SUMMARY
Visual perceptual learning is defined as performance enhancement on a sensory task and is distinguished from other types of learning and memory in that it is highly specific for location of the trained stimulus. The location specificity has been shown to be paralleled by changes in neural activity in V1 or V4 of monkeys [1, 2] and enhancement in functional magnetic resonance imaging (fMRI) signal in the trained region of the primary visual cortex (V1) [3–5] after visual training. Although recently the role of sleep in strengthening visual perceptual learning has attracted much attention, its underlying neural mechanism has yet to be clarified. Here, for the first time, fMRI activation of early visual cortex was measured and compared during sleep with and without preceding visual perceptual learning training. The fMRI measurement was conducted concurrently with polysomnogram, which indicates a subject’s sleep/wake status. As a result of predetermined region-of-interest (ROI) analysis of the human primary cortex (V1), activation enhancement during non rapid eye movement sleep after training was observed specifically in the trained region of V1. Furthermore, improvement of task-performance measured subsequently to the post-training sleep session was significantly correlated with the amount of the trained-region-specific fMRI activation in V1 during sleep. These results suggest that as far as V1 is concerned, only the trained region is involved in improving task performance after sleep.
RESULTS
Recently, the results of a number of studies suggest that sleep plays a role of improving performance of a texture discrimination task (TDT) trained before sleep [4, 6–10]. Using fMRI, previous studies compared blood oxygen level dependent (BOLD) signal while subjects were awake and performing TDT before and after sleep subsequent to training on the TDT, and found that the BOLD signal in the region in the low-level visual cortex corresponding to the trained stimulus location was enhanced after sleep [4, 9].
In order to address the question regarding what processing occurs for improvement of visual perceptual learning during sleep, it is necessary to measure brain activation during sleep subsequent to training of a visual perceptual learning task by using fMRI, which provides sufficient localization ability. The present study constitutes the first attempt to do so using this methodology. We also conducted concurrent polysomnogram (PSG) monitoring (Supplemental SFig. 1), to determine precisely the sleep or wakefulness status of the subject during fMRI measurement. Primarily, we tested the hypothesis that the sleep consolidation process in visual perceptual learning occurs specifically in the trained region. Note that, with regards to V1, activation has been found to be modulated during training on the TDT (i.e., while the subjects are awake) but only in the trained location [3, 4, 9]. Thus, using ROI (region of interest) analysis, we specifically tested whether only the trained region of V1 or other regions of V1 as well as the trained region are activated during sleep subsequent to training. The ROI analysis is an important starting point for extending future research to involve whole brain processing analysis [11].
Our main experiments involved pre-training and post-training fMRI sessions (Fig. 1). BOLD signal in humans were measured mostly during sleep (although some wakefulness periods were included), before (pre-training) and after (post-training) training of the TDT. See Supplemental data for a more detailed procedure. PGS indicated that the subjects slept more than 80% of the time during both the pre- and post-training sleep sessions (see Supplemental Result 1, SFig. 2, and STable 1).
Fig. 1.
