Abstract
Salient sounds such as those created by drumming can serve as means of nonvocal acoustic communication in addition to vocal sounds. Despite the ubiquity of drumming across human cultures, its origins and the brain regions specialized in processing such signals remain unexplored. Here, we report that an important animal model for vocal communication, the macaque monkey, also displays drumming behavior, and we exploit this finding to show that vocal and nonvocal communication sounds are represented by overlapping networks in the brain's temporal lobe. Observing social macaque groups, we found that these animals use artificial objects to produce salient periodic sounds, similar to acoustic gestures. Behavioral tests confirmed that these drumming sounds attract the attention of listening monkeys similarly as conspecific vocalizations. Furthermore, in a preferential looking experiment, drumming sounds influenced the way monkeys viewed their conspecifics, suggesting that drumming serves as a multimodal signal of social dominance. Finally, by using high-resolution functional imaging we identified those brain regions preferentially activated by drumming sounds or by vocalizations and found that the representations of both these communication sounds overlap in caudal auditory cortex and the amygdala. The similar behavioral responses to drumming and vocal sounds, and their shared neural representation, suggest a common origin of primate vocal and nonvocal communication systems and support the notion of a gestural origin of speech and music.
Keywords: gestures, speech, temporal lobe
Social living requires effective means of expression and many species communicate by using sounds. Communication sounds can be as diverse as the methods used to produce them, covering a range of temporal rates and spectral frequencies, ranging from rattlesnake rattling and stork clapping to complex human speech. Interestingly, some species communicate not only by using a variety of sounds produced by the same means, but also by using sounds produced by different means. Humans, for example, produce vocal sounds including speech and nonspeech utterances, as well as nonvocal sounds—also called acoustic gestures (1–4). Acoustic gestures encompass a range of sounds such as the discrete knocking on a door, rhythmic hand clapping, and even thumping a table in anger. Animals, too, create sounds akin to acoustic gestures in addition to their vocalizations; for example, some nonhuman primates create sounds by chest beating or hand clapping (5–7), and rodents create sounds by drumming their paws on the ground (8). The use of nonvocal acoustic signals for communication raises two fundamental questions: first whether the same brain regions are specialized for the processing of vocal and nonvocal communication sounds; and second, whether vocal and nonvocal communication systems share a common evolutionary origin (2, 3, 9–12).
Insights into the evolution of human communication systems can be revealed by comparative studies on closely related primate models, which offer the advantage of studying the underlying neuronal structures and mechanisms in greater detail (9, 10, 12–14). Indeed, recent studies uncovered evolutionary parallels between the brains of humans and macaque monkeys and provided functional homologs of human brain areas implicated in processing vocal sounds (15–17). Similar to humans, nonhuman primates produce structured periodic sounds in the form of acoustic gestures, as evidenced by reports of such behaviors in field studies (5–7, 18). However, to identify a common basis of vocal and nonvocal acoustic communication, an established model system in an evolutionarily related species and featuring both vocal and nonvocal communication would be necessary.
We here provide such a model system and demonstrate that vocal and nonvocal communication sounds are represented by overlapping neural networks in the temporal lobe. Specifically, we find that laboratory rhesus monkeys (Macaca mulatta) produce loud, structured, and periodic sounds by using artificial objects in their environment as a means of acoustic communication akin to drumming. By using behavioral tests, we confirm the behavioral impact of these sounds on listening animals. And by using functional magnetic resonance imaging (fMRI), we demonstrate that these drumming sounds and vocal communication calls activate overlapping temporal lobe networks.
Results
Drumming Behavior in Macaque Monkeys.
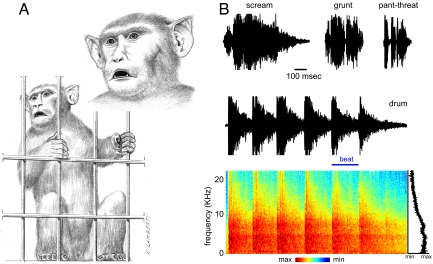
We observed that captive rhesus macaques produce loud, structured, and repetitive sounds by using artificial objects, such as cage doors, in their environment. These animals do so by slapping or banging against surfaces or shaking them vigorously to produce sounds that can be distinctly heard over the background cacophony of an animal colony (Fig. 1A). Because these sounds concord with the general definition of drumming (1, 8), we adopt this terminology in the following.
