Abstract
Objective
These studies were undertaken to characterize the subcellular localization of the two major isoforms of progesterone receptors (PR), PRA and PRB, in endometrial cancer.
Methods
Immunohistochemistry, immunoprecipitation, and confocal microscopy were performed using Hec50co and KLE endometrial cancer cell models expressing PRA or PRB as a consequence of transduction. The location of PRB compared to PRA was determined, and antibodies were tested for specificity with respect to PR isoform recognition. Immunohistochemical analyses of PR expression and subcellular compartmentalization were also performed on 20 formalin-fixed endometrial cancer tumors.
Results
Morphological and biochemical evaluations demonstrated that PRA is localized to the nucleus, even in the absence of progesterone. In contrast, a large proportion of PRB is cytoplasmic in the absence of ligand, but is rapidly translocated to the nucleus in the presence of progesterone. The differential distribution of PRA and PRB proved to be a hallmark of malignant and nonmalignant epithelia in 20 samples of archival endometrial tissue from women with the pre-operative diagnosis of endometrial cancer. All endometrial cancer specimens demonstrated cytoplasmic PRB in 50% or more of the cells, and five of the seven tumors that were moderately to poorly differentiated demonstrated no PRB staining in the nuclei. Nuclear PRB was significantly associated with increasing tumor differentiation (P = 0.031).
Conclusion
In the absence of ligand, PRA is nuclear and PRB is largely cytoplasmic. This suggests that PRA may exert ligandindependent nuclear effects, while PRB may have nongenomic cytoplasmic actions in endometrial cancer cells.
Keywords: Uterus, Endometrium, Estrogen, Progesterone, Receptors, Human, Trafficking
Introduction
Carcinoma of the uterine endometrium is the most common malignancy of the female genital tract and the fourth most common site of cancer in women. Numerous studies document that endometrial cancer is associated with estrogen-induced growth stimulation unopposed by the differentiating effects of progesterone: progesterone inhibits endometrial proliferation and can reverse endometrial hyperplasia [1]. Estrogen and progesterone act through intra-nuclear receptors, ER and PR, which belong to the superfamily of steroid hormone receptors. The expression of ER and PR are linked because transcription of the PR gene is induced by estrogen and inhibited by progestins [2,3]. PR is expressed as two major isoforms, PRA and PRB, which arise from alternative transcriptional start sites within the same gene. PRB is the full-length transcript encoding 933 amino acids. PRA encodes 769 amino acids and is identical to PRB except that it lacks the first 164 amino acids of the N-terminus (Fig. 1) [4,5]. Though initially thought to occur in equimolar amounts, studies have now demonstrated that PRA and PRB are differentially expressed and may be functionally distinct [6,7]. The unique N-termini of PRA and PRB confer different functional characteristics on the isoforms: PRB is a stronger transcriptional activator of many genes compared to PRA [7-9], but PRA counters estrogen action directly by inhibiting ER function in a dominant-negative manner [10]. In endometrial cancer cell lines, both isoforms function to enhance differentiation, with PRA inducing cell senescence and PRB inducing a secretory phenotype. Both isoforms sensitize endometrial cancer cells to apoptosis and inhibit the cell cycle at the G1 to S transition [11]. However, with respect to growth inhibition, PRB appears to have the most substantial effects in human endometrial cancer cells grown in vitro [11,12]. PRB is lost in poorly differentiated endometrial cancer cell lines such as Hec50 and KLE [7], suggesting that this isoform is important for maintenance of endometrial differentiation [7,8], and endometrial cancers appear to down-regulate PRA and PRB [13] or only PRA [14].
Fig. 1.
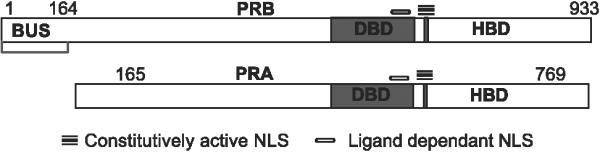
Schematic representation of PRB and PRA with nuclear localization signals (NLS). The NLS in PR is a large region extending over the hinge region and the second zinc finger. The C-terminal region is constitutively active and the N-terminal region is active only in the presence of ligand or by deletion of the hormone binding domain (HBD). DBD = DNA binding domain; BUS = B upstream segment unique to PRB.
In PR knockout mice models, PRB induces cell growth in the absence of PRA [15]. Mice deficient in both isoforms of PR, as well as those deficient in only the A isoform, demonstrate endometrial proliferation [16]. In this model, progesterone treatment causes endometrial growth through PRB in the absence of PRA. While these studies are of interest and require further consideration, differences between rodents and humans must be taken into account. Rodents do not develop endometrial cancers, and the proliferative effects of progestin through PRB do not appear to be malignant in this model, indicating differences between the potential of endometrial cells to respond to hormone stimulation between the species. Nevertheless, the possibility that PRB has proliferative effects in humans has not been entirely excluded by any studies published to date.