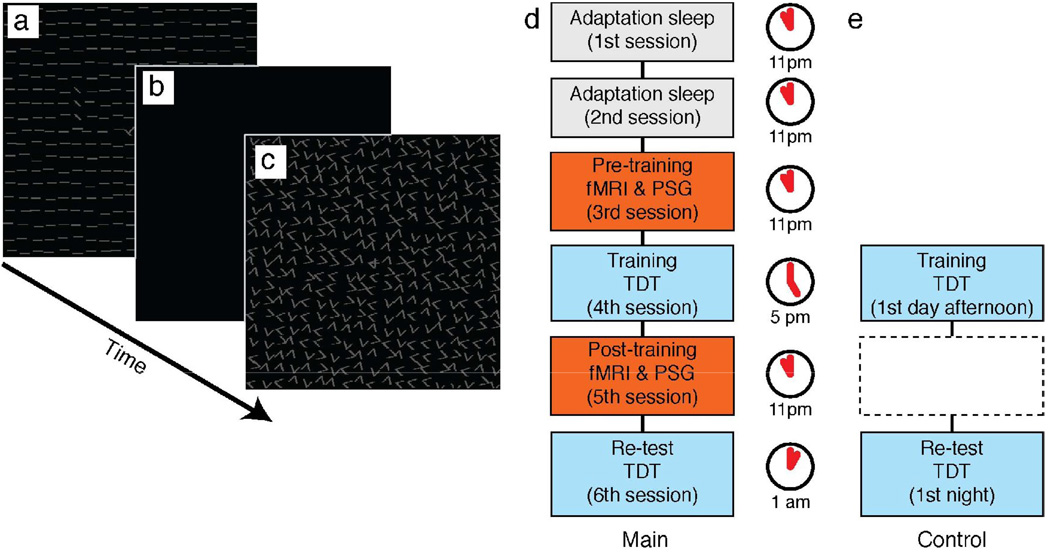
Experimental procedure. See Supplemental data for greater details. (a)–(c) Texture discrimination task (TDT). During each trial, the subjects were presented with a target display (a) which contained a triplet of diagonally orientated bars within the upper-left quadrant (n=4) or lower-right quadrant (n=3) (counter-balanced across subjects) and a letter (“T” or “L”) presented at the center fixation point of the display. The target and letter were presented against a background consisting of horizontally oriented bars. The target display was followed by a blank display (b) whose duration varied from trial to trial, followed by a masking noise display (c). In each trial, subjects were asked to report whether the central letter was a “T” or “L” and then whether the orientation of the triplet was vertical or horizontal. (d) Main experiment. The subjects were asked to spend the first night of adaptation sleep (1st session) in a mock scanner that physically mimics an actual MRI scanner. The subjects were then asked to spend the second night of adaptation sleep (2nd session) in an actual MRI scanner while scanning was conducted. All electrodes required for the real measurements were attached during the adaptation periods. On the night of the 3rd session, a pre-training fMRI session was conducted for 90 min during which polysomnogram (PSG) was concurrently obtained with fMRI. On the late afternoon of the fourth day (4th session), the subjects were trained on the TDT. Approximately 6 hours after the training session, a post-training fMRI session (5th session) was conducted with the procedure identical to that of the pre-training fMRI session. After the post-training fMRI session, a re-test of the TDT was conducted (6th session). (e) Control experiment. Here, only the training and re-test sessions were conducted with a new group of subjects (see Supplemental data). The onset times of these sessions were about the same as those of the training and re-test sessions in the main experiment.