Fig. 1.
Characteristics of drumming sounds. (A) In this example, a macaque drums by firmly grasping the cage door with his forelimbs and shaking it vigorously and repeatedly. The inset displays the typical facial expression during drumming: open-mouth threatening, staring, forward directed pinnae. (B) Drumming sounds are acoustically distinct from typical vocalizations such as screams, grunts, or pant-threats. The repetitive beat pattern of the drumming sound produced by this action is visible in the time-frequency spectrum. The black line displays the power spectrum.
Drumming sounds are acoustically distinct from typical macaque vocalizations (Fig. S1). First, drumming bouts last 1.5 s on average (mean = 1.5 ± 0.7 s, mean ± SD, n = 70), and the resulting sounds are much longer than typical vocalizations, which are typically less than half that duration (19) (Fig. 1B). Second, these drumming sounds are periodic repetitions of individual beats. The drumming frequency was 4.9 ± 2.9 beats per s, and each beat was repeated 4.8 ± 2.5 times per bout (range 2–13). Thirdly, given their artificial sources, drumming sounds differ from vocal sounds in their acoustic properties, such as pitch and timbre. All of these properties make drumming sounds salient in the acoustic environment in which these animals live.
We have observed the monkey's drumming behavior in different contexts and during interactions between individuals or between troops (groups of animals in adjacent cages). Detailed examples of these different contexts are provided in Fig. S2. Most frequent were agonistic situations, in which one animal drummed as a threatening and directed signal toward another individual. Often, drumming was accompanied by facial and postural expressions, where the drummer would stare directly at the intended recipient with his mouth slightly open and ears angled forward as in a typical threatening display (Fig. 1A) (18, 20). Drumming was also often accompanied by emotional threat vocalizations such as pant-threats, which were produced before or after drumming. In addition to such individual threat displays, we also observed drumming behavior in polyagonistic contexts, where several animals from different troops would in chorus produce drumming sounds and threatening visual displays to intimidate each other. Finally, drumming behavior was also prominent during situations of general excitement in the colony, such as before feeding. In this context, some animals produced drumming sounds and aggressive vocalizations (barks), whereas most others produced multicontext and affiliative calls (mostly, coos, grunts, and gurneys) and food-associated calls such as the harmonic-arch. These periods of general excitement lasted several tens of seconds and were characterized by an abundant number of vocalizations (see Fig. S2). All in all, we observed monkeys drumming several times each day and in different contexts, demonstrating that such displays are not a byproduct of other actions and are well established within the repertoire of the animals' expressions.
Not all animals in the colony exhibited drumming behavior. Effectively, we observed only the largest and dominant individuals drumming, although smaller individuals attempted to do so by imitating the technique. This suggests that the larger individuals drum not only because of their social status, but also because they are powerful enough to agitate bigger, heavier objects in their environment and create loud sounds. This could make drumming an effective way of conveying information about size, strength, and hence social status, as is typical for drumming displays in monkeys and apes (7, 18, 21). Furthermore, because drumming is often accompanied by facial and postural expressions, socially relevant information could be transmitted in this interaction between signaler and recipient.
Behavioral Reactions to Drumming Sounds.
To test whether the monkeys perceive and treat drumming sounds as behaviorally meaningful, we designed a preferential orienting task, which is based on the animals' natural behavior to look in the direction of interesting or relevant sounds (22–24). By using this behavioral paradigm, we compared monkeys' reactions to drumming sounds and vocalizations. Besides conspecific vocalizations and drumming sounds, this behavioral test also included other familiar cage sounds that featured some acoustic properties of drumming sounds (e.g., the metallic timbre), as well as other unfamiliar natural and animal sounds. To control for the higher sound intensity at which drumming sounds are produced naturally, we presented all sounds normalized to the same root-mean-square intensity.
