Abstract
IL-13 has a prominent role in host defense against the gastrointestinal nematode Nippostrongylus brasiliensis; however, the role of IL-13Rα2 in the immune and functional response to enteric infection is not known. In the current study, we investigated changes in smooth muscle and epithelial cell function as well as alterations in gene expression of IL-13 and IL-4 and their receptors using laser-capture microdissection of specific cell types in the small intestine of N. brasiliensis-infected mice. An infection-induced up-regulation of IL-13Rα2 gene expression was confined to smooth muscle and was dependent on STAT6 and IL-13, but not on IL-4. In contrast, expression of IL-13Rα1 was reduced, indicating that changes in IL-13α2 expression serve to limit the biological effects of IL-13. The increased availability of IL-13 in IL-13Rα2−/− mice resulted in marked changes in constitutive epithelial and smooth muscle function. In addition, maximal changes in smooth muscle hypercontractility and epithelial cell resistance peaked earlier after infection in IL-13Rα2−/− compared with wild-type mice. This did not coincide with an earlier Th2 immune response as expression of IL-4 and IL-13 was attenuated in IL-13Rα2−/− mice and worm expulsion was similar to that of wild-type mice. These data show that IL-13Rα2 plays an important role in nematode infection by limiting the availability of IL-13 during infection, thereby regulating both the immune and biological effects of IL-13.
Interleukin 13 plays a key role in the pathogenesis of bronchial asthma and is also critical to host protection against enteric helminth infection. The enteric nematode Nippostrongylus brasiliensis induced a marked elevation of Th2 cytokines in systemic (1) as well as gut tissues (2, 3). Expulsion of N. brasiliensis, a rodent gastrointestinal nematode parasite, is dependent upon this dominant Th2 response with IL-13 having a greater role than IL-4 (4). The importance of IL-13 in N. brasiliensis infection is demonstrated further by the observation that N. brasiliensis are expelled in IL-4-deficient mice while clearance is delayed in IL-13-deficient mice (1). IL-4 and IL-13 bind to receptors on the cell surface of immune and structural cells, such as epithelial cells, smooth muscle cells, and enteric nerve cells. The biological activity of IL-13 is mediated by its binding to IL-13Rα1, which is part of the heterodimer complex with IL-4Rα on the cell surface that is linked to activation of STAT6 (5). The IL-13Rα2 chain also binds IL-13 and is proposed to act as a decoy receptor that limits the activity of IL-13 (6). The IL-13Rα2 receptor has a short cytoplasmic tail and is without signal motifs (6); although there is a report that IL-13 signals through this receptor in a STAT6-independent, AP-1-dependent manner to induce activation of the TGFB1 promoter (7). Mice lacking IL-13Rα2 show enhanced IL-13 responses and exacerbation of hepatic fibrosis induced by Schistosoma mansoni (8). In contrast, treatment with soluble IL-13Rα2-Fc fusion protein inhibited expulsion of N. brasiliensis (1), and the exacerbated IL-13-dependent fibrosis in S. mansoni-infected IL-13Rα2-deficient mice was reversed by sIL-13Rα2-Fc treatment (8). Soluble cytokine receptors can act as agonists or antagonists of cytokine-induced inflammation and immune responses (9). Further information on the biological action of IL-13 and IL-13Rα2, therefore, is needed to determine the interaction of this cytokine and decoy receptor during the Th2 response to nematode infection or inflammatory disease in the gut.
We previously reported that there were region- and cell- specific differences along the gut in the expression of IL-13Rα2 (10). These data also showed that IL-13Rα2 played a physiological role in the regulation of endogenous IL-13 on smooth muscle contraction. Indeed, IL-13 mediates the hypercontractility induced by N. brasiliensis infection by a mechanism that is dependent on both STAT6 and alternatively activated macrophages (3, 11). We also showed previously that there are important functional and regional differences in the distribution of Th2 cytokine receptor expression both in small intestine and colon (10) that regulate the physiological and pathological activity of IL-4 and IL-13. Changes in the functional distribution and expression of IL-13 receptors during nematode infection and their role in controlling the effects of IL-13 on smooth muscle function are unknown. The aim of this study was to determine the contribution of IL-13Rα2 to infection-induced alterations in intestinal function. To address this, we investigated 1) the role of IL-4, IL-13, and STAT6 in the regulation of IL-13Rα2 expression and 2) the function of IL-13Rα2 on smooth muscle and epithelial cell during an infection with N. brasiliensis. Our results indicate that IL-13Rα2 plays an important role in nematode infection by limiting the availability of IL-13 during infection, thereby regulating both the immune and biological effects of IL-13.