V1 activity during sleep and performance improvement after sleep: ROI analysis
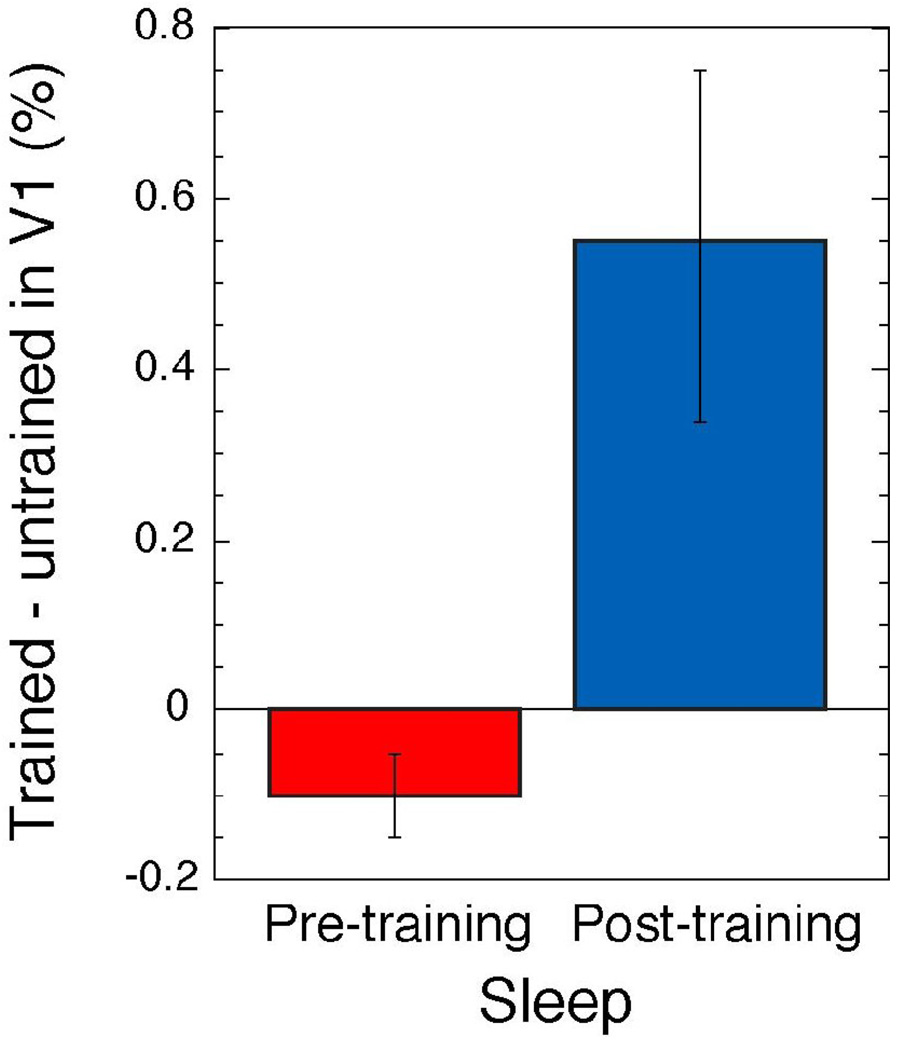
It has been found that in V1, the enhanced activation was observed specifically in the trained location while subjects were conducting the task after learning the TDT [3, 4, 9]. To test the hypothesis that a consolidation process in perceptual learning occurs during sleep specifically in the trained region, we used ROI analysis that targeted V1. We identified detailed retinotopic representation in the visual cortex including V1 for each subject by a retinotopic mapping technique [3, 12] to localize trained and untrained locations of low-level visual cortical areas. These areas were functionally demarcated for 4 quadrants of the visual field within 5-deg eccentricity (see Supplemental data). We defined sleep activation as the BOLD signal measured while the PSG indicated that the subject was awake subtracted from the BOLD signal measured while the PSG indicated that the subject was in non rapid eye movement (NREM) sleep during the pre-training and post-training fMRI sessions (see Supplemental data for more detail). During the pre-training fMRI session, sleep activation levels were the same in the trained and untrained regions of V1. However, during the post-training fMRI session, the sleep activation in the trained region of V1 was significantly higher than in the untrained regions (p<0.018, Wilcoxon signed-rank test). In addition, the amount of sleep activation in the untrained region of V1 subtracted from that in the trained region was significantly larger during the post- than during the pre-training fMRI sessions (Fig. 2, Wilcoxon signed-rank test, p<0.018).
Fig. 2.
Sleep activation. Mean subtraction (± 1 SEM) of sleep activation in the untrained region from that in the trained region of V1, for the pre- and post-training fMRI sessions.
