Summary
Prolotherapy is an alternative injection-based therapy for chronic musculoskeletal pain. Three different proliferants, D-glucose (dextrose), phenol-glucose-glycerine (P2G), and sodium morrhuate, used in prolotherapy are hypothesized to strengthen and reorganize chronically injured soft tissue and decrease pain through modulation of the inflammatory process.
Hypothesis
Commonly used prolotherapy solutions will induce inflammation (leukocyte and macrophage infiltration) in medial collateral ligaments (MCLs) compared to needlestick, saline injection, and no-injection controls.
Methods
MCLs of 84 Sprague Dawley rats were injected one time at both the tibial and femoral insertions. Immunohistochemistry (IHC) was used to determine the inflammatory response at three locations (tibial and femoral insertions and midsubstance) six, 24, and 72 hours after dextrose injection compared to saline- and no-injection controls and collagenase (positive control) (n=4). QPCR was used to analyze gene expression 24 hours post-injection (n=4). Sodium morrhuate, P2G, and needlestick control were also investigated after 24 hours (n=4).
Results
In general, inflammation (CD43+, ED1+, and ED2+ cells) increased after prolotherapy injection compared to no-injection control but did not increase consistently compared to saline and needlestick control injections. This response varied by both location and proliferant. Inflammation was observed at six and 24 hours post-injection but was resolved by 72 hours compared to no-injection controls (p < 0.05). CD43+ leukocytes and ED2+ macrophages increased compared to needlestick and saline-injection control, respectively, 24 hours post-injection (p < 0.05).
Conclusion
Prolotherapy injections created an inflammatory response, but this response was variable and overall, not uniformly different from that caused by saline injections or needlestick procedures.
Keywords: medial collateral ligament, dextrose, sodium morrhuate, P2G, saline, needlestick
Introduction
Ligament and tendon exertional injury is a common condition seen in Emergency and Primary Care Medicine. Effects to both patient and society are substantial; sprains and strains account for at least 7% of all musculoskeletal office visits1 and more than five million visits to the emergency department annually in the United States.2 Incomplete healing from these injuries is common and can lead to chronic pain,3 joint instability and laxity,4 and is a risk factor for the development of osteoarthritis (OA).5 The majority of sprains are not complete ligament ruptures6 and are treated non-surgically. Many patients are refractory to usual care therapies7 including relative rest, non-steroidal anti-inflammatory medication (NSAIDS), and corticosteroid injection. While NSAIDs and injected corticosteroids have some effect on acute pain, neither targets chronic pain mechanisms, and neither has been effective in chronic tendinopathies.8–10 In a recent study on chronic tennis elbow, corticosteroid injections performed significantly worse than physical therapy and non-specific conservative treatment at one year.10
Prolotherapy (PrT) is commonly used for a variety of musculoskeletal conditions11–13 and is becoming increasingly popular in the US, where hundreds of practitioners use PrT for a number of clinical indications.14, 15 It has been advocated as a treatment for ligament and joint laxity. Treatment involves the injection of a solution known as a ‘proliferant’ at painful ligament or tendon insertions and in adjacent joint spaces in three to five treatment sessions performed at monthly intervals, although protocols vary.16 Positive treatment effects from PrT have been documented in thirty-four case series and case reports on over 3600 patients with a variety of chronic musculoskeletal pain conditions.13 Seven randomized controlled trials have assessed PrT for low back pain,17–20 OA,21, 22 and lateral epicondylosis.23 Positive effects of PrT compared to control injections for low back pain have been reported;18, 19 equivocal results have also been reported.17, 20 Studies assessing PrT for OA report positive outcomes for knee OA22 and equivocal results in finger/thumb joints.21 Significant methodological limitations in these studies limit direct comparison and efficacy evaluation. Our group recently reported significant and clinically meaningful pain and function outcomes in subjects with severe lateral epicondylosis treated with prolotherapy.23 At four-month follow-up, prolotherapy subjects, compared to subjects receiving saline control injections, reported near-total pain resolution and a 3.6 point absolute effect size on an 11-point Likert elbow pain scale (p<0.001). They also reported improved isometric resistance strength assessed using a Baltimore Therapeutic Equipment Primus (BTE) device (p<0.01) compared to control. Clinical effect was maintained at 52 weeks.