Materials and Methods
Animals and treatments
BALB/c mice 8- to 12-wk-old (National Cancer Institute, Frederick, MD) or BALB/c mice genetically deficient in expression of IL-4 (IL-4−/−), IL-13 (IL-13−/−), Stat6 (STAT6−/−), or IL-13Rα2 (IL-13Rα2−/−; National Institute of Allergy and Infectious Diseases/Taconic Farms) were used for each experiment. The preparation and the s.c. inoculation of mice with 500 infective third-stage N. brasiliensis larvae (L3) were performed as described previously (12). Adult worm egg production was determined as described previously (12), but total adult worms were also detected qualitatively by scanning the intestinal surface with a dissecting microscope to preserve the tissue for appropriate physiological analysis. In general, mice normally expel worms by day 9 after inoculation. Some groups of mice received 10 μg/mouse of IL-13 (R&D Systems) daily for 7 days or an equal volume of normal saline and studied 7 days after the initial injection. All studies were conducted in accordance with principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Health and Human Services Publication (National Institutes of Health 85-23, revised 1996), and the Beltsville Animal Care and Use Committee 2003.
Preparation of tissue, frozen blocks, and sectioning for laser-capture microdissection (LCM)5
Sections of jejunum and mid-colon tissues were taken from each mouse at the end of the experiment. One small piece was homogenized in TRIzol (Invitrogen) and stored in −80°C until needed while the other piece was slit longitudinally, laid flat with the mucosal surface down, rolled around wood stick (Swiss-roll), and embedded in Tissue-Tek OCT compound (Sakura Finetek) in a cryomold. The tissues were frozen on dry ice-acetone, then removed from cryomold and placed at −80°C in an airtight container until sectioned. Four-micrometer tissue sections used for LCM were obtained from frozen blocks using plain uncoated slides and a HM505E cryostat (Richard-Allan Scientific); the slides were kept on dry ice immediately and stored at −80°C until needed. Sections of frozen tissue were stained with H&E to assess changes in tissue morphology. Cryosectioned tissue was stained with H&E and dehydrated and LCM was performed with a PicCell II (Arcturus Engineering) (13). Cells were captured from the region of the epithelium and muscles in small intestine and transferred to CapSure LCM Caps (Arcturus Engineering).
Gene expression analysis (RNA extraction, reverse transcription, and real-time PCR)
Total RNA extraction from whole tissue and from LCM samples and reverse transcription was performed as described previously (13, 14). Primers and probes for IL-13, IL-4, STAT6, IL-4Rα, IL-13Rα1, and IL-13Rα2 were designed using Primer Express software (Applied Biosystems) with sequences obtained from GenBank (10). Real-time PCR was performed using the SYBR Green Supermix (Bio-Rad) with iCycler (Bio-Rad). Amplification conditions were as follows: 95°C for 3 min; 40–50 cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 20 s. Fluorescence signals measured during amplification were processed after amplification. Ribosomal RNA primers were utilized and all data were normalized to constitutive ribosomal RNA values. Quantitative differences between groups were calculated according to the manufacturer’s instructions.
Smooth muscle function
Segments of mid-jejunum (1 cm) were flushed of their intestinal contents, suspended longitudinally in individual 8-ml organ baths, and maintained in oxygenated Krebs’ solution at 37°C. One end of the tissue was attached to an isometric tension transducer (model FT03; Grass Medical Instruments) and the other to the bottom of the bath. Tissues were stretched to a load of 9.9 mN (2 g). Preliminary experiments showed that this load stretched tissues to their optimal length for active contraction. Tissues were allowed to equilibrate for at least 30 min in Krebs’ buffer solution before constructing response curves to acetylcholine (1 nM-100 μM) or electric field stimulation (EFS; 1–20 Hz, 80 V). The amplitude of spontaneous contractions was measured in a 2-min period after tissue equilibration. The bath solution was replaced every 10 min throughout each study. Tension was recorded using a Grass model 79 polygraph (Grass Medical Instruments) and expressed as force per cross-sectional area (2, 3, 11).
Epithelial cell function
To determine changes in epithelial tissue resistance, muscle-free segments of small intestinal or colonic mucosae were taken from the small intestine from uninfected and N. brasiliensis-infected wild-type (WT) and IL-13Rα2−/− mice and mounted in micro-snap wells. Transepithelial electrical resistance (TEER) was measured at time 0 and at 30-min time intervals for a period of 2 h using a planar electrode (Endohm SNAP electrode attached to an Evom-G WPI analyzer; World Precision Instruments) and expressed in Ohms per cm2.
To measure glucose absorption, 1-cm segments of intestinal mucosa were stripped of muscle and mounted in Ussing chambers that exposed 0.126 cm2 to 10 ml of Krebs’ buffer. Potential difference was measured using agar-salt bridges and electrodes. Every 50 s, the tissue was short circuited at 1 V (World Precision Instruments DVC 1000 voltage clamp) and the short circuit current (Isc) was monitored continuously. Following a 15-min equilibration period, concentration-dependent (0.625–40 mM) changes in Isc were measured in response to the cumulative addition of glucose to the mucosal side. Responses from all tissue segments exposed to glucose from an individual mouse were averaged to yield a mean response per animal.
Statistics
Concentration-dependent responses in epithelium and smooth muscle were compared using MANOVA (Systat 5.2) with post hoc analysis for multiple comparisons. A p < 0.05 was considered significant. One-way ANOVA followed by Tukey’s test were used to compare TEER and mRNA expression among the different treatment groups. Appropriate vehicle and time-and age-matched controls were performed for each group (n = 3–5 each group, unless indicated otherwise). Experiments were repeated at least once to ensure consistency among the results.