In learning of a visuomotor task, it has been reported that the activation observed during the visuomotor training persisted covertly in the specific brain region during wakefulness after the training [13]. Thus, we tested whether the difference in activity between the trained and untrained regions of V1 was specific to the status of sleep in TDT perceptual learning. Immediately before sleep onset in both the pre-training and post-training fMRI sessions, we presented the checkerboard patterns (see Supplemental data) and measured the subjects’ brain activation via fMRI. The responses to the checkerboard patterns in the trained and untrained regions of V1 as compared to the responses to the fixation point were not significantly different from each other during the pre-training and post-training fMRI sessions (see Supplemental Result 2, and SFig. 3). This result suggests that excitability or general responsiveness in the trained region of V1 was equivalent to that in the untrained region while the subjects were awake with the lights on, which occurred after the intensive training during the post-training fMRI session, and that the difference in the V1 activity in the trained and untrained regions that we found in the post-training sleep fMRI session may be specific to the post-training sleep period (see Supplemental Discussion and SFig. 4 for more detail regarding V1 activation associated with TDT).
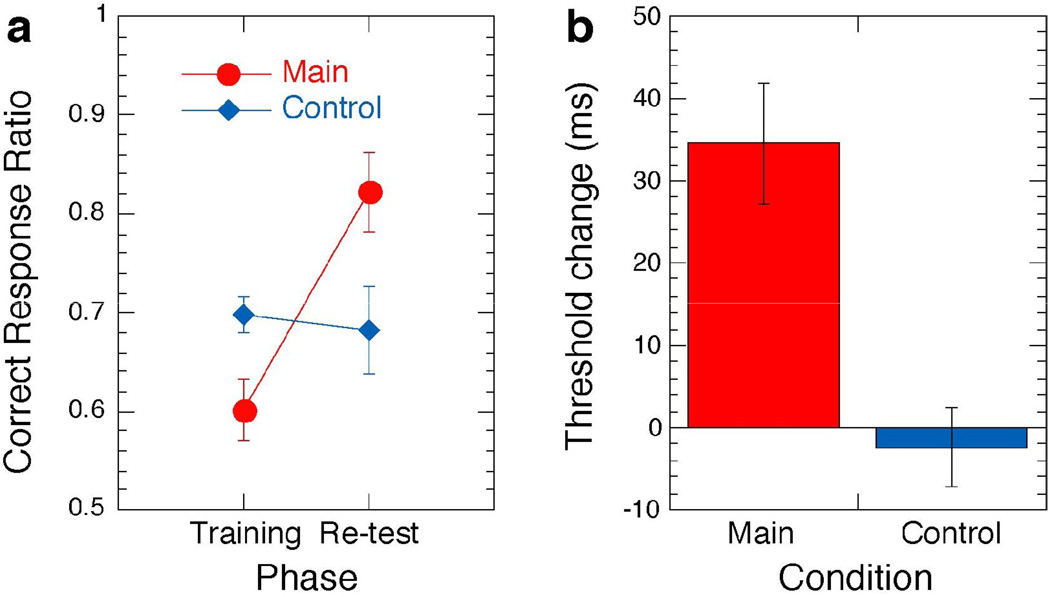
The mean correct response during the last two blocks of the re-test was significantly higher than during the corresponding two blocks of the training session (red circles in Fig. 3A, Wilcoxon signed-rank test, p<0.018). In addition, we obtained the threshold time interval between the onset of the test stimulus and following the masking noise pattern, that is, stimulus-to-mask onset asynchrony, SOA, which is another measure of learning: The shorter the interval is, the more difficult the task becomes (see [14]). The threshold time interval for 80% correct response was significantly shorter during the re-test session vs. the training session (red bar in Fig. 3B, Wilcoxon signed-rank test, p<0.03). These results indicate that performance improved after sleep subsequent to training.
Fig. 3.
Mean performance changes during the main and control experiments. (a) Mean correct response changes (± 1 SEM) for the corresponding two blocks (SOAs) for each subject in the training and re-test sessions (with the same SOAs) in the main experiment (with sleep) and the control experiment (without sleep Fig. 1e, see Supplemental data for detailed procedure for this control group and Supplemental Result 5). In the main experiment, the correct response in the re-test session was significantly higher than in the training session, whereas in the control experiment, the correct responses in the training and re-test sessions were not significantly different. (b) A threshold SOA change (mean ± 1 SEM) is defined as subtraction of the threshold SOA in the re-test session from that in the training session. Thus, a positive change in threshold SOA indicates that the threshold SOA became shorter, and that performance improved in the re-test session. Significant performance improvement was found in the main condition whereas no significant improvement was found in the control condition.
