Abstract
Earlier experiments have shown that cyclosporin A (CsA) and its non-calcineurin inhibitory analog NIM811 attenuate mitochondrial dysfunction after experimental traumatic brain injury (TBI). Presently, we compared the neuroprotective effects of previously determined mitochondrial protective doses of CsA (20 mg/kg intraperitoneally) and NIM811 (10 mg/kg intraperitoneally) when administered at 15 mins postinjury in preventing cytoskeletal (α-spectrin) degradation, neurodegeneration, and neurological dysfunction after severe (1.0 mm) controlled cortical impact (CCI) TBI in mice. In a first set of experiments, we analyzed calpain-mediated α-spectrin proteolysis at 24 h postinjury. Both NIM811 and CsA significantly attenuated the increased α-spectrin breakdown products observed in vehicle-treated animals (P < 0.005). In a second set of experiments, treatment of animals with either NIM811 or CsA at 15 mins and again at 24 h postinjury attenuated motor function impairment at 48 h and 7 days (P < 0.005) and neurodegeneration at 7 days postinjury (P < 0.0001). Delayed administration of NIM811 out to 12 h was still able to significantly reduce α-spectrin degradation. These results show that the neuroprotective mechanism of CsA involves maintenance of mitochondrial integrity and that calcineurin inhibition plays little or no role because the non-calcineurin inhibitory analog, NIM811, is as effective as CsA.
Keywords: calpain, α-spectrin, motor function, mitochondrial permeability transition pore, NIM811, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in children, young adults, and geriatric people. Every year, approximately 1.6 million people have head injury in the United States, with nearly 60,000 deaths (Marik et al, 2002). Despite recent advances in the prognoses and management of TBI, specific therapy remains elusive, in part owing to the incomplete understanding of the pathological secondary injury mechanisms that proceed from the primary shearing of neurons and blood vessels. This major unmet clinical problem has focused a majority of neurotrauma research on the discovery and development of neuroprotective agents that could ameliorate the morphological and pathophysiological effects of the disease through timely pharmacological intervention. Experimental TBI research has uncovered several injury mechanisms that are believed to be major contributing factors to the morphological and pathological features of TBI, including alterations in excitatory amino acids such as glutamate, cytosolic calcium (Ca+2) overload, reactive oxygen species (ROS) generation, activation of cysteine proteases such as calpain and activation of apoptotic cell death cascades (Buki et al, 1999b; Crompton, 1999; Faden et al, 1989; Hall et al, 1994; McIntosh et al, 1996; Okonkwo et al, 1999; Saatman et al, 1996).
It has been shown that damage to the bioenergetic integrity of mitochondria plays a critical role in posttraumatic neuronal death (Xiong et al, 1997), and that the formation and opening of the mitochondrial permeability transition pore (mPTP) is one of the core mediators of this process (Ankarcrona et al, 1995; Bernardi, 1996; Crompton, 1999; Sullivan et al, 1999). Although Ca+2 plays a pivotal role in normal neuronal function, excessive intracellular Ca+2 accumulation causes a severe reduction in mitochondrial membrane potential, which triggers the formation and opening of the mPTP, mitochondrial oxidative damage (Mbye et al, 2008; Singh et al, 2006), and eventual cell death. Mitochondrial dysfunction and the loss of synaptic homeostasis have been shown after cortical contusion injury (Sullivan et al, 1998), implicating mitochondria as key participant in TBI-induced neuropathology. Support for this conclusion comes from multiple studies showing that posttraumatic administration of cyclosporin A (CsA), a potent inhibitor of mPTP formation, attenuates cortical mitochondrial dysfunction, neuronal damage, and synaptic loss after TBI in rats or mice (Scheff and Sullivan, 1999; Sullivan et al, 2000b). Furthermore, other investigators have reported the neuroprotective role of CsA in limiting calpain activation (Ferrand-Drake et al, 2003), axonal damage (Okonkwo et al, 1999), and memory performance (Alessandri et al, 2002) after an insult to the CNS. The underlying mechanism through which CsA is believed to mediate its neuroprotective effect is through its inhibition of the mPTP, a Ca+2-dependent proteinacious pore that disrupts the mitochondrial membrane potential (ΔΨm), depletes ATP, and equilibrates small solutes and ions between the cytosol and the mitochondrial matrix, leading to cellular dysfunction (Crompton, 1999; Waldmeier et al, 2002).
However, some investigators have suggested that CsA's neuroprotective capability may be mediated in part through its inhibition of calcineurin. Calcineurin is a protein phosphatase that dephosphorylates apoptogenic proteins such as Bad, allowing it to bind other antiapoptotic Bcl-2 family members. Binding and inhibition of antiapoptotic proteins by Bad triggers the release of cytochrome c and other proapoptotic proteins from the mitochondrial inter-membrane space (Kroemer and Reed, 2000; Waldmeier et al, 2002). To show calcineurin's involvement in CsA's neuroprotective mechanism, other immunophilin ligands such as tacrolimus (FK506), which inhibit calcineurin, but lack the ability to suppress mPTP opening (Scheff and Sullivan, 1999) have been shown to produce neuroprotective effects (Marmarou and Povlishock, 2006; Reeves et al, 2007).