Results
IL-4 and IL-13 receptor expression after infection with N. brasiliensis
At 9 days after inoculation, there was a small, but significant, increase in mRNA expression for IL-4Rα (Fig. 1A), IL-13Rα1 gene expression was decreased by >50% (Fig. 1B), and there was a 5-fold increase in IL-13Rα2 (Fig. 1C). IFN-γ mRNA expression was not changed significantly by infection (1.00 ± 0.09 on day 7; 1.52 ± 0.24 on day 9).
FIGURE 1.
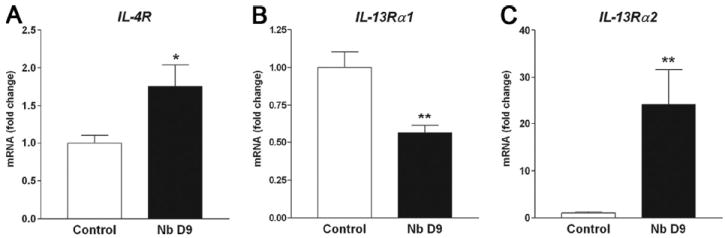
Gene expression of Th2 cytokine receptors, IL-4Rα (A), IL-13Rα1 (B), and IL-13Rα2 (C) in whole intestine from uninfected control and N. brasiliensis (Nb)-infected WT mice measured by real-time PCR (n = 5). All data are expressed relative to uninfected control. *, p < 0.05 and **, p < 0.01 vs uninfected control.
Localization of Th2 cytokine receptors using LCM
It is unclear how IL-4 and IL-13 induce immunological and physiological responses in small intestine during N. brasiliensis infection; therefore, localization and level of expression of Th2 cytokine receptors are of interest. We showed previously that the constitutive expression level of IL-4Rα and IL-13Rα1 was similar in epithelium, smooth muscle, and myenteric plexus in the small intestine (10). In contrast, the expression of IL-13Rα2 was very low with detectable levels only in smooth muscle. To identify the microenvironments in which IL-13Rα2 expression is up-regulated in N. brasiliensis-infected mice, we used LCM to isolate epithelial and smooth muscle from sections of small intestine. We first analyzed the mRNA expression of the specific molecular marker for epithelial, smooth muscle, and immune cells on respective LCM samples to confirm their purities. In LCM smooth muscle cells, the mRNA expression level of villin, a specific marker for epithelial cell, was only 2% of that in LCM epithelial cells. In LCM epithelial cells, the mRNA expression level of α smooth muscle actin, a specific marker for smooth muscle, was only 0.1% of that in LCM smooth muscle cells. In addition, the expression of leukocyte common Ag (a general marker for immune cells) was 3% in LCM epithelial cells and 14% in LCM smooth muscle cells, relative to LCM lamina propria. The data confirmed that each of our LCM samples primarily contain the specific cell population of interest. IL-4Rα gene expression in epithelium (Fig. 2A) and muscle (Fig. 2B) in N. brasiliensis-infected mice were similar to control uninfected mice. IL-13Rα1 expression was also unchanged in epithelium, but was decreased significantly in smooth muscle. In contrast to IL-4Rα and IL-13Rα1, IL-13Rα2 expression was not detected in epithelium after infection, but was up-regulated significantly in the smooth muscle. These data show that infection alters expression of receptors that specifically bind IL-13.
FIGURE 2.
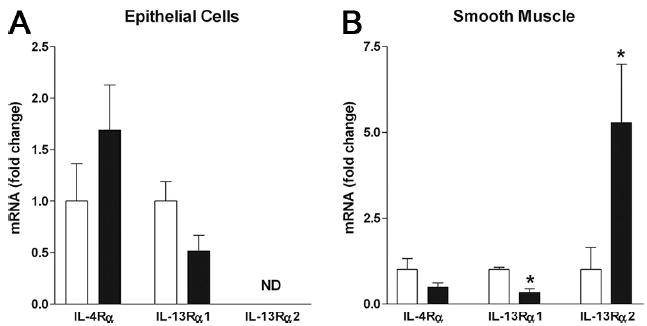
Localization of Th2 cytokine receptors in N. brasiliensis-infected intestine using LCM (n ≥ 4) to show expression in epithelium (A) and smooth muscle (B). □, Uninfected; ■, day 9 after N. brasiliensis inoculation. *, p < 0.05 vs uninfected control; ND, not detectable.
Th2 pathway regulation of IL-13Rα2 gene expression in intestinal smooth muscle
Th2 regulation of IL-13Rα2 gene expression was evaluated in IL-4−/−, IL-13−/−, and STAT6−/− mice 9 days after inoculation with N. brasiliensis. IL-13Rα2 gene was expressed constitutively only in smooth muscle cells captured with LCM followed by real-time PCR. All data were normalized to ribosomal RNA and expressed relative to muscle from uninfected WT mice. Infection up-regulated IL-13Rα2 gene expression in IL-4−/− mice to levels similar to those in WT. In contrast, IL-13Rα2 gene expression was markedly decreased in IL-13−/− mice and completely inhibited in STAT6−/− mice (Fig. 3A).
