Abstract
γδ T cells respond rapidly following West Nile virus (WNV) infection, limiting viremia and invasion of the central nervous system, and thereby protecting the host from lethal encephalitis. Here, we investigated the role of two major subpopulations of peripheral γδ T cells, Vγ1+ and Vγ4+ cells in host immunity against WNV infection. We found initially, that aged mice were more susceptible to WNV infection than young mice. Following WNV challenge, Vγ1+ cells in young mice expanded significantly, whereas Vγ4+ cells expanded modestly. In contrast, aged mice exhibited a slower and reduced response of Vγ1+ cells but maintained a higher content of Vγ4+ cells. Vγ1+ cells were the major γδ subset producing IFN-γ during WNV infection. Mice depleted of Vγ1+ cells had an enhanced viremia and higher mortality to WNV encephalitis. Vγ4+ cells had a higher potential for producing TNF-α, a cytokine known to be involved in blood brain barrier compromise and WNV entry into the brain. Depletion of Vγ4+ cells reduced TNF-α level in the periphery, accompanied by a decreased viral load in the brain and a lower mortality to WN encephalitis. These results suggest that Vγ1+ and Vγ4+ cells play distinct roles in protection and pathogenesis during WNV infection.
Keywords: West Nile, Gamma delta T cell, Aging, Pathogenesis
INTRODUCTION
West Nile virus (WNV) induced neurological disease has become a public health concern in recent years [1, 2]. The virus belongs to the family of Flaviviridae, a group of plus-sense, single stranded RNA viruses [3, 4]. Although most WNV infections in humans are asymptomatic, a small percentage of them develop encephalitis and death, mainly in the elderly and immunocompromised. Treatment is currently nonspecific and supportive [1, 3–5]. In the susceptible host, WNV is neuroinvasive (able to access the central nervous system (CNS)) and neurovirulent (able to infect the CNS, replicate in some of its cells and injure them) [6, 7]. Nevertheless, the pathogenesis of WNV induced encephalitis is not yet fully understood.
In mice and humans, γδ T cells comprise a minority of the CD3+ T cells in lymphoid tissue and blood but are well represented at epithelial and mucosal sites [8]. They can rapidly produce cytokines in response to microbial antigens [9] and have unique features, including a lack of major histocompatibility complex restriction and the potential capacity to respond to antigens without a requirement for conventional antigen processing, which together suggest a role in early pathogen control [10]. γδ T cells proliferate after parasitic [11], bacterial [12, 13] and viral infections [14, 15]. They are also involved in immune response-induced pathogenesis. In patients with Takayasu arteritis, γδ T cells are reactive to 60kD heat-shock protein and exhibit cytotoxicity to aortic endothelial cells [16]. In an experimental model of autoimmune encephalomyelitis, they were found to regulate inflammation in the CNS by a FasL-dependent mechanism [17]. Moreover, γδ T cells are divisible into functionally distinct subsets which have direct and indirect effects on host immunity to pathogen infection [18]. Splenic Vγ1-bearing T cells are important in the elimination of Listeria infection by their interferon-gamma (IFN)-γ producing activity [19]. In Coxsackievirus-infected mice, Vγ4+ T cells enhance CD4+ Th1 cell activation through IFN-γ and CD1-dependent mechanisms. The CD4+ Th1 cells further promote the activation of autoimmune CD8+ effector T cells which could ultimately lead to myocarditis [20, 21].
We have recently shown that γδ T cells are important for early control of WNV dissemination [22]. In the present study, we have further investigated the role of two major peripheral γδ T cell subsets during WNV infection. Our data suggests that Vγ1+ T cells play an important role in protecting the host against WNV infection, whereas Vγ4+ T cells are likely involved in pathogenesis.
MATERIALS AND METHODS
Mice
6–10-week-old C57BL/6 (B6) mice and 21–22-month-old B6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and National Institute of Aging (Bethesda, MD) respectively. Mice were maintained under specific pathogen-free conditions at the animal facilities at Colorado State University. Groups were age- and sex-matched for each experiment and were housed under identical conditions. All animal experiments were performed in compliance with the guidelines of the Animal Care and Use Committee at Colorado State University.
Infection in mice
A total of 100 plaque forming unit (PFU) corresponds to the LD50 and 1000 PFU represents the LD100 for WNV isolate CT 2741, as described in our previous work [22]. In this study, mice were inoculated intraperitoneally (i.p.) with either a dose close to LD50 (80PFU) or a dose close to LD100 (700 PFU) of WNV CT 2741. Infected mice were monitored twice daily for morbidity, including lethargy, anorexia and difficulty in walking.