Recently, we have shown in a mitochondrial bioenergetic mechanistic study that the CsA derivative, N-methyl-4-isoleucine-cyclosporin (NIM811), which mimics the action of CsA in preventing mPTP formation and opening, but lacks the ability to inhibit calcineurin (Waldmeier et al, 2002), can fully duplicate the mitochondrial protective efficacy of CsA, suggesting that inhibition of mPTP may be sufficient to explain CsA's protective effects (Mbye et al, 2008). To provide a more comprehensive mechanistic validation of the role of mitochondrial permeability transition inhibition in CsA's neuroprotective mechanism, in this study, we compared the ability of NIM811 and CsA to prevent calpain-mediated cytoskeletal degradation, attenuate neurological dysfunction, and reduce axonal degeneration after TBI in mice. First, we analyzed calpain-mediated proteolysis by measuring breakdown of the cytoskeletal protein, α-spectrin after TBI. Second, we examined motor function recovery at 48 h and 7 days after injury, using a composite Neuroscore. Third, we determined the extent of neuronal damage at 7 days using the de Olmos silver staining histochemical method. Specifically, this technique identifies degenerating axons and dendrites (Hall et al, 2008; Switzer, 2000). Lastly, we evaluated the therapeutic time window for NIM811 in terms of its ability to attenuate the increase in α-spectrin proteolysis observed at 24 h postinjury.
Materials and methods
Animals
The studies were performed using young adult male CF-1 mice (Charles River, Portage, MI, USA) weighing 29 to 31 g. All animals were housed in the Division of Laboratory Animal Resources sector of the University of Kentucky Medical Center, which is fully accredited by AAALAC. Procedures follow protocols approved by the University of Kentucky's Institutional Animal Care and Use Committee.
Mouse Model of Focal (Controlled Cortical Impact) Traumatic Brain Injury
The Controlled Cortical Impact (CCI) model uses a nonpenetrating, localized deformation of the cortex that histopathologically resembles closely cortical contusion seen clinically. This model also uses a pneumatic impactor with which we can independently control the contact velocity and the level of cortical deformation, thus altering the severity of the injury. In these sets of experiments, the contact velocity of the impactor was set at 3.5 ms, while inducing a severe (1.0 mm) cortical contusion. Mice were initially anesthetized in a plexiglass chamber using 4.0% isoflurane and placed in a stereotaxic frame (David Kopf, Tujunga, CA, USA). During the injury procedure, anesthesia was maintained with 2.5% isoflurane delivered via a nose cone. The head was positioned in the horizontal plane with the nose bar set at zero. After a midline incision exposing the skull, a 4.0 mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap at the craniotomy was carefully removed without damaging the underlying dura, and the exposed cortex was injured using a pneumatically controlled impactor device with a 3.0 mm flat-tip diameter, as described previously (Scheff and Sullivan, 1999; Singh et al, 2006), except that the current device uses a unique contact sensor mechanism that ensures accurate and reliable determination of cortical surface before initiating injury. This results in increased accuracy and reproducibility in regards to the CCI injury compared with that produced by earlier CCI devices. After injury, the craniotomy was closed by placement of a small disk of saline-moistened Surgicil over the dura, followed by gluing a 6.0 mm disk made of dental cement over the site with super glue. The animals were then placed in a Hova-Bator Incubator (model 1583; Randall Burkey Co., Boerne, TX, USA), which was set at 37°C, until consciousness (i.e., return of right reflex and mobility) was regained.
Experimental Design for α-Spectrin Proteolysis Studies
Our calpain-mediated α-spectrin proteolysis experiment involved four groups of mice (sham, vehicle-treated intraperitoneally, 10 mg/kg NIM811-treated intraperitone-ally, and 20 mg/kg CsA-treated intraperitoneally) with an N of 8 per group, and 1 animal per N. The doses of NIM811 and CsA were selected based on previous studies showing that these are the optimal doses for preventing posttraumatic mitochondrial dysfunction in mice subjected to CCI TBI (Mbye et al, 2008; Scheff and Sullivan, 1999). NIM811 was generously provided by Novartis Pharmaceuticals Inc. (Basel, Switzerland) and CsA was obtained commercially (Sandimmune, Novartis Pharmaceuticals Inc.). The vehicle for both drugs was the commercial CsA vehicle consisting of polyethylene glycol/sterile saline/cremaphor oil. Vehicle-treated animals received only the NIM811/CsA vehicle. An intraperitoneally bolus of vehicle, NIM811, or CsA was administered in equal volumes at 15 mins postinjury.
Tissue Extraction and Protein Assay
At the time of killing, mice were deeply anesthetized with CO2, and decapitated. The ipsilateral cortical area of interest was then rapidly dissected on an ice-chilled stage as previously described (Deng et al, 2007), and immediately transferred into precooled Triton lysis buffer (1% Triton, 20 mmol/L Tris HCl, 150 mmol/L NaCl, 5 mmol/L EGTA, 10 mmol/L EDTA, 10% glycerol) with protease inhibitors (Complete Mini Protease Inhibitor Cocktail tablet). Samples were then briefly sonicated and vortexed at 14,000 r.p.m. for 30 mins at 4°C, and supernatants were collected for protein assay. Protein concentration was determined by Bio-Rad DC Protein Assay (Hercules, CA, USA), with sample solutions diluted to contain 1 mg/mL of protein for immunoblotting.
