functional domains of the human splicing factor ASF/SF2. EMBO J., 12, 4727–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J. and Krainer,A.R. (2000) Pre-mRNA splicing in the absence of an SR protein RS domain. Genes Dev., 14, 3166–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartegni L. and Krainer,A.R. (2003) Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat. Struct. Biol., 10, 120–125. [DOI] [PubMed] [Google Scholar]
- 15.Graveley B.R., Hertel,K.J. and Maniatis,T. (1998) A systematic analysis of the factors that determine the strength of pre-mRNA splicing enhancers. EMBO J., 17, 6747–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blencowe B.J., Bowman,J.A.L., McCracken,S. and Ronina,E. (1999) SR-related proteins and the processing of messenger RNA precursors. Biochem. Cell Biol., 77, 277–291. [PubMed] [Google Scholar]
- 17.Kohtz J.D., Jamison,S.F., Will,C.L., Zuo,P., Lührmann,R., Garcia-Blanco,M.A. and Manley,J.L. (1994) Protein-protein interactions and 5′ splice site recognition in mammalian mRNA precursors. Nature, 368, 119–124. [DOI] [PubMed] [Google Scholar]
- 18.Cao W. and Garcia-Blanco,M.A. (1998) A serine/arginine-rich domain in the human U1-70k protein is necessary and sufficient for ASF/SF2 binding. J. Biol. Chem., 273, 20629–20635. [DOI] [PubMed] [Google Scholar]
- 19.Valcárcel J., Gaur,R.K., Singh,R. and Green,M.R. (1996) Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science, 273, 1706–1709. [DOI] [PubMed] [Google Scholar]
- 20.Lowary P.T. and Uhlenbeck,O.C. (1987) An RNA mutation that increases the affinity of an RNA-protein interaction. Nucleic Acids Res., 15, 10483–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dauwalder B. and Mattox,W. (1998) Analysis of the functional specificity of RS domains in vivo. EMBO J., 17, 6049–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazalla D., Zhu,J., Manche,L., Huber,E., Krainer,A.R. and Cáceres,J.F. (2002) Nuclear export and retention signals in the RS domain of SR proteins. Mol. Cell. Biol., 22, 6871–6882. [DOI] [PMC free article] [PubMed] [Google Scholar]