Quantitative PCR (Q-PCR) for viral load and cytokine production
RNA was extracted from the blood and brain tissues using RNAeasy extraction kit (Qiagen, Valencia, CA). RNA was used to synthesize complementary (c)DNA using the ProSTAR First-strand RT-PCR kit (Stratagene, Cedar Creek, TX). The sequences of the primer-probe sets for WNV envelope (WNVE) and TNF-α cDNA and PCR reaction conditions were described previously [23, 24]. Probes contained a 5′ reporter, FAM, and a 3′ quencher, TAMRA (Applied Biosystems, Foster City, CA). The assay was performed on an iCycler (Bio-Rad, Hercules, CA). To normalize the samples, the same amount of cDNA was used in a Q-PCR for β-actin. The ratio of the amount of amplified gene compared with the amount of β-actin cDNA represented the relative levels in each sample.
Plaque assay
Vero cells were seeded in 6-well plates in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO) 24h before infection. Serial dilutions of sera from infected mice were added and incubated for 2h. Subsequently, DMEM containing 5% FBS and 1% low-melting-point agarose were added and the plates were incubated for 4 days. A second overlay of 2.5ml 1% agarose-medium containing 0.01% neutral red was added to visualize plaques. Virus concentrations were determined as PFU/ml.
In vivo depletion of γδ subpopulation T cells
Hamster Vγ1 mAb 2.11 and Vγ4 mAb UC3 were purified from hybridoma culture supernatants as described earlier [25]. T cell depletion was achieved by two consecutive injections of 100 μg of hamster anti-Vγ1 or -Vγ4 i.p. at 2 days and 24h before WNV challenge. Sham Ab treatments were performed with the same amount of hamster IgG isotype (Innovative Research, Southfield, MI).
T cell purification from spleen
Single cell suspensions of T cells were isolated from spleens by a positive selection method, using anti-CD90magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions.
Flow cytometry
γδ-T-cell sub-populations were identified by applying a 3-step staining procedure: 1×106 cells were first stained with mAb GL3-FITC (hamster anti-mouse TCRδ, BD Biosciences, San Diego, CA), followed by incubation with biotinylated mAb 2.11 [26] or mAb UC3 [27], and finally staining with Phycoerythrin (PE)-streptavidin (e-Bioscience, San Diego, CA). After staining, cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) and examined using a Coulter XL instrument (Beckman Coulter, Fullerton, CA). Dead cells were excluded on the basis of forward and side light scatter. Data were analyzed using FCS express 2 (De Novo Software Ontario, Canada).
Intracellular cytokine staining
To measure cytokine production, splenic T cells from WNV-infected mice were isolated and stimulated at 2 × 106 cells/well with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 h at 37°C. Golgi-plug (BD Biosciences) was added during the final 2.5 h. The cells were harvested, stained with FITC-labeled mAb to mouse TCRδ and biotinylated Vγ1 or Vγ4 mAbs, followed by Streptavidin-PE-Cy5, then fixed in 2% paraformaldehyde. The cells were next permeabilized with 0.5% saponin before adding PE-conjugated antibodies to IFN-γ, tumor necrosis factor-α (TNF-α) or rat IgG1 (BD Biosciences). Cells were analyzed using a Coulter XL instrument as described above.
Statistical analysis
Survival curve comparisons were performed using Prism software (GraphPad Software, San Diego, CA) statistical analysis, which uses the log rank test (equivalent to the Mantel-Haenszel test). Values of p to compare viral burden, cytokine levels and γδ T cell numbers were calculated with a non-paired Student’s t test or Mann-Whitney test.