Western Blotting Analysis of Calpain-Mediated α-Spectrin Degradation
To measure calpain-mediated α-spectrin proteolysis, aliquots of each cortical sample (a total of 5 μg) were run on a SDS/polyacrylamide gel electrophoresis Precast gel (3% to 8% Tris-Acetate Criterion XT Precast gel, Bio-Rad) and transferred onto a nitrocellulose membrane using a semidry electrotransferring unit set at 15 V for 15 mins as previously described (Deng et al, 2007). After gel transfer, membranes were incubated in a Tris-buffered saline blocking solution with 5% milk for 1 h at room temperature. The blots were then probed with an anti-α-spectrin antibody (monoclonal, Affiniti FG6090) at a dilution of 1:5000 in Tris-buffered saline with Tween 20 blocking solution with 5% milk, followed by a goat antimouse secondary conjugated to an infrared dye (1:5000, IR-Dye800CW, Rockland). Densitometric analysis of western blots was performed using a Li-Cor Odyssey Infrared Imaging System, to quantify the level of the 145 and 150 kDa α-spectrin breakdown products (SBDPs 145 and 150). A standardized protein loading control was included on each blot to normalize the band densities so that comparisons could be made across multiple blots. This was made up of pooled brain tissue protein collected from previously run TBI mice that gave strong bands corresponding to the 280 kDa parent α-spectrin, the 150 kDa, and the 145 kDa breakdown products. The amount of protein in the loading control had been previously determined and shown to be within the linear range as measured with the Li-Cor Odyssey Infrared Imaging System. All the samples were then averaged as mean (±s.e.m.).
Experimental Design for Motor Functional Recovery and Neurodegeneration Studies
Our neurological motor function experiments involved four groups of mice (sham, vehicle-treated intraperitone-ally, 10 mg/kg NIM811-treated intraperitoneally, and 20 mg/kg CsA-treated intraperitoneally) with an N of 12 per group, and 1 animal per N. Depending on group designation, animals received either no injury and no drug administration; a bolus dosage (10 mg/kg intraperitoneally) of NIM811 in a solution of polyethylene glycol/sterile saline/cremaphor oil; a bolus dosage (20 mg/kg intraperitoneally) of CsA in a solution of polyethylene glycol/sterile saline/cremaphor oil; or an equivalent volume of vehicle (as described above) at 15 mins and again at 24 h postinjury. In our neurodegeneration experiments, eight animals/group were randomly selected from those used to conduct the Neuroscore motor function analysis and killed at 7 days after TBI for de Olmos aminocupric silver staining (i.e., neurodegeneration) measurement.
Neuroscore Motor Function Analysis
A composite Neuroscore test was conducted to assess the neurological motor function of sham and brain-injured animals at 48 h and 7 days post-TBI as previously described (McIntosh et al, 1989), with slight modifications for use in mice (Raghupathi et al, 1998). This motor test has been shown to correlate with injury severity (Nakamura et al, 1999), and is known to be sensitive to pharmacological manipulation (Saatman et al, 1997). Animals were tested at 48 h and 7 days postinjury and were given an integer score from 0 (severely impaired) to 4 (normal) for each of the following indices: fore-limb function, hind-limb function, and resistance to lateral pulsion. For fore-and hind-limb function analysis, animals were either suspended by the tail for measurement of grip strength of fore limbs, and extension and flexion of hind limbs, or subjected to a grid-walk test for evaluation of foot falls. In the lateral pulsion analysis, animals were tested on their ability to resist lateral pulsion toward left and right. Animals that performed normally received as much as 12 points (4 points multiplied by 3 indices). Evaluation of neurological motor function was conducted by an experienced investigator, who was blinded as to treatment groups.
de Olmos Silver Staining Analysis of Neurodegeneration
After neurobehavioral analysis at 7 days postinjury, we examined the extent of neuronal damage using the de Olmos aminocupric silver staining histochemical technique as previously described (de Olmos et al, 1994; Hall et al, 2008; Switzer, 2000). As we have recently observed (Hall et al, 2008), this method selectively stains degenerating neuronal axons and dendrites, but not cell bodies. However, we have shown that histological methods that simply reveal either surviving or degenerating neurons greatly understate the major aspect of posttraumatic neuronal damage that involves the neuronal processes. Considering the important role of axonal injury in TBI pathologic study and pathophysiology (Buki et al, 1999a; Buki et al, 2000; Povlishock et al, 1999), the assessment of neuroprotection by this method seems very appropriate. Moreover, we have successfully validated the technique for assessment of neuroprotection in previous studies (Deng-Bryant et al, 2008; Kupina et al, 2003; Kupina et al, 2002).