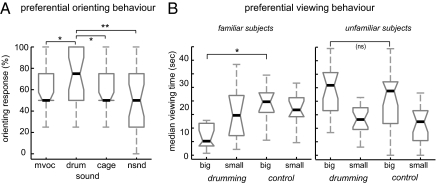
Of all sounds, the animals oriented most frequently to the drumming sounds (Fig. 2A). In fact, the orienting response to drumming sounds (drum) was significantly higher than to unfamiliar natural sounds (nsnd, sign-rank test; P < 0.01, n = 27) and to other cage sounds (cage, P < 0.05). Moreover, responses to drumming sounds were also more frequent than to vocalizations (mvoc, P < 0.05), demonstrating that drumming sounds are indeed as important as conspecific vocalizations to the animals and that these sounds do not attract attention simply because they are louder than other natural sounds.
Fig. 2.
Behavioral tests. (A) Spontaneous orienting behavior toward different sounds. Behavior was scored as the percentage of trials for which the subjects oriented in the direction of the sound. Monkeys oriented more frequently to drumming sounds (drum) than to other environmental sounds (nsnd), cage sounds (cage), or conspecific vocalizations (mvoc). Stars denote significant differences (sign-rank tests): *, P < 0.05, **, P < 0.01. (B) Preferential looking toward either a larger (big) or smaller (small) conspecific during the presentation of drumming or control (cage) sounds. When the subjects were familiar with the stimulus monkeys onscreen, drumming sounds affected the time spent viewing the larger but not the smaller monkey onscreen. When the conspecifics displayed onscreen were unfamiliar to the subjects, the time spent viewing either the larger or smaller stimulus monkeys did not differ between sounds. Stars denote significant differences (rank-sum tests): *, P < 0.05; ns, not significant.
By using a second behavioral test, we confirmed that drumming sounds indeed convey socially relevant information, and modify the way animals survey other conspecifics. In a preferential looking paradigm (23, 24), subjects were presented two simultaneously displayed movies, one showing a larger and the other showing a smaller conspecific, both quietly sitting in their cages. These movies were either accompanied by the binaural presentation of drumming or other cage sounds. To dissociate affects introduced by (visual) acquaintance of individual animals, we analyzed the data depending on whether the subject was familiar (had prior acquaintance) or unfamiliar with the monkeys presented as visual stimuli (Fig. 2B).
When subjects were familiar with the monkeys onscreen, they clearly avoided looking at the larger animal onscreen during the presentation of drumming sounds, but not during the presentation of control cage sounds. An analysis of variance (Scheirer-Hare's nonparametric ANOVA) revealed an overall effect of animal size [F (1, 43) = 4.0, P < 0.05; n = 11], and posthoc tests confirmed significantly reduced looking times toward the larger monkey onscreen during the presentation of drumming sounds (rank-sum test, P < 0.05); looking times toward the smaller monkey did not differ between sounds (P = 0.79). When subjects were unfamiliar with the monkeys onscreen, there was again an overall effect of animal size [F (1, 79) = 33, P < 10−6; n = 20], but the subjects looked longer at the larger monkeys onscreen regardless of the sound (no effect of sound, P = 0.35). This demonstrates that drumming sounds alter the way that listening monkeys survey other conspecifics: drumming sounds likely are directional indicators of the drummer's size, which in the presence of a large and familiar conspecific enhances submissive social behavior.
That the subjects took the experimental setting for real is demonstrated by their spontaneous use of behaviorally relevant expressions. Many subjects attempted to communicate with the individuals shown onscreen, as demonstrated by social gestures like lip smacking (25–27). Indeed, lip smacking was more frequent during the presentation of drumming sounds (total time of lip-smacking across all animals tested: 240.57 s for drumming sounds and 180.85 s for cage sounds), suggesting that listeners escalate their affiliative and appeasing gestures to offset the threat signaled by drumming.
Brain Networks Activated by Communication Sounds.
Having established that macaque monkeys produce drumming sounds as a means to broadcast and convey social information, we investigated the auditory networks involved in processing these sounds. Specifically, we asked whether and how brain regions preferentially responding to drumming sounds relate to those networks specifically activated by conspecific vocal communication sounds. Previous studies have revealed specialized networks for the processing of vocal communication calls (14–17), but whether specialized networks for processing nonvocal communication sounds exist remains unclear. To do so, we performed high-resolution fMRI studies by using a paradigm that contrasted fMRI activations to drumming sounds with those to conspecific vocalizations (mvoc) and other natural sounds (nsnd; including environmental sounds and vocalizations of other animals). fMRI measurements were done on anesthetized animals by using established auditory sparse-imaging paradigms (16, 28, 29).