FIGURE 3.
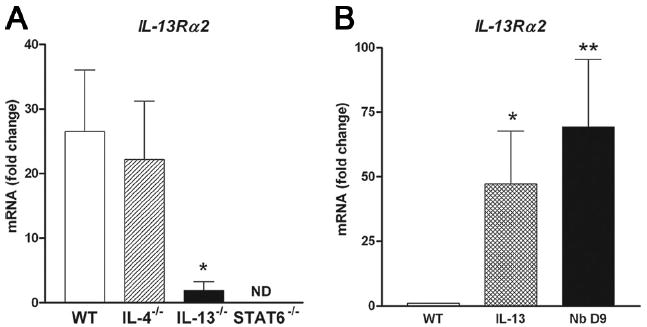
A, IL-13Rα2 gene expression in the smooth muscle cells at day 9 after inoculation with N. brasiliensis (Nb) in the small intestine of WT, IL-4−/−, IL-13−/−, and STAT6−/− (n ≥ 3) mice using LCM followed by real-time PCR. All data are expressed relative to WT uninfected muscle. *, p < 0.01 vs infected WT control. B, IL-13Rα2 expression in mice treated for 6 days with IL-13 (10 μg/mouse) and studied on day 7 or mice studied 9 days after inoculation with N. brasiliensis. *, p < 0.05 and **, p < 0.01 vs WT control.
To determine whether IL-13 alone could alter IL-13Rα2 gene expression, we treated WT mice with IL-13 for 6 days and collected tissue at day 7. Exogenous IL-13 significantly increased IL-13Rα2 expression in the small intestine (whole tissue) in uninfected WT mice (Fig. 3B), indicating that expression of IL-13Rα2 is regulated by IL-13 through STAT6 signaling.
IL-13Rα2 deficiency alters N. brasiliensis-induced up-regulation of Th2 cytokine expression, but not protective immunity
To examine the contribution of IL-13Rα2 to the host defense against N. brasiliensis, the expression of Th2 cytokines and their receptors was assessed in full-thickness sections of the small intestine from WT and IL-13Rα2−/− mice. In WT mice, IL-4 and IL-13 gene expression peaked at day 7 and was lower at day 9, although the levels remained significantly elevated above controls. In contrast, the up-regulation of IL-4 gene expression was attenuated significantly at day 7 and 9 after inoculation with N. brasiliensis in IL-13Rα2−/− mice when compared with WT mice (Fig. 4A). IL-13 gene expression in IL-13Rα2−/− mice was similar to that of WT mice at day 7 (Fig. 4B), but was reduced significantly from WT at day 9 after inoculation. There was a similar level of expression of IFN-γ, IL-4Rα, IL-13Rα1, and STAT6 in control and N. brasiliensis-infected WT and IL-13Rα2−/− mice (data not shown). Infection also caused a modest up-regulation in TGF-β expression in WT mice that was not appreciably different from expression in IL-13Rα2−/− mice (Table I).
FIGURE 4.
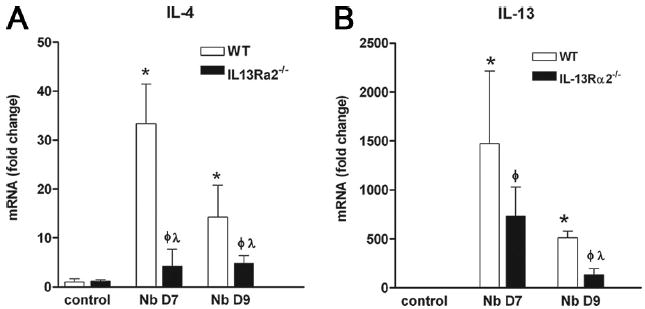
IL-4 (A) and IL-13 (B) gene expression in control uninfected WT and IL-13Rα2−/− mice and at days 7 and 9 after inoculation with N. brasiliensis (Nb). □, WT; ■, IL-13Rα2−/− mice. *, p < 0.05 vs WT control; ϕ, p < 0.05 vs control IL-13Rα2−/− and λ, p < 0.05 vs respective WT.
Table I.
TGF-β gene expression in small intestine in response to N. brasiliensis infection
| Control | Nb D7 | Nb D9 | |
|---|---|---|---|
| WT | 1.0 ± 0.15 | 3.3 ± 0.9 | 3.3 ± 0.31 |
| IL-13Rα2−/− | 1.4 ± 0.08 | 2.2 ± 0.25 | 2.4 ± 0.29 |
To determine whether the changes in Th2 cytokine expression in IL-13Rα2−/− mice altered protective immunity, worm and egg counts were performed in WT and IL-13Rα2−/− mice at days 7 and 9 after infection. Both strains had a similar number and distribution of adult worms in the small intestine at day 7 by visual observation and had no worms in the small intestine by day 9. There were no significant differences in fecal egg counts at day 7 or 9 between the two strains. A separate set of WT and IL-13Rα2−/− mice tested only for quantitative counts of adult worms and eggs at day 7 after inoculation had similar numbers, indicating no appreciable change in migration of N. brasiliensis larvae to the small intestine (data not shown). These data suggest that larval migration and adult clearance of N. brasiliensis is unabated despite the reduced expression of IL-4 (Fig. 4A), the increased availability of IL-13 due to the lack of IL-13Rα2 in the deficient mice. In addition, the absence of IL-13Rα2 does not alter gene expression of TGF-β.