At 7 days, mice (N = 8/group) were overdosed with sodium pentobarbital (200 mg/kg intraperitoneally) and transcardially perfused with 0.9% sodium chloride, followed by a fixative solution containing 4% paraformaldehyde. After decapitation, the heads were stored in a fixative solution containing 15% sucrose for 24 h after which the brains were removed, placed in fresh fixative, and shipped for histological processing to Neuroscience Associates Inc. (Knoxville, TN, USA). Brains used for this study were embedded into one gelatin block (Multiblock Technology, Neuroscience Associates). The block was then frozen and 13 35 μm coronal sections of 420 μm apart between 1.1 mm anterior and 4.4 mm posterior to bregma were silver stained and counterstained with Nuclear Fast Red, to reveal degenerating neuronal processes. Brain sections were then photographed on an Olympus Provis A70 microscope at × 1.25 magnification using an Olympus Magnafire digital camera, and analyzed by Image-Pro Plus (4.0). The percentage area of silver staining for each brain section was calculated by dividing the area of ipsilateral hemispheric silver staining in each section by the area of the contralateral hemisphere and multiplying by 100. The volume of silver staining in the ipsilateral hemisphere as a percentage of the contralateral hemispheric volume was estimated by the equation %V = (t × Σ ai(s))/(t × Σ ac(s)) × 100, where % V is percent silver stain volume, t = the distance between sections analyzed (420 μm), and Σ a(s) is the sum of area of silver staining for ipsilateral (i) and contralateral (c) hemispheres in all sections examined (13 for each brain). As explained in our previous work (Hall et al, 2008 no. 1228), the cortical contusion volume is added into the silver staining volume even though the contusion site has cavitated by 7 days as it is part of the overall volume of neurodegeneration.
Experimental Design for Therapeutic Window Study Using α-Spectrin Proteolysis as an End Point
Our final therapeutic window experiments for NIM811 involved seven groups of mice with an N of 8 per group, and 1 animal per N. Depending on group designation, animals received either no injury and no drug administration; a bolus dosage (10 mg/kg intraperitoneally) of NIM811 administered at either 15 mins, 1, 3, 6, or 12 h postinjury; or an equivalent volume of vehicle (as described above) administered at 15 mins postinjury. Animals were then killed at 24 h after TBI, and calpain-mediated α-spectrin breakdown was measured as described above.
Statistical Analysis
For the spectrin and neurodegeneration studies, results were expressed as the mean±s.e., using a Statview 5.0 statistical program. For treatment group analyses, we performed a one-way analysis of variance (ANOVA), followed by Student–Newman–Keuls post hoc analysis to determine the significance of differences between groups. For the motor function behavioral studies, we performed a one-way Kruskal–Wallis ANOVA, followed by Mann–Whitney U-post hoc analysis, using a Statistica statistical program. For all ANOVAs, a P < 0.05 was required to establish a statistically significant difference across the groups. In addition, for both post hoc analyses, the programs determined significance based on a correction for multiple comparisons among groups.
Results
Ability of NIM811 and CsA to Attenuate Posttraumatic Calpain-Mediated Cytoskeletal Degradation
In a first set of experiments, we compared the ability of a 10 mg/kg intraperitoneal dose of NIM811 and a 20 mg/kg intraperitoneal dose of CsA to preserve cellular cytoskeleton by preventing calpain-mediated α-spectrin breakdown after TBI. In an earlier study, we showed that 10 mg/kg NIM811 represents the optimum dosage in preserving mitochondrial bioenergetics at 12 h postinjury (Mbye et al, 2008). Furthermore, previous studies by Sullivan and colleagues have established that 20 mg/kg CsA is the optimum dosage in regards to attenuating cortical lesion in mice after CCI injury (Scheff and Sullivan 1999). Thus, we adopted the 10 and 20 mg/kg doses of NIM811 and CsA, respectively, to compare their protective effects on cytoskeletal degradation (α-spectrin breakdown) after CCI-mediated injury in mice. Densitometric western blot analysis of SBDPs was conducted at 24 h (Figure 1A) after injury in cortical tissues isolated from sham (craniotomy, but no injury) and injured animals treated with either NIM811, CsA, or equivalent volume of vehicle. The 24 h time point was chosen based on it being the peak of posttraumatic α-spectrin breakdown in the mouse CCI model (Deng et al, 2007).
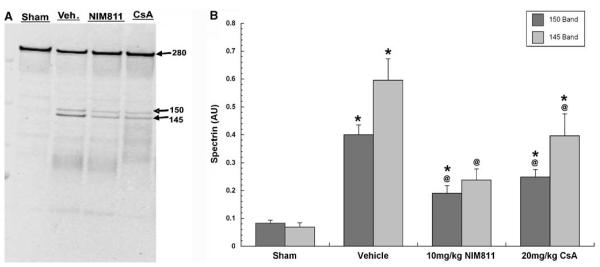
Figure 1.
Representative blot for spectrin breakdown products (A) and quantitative effects of NIM811 and CsA on post-traumatic calpain-mediated cytoskeletal degradation at 24 h after severe CCI (B). Plotted is the mean (±s.e.m.) level of SBDPs in the injured cortex for each group. TBI caused significant increase in both 145 and 150 SBDPs in vehicle-treated animals, and treatment with either 10 mg/kg of NIM811 or 20 mg/kg of CsA significantly ameliorated α-spectrin breakdown. Statistical differences (one-way ANOVA and Student–Neuman–Keuls post hoc test): *P < 0.05 versus sham, @P < 0.05 versus vehicle, n =8.