Importantly, sleep activation in the trained region of V1 was highly correlated with the level of performance improvement (r=0.82 for threshold SOA reduction, r=0.83 for correct response increase, see Supplemental Result 3 and SFig. 5). However, sleep activation in the untrained region of V1 was not significantly correlated with the level of performance improvement. These results indicate that this trained region specific activation of V1 during sleep after training is highly involved in the performance improvement observed after sleep and reflects processing specifically for improving learning rather than residual activation after training while subjects were awake. Taken together, the results indicate that a highly localized activation specific to the trained region of V1 is involved in improving PL during sleep.
DISCUSSION
The present study is the first to measure brain activation with and without preceding training of a visual task during sleep that utilized concurrent fMRI and PSG recordings. The latter allows for precise identification of on-going sleep status. The results revealed significantly increased activation specifically in the trained location of V1 during NREM sleep subsequent to the training of a visual task. This increased activation in the trained region was specific to the sleep status. Notably, the amount of activation in the trained location of V1 during sleep in the post-training fMRI session was highly correlated with performance improvement after sleep. These results indicate that activity enhancement specifically in the trained location of V1 during sleep reflects processing that improves visual perceptual learning and are in accord with the hypothesis that consolidation of learning occurs during sleep after training.
Two models have been proposed to account for performance enhancement after sleep subsequent to training for learning in general; synaptic homeostasis model [15] and reactivation model [16–18]. The reactivation model indicates that neurons that are involved in learning acquisition are covertly reactivated during sleep to strengthen neuronal connections [16–18]. For example, firing-rate patterns during training of episodic memory in cortical areas including the hippocampus and the medial prefrontal cortex in rats [19, 20]. In addition, it has been shown that the brain regions, which were recruited during the training showed enhanced brain activation during sleep in human PET studies [21, 22]. The reactivation model predicts activation in the area highly related to trained memory/learning during sleep and is in accord with the present finding of BOLD signal enhancement in the trained area of V1 during sleep and performance enhancement after the sleep. The synaptic homeostasis model [15] indicates that slow-wave activity, which is prominent during early NREM sleep, plays a role in scaling down synapses including those that are excessively increased or strengthened by a learning acquisition process during wakefulness. This model is supported by increased slow-wave activity near the motor and parietal areas in the right hemisphere during sleep after implicit motor learning [23]. While this model is highly intriguing, from our results, which are not based on any spectral analysis, it is difficult to judge the validity of the homeostasis model. If a downscaling requires an active molecular process (cf. [24, 25]) resulting in increased metabolism, the present result would be in accordance with synaptic homeostasis model.
In the current study, we had an apriori anatomical hypothesis that in V1 the sleep consolidation process in visual perceptual learning occurs specifically in the trained region. To test the hypothesis, we made a pre-determined ROI analysis that targeted V1 and obtained results supporting the hypothesis. Although the result was in accord with our hypothesis, note that this does not indicate that other areas are not involved in consolidation of sleep. Some suggest that post-sleep changes on this task have been identified in the later visual cortical regions - both within the occipital, temporal and parietal areas [9]. In addition, the dramatic connectivity changes during NREM sleep [11, 26] may suggest that a learned representation during wakefulness may lead to plasticity processes not only in the trained location, but in reciprocal areas connected to it as well. For example, Schwartz et al. indicate that connectivity of other areas including the left frontal cortex, to V1 that was observed at the beginning of training disappeared 24 hours after training of TDT in visual perceptual learning [4]. Thus, examining connectivity during sleep after training would constitute an important future study. Since location of target presentation was counterbalanced across the subjects in the present experiment, this design is not suitable for analysis of multiple brain areas. However, to obtain some idea, sleep activation in the whole cortex is shown in Supplemental SFig. 6. This shows enhanced activity in the trained region of V1 that was found to be significant when predetermined ROI analysis was applied as aforementioned. Though less clearly, some activity in the left dorsolateral prefrontal area was also observed (see Supplemental Result 4 for the limitation of this analysis to our data). A future study with an experimental design suitable for multiple areas analyses would clarify whether the left dorsolateral prefrontal area activation is significantly involved in consolidation during sleep.