Smooth muscle function in naive and N. brasiliensis-infected IL-13Rα2−/− mice
To investigate whether IL-13Rα2 is involved in the control of intestinal smooth muscle function during nematode infection, groups of WT and IL-13Rα2−/− mice were infected with N. brasiliensis and examined at days 7 and 9 after inoculation. Smooth muscle responses to acetylcholine were significantly higher in naive IL-13Rα2−/− mice than in WT mice, consistent with our previous findings (Fig. 5) (10). Of interest was that constitutive responses to EFS were similar in both WT and IL-13Rα2−/− mice, suggesting that the increased availability of IL-13 in the uninfected IL-13Rα2−/− mice altered responses at the level of smooth muscle rather than enteric nerves. In WT mice, responses to acetylcholine were elevated by day 7 after inoculation and were maximal at day 9 (Fig. 5A). Responses to EFS remained similar to vehicle-treated mice at day 7 after inoculation, but were increased significantly by day 9 (Fig. 5B). In IL-13Rα2−/− mice, however, the intestinal smooth muscle responses to both acetylcholine and EFS peaked at day 7 after inoculation (Fig. 5, C and D).
FIGURE 5.
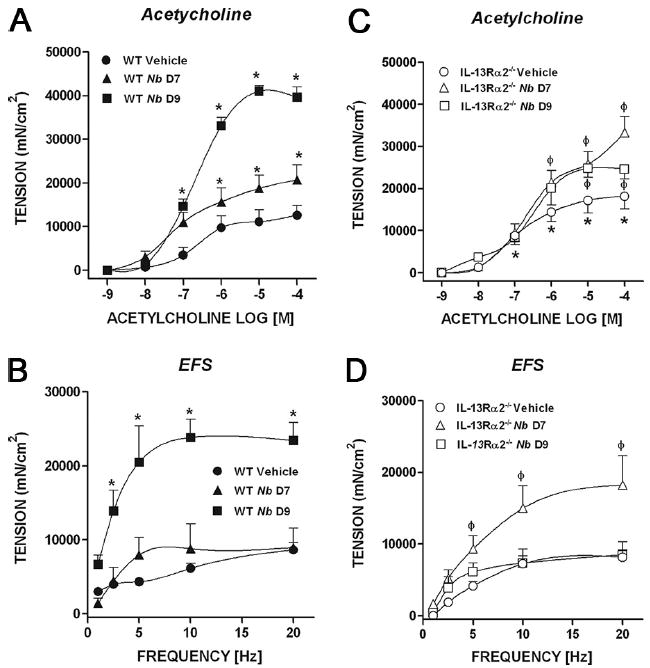
Concentration-dependent in vitro smooth muscle responses to acetylcholine (A and C) and frequency-dependent responses (EFS) to nerve stimulation (B and D) in both WT mice (A and B) and IL-13Rα2−/− mice (C and D). *, p < 0.05 vs WT vehicle and ϕ, p < 0.05 vs IL-13Rα2−/− vehicle. Nb, N. brasiliensis.
Epithelial function in naive and N. brasiliensis-infected IL-13Rα2−/− mice
To determine the role of IL-13Rα2 in the epithelial response to nematode infection, WT and IL-13Rα2−/− mice were inoculated with N. brasiliensis and studied 7 and 9 days later. Changes in epithelial resistance were determined by TEER, an index of epithelial paracellular permeability. We reported earlier that N. brasiliensis infection increased intestinal mucosal permeability measured in Ussing chambers, and this effect was mimicked by administration of exogenous IL-13 (15, 16). In the present study, there was a significant decrease in TEER in the small intestine of vehicle (media)-treated IL-13Rα2−/− mice compared with vehicle-treated WT mice (Fig. 6), suggesting a constitutive effect on normal barrier function as a result of the increased availability of IL-13 in mice lacking IL-13Rα2. This effect appeared to be region-specific since there were no significant changes in TEER in the colon of WT and IL-13Rα2−/− mice (18.8 ± 0.5 vs 14.4 ± 1.6 Ω · cm2, respectively). The TEER measurements of small intestine taken from WT and IL-13Rα2−/− mice at 7 and 9 days after inoculation with N. brasiliensis were decreased significantly from that of their respective uninfected and vehicle-treated control mice (Fig. 6).
FIGURE 6.