RESULTS
A major risk factor for fatality of WNV infection in humans is aging [5, 28]. To address this risk factor in the murine model, aged mice (21–22 month-old) and young adult controls (6–10-week-old) were first infected with 80 PFU (a dose close to LD50) of WNV strain CT2741 and monitored daily for survival. Aged mice were more susceptible to WNV infection than young mice (13% survival vs. 40% survival; P < 0.05) (Fig. 1A). We next measured viremia in both groups of mice following WNV challenge. As measured by Q-PCR and plaque assay (Fig. 1B, left & right panels), there was a higher viremia in aged mice than in young mice at day 3 post-infection. By day 7, viral levels in blood of young mice were no longer detectable, but viral burden remained elevated in aged mice. Our previous results have demonstrated an important role of γδ T cells in protection of the host from WNV infection [22]. Here, to understand the underlying mechanisms of the enhanced susceptibility to WN encephalitis in aged mice, we studied the responses of γδ T cells in these mice following WNV infection. Splenocytes were isolated and examined for the percentage and total numbers of selected populations. Samples were assessed before infection (control)and at early (day 3) and later (day 5) intervals post-infection. Among enriched splenic T cells, the percentage (Fig. 2A) and total number of γδ+ cells (Fig. 2B) in young mice increased significantly at day 3 post-infection, and decreased though remained higher than non-infected controls at the later stage (day 5). In contrast, the percentage and total number of γδ+ T cells in aged mice was unchanged at day 3, but showed some increase at day 5 following infection (Figs. 2A & 2B). These data suggest that a difference in γδ T cell response in aged mice might contribute to the enhanced susceptibility to WN encephalitis. In further investigating the role of γδ T cell subsets during WNV infection, we found that the percentage and total number of Vγ1+ γδ+ cells in young mice increased substantially at day 3 and remained higher at day 5 following infection (Figs. 2C & 2D). The percentage of the Vγ4+ subset in young mice decreased at day 3 and remained at the baseline level at day 5 (Fig. 2C), though total number of Vγ4+ T cells increased modestly (Fig. 2D). In WNV infected aged mice, the percentage and total number of Vγ1+ cells increased only at day 5 (Figs. 2C & 2D). The percentage of Vγ4+ subset remained unchanged at day 3 and was decreased at day 5 (Fig. 2C). The total number of Vγ4+ T cells in aged mice remained unchanged (Fig. 2D). In comparison to young mice, Vγ1+ T cells of aged mice responded more slowly and less strongly to WNV infection, whereas number of Vγ4+ T cells was higher in aged mice at early stage of infection.
Figure 1. Aged mice are more susceptible to WNV infection.

(A) Young and aged mice were infected with 80 PFU of WNV and monitored twice daily for mortality. Data shown are pooled from five independent experiments. * P < 0.05 for young mice (n = 25) vs. aged mice (n = 22). (B) Aged mice have a higher viremia during WNV infection than young mice. Viral load was determined in blood of WNV infected mice at the indicated days using Q-PCR (left panel) and plaque assay (right panel). The y-axis depicts the ratio of the amplified WNV-E cDNA to β-actin cDNA of each sample (unitless ratio ± 1 SEM) or PFU/ml. * P < 0.05 for young mice vs. aged mice. Data reflects 3–4 mice per condition, performed in duplicates and are representative of three separate experiments.