Further studies on the expression of PRA relative to PRB and other hormone-dependent genes that may be biomarkers of clinical response to therapy are warranted to clarify the role of receptor expression and endometrial cancer recurrence. Studies now indicate that commercially available antibodies may not recognize PRA and PRB with equal affinity by immunohistochemistry (IHC) despite findings to the contrary using immunoblotting. Mote et al. [17] have found that PRB is not recognized by many commonly used antibodies, raising the possibility that PRB expression may have been under-reported in the past. In these studies, we confirm the findings of others with respect to differential PR isoform recognition by commercially available antibodies and use these findings to study the unique expression patterns and subcellular location of PRA, PRB, and ER-α. Although ER and PR have been considered to be predominantly nuclear proteins, a recent study has shown that when expression vectors for PRA and PRB are transfected into four cell lines, 82% of PRA localizes to the nucleus while only 59% of PRB localizes to the nucleus [18]. This finding as well as the studies reported herein suggest a functional and subcellular localization difference between PRA and PRB that may have clinical significance.
Materials and methods
Materials and antibodies
Detection kits (Cat. 760-001) for the Ventana Nex ES automated IHC stainer were purchased from the Ventana Corporation (Tucson, AZ). Monoclonal antibodies against ER-α (clone 6F11) and PRA+B (to common regions of PRA and PRB but preferentially recognizing PRA, clone 1A6) were also purchased from Ventana and Dakocytomation (Carpinteria, CA). Another monoclonal antibody, AB52, recognizing both PRA and PRB on immunoblots was a gift of Dr. Kathryn Horwitz, the University of Colorado Health Sciences Center, Denver, CO. Confocal experiments were carried out with Ab-8 (clone hPRa2+hPRa3) from Neo-Markers (Fremont, CA), recognizing both PR isoforms. Differential detection of PRB was accomplished using antibodies to the unique 164 amino acids of the N-terminus that is absent in PRA. Three PRB specific antibodies were used, clone hPRa2 and hPRa6, purchased from Lab Visions (Fremont, CA), and B30, a gift from Dr. Kathryn Horwitz. B30 is useful for immunoblotting, while hPRa2 and hPRa6 are used for immunoblotting as well as potentially, for IHC. ER was localized by IHC using antibody clone 1D5 (Ventana), which is specific for ER-α. All primary antibodies were mouse anti-human and are listed in Table 1.
Table 1.
Antibodies used in these studies including their manufacturer and target specificity
| Antibody | Manufacturer | Target | Specificity |
|---|---|---|---|
| PR | Dakocytomation | PR | Preferentially PRA |
| PR | Ventana | PR | Preferentially PRA |
| AB52 | K. Horwitz | PR | PRA and PRB |
| B30 | K. Horwitz | N-terminus PR | PRB |
| hPRa2 | Lab Visions | N-terminus PR | PRB |
| hPRa6 | Lab Visions | N-terminus PR | PRB |
Cell models
Hec50co cells (obtained from Dr. Erlio Gurpide) and KLE cells (American Type Culture Collection) are poorly differentiated endometrial cancer with very low levels of endogenous PRA and no PRB [7]. T47D and MCF-7 breast cancer cells were obtained from Kathryn Horwitz, the University of Colorado. Cells were grown and maintained in the laboratory as previously described [7]. To study the PR isoforms, the cells were infected with adenoviral vectors encoding either PRA or PRB along with green fluorescent protein (GFP) under the control of separate promoters using a multiplicity of infection (MOI) of 1 to 10 viral particles per cell, as previously published [19].
Adenoviral vectors
As previously described [19], adenoviral vectors were constructed using the pShuttle vector (a gift from Dr. T. C. He), encoding green fluorescent protein (GFP) and containing a polylinker for cloning other genes of interest that are under the control of a second, independent cytomegalovirus (CMV) promoter. To make the PR-encoding vectors AdPRA and AdPRB, the PRA and the PRB gene coding sequences were cloned from the vector pSG5-hPR1 (a gift from Dr. Pierre Chambon) using BamHI and EcoRI restriction sites, respectively. pShuttle-PR was generated by insertion of PRA or PRB into the polylinker sequence in the shuttle vector through blunt end ligation. The recombinant adenoviral plasmids were generated by homologous recombination between the adenovirus backbone plasmid (a gift of Dr. T. C. He) and pShuttle-PR in E. coli BJ5183 cells. The resultant supercoiled plasmid DNA was transformed into DH10B cells for amplification after confirmation of the plasmid construction. Transfection of 293 cells by the recombinant adenovirus was carried out using a mixture of linearized plasmid DNA, Lipofectamine (Life Technologies) and OptiMEM I (Life Technologies) according to the manufacturer's instructions. Transfected cells were monitored for GFP expression, and the viruses expressing PRA (AdPRA) or PRB (AdPRB) were harvested 7-10 days after transfection. For these experiments, MOIs up to 10 viral particles per cell were employed to obtain PR expression levels roughly equivalent to the late proliferative phase of the menstrual cycle.
Patient samples
Paraffin-embedded endometrial tissues from pre- and post-menopausal women who had undergone a hysterectomy for endometrial cancer over the last 4 years were obtained from the University of Colorado's archival tissue bank. Representative samples of normal proliferative and secretory endometria were used as comparisons. The University of Colorado and the University of New Mexico Institutional Review Boards approved the use of the tissue samples. The most suitable tissue blocks were chosen, and when possible, the blocks included nonmalignant adjacent endometrium and stroma as well as the tumor. Each tumor was graded as well, moderately, or poorly differentiated, and the nonmalignant glandular epithelium was classified as normal, hyperplastic, or atrophic. Twenty patients with the pre-operative diagnosis of endometrial cancer were originally selected for evaluation; however, four of these had no residual tumor at the time of hysterectomy, leaving 16 endometrial cancer specimens for study. Also, of the 20 original patients, only 13 had surrounding, nonmalignant endometrium for evaluation.