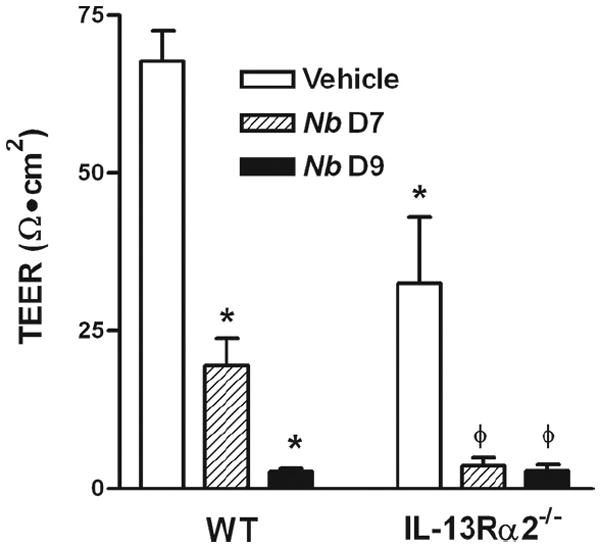
TEER in muscle-free mucosa mounted in micro-snap wells in uninfected (vehicle) WT and IL-13Rα2−/− mice (n = 5–7) as well as days 7 and 9 after inoculation with N. brasiliensis (Nb). TEER values are at 60 min after mounting. *, p < 0.05 vs WT vehicle and ϕ, p < 0.05 vs IL-13Rα2−/− vehicle.
Another stereotypic feature of enteric nematode infection is reduced sodium-linked glucose absorption that is also mimicked by exogenous IL-13 administration (15, 16). The constitutive level of glucose absorption in uninfected vehicle-treated IL-13Rα2−/− mice was reduced relative to that of uninfected WT mice (Fig. 7, A vs B), suggesting a constitutive reduction in epithelial glucose absorption due to the elevated levels of IL-13 circulating in the absence of functional IL-13Rα2 receptors. In WT mice, there was a significant decrease in sodium-linked glucose absorption at day 7 after inoculation that was further decreased at day 9. In the IL-13Rα2 receptor-deficient mice, there was an almost complete inhibition of glucose absorption at days 7 and 9 after inoculation with N. brasiliensis.
FIGURE 7.
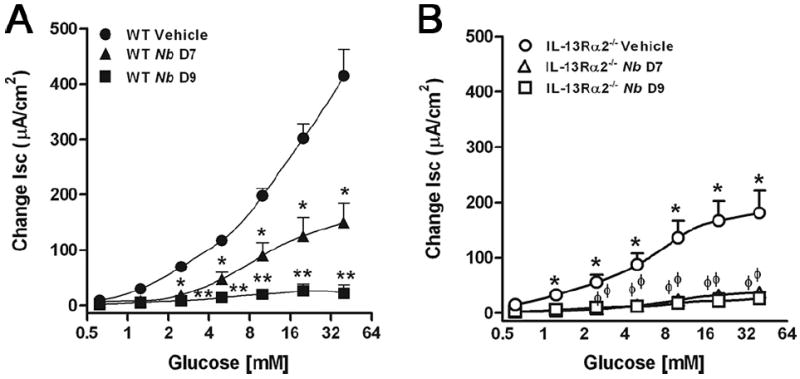
Concentration-dependent changes in sodium-linked glucose absorption in muscle-free mucosa mounted in Ussing chambers in uninfected (vehicle) WT (A) and IL-13Rα2−/− (B) mice (n = 5–7) as well as days 7 and 9 after inoculation with N. brasiliensis. Values represent changes in short circuit current (Isc). *, p < 0.05 vs WT vehicle and ϕ, p < 0.05 vs IL-13Rα2−/− vehicle.
Discussion
Th2 immune responses are associated with atopic disease, such as allergy and asthma, as well as enteric nematode infection. It is well known that Th2 cytokines regulate expansion of Th1 cytokines and vice versa to maintain homeostasis in vivo and the imbalance between the Th1 and Th2 profiles is implicated in a number of gastrointestinal pathologies. Recent reports propose a role for Th2 cytokines in pathogenesis of inflammatory bowel diseases with dysregulation of IL-13 emerging as a key factor in the development of chronic mucosal inflammation in animal models (17, 18). This further emphasizes the importance of determining the mechanisms involved in the regulation of Th2 cytokine expression as well as the role of their receptors in biological effects of Th2 cytokines on gut function. N. brasiliensis is a well-studied enteric nematode parasite that induces polarized Th2 responses characterized by elevation of IgE and Th2 cytokines such as IL-4, IL-5, and IL-13. IL-4 and IL-13 share receptor components, in part, and their biological effects are linked to STAT6 signaling. IL-13, IL-4Rα, and STAT6 are all required for the expulsion of N. brasiliensis (1). In this study, we showed for the first time that IL-13Rα2 has a constitutive role in the regulation of the effects of IL-13 on intestinal epithelial function. In addition, we observed that the IL-13Rα2 contributes to the development of Th2-mediated immunity as well as to the control of the biological effects of IL-13 on intestinal function.