One-way ANOVA for 145 and 150 kDa SBDPs revealed a statistically significant effect across experimental groups (F = 14.4, 24.1; d.f. = 3.28; P < 0.0001). Subsequent Student–Neuman–Keuls post hoc comparison of intergroup differences showed that mean 145 kDa SBDP in ipsilateral cortical tissue isolated from injured vehicle-treated animals was 760% higher (P < 0.0001) compared with the levels in sham, noninjured animals (Figure 1B). The 150 kDa SBDP was 380% higher. In contrast, mean 145 kDa SBDP in cortical tissue isolated from either NIM811- or CsA-treated groups were significantly (P < 0.02) decreased with a 60% decrease in the case of NIM811 and a 33% decrease in the case of CsA, compared with the levels in vehicle-treated animals. For the 150 kDa SBDP, the reduction by NIM811 was 52% whereas that by CsA was 37%. Although the NIM811 effects seem to be greater than those for CsA in the case of both SBDPs, the differences in the effect size between the two compounds were not significant.
Ability of NIM811 and CsA to Improve Motor Functional Recovery after TBI
In a second set of experiments, we evaluated the ability of two-bolus injections of NIM811 and CsA at 15 mins and 24 h postinjury to improve motor function recovery of animals at 48 h (Figure 2A), by comparing the Neuroscore measurement of sham and injured animals treated with either vehicle, 10 mg/kg NIM811, or 20 mg/kg CsA. The ANOVA showed a statistically significant difference across groups (control subjects = 9, d.f. = 3; P < 0.005). Post hoc analysis of composite Neuroscore revealed that CCI-mediated TBI caused significant (P < 0.005) motor impairment in vehicle-treated animals relative to sham, noninjured controls. However, the motor function of animals treated with either NIM811 or CsA was significantly (P < 0.005) improved compared with their vehicle-treated counterparts. Moreover, the motor performance of animals treated with either NIM811 or CsA was not significantly different than that of the sham group.
Figure 2.
Effects of NIM811 and CsA on motor dysfunction at 48 h (A) and 7 days (B) after severe CCI. Plotted is the mean (±s.e.m.) composite Neuroscore value for each group, including sham, noninjured animals. TBI causes significant decrease in the composite Neuroscore of vehicle-treated animals. Treatment with either 10 mg/kg of NIM811 or 20 mg/kg of CsA at 15 mins and 24 h postinjury significantly improved the motor function at both 48 h and 7 days. Statistical differences (one-way ANOVA and Student–Neuman—Keuls post hoc test): *P < 0.05 versus sham, @P < 0.05 versus vehicle, n = 12.
Further evaluation of motor function at 7 days postinjury (Figure 2B) show similar results to those obtained from at 48 h. The ANOVA revealed a significant difference across experimental groups (control subjects = 11.6, d.f. = 3; P < 0.008). Likewise, the TBI-induced motor deficits in the vehicle-treated brain-injured animals relative to sham were still significant (P < 0.0005). In contrast, motor function of animals treated with two injections of either NIM811 or CsA remained significantly (P < 0.0005) improved compared with the vehicle-treated group, and neither drug-treated group showed a significant difference compared with sham. Furthermore, at both the 48 h and 7-day time points, there was no difference between NIM811 and CsA-treated groups.
Effect of NIM811 and CsA on Neuronal Damage as Shown by de Olmos Silver Staining
After measurement of motor function at 7 days postinjury, we extracted brains and evaluated the extent of neuronal degeneration using the de Olmos silver staining histochemical technique. Figure 3 displays the effect of NIM811 and CsA on the volume of silver staining (i.e., neurodegeneration) in the ipsilateral hemisphere at 7 days postinjury. The one-way ANOVA revealed a statistically significant difference across experimental groups (F = 59.9; d.f. = 3,28; P < 0.0001). Subsequent Student–Newman–Keuls post hoc comparisons of group differences showed that mean volume of silver staining for vehicle-treated animals, as a percent of contralateral hemisphere was significantly (P < 0.0001) higher with 528% increase, compared with that of tissue isolated from sham, noninjured animals. However, mean volumes of neurodegeneration were significantly (P < 0.0001) decreased with a 38% decrease in the case of NIM811 and 36% decrease in the case of CsA, relative to the vehicle-treated group. Figure 3 includes typical photomicrographic examples of silver staining at the injury epicenter in vehicle-, NIM811-, and CsA-treated mice.
Figure 3.
Effects of NIM811 and CsA on de Olmos silver staining (i.e., neurodegeneration) in the ipsilateral hemisphere at 7 days after severe CCI. Plotted is the mean (±s.e.m.) volumetric percentage of silver staining for each group, including sham, noninjured animals. TBI caused a significant increase in silver staining in the ipsilateral hemisphere of vehicle-treated animals, relative to sham, and treatment with either 10 mg/kg of NIM811 or 20 mg/kg of CsA at 15 mins and 24 h postinjury significantly reduced neurodegeneration compared with vehicle-treated group. Photomicrographs show 4 x magnification of injury and treatment effects on silver staining after severe CCI, as shown by enlargement of injury cavity in the vehicle-treated group, and a reduction in cavity in the NIM811- and CsA-treated groups compared with vehicle-treated groups. Statistical differences (one-way ANOVA and Student–Neuman–Keuls post hoc test): *P < 0.05 versus sham, @P < 0.05 versus vehicle, n = 8. Photomicrographs show typical cross-sections through the epicenter of the injury in sham, vehicle-, NIM811 and CsA-treated mice.