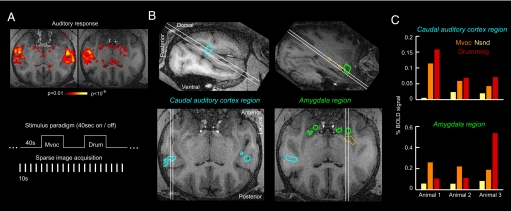
Stimulation with naturalistic sounds evoked robust activations in the temporal lobe (Fig. 3A). By contrasting the activations to the different sound categories, we localized regions preferentially responding to vocalizations compared to other natural sounds and regions preferentially responding to drumming sounds compared to natural sounds (Fig. S3). The former contrast reveals regions involved in processing vocal communication sounds, the latter reveals regions involved in processing drumming sounds. Strong responses to vocalizations occurred at several locations along the rostro-caudal axis of the lower bank of the lateral sulcus (auditory cortex), on the superior temporal gyrus, and in the amygdala region, in good concordance with previous findings (16). Strong responses to drumming sounds were found in caudal auditory cortex, in the amygdala, and other subcortical structures (Fig. S3).
Fig. 3.
Functional mapping of brain regions preferentially responding to drumming sounds and vocalizations. (A) fMRI-BOLD response to overall acoustic stimulation for one experiment. Sounds were presented in a block design, and functional images were acquired by using a sparse imaging sequence. (B) Overlap of regions responding preferentially to vocalizations and drumming sounds, both compared to other naturalistic sounds (red lines). The full preference maps for both conditions are shown in Fig. S3. Across monkeys, consistent overlap is found in the caudal auditory cortex (Left) and the amygdala (Right). Yellow lines represent additional regions of overlap that are not consistent across animals. (C) fMRI-BOLD response amplitudes in these regions of overlap for all three animals (left auditory cortex, right amygdala).
To determine networks preferentially responding to both vocal and nonvocal macaque communication sounds, we computed the overlap of the preferences to vocalizations and drumming sounds and determined those regions consistently appearing in all three animals tested. As in the example shown in Fig. 3B, there were two regions responding preferentially to conspecific vocalizations and drumming sounds: one in caudal auditory cortex and another in the amygdala. Importantly, these two regions were consistently found in all animals (shown in Fig. S4A). It should be noted that individual experiments also revealed other regions of overlap as well, which were not consistent across animals (see Fig. S4A). To further localize the caudal auditory region with respect to functionally defined auditory fields, we exploited a described mapping procedure, which provides a functional parcellation of auditory cortex based on the tonotopic organization of auditory fields (30). This positioned the region of overlapping activations as within the caudal belt (caudo-medial and caudo-lateral fields), but also extending into primary field A1 and the caudal parabelt (including region Tpt), a location that is consistent with a vocalization-sensitive region reported in a previous study (16). These functional imaging results demonstrate that vocal and nonvocal communication sounds are partly processed by the same overlapping and dedicated auditory and emotional networks.
Discussion
Our findings reveal that macaque monkeys produce acoustic gestures by drumming that differ acoustically from vocalizations, yet attract the attention of the listener and alter the way monkeys survey their conspecifics. These drumming sounds hence serve as directional communication signals used in specific contexts, because they convey information about the social status or the emotional state of mind of the drummer, similar to the typical vocal communication sounds known for this species. In addition, drumming sounds engage temporal lobe networks that overlap with those specifically activated by vocal communication calls, demonstrating the existence of common networks specialized for the processing of vocal and nonvocal communication sounds in an old-world primate.
Drumming as a Means to Communicate.
Different forms of drumming behavior have been described for nonhuman primates, such as gorillas performing chest beating and hand clapping (5, 6), chimpanzees drumming on tree buttresses (7), gibbons slamming doors of their enclosures (31), or macaques shaking branches or thumping on dead logs (18, 21). Noteworthy, there are many similarities between those drumming behaviors and these described here for captive macaque monkeys. First, often these sounds are produced in conjunction with vocalizations, hence likely serve to support or extend other means of communication. Second, many of these drumming behaviors convey a sign of dominance or a threat used by the drummer to intimidate the recipient, suggesting that drumming is an expression of an emotional state of the drummer. Thirdly, drumming sounds direct attention toward the drummer and evoke aggressive or affiliative-submissive behaviors in the recipient. Therefore, it is possible that drumming is a form of communication that evolved in a common ancestor and has since propagated throughout primate evolution (1). This line of reasoning is further supported by the observation that also small animals like rodents drum by striking parts of their bodies on a substrate (8). Although in this context communication is mediated by seismic vibrations, this preference may well correlate to the ethological frequencies that are representative of the animal's environmental niche.