There are a number of cytokines with soluble receptors that act as agonists or antagonists to attenuate or promote cytokine signaling and to regulate inflammation and immunity (9, 19, 20). There is considerable evidence that IL-13Rα2 acts as a decoy receptor. There are also reports that in the presence of TNF-α, IL-13 binding to IL-13Rα2 signals though AP-1 to increase TGF-β1 expression in monocytes and macrophages and promote fibrosis (7). In the current study, IL-13Rα2 gene expression was highly up-regulated in N. brasiliensis-infected WT mice and was dependent on both IL-13 and STAT6, but not IL-4. Previous studies report that both IL-4 and IL-13 increased levels of IL-13Rα2 based on studies using exogenous IL-4 or IL-13 in WT, IL-4Rα−/−, and STAT6−/− mice (8, 20). In the present study, we used whole intestinal tissue from WT and mice deficient in IL-4, IL-13, and STAT6 genes to study IL-13Rα2 gene expression. Although we found that IL-13Rα2 was independent of IL-4 gene expression, the requirement for STAT6 is consistent with the requirement of an intact type 2 IL-4R for N. brasiliensis-induced up-regulation of IL-13Rα2 in the small intestine. We did not find evidence for IL-13Rα2-dependent TGF-β1 gene expression because the increased bioavailability of IL-13 in both uninfected and N. brasiliensis-infected IL-13Rα2−/− mice did not lead to changes in TGF-β1 compared with levels induced in WT mice. This is consistent with previous reports showing that IL-13 can induce liver fibrosis independently of TGF-β (21).
We demonstrated previously the location and distribution of Th2 cytokine receptors expressed in intact gastrointestinal tract using LCM (10). Although IL-13 is known to be essential for worm expulsion, infection-induced changes in the distribution and expression of Th2 cytokine receptors in the gut tissues are not known. The IL-13Rα1-binding chain forms a high-affinity receptor for IL-13 when coexpressed with IL-4-Rα. IL-4Rα gene expression was up-regulated in whole tissue, but not in structural cells, suggesting that the increased expression is attributed to the type 1 receptor in hematopoietic cells. Infection with N. brasiliensis significantly down-regulated IL-13Rα1 gene expression in whole tissue as well in smooth muscle, but expression was unaltered in epithelial cells. We showed previously that there is a regional- and cell-specific distribution of IL-13Rα2 along the gut with a constitutive expression only in smooth muscle (10). Infection markedly increased IL-13Rα2 expression in smooth muscle cells while there was no IL-13Rα2 expression detected in epithelial cells, even after infection. Receptor expression, therefore, is important to the coordination and localization of the immune response and the biological activity of IL-13. The infection-induced changes in IL-13α1 receptor expression may serve to limit the effects of IL-13 acting at the type 2 IL-4R to avoid exaggerated responses. Simultaneously, the increase in IL-13Rα2 expression serves to limit the biological availability of IL-13 to exert its effects through the IL-13Rα1. These changes appear to be particularly important for regulating the effects of IL-13 on smooth muscle, confining the activity to this cell type, and/or sequestering IL-13 away from other cells such as epithelial cells.
We showed earlier that there was a increased contractility to acetylcholine in IL-13Rα2−/− mice, indicating that the receptor has a constitutive role in limiting the effects of IL-13 on intestinal and colonic smooth muscle contractility (10). Of interest is the exclusive expression of IL-13Rα2 in smooth muscle during N. brasiliensis infection, suggesting a specific need to regulate the biological effects of IL-13 in this cell type. When compared with WT, N. brasiliensis-infected IL-13Rα2−/− mice exhibited an earlier increase of contractility to both acetylcholine and nerve stimulation that is consistent with an increased availability of IL-13. This demonstrated that IL-13Rα2 plays a major role controlling the effects of IL-13 on smooth muscle function both constitutively and during a gastrointestinal nematode infection.
Nematode infection also induces stereotypic STAT6-dependent changes in epithelial cell function including increased mucosal permeability and inhibition of sodium-linked glucose absorption (16, 22). This impaired resistance and absorption contribute to the net increase in intraluminal fluid that helps facilitate worm expulsion. IL-13 has been identified as a critical effector cytokine for epithelial permeability in inflammatory bowel disease (23). The increased availability of IL-13 in IL-13Rα2−/− mice resulted in a constitutive decrease in tissue resistance in the small intestine, but not in the colon, indicating a regional specificity of IL-13. The lack of an effect of IL-13 on colonic mucosal permeability in vivo is at odds with previous data showing that IL-13 impaired epithelial barrier function in the HT29 colorectal cancer cell line (18). This is likely due to differences in the effects of IL-13 in an integrated explanted tissue system with multiple cell types vs monolayer cell cultures or that colonic cells may not act like small intestinal cells, which exhibit a marked decrease in resistance in response to IL-13 (15). The significant decrease in resistance and glucose absorption in the small intestine of control IL-13Rα2−/− mice indicate that increased availability of IL-13 markedly affects epithelial function. This is important given the proposed role of soluble IL-13Rα2 as a mechanism to localize the effects of IL-13 to the site of secretion (20) or to provide a reservoir for IL-13 that may extend the duration of its activity.