Therapeutic Window of NIM811, as Measured by Calpain-Mediated α-Spectrin Breakdown
In a final set of experiments, we evaluated the therapeutic window for NIM811 in regards to its ability to attenuate posttraumatic calpain-mediated α-spectrin proteolysis after CCI-TBI in mice. Mice were treated with NIM811 (10 mg/kg, intraperitoneally) beginning at 15 mins, 1, 3, 6, or 12 h postinjury and the levels of the 145 and 150 kDa SBDPs were measured at 24 h postinjury compared with sham and vehicle-treated groups. Figure 4A shows a sample western blot of the intact 280 kDa parent spectrin, and the 150 and 145 kDa SBDP bands, which show a dramatically increased band density after injury with only vehicle treatment, and a decrease in both the 150 and 145 kDa bands, relative to vehicle, after NIM811 treatment at all of the postinjury treatment delays. The one-way ANOVA revealed a statistically significant difference across experimental groups (F = 3.25, 3.04; d.f. = 6,49; P < 0.01) in the level of the calpain-specific 145 kDa (Figure 4B) and the calpain/caspase 3-mediated 150 kDa (Figure 4C) SBDPs. Similar to the earlier findings in our previous spectrin breakdown study, subsequent post hoc analysis revealed that animals treated with vehicle had a significantly (P < 0.0001) higher level of both SBDPs compared with sham, noninjured animals (+ 1160% for the 145 kDa and + 950% for the 150 kDa). In contrast, animals treated with 10 mg/kg NIM811 at 15 mins, 1, 3, 6, or 12 h postinjury all had a significantly (P < 0.05) decrease levels of SBDPs in comparison to vehicle-treated animals. No difference in SBDP levels between NIM811-treated groups were observed, indicating that a 12 h delay in NIM811 dosing regiment is just as effective in reducing α-spectrin proteolysis as when the compound is administered only 15 mins after injury.
Figure 4.
Therapeutic window of NIM811, as measured by calpain-mediated α-spectrin breakdown 24 h after severe CCI. Representative western blot of spectrin breakdown products (145 and 150 kDa SBDPs) after administration of NIM811 at either 15 mins, 1, 3, 6, or 12 h postinjury (A). TBI increased both 145 and 150 SBDPs. Delayed treatment with 10 mg/kg of NIM811 out to 12 h attenuated α-spectrin degradation relative to vehicle treatment. A one-way ANOVA revealed a statistically significant difference across experimental groups in the amount of SBDPs for both the 145 kDa (B) and 150 kDa (C) SBDPs. Subsequent Student-Newman-Keuls post hoc analysis revealed that animals treated with vehicle had a significantly (P < 0.0001) higher mean of SBDPs compared with sham, noninjured animals. In contrast, animals treated with one bolus of 10 mg/kg of NIM811 at 15 mins, 1, 3, 6, or 12 h postinjury all had a significantly (P < 0.05) decrease mean of SBDPs than vehicle-treated animals. *P < 0.05 versus sham, @P < 0.05 versus vehicle, n =8.
Discussion
Previous studies have shown that administration of cyclosporin A (CsA), a potent modulator of the mPTP, attenuates mitochondrial dysfunction and neuronal damage after experimental TBI in rodents (Mbye et al, 2008; Scheff and Sullivan, 1999; Sullivan et al, 2000a; Sullivan et al, 2000b; Sullivan et al, 1999; Buki et al, 2000; Okonkwo et al, 1999; Okonkwo and Povlishock, 1999). Moreover, other investigators have reported that the mitochondrial and neuroprotective efficacy of CsA after an insult to the CNS includes a reduction in calpain activation (Ferrand-Drake et al, 2003), axonal damage (Okonkwo et al, 1999), and an improvement in memory performance (Alessandri et al, 2002). Cyclosporin A's neuroprotective mechanism is believed to be mediated through inhibition of mitochondrial permeability transition by binding to cyclophilin D (Cyp-D), which is a necessary component of the mPTP. However, other investigators (Singleton et al, 2001) have suggested that the compound's neuroprotective capability may also be mediated through a mechanism related to its immunosuppressive property that involves inhibition of the cellular phosphatase calcineurin that in turn leads to decreased levels of the phosphorylated form of the nuclear factor of activated T cells and a resulting decrease in the levels of certain interleukins and cytokines (Pattison et al., 1997). However, we have recently shown that the posttraumatic mitochondrial protection seen with CsA can be duplicated by administration of the CsA analog NIM811 (Mbye et al, 2008) that has no interaction with calcineurin (Waldmeier et al, 2002).
In an attempt to further explore the neuroprotective efficacy of CsA, we compared the ability of a 10 mg/kg intraperitoneally dose of NIM811 and a 20 mg/kg intraperitoneally dose of CsA to preserve the cytoskeleton by preventing calpain-mediated α-spectrin breakdown after TBI. Densitometric analyses of 145 and 150 kDa breakdown products indicated that SBDP levels in tissue isolated from vehicle-treated animals were significantly higher compared with those from sham, noninjured animals. Injury-induced spectrin proteolysis was significantly decreased by treatment with either NIM811 or CsA. Although the reduction in calpain-mediated cytoskeletal degradation was only partial for both drugs, and the levels remained significantly higher than those in the sham animals, NIM811 decreased both SBDPs by 60% compared with the vehicle-treated group. Furthermore, although NIM811's effect size appeared to be larger than that for CsA, the efficacy difference was not statistically significant, indicating equal cytoskeletal protective efficacy for NIM811 and CsA. These results are consistent with previous findings where we showed that NIM811 was as effective as CsA in attenuating mitochondrial oxidative damage and respiratory dysfunction (Mbye et al, 2008).