Immunohistochemistry
Tissues were fixed in paraffin, cut into 4-μ sections and mounted onto polylysine-coated slides. Slides were deparaffinized through three changes of xylene and through graded alcohols to water. Antigen retrieval was performed by microwaving slides in 1 mM EDTA pH 8.0 in a pressure cooker for 15 min. Following a cooling period at room temperature for 5 min, slides were rinsed twice in Tris wash buffer pH 7.6 (0.5 M Tris HCl and 0.15 M NaCl) for 3 min. Endogenous peroxidase was quenched with 1% hydrogen peroxide in deionized water for 3 min. IHC was performed on the Ventana Nex ES automated stainer using the avidin-biotin detection method. All steps were performed at 37°C. The monoclonal primary antibodies for ER-α, PRA (both prediluted), and PRB at a concentration of 1:25 or 8 μg/ml were incubated for 32 min. The secondary antibody utilized was a goat anti-mouse and was added to the incubation mixture for 10 min. An amplification step was then performed using a reagent provided by Ventana (Cat # 760-080). The labeled streptavidin-horseradish peroxidase used for detection was incubated for 10 min. Finally, the slides were incubated with diaminobenzidine solution, the chromogen substrate. Slides were rinsed with wash buffer and counterstained with hematoxylin, dehydrated through graded alcohols, and permounted with Cytoseal 60 (Stephens Scientific). Isotype-matched negative controls were included in all cases. MCF-7 breast cancer cells treated with estrogen to down-regulate ER-α served as an additional negative control. Ishikawa endometrial cancer cells served as positive controls for ER-α, PRA, and PRB. T47D breast cancer cells, expressing high levels of both PR isoforms, served as an additional positive control for PRA and PRB. A pathologist reviewed all slides, and the percentage of cells staining positive was calculated over five high power fields. Statistical analysis of differences in PRB subcellular localization as a function of tumor differentiation was carried out using the Mantel-Haenszel test with modified ridit scores.
Immunoprecipitation
Hec50co or KLE endometrial cancer cells expressing either PRA or PRB as a consequence of PRA or PRBencoding adenoviral (Ad-PRA or Ad-PRB) infection were grown in the laboratory, and a total protein extraction was performed [19]. Five micrograms of immunoprecipitation antibody was added to 1 mg of protein cell lysate. The mixture was rocked at 4°C for 2 h. Fifty microliters of Protein G agarose bead slurry (Gibco Invitrogen Corp., Carlsbad, CA) was added to the immune complexes, and the mixture was rocked for 3 h at 4°C. The beads were pulsed in a microfuge for 10 s, centrifuged at 14,000 × g, and the supernatant was aspirated and set aside without disturbing the beads. The beads were washed and resuspended in 50 μl of Laemmli sample buffer and boiled for 5 min. The beads were collected by microfuge pulse, and the supernatant was aliquoted, run on SDS PAGE gels, and immunoblotted as previously described [7]. Total cell lysate that had not undergone immunoprecipitation was loaded for comparison.
Immunoblotting
ECL Western blotting kits were purchased from Amersham (Arlington Heights, IL), and the kit instructions were followed. Briefly, 800 μg of protein extract was loaded per lane on a 7.5% SDS-polyacrylamide gel. This was run at 8 mV overnight in Tank buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3). The proteins were transferred to nitrocellulose membranes (Amersham). Following primary antibody incubation, the membranes were incubated with a goat anti-mouse secondary antibody (Cappel, Organon Teknika Corp., West Chester, PA) followed by Luminol reagent (Amersham), and chemiluminescence was detected by autoradiography.
Confocal microscopy
Hec50co and KLE cells were grown to 50% confluence on cover slips and infected with the adenoviral vectors Ad-PRA and Ad-PRB (MOI = 10). Twenty-four hours after infection, the cells were treated with 10-7 M progesterone (P4) dissolved in ethanol as a vehicle or with vehicle alone for 30 min. The cells were washed three times with phosphate buffered saline (PBS), fixed in 3% paraformaldehyde in PBS, and quenched in 50 mM NH4Cl/PBS. Cells were then permeabilized in 0.1% Triton X-100, washed, and incubated with primary antibody Ab-8 (NeoMarkers), which recognizes either PR isoform, diluted 1/500 in PBS with 1% bovine serum albumin (BSA) for 1 h. This was followed by incubation with the secondary antibody (donkey antimouse, Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min. The number of cells expressing nuclear and cytoplasmic PRA and PRB was determined by scoring the location of the receptor in 200 cells over multiple representative fields. Cells infected with the PR-encoding vectors were easily tracked because the coding region for GFP is also present in the vectors, and GFP is expressed as a separate protein. The infection efficiency of the adenoviral vectors is very high, with total infectivity exceeding 95%.