In the present study, we did not investigate brain activation during REM sleep. While we found trained region specific brain activation in V1 associated with consolidation of visual perceptual learning during NREM sleep, it is possible that REM sleep also plays a role in consolidation of visual perceptual learning [16, 27, 28]. Previous studies have indicated the involvement of REM sleep in consolidation of the visual task used in the present study [6, 8]. PET studies in humans have also shown involvement of REM sleep: Activation in the brain regions, which were recruited for the visuomotor training before the sleep was enhanced [21, 29], with changed connectivity between the frontal and parietal regions [30] during REM sleep. Thus, it is possible that the trained region specific activation in V1 during NREM sleep, which was found in our study, is just a part of multiple stages of consolidation processing during sleep which usually lasts several hours in adults [11]. Future studies are required to test this possibility.
In the present study, we measured and compared BOLD signal during sleep with and without preceding visual training with concurrent PSG measurement. For the first time, we observed a significant amount of activation specifically in the trained region of V1 during sleep after training of a visual task that was highly correlated with performance increase after sleep. In this initial study, we utilized ROI analysis and concentrated on examining activation in V1. Future studies should clarify whether brain areas other than V1 are also involved and how cortical connectivity of the trained area of V1 to other areas may change during sleep after training, as well as how REM sleep is involved in sleep consolidation.
Supplementary Material
Supplemental Data including detailed experimental procedures, additional results and figures are provided in Supplemental data.
ACKNOWLEDGEMENTS
This work was supported by the National Eye Institutes (R21EY018925, R01EY019466, and R01EY015980), NIH National Center for Research Resources (P41RR14075), the Mental Illness and Neuroscience Discovery (MIND) Institute, the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Med School, the ERATO Shimojo Implicit Brain Function Project from Japan Science and Technology, and Japan Society for the Promotion of Science. The authors thank Patrick Purdon, and Leonardo Angelone for assistance in simultaneous measurements of EEG and fMRI, and Kristina Vissher and Aaron Seitz for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 2.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci U S A. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 7.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 8.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 9.Walker MP, Stickgold R, Jolesz FA, Yoo SS. The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex. 2005;15:1666–1675. doi: 10.1093/cercor/bhi043. [DOI] [PubMed] [Google Scholar]
- 10.Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- 11.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fize D, Vanduffel W, Nelissen K, Denys K, Chefd’Hotel C, Faugeras O, Orban GA. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J Neurosci. 2003;23:7395–7406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peigneux P, Orban P, Balteau E, Degueldre C, Luxen A, Laureys S, Maquet P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 17.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 18.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 19.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 21.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 22.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 24.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 26.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 27.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nissen C, Kloepfer C, Nofzinger EA, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia--a pilot study. Sleep. 2006;29:1068–1073. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- 29.Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20:125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- 30.Laureys S, Peigneux P, Phillips C, Fuchs S, Degueldre C, Aerts J, Del Fiore Fiore, Petiau C, Luxen A, van der Linden M, et al. Experience-dependent changes in cerebral functional connectivity during human rapid eye movement sleep. Neuroscience. 2001;105:521–525. doi: 10.1016/s0306-4522(01)00269-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data including detailed experimental procedures, additional results and figures are provided in Supplemental data.