It is worth noting that drumming sounds differ acoustically from vocalizations by their intensity and repetitive structure, and thus constitute highly salient sounds over the background of a monkey colony. As a consequence, monkeys might drum because such sounds attract wide attention and because they have learned to associate intense sounds with social power. The auditory system might hence exploit similar saliency principles to highlight an animal's strength and health as those that make visual social information apparent, such as a bright scarlet or blue facial and hindquarter colorations (32, 33). In addition, low frequency sounds produced by large objects (e.g., branches) can travel over long distances, and hence serve as an ideal means to communicate with distant and invisible members of a group. Indeed, studies in the wild have shown that some macaque species do exploit such nonvocal sounds for long-distance communication in large and arboreous habitats. The drumming behavior observed in our captive animals might hence reflect a similar local behavioral adaptation of branch shaking or log thumping in the wild (18, 21).
Common Networks Activated by Vocal and Nonvocal Sounds.
Our fMRI activations show that vocal calls and nonvocal drumming sounds are processed by overlapping networks. Although previous studies have shown that vocalizations preferentially activate a network of several caudal and rostral regions within the auditory cortex (9, 10, 12, 14–16), it was unknown whether nonvocal communication sounds engage overlapping or distinct networks. Our results make a strong case for such an overlap.
We found that preferential responses to vocalizations and drumming sounds occur in a identified region in caudal auditory cortex, which mostly covers the caudal belt, but also reaches into primary field A1 and the parabelt (16). Although the exact function of this region still remains elusive, current evidence promotes several speculations. For example, previous studies have shown that caudal auditory cortex is highly susceptible to cross-modal influences, with the strongest multisensory influences also occurring in the caudal belt (34–36). Overlapping representations of vocal and nonvocal communication sounds hence may be formed in a region receiving information from different sensory modalities. This may not be surprising, given the multisensory nature of the vocal or drumming displays, which consist of defined facial expressions, visible body motion, as well as acoustic signals. Given the great behavioral importance of correctly perceiving and identifying these displays, it would be of great benefit for primate brains to directly combine communication signals from different sensory modalities in regions specialized for processing these (34). In addition, the caudal auditory cortex has been suggested to form a key processing stage that segregates and routes acoustic information to higher regions depending on their acoustic structure, hence functioning as a gatekeeper that facilitates the processing of novel salient sounds—the so called “computational hub” hypothesis (9, 37–39). Given the social importance of communication sounds for primates, they are certainly the most imminent to be segregated and recognized. Hence an overlap of auditory gatekeeper functions, multisensory influences, and representations of vocal and nonvocal acoustic communications may simply reflect an adaptation to the need to quickly and reliably react to social signals.
The second overlap of strong activations for vocalizations and drumming sounds was found in the amygdala. Because both kinds of sounds broadcast social or emotional information, they are expected to activate the emotional system. In fact, previous studies have shown that different emotional sounds, both of vocal or nonvocal nature, activate the amygdala (40–42). The present findings hence well agree with this prior expectation.
Acoustic Gestures, Music and Language.
According to the gestural theory of communication speech and language developed from gestures produced by hands, body posture, or facial expressions (3, 43, 44). In fact, drumming gestures of nonhuman primates such as chest beating, buttress drumming, or branch shaking not only result in the production of sounds but are accompanied by vivid facial expressions and body movements. This raises the possibility that acoustic communication originated to complement expressive visual gestures, or actions performed for nonauditory purposes. Noteworthy, because multisensory integration enhances the recognition of facial or body expressions (34, 36, 45), the notion that acoustic communication emerged from multisensory gestures (4) fits well with our finding that vocal and nonvocal communication sounds preferentially activate overlapping regions in multisensory caudal auditory cortex.