Results
Immunoprecipitation (IP) was performed to determine which isoforms of PR are recognized by commercially available antibodies using KLE and Hec50co endometrial cancer cells in which high levels of either PRA or PRB were expressed following AdPRA or AdPRB infection. To determine whether the presence of progesterone affected PR recognition by antibodies, cells were treated with hormone or with vehicle alone prior to protein extraction. Following IP with the PR antibody to be tested, cell extracts were run on a Western blot, and both PR isoforms A and B were identified by immunoblotting using AB52. AB52 recognizes PRA and PRB with equal affinity on Western blots. Fig. 2 shows a series of experiments where PR antibodies have been used to immunoprecipitate proteins in extracts expressing both PR isoforms, the presence of which is confirmed by immunoblotting of the extracts prior to immunoprecipitation. The antibodies used for immunoprecipitation were AB52, Dakocytomation PR and Ventana PR. The antibody used as the probe on the immunoprecipitation and Western blots was AB52. Fig. 2A, demonstrates the results when AB52 is used to immunoprecipitate extract containing no PR (lanes 1 and 2) PRA (lanes 3 and 4), or PRB (lanes 5 and 6). Approximately equimolar amounts of PRA and PRB are present on the IP, demonstrating a near equal affinity of AB52 for both isoforms. This is in contrast to 2B and 2C where the Dakocytomation antibody against PR fails to recognize PRB and the Ventana antibody demonstrates an eightfold lower affinity for PRB compared to PRA. These results were confirmed by IHC on KLE and Hec50co cells expressing PRA or PRB (data not shown) and indicate that some commercially available antibodies fail to recognize PRB with adequate sensitivity.
Fig. 2.
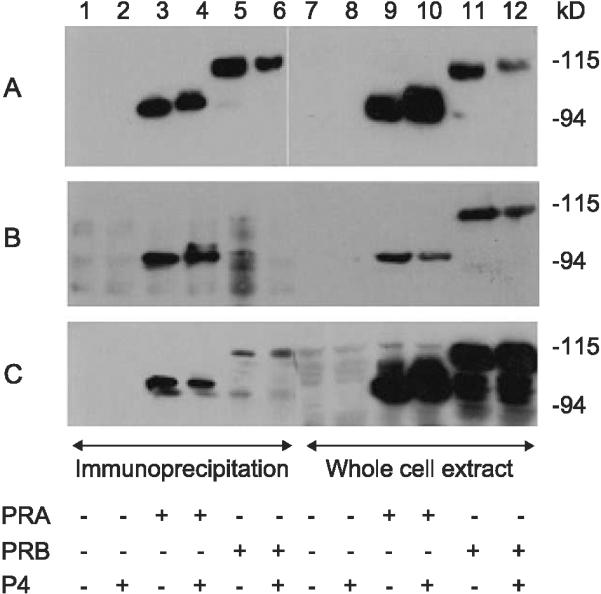
Immunoprecipitation and immunoblot recognition of PR by antibodies purported to recognize both PRA and PRB. (A) The antibody AB52 was used to IP extracts containing no PR (lanes 1 and 2), PRA (lanes 3 and 4), and PRB (lanes 5 and 6). Progesterone or vehicle alone was added to the media as indicated. Lanes 7-13 demonstrate the companion immunoblot indicating the presence of PR isoforms in the extracts prior to IP. (B) The Dako PR antibody was used to IP the extracts as indicated (lanes 1-6), and the immunoblot (lanes 7-12) confirms the presence of PRA (lanes 9 and 10) and PRB (lanes 11 and 12) prior to IP. (C) The Ventana PR antibody was used to IP extracts (lanes 1-6) containing no PR, PRA, or PRB as indicated. The companion immunoblot (lanes 7-12) confirms the presence of PRA and PRB prior to IP. For all immunoblots, AB52 was used to recognize PRA and PRB.
Concerns for the lower antibody affinity for PRB with the Dakocytomation and Ventana PR antibodies can be overcome by performing IHC with PRB specific antibodies. Three PRB-specific antibodies were used for these experiments, B30, hPRa2, and hPRa6. AB52 served as the control antibody on all Western blots, and hPRa2 and hPRa6 served as the IP antibodies in Figs. 3A and B, respectively. Figs. 3A and B illustrate that by IP, these antibodies are specific for PRB, as predicted. We found that hPRa6 provided superior intensity of staining for PRB for IHC (data not shown), and this antibody was used for IHC on human tissues in the experiments that follow (Figs. 5-7).
Fig. 3.
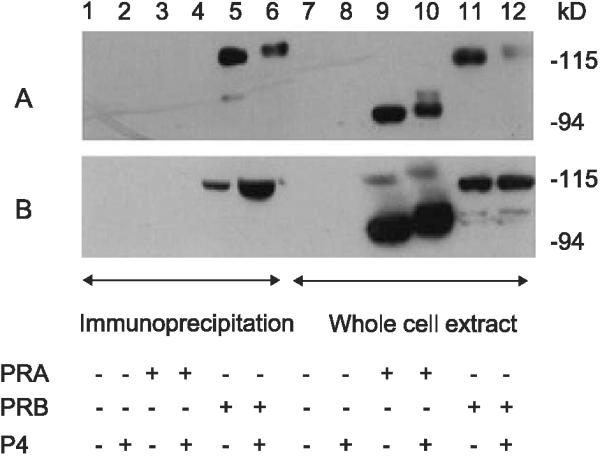
Immunoprecipitation and immunoblot recognition of PRB by antibodies to the unique N terminus of PRB. (A) hPRab2 was used to IP cell extract containing no PR, PRA, and PRB as indicated. (B) hPRab6 was used to IP cell extract containing no PR, PRA, and PRB. For A and B, the cell extract prior to IP was immunoblotted using AB52, which recognizes both PRA and PRB and is used as a control to demonstrate the presence of both isoforms of PR in the extract prior to IP.
Fig. 5.