Figure 2. Aged mice exhibited a slower and reduced response of Vγ1+ cells but maintained higher content of Vγ4+ cells.
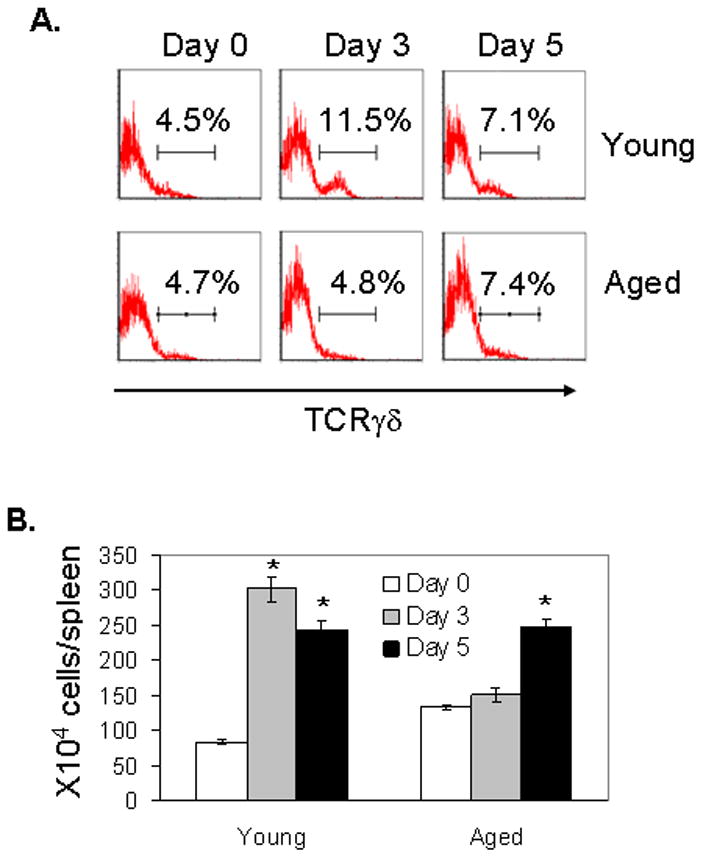
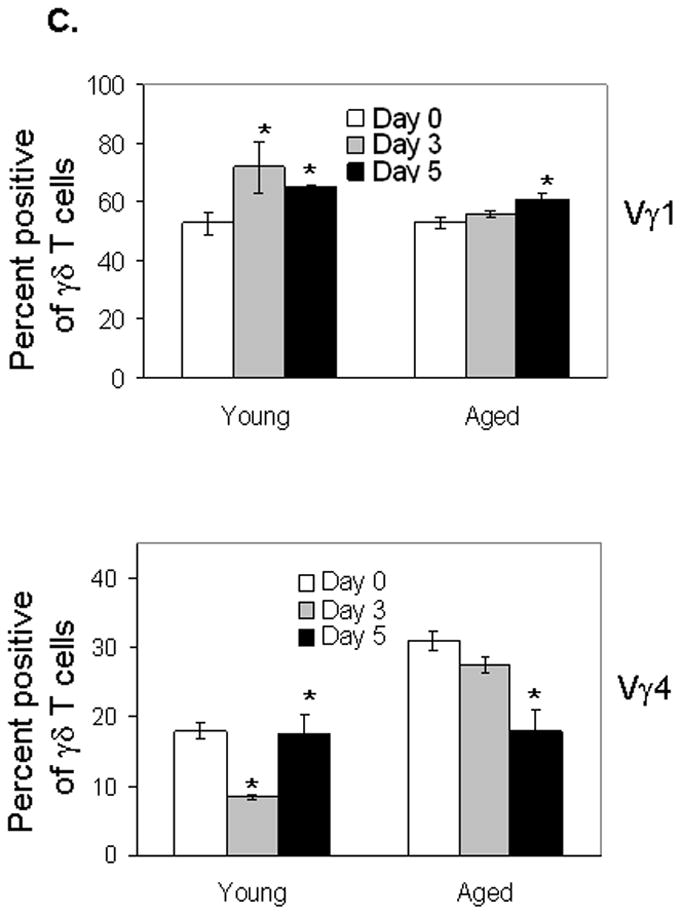

Splenic T cells were isolated before infection (day 0) and at days 3 and 5 post-infection and stained for TCRγδ and Vγ1 or Vγ4. (A) Total γδ+ percentage among splenic T cells. Data shown are representative of three independent experiments. (B) Total number of γδ T cells. (C) Vγ1+ and Vγ4+ percentages of all splenic γδ T cells. (D) Total number of TCRγδ+Vγ1+ and TCRγδ+Vγ4+ subsets. In panels B–D, 6–10 samples per condition pooled from three experiments were used. * P < 0.05 for infected vs. non-infected mice.
It is known that γδ T cells help to control virus dissemination and prevent mortality from WN encephalitis partially due to their IFN-γ producing activity [22]. To further determine the role of γδ T cell subsets during WNV infection, we measured IFN-γ producing activity of γδ T cell subsets after ex vivo treatment with PMA and ionomycin. At day 3 post-infection, about 27% of γδ T cells produce IFN-γ. Among them, a vast majority are Vγ1+ IFN-γ+ (22%), whereas 2.4% of them are Vγ4+IFN-γ+ (Fig. 3A). Therefore, Vγ1+ T cells are the major γδ T cell subsets producing IFN-γ at the early stage of WNV infection. We next analyzed IFN-γ production by Vγ1+ T cells in young and aged mice at day 3 post-infection. The percentage of IFN-γ producing Vγ1+ T cells in young mice (34%) was shown to be similar to that of aged mice (35%) as shown in Fig. 3B (Vγ1+γδ+ cells were gated for analysis). However, total number of Vγ1+IFN-γ+ cells in young mice was still three-fold higher than in aged mice due to the differences in T cell expansion (Fig. 2D). To verify the contribution of Vγ1+ cells to protective immunity against WNV infection, we depleted these cells from 6-week-old B6 mice. Mice were treated with an i.p. injection of 100 μg of hamster anti-mouse Vγ1 daily for 2 days. Controls were treated with hamster IgG. The efficiency of the depletion at 24 h post-treatment was analyzed by using both antibodies to TCR γδ and Vγ1 in flow cytometry. As shown in Fig. 3C, total γδ+ T cells were reduced over 56% after Vγ1+ depletion (3.9% vs. 1.68%), and Vγ1+γδ+ population dropped over 86% (2.3% vs. 0.32%). Twenty-four hours after the depletion of Vγ1+ T cells, mice were challenged with a dose close to LD50 for WNV. We noted a nearly 3-fold higher viremia in Vγ1+ T cell depleted mice than in control mice at day 3 post-infection (Fig. 3D). Moreover, B6 mice that were depleted of Vγ1+ T cells were found to be more susceptible to WNV infection than control mice (Fig. 3E, P < 0.01), suggesting a role of Vγ1+ T cells in limiting virus replication and protection mice from mortality
Figure 3. Vγ1+ T cells are protective during WNV infection.