This idea fits also with influential models of speech processing, in which regions in caudal auditory cortex are part of an “internal model” involved in the online processing of sensorimotor data, comparing an efference copy from premotor areas with incoming sensory information from primary auditory cortex (9). Such a model has access to both the gestural-motor aspect as well as to the acoustical information, and hence provides a link to influence both sensory to motor and motor to sensory transformation loops (9). As a result, these caudal auditory regions could have played an important role in shaping the evolution of acoustic communication by using information about gestural motor signals.
It has also been recognized that language emerged in close association with music (1, 46–48), and it is likely that any underlying common neural substrate would have propagated through primate evolution. Although monkeys have neither language nor musical abilities (49, 50), they communicate by using vocalizations, and their drumming behavior may well be homologous to that used by humans in the context of instrumental music (1). Hence primate drumming might represent a precursor of musical abilities in humans, a notion that is also underscored by the ubiquity of drumming across human cultures (51) and its innate nature (52, 53). Our finding of overlapping brain networks involved in the processing of vocalizations and drumming sounds can hence be interpreted as revealing functional mechanisms to support the theory that vocal communication and drumming may have coevolved into the human faculties of language and music.
Materials and Methods
Behavioral and fMRI studies were performed with male rhesus macaques (Macaca mulatta) that are part of a colony housed at the Max Planck Institute for Biological Cybernetics. All procedures were approved by the local authorities (Regierungspräsidium) and are in full compliance with the guidelines of the European Community (EUVD 86/609/EEC) for the care and use of laboratory animals.
Behavioral observations were conducted in our animal facilities, where animals are socially housed in troops of two to four animals per enclosure, which established hierarchies and interactions within a troop. Behavioral tests were based on preferential looking techniques, which are ideally suited to test perception in prelinguistic children and nonhuman primates (23, 24, 54, 55). Functional imaging experiments were performed by using anesthetized animals and on a high-field magnet (4.7 T) by using established protocols (16, 28, 29, 56). Sounds were presented at an average intensity of 80 dB by using MR-compatible headphones. We acquired functional data from three animals (two imaged twice) by using whole-head volume coils and a multishot (two segments) gradient-recalled echo planar imaging sequence with parameters as used in previous reports. The image slices were oriented parallel to the lateral sulcus to capture auditory cortex within the smallest number of slices. Functional images were acquired by using a sparse imaging sequence every 10 s. Functional data were analyzed in Matlab (MathWorks), and two contrasts for voxels preferentially responding to vocalizations or drumming sounds were constructed. Voxels with significant effects in either contrast were identified by using spatial clustering, and the corresponding P values were computed (57). More detailed descriptions of the methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Mark Augath for providing expertise and help in acquiring the MRI data, Mirko Lindig and Denise Ipek for technical assistance during MRI experiments, our animal care staff for help with the monkey subjects and Christopher Petkov for inspiring discussions. We also thank the referees for their helpful comments. This work was funded by the Max-Planck Society, Munich, Germany.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909756106/DCSupplemental.
References
- 1.Fitch WT. The biology and evolution of music: A comparative perspective. Cognition. 2006;100:173–215. doi: 10.1016/j.cognition.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Tomasello M. The Origins of Human Communication. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 3.Corballis MC. The Evolution of Language. Ann NY Acad Sci. 2009;1156:19–43. doi: 10.1111/j.1749-6632.2009.04423.x. [DOI] [PubMed] [Google Scholar]