N. brasiliensis induces a strong Th2 response and it is known that IL-13 and IL-4 contribute to expulsion of worms. In the present study, IL-13 gene expression was highly up-regulated compared with IL-4, consistent with the higher IL-13 production reported in other infections (24). In contrast, expression of both IL-4 and IL-13 were markedly lower in mice lacking the IL-13Rα2, supporting a role for this receptor in controlling the Th2 immune response. The increased availability of IL-13 in the absence of IL-13Rα2 may act to “jump start” the immune response evidenced by the earlier onset and greater intensity of the physiological responses of structural cells in the intestine. Surprisingly, this did not coincide with changes in larval migration to the intestine at day 7 after inoculation or earlier worm expulsion from the intestine. The attenuated IL-4 and IL-13 expression in IL-13Rα2−/− mice in response to infection, however, suggests that the constitutively elevated levels of IL-13 cytokines impact both the timing and/or magnitude of the Th2 response to N. brasiliensis infection even if worm expulsion is not markedly altered. These results differ from the dominant role of IL-13Rα2 in limiting the IL-13-mediated pathological responses of IL-13 in S. mansoni infection (8), suggesting that increased availability of IL-13 in N. brasiliensis infection primarily augments IL-13 effector functions. Nevertheless, persistent and increased intraluminal fluid and decreased glucose absorption following nematode infection of the intestine in IL-13Rα2−/− mice would likely compromise the longer term nutritional and metabolic activity of these mice compared with intact mice that more quickly sequester IL-13 following its contribution to worm expulsion and resume normal nutrient absorption.
In conclusion, Th2 cytokines and their receptors are important for the orchestration of cytokine effects on structural cells that play a key role in host defense. IL-13Rα2 gene expression was dependent on both IL-13 and STAT6, but not on IL-4 gene expression. The absence of the IL-13Rα2 blunted the up-regulation of both IL-4 and IL-13 gene expression, demonstrating that the increased availability of IL-13 can influence the timing as well as the magnitude of the IL-13-mediated actions on effector cells without altering larval migration and worm clearance. The specific expression of IL-13Rα2 in smooth muscle in both uninfected and N. brasiliensis-infected mice emphasized the important role of controlling IL-13 activity given its marked effects on smooth muscle and its role in fibrosis. IL-13Rα2 could have therapeutic importance because it could regulate IL-13-dependent effects on allergy or several inflammatory diseases. The present study is the first to demonstrate changes in both the expression and distribution of Th2 cytokines and their receptors that can be linked to N. brasiliensis-induced alterations in gut function.
Footnotes
This work was supported by National Institutes of Health Grants R01-AI/DK49316 (to T.S.-D), by U.S. Department of Agriculture CRIS Project 1235-52000-053 (to J.F.U.), by Miyagi University Overseas Research Fund (to M.M.), and by the intramural research program of National Institute of Allergy and Infectious Diseases/National Institutes of Health.
The opinions and assertions in this article are those of the authors and do not necessarily represent those of the U.S. Department of Agriculture or Department of Defense.
Abbreviations used in this paper: LCM, laser-capture microdissection; EFS, electric field stimulation; WT, wild type; TEER, transepithelial electrical resistance.
Disclosures The authors have no financial conflict of interest.
References
- 1.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite. Nippostrongylus brasiliensis Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune regulation of protease-activated receptor-1 expression in murine small intestine during Nippostrongylus brasiliensis infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao A, Urban JF, Jr, Anthony RM, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135:217–225. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 5.Zurawski SM, Chomarat P, Djossou O, Bidaud C, McKenzie ANJ, Miossec P, Banchereau J, Zurawski G. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–13878. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O’Hara RM, Jr, Beier DR, Turner KJ, Wood CR, Collins M. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor α1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 7.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 8.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ, Wynn TA. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. 2008;283:14177–14181. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto M, Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, Urban JF, Jr, Wynn TA, Shea-Donohue T. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 12.Urban JF, Jr, Madden KB, Cheever AW, Trotta PP, Katona IM, Finkelman FD. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus brasiliensis. J Immunol. 1993;151:7086–7094. [PubMed] [Google Scholar]
- 13.Morimoto M, Morimoto M, Whitmire J, Xiao S, Anthony RM, Mirakami H, Star RA, Urban JF, Jr, Gause WC. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto M, Morimoto M, Whitmire J, Star RA, Urban JF, Jr, Gause WC. In situ localization of gene expression using laser capture microdissection. In: Dieffenbach CW, Dveksler GS, editors. PCR Primer. 2. Cold Spring Harbor Lab. Press; Plainview, NY: 2003. pp. 135–148. [Google Scholar]
- 15.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 16.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 17.Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. Induction of IL-13 triggers TGF-β1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 19.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 20.Khodoun M, Lewis C, Yang JQ, Orekov T, Potter C, Wynn T, Mentink-Kane M, Khurana Hershey GK, Wills-Karp M, Finkelman FD. Differences in expression, affinity, and function of soluble (s)IL-4Rα and sIL-13Rα2 suggest opposite effects on allergic responses. J Immunol. 2007;179:6429–6438. doi: 10.4049/jimmunol.179.10.6429. [DOI] [PubMed] [Google Scholar]
- 21.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 22.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF., Jr The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 23.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]