Splenic T cells were isolated from WNV-infected mice at day 3 post-infection and were cultured ex vivo with PMA plus ionomycin and stained for TCRγδ, Vγ1 or Vγ4 and IFN-γ. (A) Percent positive of γδ T cells producing IFN-γ in young mice. γδ T cells were gated for analysis of the percentage of IFN-γ+, Vγ1+ IFN-γ+ or Vγ4+ IFN-γ +. Data reflects 3–4 mice per condition, and are representative of three separate experiments. (B) Potential of Vγ1+ T cells producing IFN-γ in young and aged mice. γδ + Vγ1+ cells were gated for analysis. One representative from three independent experiments was shown. (C)–(E) 6–10-week-old B6 mice were given an i.p. injection of 100 μg of anti-Vγ1 on two consecutive days. Control mice were treated with the same amount of hamster IgG. At 24h post-depletion, splenic T cells were isolated and stained with antibodies to TCRγδ and Vγ1 to confirm depletion via flow cytometry (C). 24hours post-treatment, mice were challenged with 80 PFU of WNV. (D) Vγ1 cell depleted mice have a higher viremia at day 3 post-infection as determined by Q-PCR. The y-axis depicts the ratio of the amplified WNV-E cDNA to β-actin cDNA of each sample. 4–5 mice were used per condition performed in duplicates and are representative of two independent experiments. * P < 0.05 for control vs. Vγ1+ cell depleted mice. (E) Mice were monitored for survival following infection. ** P = 0.0005 < 0.01 for control mice (n = 10) vs. Vγ1+ cell depleted group (n = 10).
Aged mice maintain a higher content of Vγ4+ T cells following WNV infection, suggesting a potential role of these cells in viral pathogenesis. Vγ4+ T cells have been reported to induce inflammatory responses directly or bias CD4+ T cells toward dominant Th1 or Th17 responses [20, 29]. In study of Vγ4+ T cell functions during WNV infection, we found that the percentage of Vγ4+ T cells producing TNF-α (54%) is considerably higher than that of Vγ1+ T cells (37%) at day 3 post-infection (Fig. 4A). To verify these results, we next depleted Vγ4+ T cells from 6-week-old B6 mice. Mice were treated with an i.p. injection of 100 μg of hamster anti-mouse Vγ4 daily for 2 days. Controls were treated with hamster IgG. Similar to Vγ1 depletion, the efficiency of the depletion at 24 h post-treatment was analyzed by using both antibodies to TCR γδ and Vγ4 in flow cytometry. As shown in Fig. 4B, total γδ+ T cells were reduced over 13% after Vγ4+ depletion (3.55% vs. 3.08%), and Vγ4+γδ+ cells dropped over 82% (0.65% vs. 0.12%). As these cells are potentially pathogenic, we next used a higher dose to study host resistance to WNV infection in the depleted mice. Twenty-four hours after the depletion of Vγ4+ cells, mice were challenged with 700 PFU, a dose close to LD100 for WNV. Depletion of Vγ4+ T cells leads to a significant reduction of TNF-α production from splenic γδ T cells (Fig. 4C) and in blood (Fig. 4D) at day 3 post-infection. TNF-α receptor 1 signaling is known to be vital for blood brain barrier (BBB) compromise and virus entry into CNS [24]. We also noted that viral load in brains of Vγ4+ T cell depleted mice was significantly lower than in control mice at day 7 post-infection (Fig. 4E, P < 0.01). In consistent with these results, mice depleted of Vγ4+ T cells were more resistant to WNV infection than controls (Fig. 4F, P < 0.05).
Figure 4. Vγ4+ T cells are pathogenic during WNV infection.