- 4.Tomasello M, Call J. In: The Gestural Communication of Apes and Monkeys. Call J, Tomasello M, editors. New York, NY: Oxford Univ Press; 2007. pp. 221–240. [Google Scholar]
- 5.Schaller G. The Mountain Gorilla. Chicago, IL: University of Chicago Press; 1963. [Google Scholar]
- 6.Kalan A, Rainey H. Hand-clapping as a communicative gesture by wild female swamp gorillas. Primates. 2009;50:273–275. doi: 10.1007/s10329-009-0130-9. [DOI] [PubMed] [Google Scholar]
- 7.Arcadi AC, Robert D, Boesch C. Buttress druming by wild chimpanzees: Temporal patterning. Phrase integration into loud calls, and preliminary evidence for individual distinctiveness. Primates. 1998;39:505–518. [Google Scholar]
- 8.Randal JA. Evolution and function of drumming as communication in mammals. Amer Zool. 2001;41:1143–1156. [Google Scholar]
- 9.Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petkov CI, Logothetis NK, Obleser J. Where are the human speech and voice regions, and do other animals have anything like them? Neuroscientist. 2009 Jun 10; doi: 10.1177/1073858408326430. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Pollick AS, de Waal FB. Ape gestures and language evolution. Proc Natl Acad Sci USA. 2007;104:8184–8189. doi: 10.1073/pnas.0702624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanwal JS, Rauschecker JP. Auditory cortex of bats and primates: Managing species-specific calls for social communication. Front Biosci. 2007;12:4621–4640. doi: 10.2741/2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghazanfar AA, Miller CT. Language evolution: Loquacious monkey brains? Curr Biol. 2006;16(20):R879–881. doi: 10.1016/j.cub.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Gil-da-Costa R, et al. Toward an evolutionary perspective on conceptual representation: Species-specific calls activate visual and affective processing systems in the macaque. Proc Natl Acad Sci USA. 2004;101:17516–17521. doi: 10.1073/pnas.0408077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil-da-Costa R, Martin A, Lopes MA, Munoz M, Fritz JB, Braun AR. Species-specific calls activate homologs of Broca's and Wernicke's areas in the macaque. Nat Neurosci. 2006;9:1064–1070. doi: 10.1038/nn1741. [DOI] [PubMed] [Google Scholar]
- 16.Petkov CI, et al. A voice region in the monkey brain. Nat Neurosci. 2008;11:367–374. doi: 10.1038/nn2043. [DOI] [PubMed] [Google Scholar]
- 17.Taglialatela JP, Russell JL, Schaeffer JA, Hopkins WD. Communicative signaling activates ‘Broca's’ homolog in chimpanzees. Curr Biol. 2008;18:343–348. doi: 10.1016/j.cub.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehlman PT. In: Evolution and Ecology of Macaque Societies. Fa JE, Lindburg DG, editors. Cambridge, UK: Cambridge Univ Press; 1996. [Google Scholar]
- 19.Hauser MD, Marler P. Food-associated calls in rhesus macaques (Macaca mulatta): I. Socioecological factors. Behav Ecol. 1993;4:194–205. [Google Scholar]
- 20.Maestripieri D. Gestural communication in macaques: Usage and meaning of nonvocal signals. Evol Comm. 1997;1:193–222. [Google Scholar]
- 21.Zhao Q-K. Intergroup interactions in Tibetan macaques at Mt. Emei, China. Am J Phys Anthropol. 1997;104:459–470. doi: 10.1002/(SICI)1096-8644(199712)104:4<459::AID-AJPA3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Kayser C, Petkov CI, Lippert M, Logothetis NK. Mechanisms for allocating auditory attention: An auditory saliency map. Curr Biol. 2005;15:1943–1947. doi: 10.1016/j.cub.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Ghazanfar AA, Logothetis NK. Neuroperception: Facial expressions linked to monkey calls. Nature. 2003;423:937–938. doi: 10.1038/423937a. [DOI] [PubMed] [Google Scholar]
- 24.Ghazanfar AA, et al. Vocal-tract resonances as indexical cues in rhesus monkeys. Curr Biol. 2007;17:425–430. doi: 10.1016/j.cub.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterck EHM, Goossens BMA. The meaning of “macaque” facial expressions. Proc Natl Acad Sci USA. 2008;105:E71. doi: 10.1073/pnas.0806462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maestripieri D, Wallen K. Affiliative and submissive communication in rhesus macaques. Primates. 1997;38:127–138. [Google Scholar]
- 27.De Marco A, Visalberghi E. Facial displays in young tufted capuchin monkeys (Cebus apella): Appearance, meaning, context and target. Folia Primatol. 2007;78:118–137. doi: 10.1159/000097061. [DOI] [PubMed] [Google Scholar]