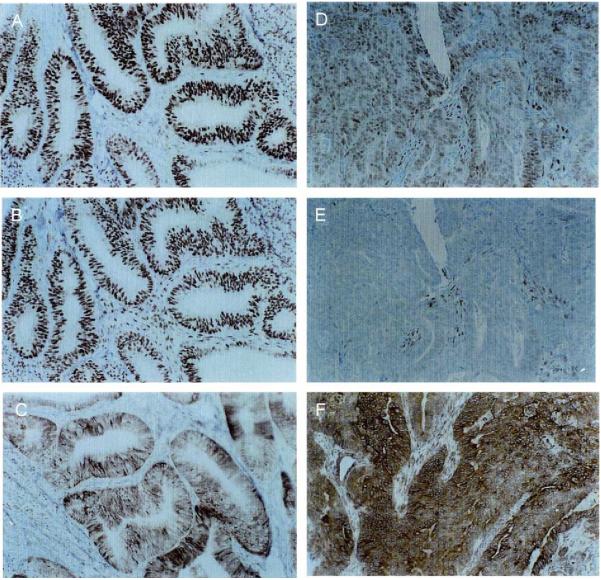
ER, PRA, and PRB expression in two representative patients, one with a well-differentiated endometrial cancer (A, B, C) and the other with a poorly differentiated endometrial cancer (D, E, F). IHC for ER-α (A, D), PRA (B, E), and PRB (C, F) was performed as described in Materials and methods.
Fig. 7.
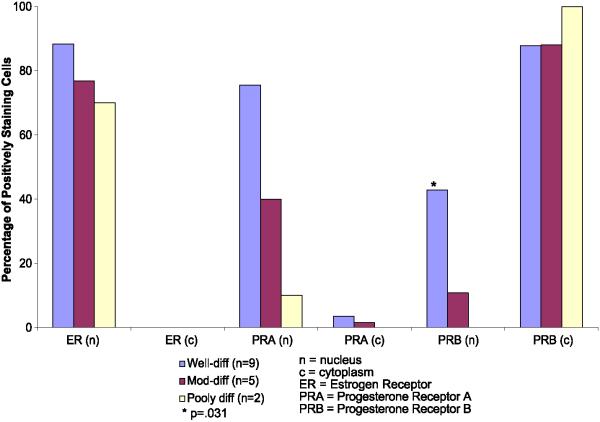
Percentage of tumor cells demonstrating positive staining for nuclear and cytoplasmic receptors in well-, moderately- and poorly-differentiated endometrial cancers. ER = estrogen receptors and PRB = progesterone B receptors. Blue bars = well-differentiated tumors (n = 9), red bars = moderately differentiated tumors (n = 5), and beige bars = poorly differentiated tumors (n = 2). *The presence of PRB in the nucleus is significantly correlated with tumor differentiation using the Mantel-Haenszel test, P = 0.031. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
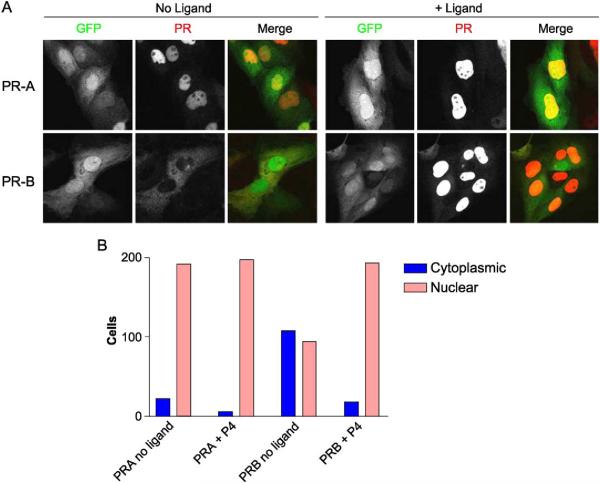
Cell models expressing high levels of either the PRA or PRB receptors were used to unequivocally evaluate the subcellular distribution of each receptor in the presence and absence of ligand. Using confocal microscopy, the differences in PRA compared to PRB compartmentalization were readily evident (Fig. 4A). In the absence of ligand, PRB is mainly cytoplasmic, while PRA is >90% nuclear (Fig. 4B). In the presence of progesterone, PRB synchronously translocates to the nucleus within 30 min of ligand addition and becomes exclusively nuclear (Fig. 4B, PRAB + P4). PRA is nuclear irrespective of whether or not progesterone is present (Fig. 4B, PRA + P4).
Fig. 4.