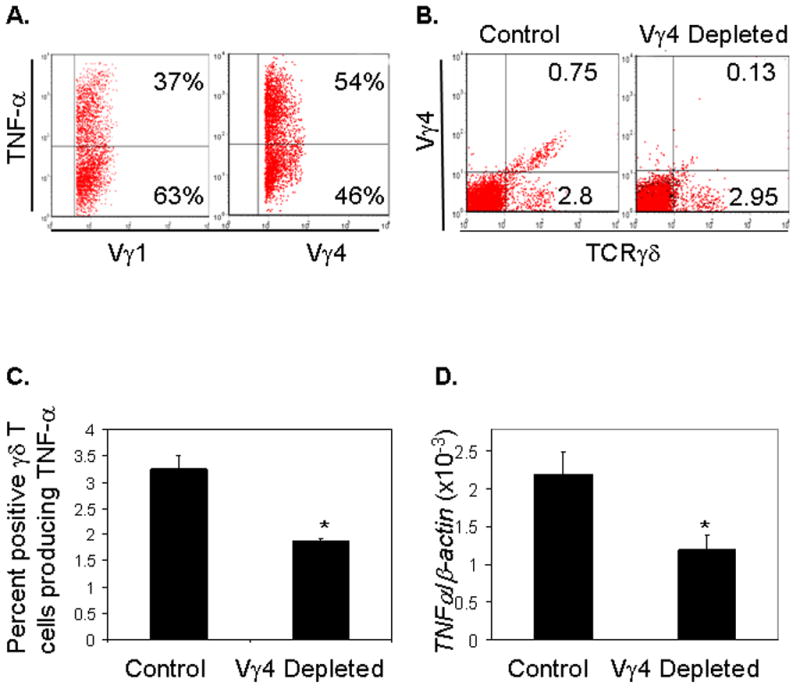

Splenic T cells were isolated from WNV-infected young mice at day 3 post-infection and were cultured ex vivo with PMA plus ionomycin and stained for TCRγδ, Vγ4 or Vγ1, and TNF-α. (A) Percent positive Vγ1+ or Vγ4+ T cells producing TNF-α. γδ+Vγ1+ T cells or γδ+Vγ4+ cells were gated for analysis. Data reflects 3 mice per condition, and are representative of three separate experiments. (B)– (F) Mice were given an i.p. injection of 100 μg of anti-Vγ4 on two consecutive days. Control mice were treated with the same amount of hamster IgG. At 24h post-depletion, splenic T cells were isolated and stained with antibodies against TCRγδ and Vγ4 to confirm depletion via flow cytometry (B). 24hours post-treatment, mice were challenged with 700 PFU of WNV. (C) Percent positive γδ T cells producing TNF-α in controls or Vγ4+ T cell depleted mice at day 3 post-infection. * P < 0.05 for controls vs. Vγ4+ T cell depleted mice. 4 mice per condition were used. Similar experiments were repeated twice. (D) Vγ4+ cell depleted mice have a reduced TNF-α production in the blood at day 3 post-infection as determined by Q-PCR. The y-axis depicts the ratio of the amplified TNF-α cDNA to β-actin cDNA of each sample. 4–5 mice per condition was used, performed in duplicates and are representative of two independent experiments. * P < 0.05 for control vs. Vγ4+ T cell depleted mice. (E) Vγ4+ cell depleted mice have a reduced viral load in the brain at day 7 post-infection as determined by Q-PCR. The y- axis depicts the ratio of the amplified WNVE cDNA to β-actin cDNA of each sample (8–10 samples per condition from two experiments were pooled). ** P < 0.01 for control vs. Vγ4+ T cell depleted mice. (F) Mice were monitored daily for survival following infection. * P = 0.03 < 0.05 for control mice (n = 9) vs. Vγ4+ T cell depleted mice (n = 9).
DISCUSSION
Following the initial subcutaneous or i.p. infection, WNV induces a systemic infection and eventually invades the CNS [30, 31]. Although how WNV crosses the BBB and enters CNS is not clearly understood, it was suggested that WNV infects the CNS in part via hematogenous spread, as an increased viral burden in serum correlates with earlier viral entry into the brain [32]. Moreover, systemic WNV replication induces a proinflammatory response that modulates BBB permeability, which in turn may enable viral entry into the brain and induce lethal encephalitis [24, 33]. Therefore, it appears critical to control virus dissemination in the periphery during early stage of WNV infection.