Controlled cortical impact TBI caused significant motor impairment in terms of fore-limb and hind-limb extension, grid-walk footfalls, and the ability to resist lateral pulsion. Treatment of mice with either 10 mg/kg NIM811 or 20 mg/kg CsA at 15 mins and again at 24 h postinjury improved motor function to the same level seen in the sham, noninjured animals at both 48 h and 7 days after injury. This normalization of motor function by both compounds was associated with a neuroprotective effect of both NIM811 and CsA in terms of attenuating posttraumatic neurodegeneration at 7 days postinjury, as measured by the de Olmos silver staining histo-chemical technique, which selectively stains degenerating neuronal processes (de Olmos et al, 1994; Switzer, 2000). Post hoc comparison of group differences from this study showed that mean volume of silver staining as a percentage of contralateral hemisphere from animals treated with vehicle alone was still significantly higher than that of sham, noninjured animals. However, mean volumes of neurodegeneration were significantly decreased by 38% in the NIM811- and 36% in the CsA-treated animals, relative to vehicle-treated animals. This suggests that a partial neuroprotective effect combined with intrinsic neurological recovery processes may be able to completely restore neurological function. However, the essentially complete motor functional recovery-promoting effects of NIM811 need to be further studied using behavioral methods that may be more sensitive for the demonstration of residual neurological deficits than the Neuroscore motor function analysis method.
To conduct a more comprehensive analysis of NIM811, we determined the extent of its neuroprotective therapeutic time window by administering a single dose of 10 mg/kg NIM811 at 15 mins, 1, 3, 6, or 12 h postinjury, followed by evaluation of post-traumatic α-spectrin breakdown at 24 h postinjury. The results of these experiments showed that NIM811 therapy retained its protective efficacy even when treatment initiation is delayed until 12 h after TBI. The fact that the onset of NIM811 treatment 12 h postinjury is as effective as initiation at 15 mins illustrates that the secondary injury process is still inhibitable by pharmacological neuroprotective intervention at longer periods at least in the case of NIM811. Previous studies with CsA in the rat CCI-TBI model have shown that the compound is still able to reduce posttraumatic brain damage even when delayed to 24 h postinjury (Sullivan et al, 2000a). The current therapeutic experiments with NIM811 using 24 h cytoskeletal degradation as the neuroprotective end point did not allow for a full definition of the maximum possible treatment delay since 24 h represents the peak of posttraumatic α-spectrin degradation in the currently used mouse CCI-TBI model (Deng et al, 2007). However, it seems unlikely that cytoskeletal protective efficacy could extend much beyond 12 h as there is a significant increase (doubling) in α-spectrin degradation between 12 and 24 h (Deng et al, 2007). Moreover, even though spectrin degradation provides a reasonable surrogate for posttraumatic neurodegeneration, additional experiments are needed to confirm that NIM811 can lessen histological damage and improve behavioral recovery when its administration is delayed until 12 h postinjury.
The neuroprotective property that is shared by CsA and NIM811 is the ability to prevent mitochondrial permeability transition, by binding to Cyp-D and blocking its association with the adenine nucleotide translocase (ANT), which in turn prevents the formation and opening of the mPTP (Albensi et al, 2000; Crompton, 1999; Sullivan et al, 1999). By preventing the opening of the mPTP, CsA, and NIM811 preserve the Ca+2 buffering and respiratory capacities of mitochondria (Mbye et al, 2008; Okonkwo and Povlishock, 1999; Sullivan et al, 1999), which prevents further cytosolic Ca+2 overload, and subsequent calpain and caspase 3-mediated cytoskeletal degradation that has been associated with TBI.
As noted earlier, it has been suggested that calcineurin inhibition may contribute to CsA's neuroprotective effects as it does in the case of the neuroprotective actions of another immunophilin, FK506 (Marmarou and Povlishock, 2006; Reeves et al, 2007; Singleton et al, 2001). Calcineurin is a protein phosphatase that dephosphorylates, and thus activates nitric oxide synthase (Heales et al, 1999). Conceivably, by inhibiting calcineurin, CsA would keep NOS in its inactive state and thereby, reduce nitric oxide radical (NO) production. This would lessen formation of the NO-derived reactive oxygen species peroxynitrite, which is known to be important the pathophysiology of posttraumatic secondary injury (Deng et al, 2007). Consistent with that possibility, we have previously showed that CsA attenuates peroxynitrite-induced oxidative damage to mitochondrial proteins (Mbye et al, 2008). However, this mechanistic explanation cannot play a role in NIM811's neuroprotective effects as the CsA analog does not inhibit calcineurin. Moreover, because the neuroprotective efficacy of NIM811 is comparable to that of CsA and the common mechanism of action between the two compounds is Cyp-D binding and preservation of mitochondrial function (Waldmeier et al, 2002), it is likely that mPTP inhibition is responsible for the neuroprotective activity of both compounds.
In summary, the findings in our earlier set of studies showing an impressive mitochondrial protective effect of NIM811 (Mbye et al, 2008), coupled with the current results showing the ability of NIM811 to fully duplicate the neuroprotective and motor functional efficacy of CsA support the conclusion that the mechanism of action for the two compounds involves a maintenance of mitochondrial bioenergetic integrity, and that calcineurin inhibition appears to play a little or no role. Furthermore, because of its lack of calcineurin inhibition and immunosuppressive properties (Waldmeier et al, 2002), NIM811 may provide a safer alternative for post-TBI neuroprotection than CsA.