Subcellular localization of PRA and PRB with and without ligand. (A) Hec50co cells were infected with recombinant adenoviruses encoding GFP and PRA or PRB. Cells expressing GFP and PRA (top) or PRB (bottom) were analyzed by confocal microscopy. The green fluorescence identifies infected cells expressing GFP in the cytoplasm and the nucleus. The red fluorescence identifies the localization of the indicated PR isoform. Left panels: In the absence of ligand, PRA is nuclear and PRB is cytoplasmic in the cells shown. Right panels: In the presence of ligand, both PRA and PRB are nuclear. (B) Quantification of cytoplasmic vs. nuclear distributions of PRA and PRB. The subcellular location of PRA and PRB was scored in 200 cells by confocal microscopy. The number of cells expressing nuclear as compared to cytoplasmic PRA and PRB are plotted for control cells (no ligand) or samples treated with progesterone (+P4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
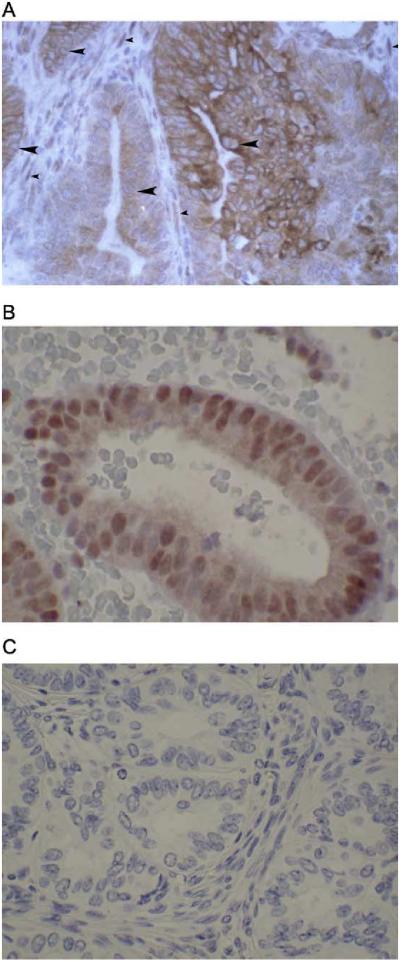
The expression and subcellular localization of receptors in tissue samples was evaluated next to address whether ER-α, PRA, and PRB were differentially expressed and/or localized in the malignant glands compared to the stroma. Receptor expression and localization graded according to tumor differentiation were also evaluated. Fig. 5 shows representative immunostaining for ER-α, PRA, and PRB in a well-differentiated endometrial cancer (left panels) compared to a poorly differentiated endometrial cancer (right panels). In the well-differentiated tumor on the left, ER-α (Fig. 5A) and PRA (Fig. 5B) are present and nuclear in 70-100% of the epithelial cells and the majority of the stromal cells. However, in Fig. 5C, it is clear that PRB is predominantly cytoplasmic in much of the malignant glandular epithelium. In contrast, the majority of PRB in nonmalignant proliferative endometrial glandular epithelium is nuclear (data not shown), just as it is in the stroma underlying the malignant epithelium (Fig. 5C). The tumor on the right is a case of poorly differentiated endometrial cancer in which ER-α and PRA have been downregulated (Figs. 5D and E, respectively), but somewhat surprisingly, intense staining for cytoplasmic PRB is present (Fig. 5F). Fig. 6A is a higher magnification to illustrate the extensive cytoplasmic staining for PRB in this tumor. The nuclei of the malignant epithelial cells (indicated by the large arrowheads) are counter-stained blue and are devoid of receptor. In comparison, nuclei of stromal cells are brown (the small arrowheads), indicating the continued presence of nuclear PRB in these cells. Fig. 6B is provided for comparison and is a relatively well-differentiated endometrial tumor in which nuclear PRB is preserved. Fig. 6C is a negative control for the IHC staining. These cases serve as examples, and Fig. 7 compares the nuclear versus cytoplasmic location of ER-α, PRA, and PRB by immunohistochemistry in well (n = 9), moderately (n = 5), and poorly (n = 2) differentiated additional cancers. ER-α and PRA, when present, reside mainly in the nucleus regardless of the grade of the tumor. This is evident by comparing the bars labeled ER (n) and PRA (n) to ER (c) and PRA (c) in Fig. 7. On the other hand, PRB are lost from the nucleus as tumors progress towards a poorly differentiated phenotype. The association between nuclear staining for PRB and a well-differentiated tumor compared to a poorly differentiated one is statistically significant (Mantel-Haenszel test with modified ridit scores = 4.936, df = 1, P = 0.031), and is indicated by the asterisk in Fig. 7. Hence, the cytoplasmic localization of PRB is not universal, but is a common finding in more poorly differentiated cancers. The fact that the anomalous PRB distribution is mimicked in KLE and Hec50co cells expressing the receptor suggests these cells may serve as models for identifying the cause and effect of such altered PRB distribution.
Fig. 6.
Detail of PRB staining and nuclear exclusion at high magnification. (A) PRB is cytoplasmic in some poorly differentiated tumors. Note the central blue staining of the nucleus of the tumor cells (large arrowheads), with surrounding brown staining in the cytoplasm. In comparison, the stromal cells surrounding the tumor demonstrate brown nuclear staining for PRB (small arrowheads). (B) PRB retains nuclear staining in some well-differentiated endometrial tumors. (C) Negative control for IHC staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Discussion
These studies have documented differential expression and subcellular localization of PRA and PRB in endometrial cancer cells and tissues. The investigations were restricted to endometrial cancer; however, work by others using breast cancer cells indicates that our findings may have general applicability [18,20]. One interesting finding is that some PR antibodies in widespread use do not recognize PRA and PRB with equal affinity, particularly for IHC. This is likely due to conformational differences between the isoforms induced by the unique N-terminus of PRB. Our data suggest that older IHC studies utilizing “common” PR antibodies (that actually bind preferentially to PRA) may have underestimated the amount of PRB present in tissues.
In general, two experimental paradigms were used for these studies: in vitro cell models expressing either PRA or PRB as a consequence of gene transduction, and formalin-fixed paraffin-embedded surgical specimens from women with endometrial cancer where malignant and adjacent nonmalignant endometrial tissues were evaluated. The in vitro experiments allowed a detailed microscopic analysis of PR isoform subcellular localization in endometrial cancer cells using confocal microscopy that would not have been possible using IHC alone. The consistent differences in localization between the nucleus and the cytoplasm found for the PR isoforms, with PRA being uniquely nuclear and PRB distributed between the nucleus and the cytoplasm, was then confirmed by IHC in tissues from patients with endometrial cancer.