In this study, we have defined aging as a risk factor in the murine model of WNV infection. Aged mice are found to be more susceptible to WNV infection, and show a persistently elevated systemic infection. Our previous work has demonstrated that γδ T cells protect the host from lethal encephalitis by limiting the viral load and invasion of the CNS within the first few days following infection [22]. Here, consistent with these findings, we have shown a slow and reduced γδ T cell expansion in aged mice in comparison to young mice. In further investigation of the role of discrete subpopulations of γδ T cells during WNV infection, we found Vγ1+ T cells of young mice expand dramatically at early stage of WNV infection, whereas Vγ4+ T cells expand only modestly. In contrast, Vγ1+ T cells of aged mice responded to WNV infection in a slower and reduced manner. Vγ4+ T cells of aged mice maintained at higher level than young mice at day 3 post-infection. Collectively, these data suggest that the differences in γδ subsets response to WNV infection could explain the enhanced susceptibility of aged mice to WN encephalitis.
γδ T cells have been shown to control virus dissemination and prevent mortality from WNV infection partially due to their IFN-γ producing activity [22]. Vγ1+ T cells are the major γδ T cell subset producing IFN-γ during WNV infection. Although the percentage of Vγ1+ T cells producing IFN-γ is similar in young and aged mice, the total number of Vγ1+lFN-γ+ cells in young mice is higher than that in aged mice. Moreover, mice depleted of Vγ1+ cells had an enhanced viremia and higher mortality to WNV encephalitis, suggesting a role of these T cells in protection. Our previous studies have shown that TNF-α receptor 1 signaling is vital for BBB compromise and virus entry into CNS [24]. We found that Vγ4+ T cells have a greater potential for TNF-α production than Vγ1+ T cells. Depletion of Vγ4+ T cells caused a significant reduction in TNF-α production in the periphery at day 3 post-infection. This was followed by a lower viral burden in the brains of Vγ4+ T cell depleted mice at day 7 post-infection and a higher mortality as well. Overall, it seems likely that the higher content of Vγ4+ T cells in aged mice could contribute to more virus entry into CNS, thereby inducing more severe encephalitis and higher mortality. Immune factors are reported to modify with advancing age, including both cellular and humoral immune responses. Human γδ T cells are known to display numerical and functional alteration in the elderly [34–40]. Overall, these results revealed a major difference in γδ T cell subsets between young adult and aged mice in response to WNV infection. Our findings may provide important clues as to the pathogenesis of human WNV infection.
Evidence for opposite roles of Vγ1+ T cells and Vγ4+ T cells in the resolution of pathogen infection has been reported during Coxsackie virus [20] and murine cytomegalovirus infection [41]. Our data provide the first evidence that Vγ1+ T cells play an important role in protecting host against WNV infection and that Vγ4+ T cells are involved in WNV pathogenesis. The induction of proinflammatory cytokines by Vγ4+ T cells was exacerbated in Vγ1-depleted mice, suggesting that Vγ1+ T cells counteract or prevent the detrimental actions of Vγ4+ T cells, possibly via their cytotoxic effect on macrophages [42]. Thus the maintenance of a balance between Vγ1+ and Vγ4+ T cell function appears to be critical to the outcome of an infection. Activation of γδ T cells by nonspecific immunomodulators offers a possible avenue of boosting the immune response to acute infection, as demonstrated in other viral models [43]. Combined with the findings in the aged mouse model, our findings also imply that γδ T cells could be important in WNV treatment, and in vaccine development as a potential target population.
Acknowledgments
The authors thank Matt Whitney for technical assistance. This worked is supported by grant from American Federation for Aging Research (to T.W.), the National Institutes of Health 2R01AI44920 and R21AI063400 (to R.L.O.), 2R01HL65410 (to W.K.B.) and 1R01 AI072060-01A2 (to T.W.).