Acknowledgements
This research was supported by funding from the NIH (1R01 NS046566, 1P30 NS051220) and the Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT).
References
- Albensi BC, Sullivan PG, Thompson MB, Scheff SW, Mattson MP. Cyclosporin ameliorates traumatic brain-injury-induced alterations of hippocampal synaptic plasticity. Exp Neurol. 2000;162:385–9. doi: 10.1006/exnr.1999.7338. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Rice AC, Levasseur J, DeFord M, Hamm RJ, Bullock MR. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J Neurotrauma. 2002;19:829–41. doi: 10.1089/08977150260190429. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–73. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Bernardi P. The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. doi: 10.1016/0005-2728(96)00041-2. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Povlishock JT. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J Neurotrauma. 1999a;16:511–21. doi: 10.1089/neu.1999.16.511. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–34. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Siman R, Trojanowski JQ, Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999b;58:365–75. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Part 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS, Beltramino CA, de Olmos de Lorenzo S. Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol Teratol. 1994;16:545–61. doi: 10.1016/0892-0362(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205:154–65. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–26. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Ferrand-Drake M, Zhu C, Gido G, Hansen AJ, Karlsson JO, Bahr BA, Zamzami N, Kroemer G, Chan PH, Wieloch T, Blomgren K. Cyclosporin A prevents calpain activation despite increased intracellular calcium concentrations, as well as translocation of apoptosis-inducing factor, cytochrome c and caspase-3 activation in neurons exposed to transient hypoglycemia. J Neurochem. 2003;85:1431–42. doi: 10.1046/j.1471-4159.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Hall ED, Andrus PK, Yonkers PA, Smith SL, Zhang JR, Taylor BM, Sun FF. Generation and detection of hydroxyl radical following experimental head injury. Ann N Y Acad Sci. 1994;738:15–24. doi: 10.1111/j.1749-6632.1994.tb21785.x. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–47. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Bolanos JP, Stewart VC, Brookes PS, Land JM, Clark JB. Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta. 1999;1410:215–28. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kupina NC, Detloff MR, Bobrowski WF, Snyder BJ, Hall ED. Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Kupina NC, Detloff MR, Dutta S, Hall ED. Neuroimmunophilin ligand V-10,367 is neuroprotective after 24-h delayed administration in a mouse model of diffuse traumatic brain injury. J Cereb Blood Flow Metab. 2002;22:1212–21. doi: 10.1097/01.wbc.0000037994.34930.bc. [DOI] [PubMed] [Google Scholar]
- Marik PE, Varon J, Trask T. Management of head trauma. Chest. 2002;122:699–711. doi: 10.1378/chest.122.2.699. [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Povlishock JT. Administration of the immunophilin ligand FK506 differentially attenuates neurofilament compaction and impaired axonal transport in injured axons following diffuse traumatic brain injury. Exp Neurol. 2006;197:353–62. doi: 10.1016/j.expneurol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, Sullivan PG, Springer JE, Hall ED. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp Neurol. 2008;209:243–53. doi: 10.1016/j.expneurol.2007.09.025. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–42. [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–44. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Saatman KE, Galvin JE, Scherbel U, Raghupathi R, Trojanowski JQ, McIntosh TK. Increased vulnerability of NFH-LacZ transgenic mouse to traumatic brain injury-induced behavioral deficits and cortical damage. J Cereb Blood Flow Metab. 1999;19:762–770. doi: 10.1097/00004647-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Buki A, Siman R, Povlishock JT. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport. 1999;10:353–8. doi: 10.1097/00001756-199902050-00026. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab. 1999;19:443–51. doi: 10.1097/00004647-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Pattison J, Sibley RK, Krensky AM. Immunologic Renal Diseases. Lippincott-Raven; Philadelphia: 1997. Mechanisms of allograft rejection; pp. 331–54. [Google Scholar]
- Povlishock JT, Buki A, Koiziumi H, Stone J, Okonkwo DO. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir Suppl. 1999;73:15–20. doi: 10.1007/978-3-7091-6391-7_3. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Fernandez SC, Murai H, Trusko SP, Scott RW, Nishioka WK, McIntosh TK. BCL-2 overexpression attenuates cortical cell loss after traumatic brain injury in transgenic mice. J Cereb Blood Flow Metab. 1998;18:1259–69. doi: 10.1097/00004647-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Lee NN, Povlishock JT. Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury. Brain Res. 2007;1154:225–36. doi: 10.1016/j.brainres.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996;55:850–60. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418–27. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16:783–92. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–18. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Stone JR, Okonkwo DO, Pellicane AJ, Povlishock JT. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J Neurotrauma. 2001;18:607–14. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Mattson MP, Scheff SW. Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J Neurotrauma. 1998;15:789–98. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience. 2000a;101:289–95. doi: 10.1016/s0306-4522(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson M, Scheff SW. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol. 2000b;161:631–7. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Switzer RC., III Application of silver degeneration stains for neurotoxicity testing. Toxicol Pathol. 2000;28:70–83. doi: 10.1177/019262330002800109. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–9. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]