Confocal microscopic evaluation of endometrial cancer cells expressing either PRA or PRB, as described herein, highlight the difference in cellular compartments occupied by PRA and PRB when ligand is not present. However, when progesterone is added, the entire population of PRB in the cytoplasm rapidly translocates to the nucleus. Conversely, PRA resides in the nucleus whether or not progesterone is present, and endometrial cancer cells expressing exclusively PRA demonstrate little, if any, cytoplasmic fluorescence. Our work is consistent with the studies of others, who demonstrated that when an expression vector encoding PRB was introduced into cells grown in vitro, the subcellular location of the receptor was cytoplasmic until progestin was added [18,20]. In their studies as in ours, addition of progestin caused the rapid nuclear translocation of PRB. This is in contradistinction to PRA, which was predominantly nuclear whether or not hormone was present.
Our work adds to the body of evidence by extending the in vitro findings to human tissues. The PRB distribution documented in the poorly differentiated Hec50co and KLE cell lines mimics its subcellular compartmentalization in endometrial cancer tissues. With the appropriate antibodies in hand, we show that endometrial adenocarcinomas of all grades (well, moderately and poorly differentiated) express ER-α, PRA, and PRB. PRA, like ER-α, is nuclear. On the other hand, PRB is distributed both in the nuclear compartment as well as the cytoplasm and appears to be excluded from the nucleus entirely in some poorly differentiated cases. Complete nuclear exclusion of PRB was found in five of the seven moderately to poorly differentiated cancers studied, and PRB was cytoplasmic in more than 50% of the tumor cells. It should be noted that the tissues evaluated were endometrial adenocarcinomas, and these findings may not apply to other histologic subtypes of endometrial cancer such as clear cell and papillary serous.
What are the potential biological implications of our findings? Like glucocorticoid receptors (GRs), PRs are known to shuttle in and out of the nucleus. PR may cross talk with signal transduction pathways that could initiate or enhance responses in conjunction with membrane bound growth factor receptors or G-protein receptors. PRs contain a proline-rich motif within the shared A domain of PRA and PRB, which interacts directly with SH3 domains of c-Src and its family member Hck [21]. Both PRA and PRB demonstrate this interaction in vitro, but since PRA is largely restricted to the nucleus in endometrial cancer cells, we predict that cytoplasmic signaling events depend upon PRB. PRB has also been reported to localize to the cytoplasm in breast cancer cells in the absence of ligand and is driven into the nucleus in the presence of progesterone or EGF by two distinct mechanisms [22]. This indicates that growth factor signaling as well as hormone may activate PRB as a transcription factor by targeting it to the nucleus. Could potential interactions between cytoplasmic PRB and other signaling molecules produce a growth modulatory effect, even a growth-stimulatory effect, in endometrial cancer cells? We speculate that this question could have bearing on the proliferative effects of PRB in knockout mice models; however, these questions remain to be determined by future studies. Better established are the effects of EGF on PRB subcellular localization. The EGF pathway to nuclear localization requires phosphorylation of PRB through the MAP kinase cascade at serine 294 [23]. Since PRA appears to reside in the nucleus constitutively, phosphorylation by MAP kinase is not required for its nuclear localization. The difference in tertiary structure of the two isoforms must account for the requirement that PRB, but not PRA, be phosphorylated by MAP kinase or progesterone to enter the nucleus. The constitutive nuclear location of PRA make it the logical candidate to initiate hormone-independent gene transcription, as has recently been proposed for PR [24]. The ability of PR (particularly PRA) to promote gene transcription in the absence of ligand is a new concept that deserves further study.
We are now investigating the mechanism(s) underlying PRB cytoplasmic to nuclear shuttling, the molecular details of which are incompletely understood. It has been suggested that a dynamic situation exists whereby receptors diffuse into the cytoplasm and are constantly and actively transported back to the nucleus [25]. Nuclear import and export control the functional activity and the cellular concentration of the receptors [26]. For PR, nuclear translocation depends upon two intact nuclear localization signal (NLS) sequences, one that is constitutive and is located in the hinge region and another located in the DNA binding domain (Fig. 1). The latter can be activated by the binding of hormone or by the deletion of the hormone-binding domain. Therefore, either mutations in the NLS sequences or a lack of hormone could render PR cytoplasmic, and both abnormalities have been shown to result in loss of PR from the nucleus in cell models.
Nuclear shuttling also requires appropriate interactions with the chaperone heat shock protein (HSP) 90 as well as an intact NLS. The functions of heat shock protein 90 with respect to PR and/or GR include (1) the assembly of the receptor into a form capable of productive interactions with hormone, (2) the release of the receptor from chromatin following hormone withdrawal, and (3) the attachment of cytoplasmic receptor to the cytoskeleton to promote rapid nuclear transport [27,28]. It is possible that the PR isoforms interact differently with chaperone proteins such as HSP 90, thus controlling nuclear import; however, no published studies have directly addressed this interesting question. Future investigations should seek to clarify which of a number of mechanisms underlie the different cellular compartmentalization of the PR isoforms. We predict that the distinct subcellular localization of the PR isoforms relates to their unique tertiary structures and potentially involves differential phosphorylation, interactions with heat shock protein 90, and NLS function for PRA compared to PRB.