References
- 1.Solomon T, Ooi MH, Beasley DW, Mallewa M. West Nile encephalitis. Bmj. 2003;326:865–869. doi: 10.1136/bmj.326.7394.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, Garmendia AE, Van Kruiningen HJ. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Fikrig E. Immunity to West Nile virus. Curr Opin Immunol. 2004;16:519–523. doi: 10.1016/j.coi.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- 7.Halevy M, Akov Y, Ben-Nathan D, Kobiler D, Lachmi B, Lustig S. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch Virol. 1994;137:355–370. doi: 10.1007/BF01309481. [DOI] [PubMed] [Google Scholar]
- 8.Hayday A, Pao W. T cell receptor gd. Encyclopedia of Immunology. 1997 in press. [Google Scholar]
- 9.Ferrick DA, King DP, Jackson KA, Braun RK, Tam S, Hyde DM, Beaman BL. Intraepithelial gamma delta T lymphocytes: sentinel cells at mucosal barriers. Springer Semin Immunopathol. 2000;22:283–296. doi: 10.1007/s002810000047. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera M, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. Journal of Experimental Medicine. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin R. γδ T lymphocytes in human tuberculosis. Journal of Infectious Diseases. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, et al. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabelitz D, Wesch D. Role of gamma delta T-lymphocytes in HIV infection. Eur J Med Res. 2001;6:169–174. [PubMed] [Google Scholar]
- 16.Chauhan SK, Singh M, Nityanand S. Reactivity of gamma/delta T cells to human 60-kd heat-shock protein and their cytotoxicity to aortic endothelial cells in Takayasu arteritis. Arthritis Rheum. 2007;56:2798–2802. doi: 10.1002/art.22801. [DOI] [PubMed] [Google Scholar]
- 17.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 18.Bank I, DePinho RA, Brenner MB, Cassimeris J, Alt FW, Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986;322:179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki G, Yamada H, Kishihara K, Yoshikai Y, Nomoto K. Mechanism of murine Vgamma1+ gamma delta T cell-mediated innate immune response against Listeria monocytogenes infection. Eur J Immunol. 2002;32:928–935. doi: 10.1002/1521-4141(200204)32:4<928::AID-IMMU928>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 21.Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. J Virol. 2002;76:10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-gamma-producing gammadelta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 23.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field- collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 25.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 26.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 28.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 29.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of Collagen-Induced Arthritis by Oligoclonal, IL-17-Producing {gamma}{delta} T Cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. doi: 10.1111/j.1749-6632.2001.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Anderson JF, Magnarelli LA, Wong SJ, Koski RA, Fikrig E. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167:5273–5277. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 32.Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, Wong SJ, Montgomery RR, Fikrig E, Bucala R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest. 2007;117:3059–3066. doi: 10.1172/JCI32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argentati K, Re F, Donnini A, Tucci MG, Franceschi C, Bartozzi B, Bernardini G, Provinciali M. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J Leukoc Biol. 2002;72:65–71. [PubMed] [Google Scholar]
- 35.Cardillo F, Falcao RP, Rossi MA, Mengel J. An age-related gamma delta T cell suppressor activity correlates with the outcome of autoimmunity in experimental Trypanosoma cruzi infection. Eur J Immunol. 1993;23:2597–2605. doi: 10.1002/eji.1830231033. [DOI] [PubMed] [Google Scholar]
- 36.Colonna-Romano G, Potestio M, Aquino A, Candore G, Lio D, Caruso C. Gamma/delta T lymphocytes are affected in the elderly. Exp Gerontol. 2002;37:205–211. doi: 10.1016/s0531-5565(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 37.Ku CC, Kappler J, Marrack P. The growth of the very large CD8+ T cell clones in older mice is controlled by cytokines. J Immunol. 2001;166:2186–2193. doi: 10.4049/jimmunol.166.4.2186. [DOI] [PubMed] [Google Scholar]
- 38.Turner J, Frank AA, Brooks JV, Marietta PM, Vesosky B, Orme IM. Tuberculosis in aged gammadelta T cell gene disrupted mice. Exp Gerontol. 2001;36:245–254. doi: 10.1016/s0531-5565(00)00199-6. [DOI] [PubMed] [Google Scholar]
- 39.Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ, Staal FJ. Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol. 2005;115:834–840. doi: 10.1016/j.jaci.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J Exp Med. 2002;195:283–293. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ninomiya T, Takimoto H, Matsuzaki G, Hamano S, Yoshida H, Yoshikai Y, Kimura G, Nomoto K. Vgamma1+ gammadelta T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology. 2000;99:187–194. doi: 10.1046/j.1365-2567.2000.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrew EM, Newton DJ, Dalton JE, Egan CE, Goodwin SJ, Tramonti D, Scott P, Carding SR. Delineation of the function of a major gamma delta T cell subset during infection. J Immunol. 2005;175:1741–1750. doi: 10.4049/jimmunol.175.3.1741. [DOI] [PubMed] [Google Scholar]
- 43.Hoq MM, Suzutani T, Toyoda T, Horiike G, Yoshida I, Azuma M. Role of gamma delta TCR+ lymphocytes in the augmented resistance of trehalose 6,6′-dimycolate-treated mice to influenza virus infection. J Gen Virol. 1997;78:1597–1603. doi: 10.1099/0022-1317-78-7-1597. [DOI] [PubMed] [Google Scholar]