- 28.Kayser C, Petkov CI, Augath M, Logothetis NK. Functional imaging reveals visual modulation of specific fields in auditory cortex. J Neurosci. 2007;27:1824–1835. doi: 10.1523/JNEUROSCI.4737-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- 30.Petkov CI, Kayser C, Augath M, Logothetis NK. Functional imaging reveals numerous fields in the monkey auditory cortex. PLOS Biol. 2006;4:e215. doi: 10.1371/journal.pbio.0040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geissmann T. Door slamming: Tool-use by a captive white-handed gibbon (Hylobates lar) Gibbon J. 2009;5:53–60. [Google Scholar]
- 32.Ghazanfar AA, Santos LR. Primate brains in the wild: The sensory bases for social interactions. Nat Rev Neurosci. 2004;5:603–616. doi: 10.1038/nrn1473. [DOI] [PubMed] [Google Scholar]
- 33.Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm Behav. 1976;7:481–494. doi: 10.1016/0018-506x(76)90019-2. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kayser C, Petkov CI, Logothetis NK. Multisensory interactions in primate auditory cortex: fMRI and electrophysiology. Hear Res. 2009 Mar 6; doi: 10.1016/j.heares.2009.02,011. [DOI] [PubMed] [Google Scholar]
- 36.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 38.Obleser J, Zimmermann J, Van Meter J, Rauschecker JP. Multiple stages of auditory speech perception reflected in event-related FMRI. Cereb Cortex. 2007;17:2251–2257. doi: 10.1093/cercor/bhl133. [DOI] [PubMed] [Google Scholar]
- 39.Jaaskelainen IP, et al. Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci USA. 2004;101:6809–6814. doi: 10.1073/pnas.0303760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. NeuroImage. 2007;36:480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 41.Morris JS, Scott SK, Dolan RJ. Saying it with feeling: Neural responses to emotional vocalizations. Neuropsychologia. 1999;37:1155–1163. doi: 10.1016/s0028-3932(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 42.Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12:181–198. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- 43.Maestripieri D. Gestural communicationin three species of macaques (Macaca mulatta, M. nemestrina, Marctoides) Gesture. 2005;5:57–73. [Google Scholar]
- 44.Feyereisen P, Lannoy de J-D. Gestures and Speech: Psychological Investigations. Cambridge, UK: Cambridge Univ Press; 1991. [Google Scholar]
- 45.Collignon O, et al. Audio-visual integration of emotion expression. Brain Res. 2008;1242:126–135. doi: 10.1016/j.brainres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Hauser MD, McDermott J. The evolution of the music faculty: A comparative perspective. Nat Neurosci. 2003;6:663–668. doi: 10.1038/nn1080. [DOI] [PubMed] [Google Scholar]
- 47.McDermott J. The evolution of music. Nature. 2008;453:287–288. doi: 10.1038/453287a. [DOI] [PubMed] [Google Scholar]
- 48.Fitch WT. The evolution of music in comparative perspective. Ann NY Acad Sci. 2005;1060:29–49. doi: 10.1196/annals.1360.004. [DOI] [PubMed] [Google Scholar]
- 49.McDermott J, Hauser M. Are consonant intervals music to their ears? Spontaneous acoustic preferences in a nonhuman primate. Cognition. 2004;94:B11–B21. doi: 10.1016/j.cognition.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 50.McDermott J, Hauser MD. Nonhuman primates prefer slow tempos but dislike music overall. Cognition. 2007;104:654–668. doi: 10.1016/j.cognition.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Blades J. Percussion Instruments and Their History. Westport, CT: Bold Strummer Ltd; 1992. [Google Scholar]
- 52.Winkler I, Háden GP, Ladinig O, Sziller I, Honing H. Newborn infants detect the beat in music. Proc Natl Acad Sci USA. 2009;106:2468–2471. doi: 10.1073/pnas.0809035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirschner S, Tomasello M. Joint drumming: Social context facilitates synchronization in preschool children. J Exp Child Psychol. 2009;102:299–314. doi: 10.1016/j.jecp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982;218:1138–1141. doi: 10.1126/science.7146899. [DOI] [PubMed] [Google Scholar]
- 55.Patterson ML, Werker JF. Matching phonetic information in lips and voice is robust in 4.5 month-old infants. Infant Behav Dev. 1999;22:237–247. [Google Scholar]
- 56.Kayser C, Petkov CI, Augath M, Logothetis NK. Integration of touch and sound in auditory cortex. Neuron. 2005;48:373–384. doi: 10.1016/j.neuron.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Tolias AS, et al. Mapping cortical activity elicited with electrical microstimulation using fMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.