References
- [1].Persson I, Adami HO, Bergkvist L, Lindgren A, Pettersson B, Hoover R, et al. Risk of endometrial cancer after treatment with oestrogens alone or in conjunction with progestogens: results of a prospective study. BMJ. 1989;298(6667):147–51. doi: 10.1136/bmj.298.6667.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Savouret JF, et al. The progesterone receptor. Biological effects of progestins and antiprogestins. Hum Reprod. 1994;9(Suppl 1):7–11. doi: 10.1093/humrep/9.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- [3].Savouret JF, Rauch M, Redeuilh G, Sar S, Chauchereau A, Woodruff K, et al. Interplay between estrogens, progestins, retinoic acid and AP-1 on a single regulatory site in the progesterone receptor gene. J Biol Chem. 1994;269(46):28955–62. [PubMed] [Google Scholar]
- [4].Horwitz KB, Alexander PS. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983;113(6):2195–201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- [5].Krett NL, Wei LL, Francis MD, Nordeen SK, Gordon DF, Wood WM, et al. Human progesterone A-receptors can be synthesized intracellularly and are biologically functional. Biochem Biophys Res Commun. 1988;157(1):278–85. doi: 10.1016/s0006-291x(88)80044-5. [DOI] [PubMed] [Google Scholar]
- [6].Graham MLD, Smith JA, Jewett PB, Horwitz KB, et al. Heterogeneity of progesterone receptor content and remodeling by tamoxifen characterize subpopulations of cultured human breast cancer cells: analysis by quantitative dual parameter flow cytometry. Cancer Res. 1992;52(3):593–602. [PubMed] [Google Scholar]
- [7].Kumar NS, et al. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: implications for unopposed estrogen action. Cancer Res. 1998;58(9):1860–5. [PubMed] [Google Scholar]
- [8].Leslie KK, Kumar NS, Richer J, Owen G, Takimoto G, Horwitz KB, et al. Differential expression of the A and B isoforms of progesterone receptor in human endometrial cancer cells. Only progesterone receptor B is induced by estrogen and associated with strong transcriptional activation. Ann N Y Acad Sci. 1997;828:17–26. doi: 10.1111/j.1749-6632.1997.tb48520.x. [DOI] [PubMed] [Google Scholar]
- [9].Jacobsen BM, Richer JK, Schittone SA, Horwitz KB, et al. New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. J Biol Chem. 2002;277(31):27793–800. doi: 10.1074/jbc.M202584200. [DOI] [PubMed] [Google Scholar]
- [10].Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP, et al. Human progesterone receptor A form is a cell-and promoter-specific repressor of human progesterone receptor B function [see comments] Mol Endocrinol. 1993;7(10):1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- [11].Dai D, Wolf DM, Litman ES, White MJ, Leslie KK, et al. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res. 2002;62(3):881–6. [PubMed] [Google Scholar]
- [12].Smid-Koopman E, Blok LJ, Kuhne LC, Burger CW, Helmerhorst TJ, Brinkmann AO, et al. Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J Soc Gynecol Investig. 2003;10(1):49–57. [PubMed] [Google Scholar]
- [13].Arnett-Mansfield RL, deFazio A, Wain GV, Jaworski RC, Byth K, Mote PA, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61(11):4576–82. [PubMed] [Google Scholar]
- [14].Fujimoto J, Ichigo S, Hori M, Nishigaki M, Tamaya T, et al. Expression of progesterone receptor form A and B mRNAs in gynecologic malignant tumors. Tumour Biol. 1995;16(4):254–60. doi: 10.1159/000217942. [DOI] [PubMed] [Google Scholar]
- [15].Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM, et al. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- [16].Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- [17].Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL, et al. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;54(8):624–30. doi: 10.1136/jcp.54.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lim CS, Baumann CT, Htun H, Xian W, Irie M, Smith CL, et al. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol Endocrinol. 1999;13(3):366–75. doi: 10.1210/mend.13.3.0247. [DOI] [PubMed] [Google Scholar]
- [19].Dai D, Kumar NS, Wolf DM, Leslie KK, et al. Molecular tools to reestablish progestin control of endometrial cancer cell proliferation. Am J Obstet Gynecol. 2001;184(5):790–7. doi: 10.1067/mob.2001.113844. [DOI] [PubMed] [Google Scholar]
- [20].Kanwal C, Li H, Lim CS. Model system to study classical nuclear export signals. AAPS PharmSci. 2002;4(3):E18. doi: 10.1208/ps040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8(2):269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- [22].Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA, et al. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17(4):628–42. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- [23].Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97(3):1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- [25].Guiochon-Mantel A, Lescop P, Christin-Maitre S, Loosfelt H, Perrot-Applanat M, Milgrom E, et al. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 1991;10(12):3851–9. doi: 10.1002/j.1460-2075.1991.tb04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu J, DeFranco DB. Protracted nuclear export of glucocorticoid receptor limits its turnover and does not require the exportin 1/CRM1-directed nuclear export pathway. Mol Endocrinol. 2000;14(1):40–51. doi: 10.1210/mend.14.1.0398. [DOI] [PubMed] [Google Scholar]
- [27].Galigniana MD, et al. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12(12):1903–13. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- [28].Liu J, DeFranco DB. Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol Endocrinol. 1999;13(3):355–65. doi: 10.1210/mend.13.3.0258. [DOI] [PubMed] [Google Scholar]