Three solutions are commonly used in PrT,15 D-glucose (dextrose), phenol-glucose-glycerin (P2G), and sodium morrhuate. These three PrT solutions are generally used individually and are hypothesized to create local irritation and subsequent inflammation and anabolic response,24 though these effects have not been rigorously assessed. In vitro and a few in vivo animal studies have analyzed some aspects of PrT solutions. Increased glucose concentration (D-glucose) causes an increase in cell protein synthesis,25, 26 DNA synthesis,25 and cell volume.25 The effects on cell proliferation are conflicting; some studies report increased proliferation25 and others report increased apoptosis.26 Phenol – glucose – glycerin (P2G, 1.25% – 12.5% – 12.5%) is hypothesized to be a stronger inflammatory stimulator.24 Phenol has been used in animal models to study acute irritant dermatitis by creating an inflammatory response.27 However, it has also been shown to be toxic to human colonic epithelial cells.28 In addition, phenol can temporarily block peripheral nerves in humans29 and damage the sciatic nerve in rats resulting in partial hind limb paralysis.30 Toxicity in the context of PrT has been studied in a rat model; the injectant Proliferol contains phenol and is similar to P2G (Proliferol – 0.25% lidocaine hydrochloride, 1% phenol, 12.5% dextrose, and 12.5% glycerin). Proliferol was found to create a temporary elevation in AST (aspartate aminotransferase) and ALT (alanine aminotransferase), suggesting skeletal muscle trauma or hepatic insult.31 A prior pilot study suggested that Proliferol creates an acute inflammatory reaction.32 Sodium morrhuate is an extract of cod liver oil and a sclerosing agent.33 In vitro, sodium morrhuate is toxic to granulocytes, red blood cells, and endothelial cells.34 Four animal studies have investigated sodium morrhuate as a prolotherapy agent. These studies found an increase in ligament strength,35, 36 mass, thickness,36 and a trend toward an increase in cell number, glycosaminoglycan content, and water content37 in ligaments injected with sodium morrhuate compared to saline36, 37 or no injection.35 The injection of Pomeroy solution, which includes sodium morrhuate, dextrose, mepivacine, and cyanocobalamine, did not alter strength or elastic modulus of crush-injured Achilles tendons.38 These in vitro and in vivo reports suggest that PrT solutions might produce an inflammatory response when delivered in vivo.
The aim of this study was to test the hypothesis that PrT injections cause an inflammatory response in knee ligaments. Specifically, we hypothesized that the three commonly used PrT solutions will induce an inflammatory response as assessed by leukocyte and macrophage infiltration into rat medial collateral ligaments (MCLs) compared to saline injection, needlestick, and uninjected controls. We also hypothesized that gene expression of two cytokines related to inflammation, platelet-derived growth factor (PDGF) and interleukin-1β (IL-1β), will be up-regulated following PrT injections. Needle and saline control groups were used in order to isolate the effects of the prolotherapy injectant. Needle trauma alone might induce inflammation due to trauma and subsequent bleeding and has been reported to improve symptoms of myofascial pain.39 A saline injection group was used to control for possible effects of added pressure or volume.
Methods
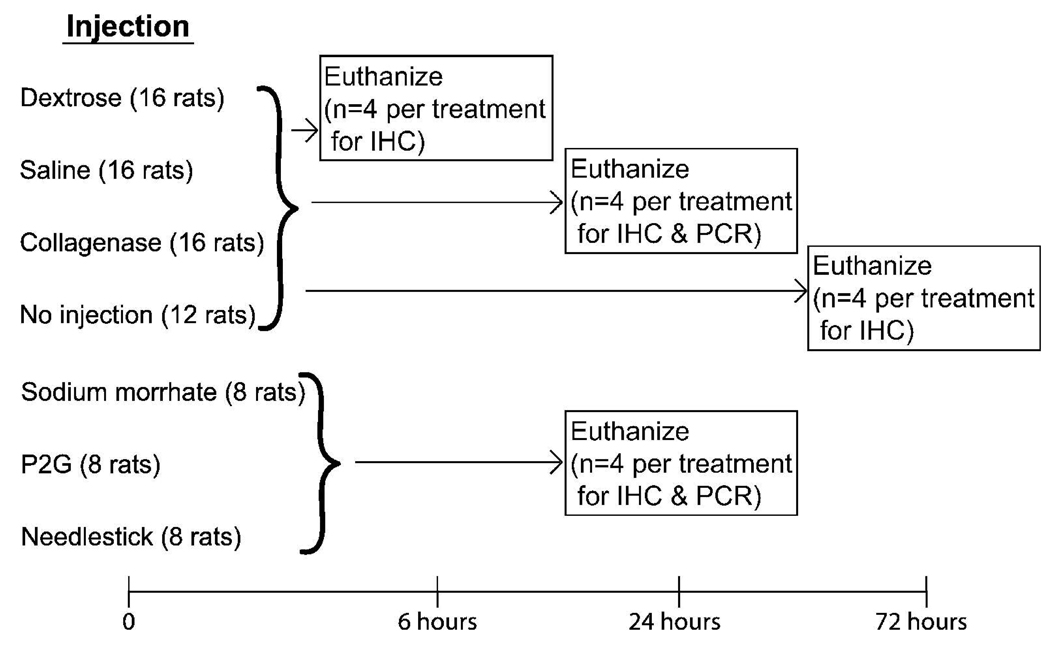
This study was approved by the University of Wisconsin Institutional Animal Use and Care Committee. Injections are expected to produce an inflammatory response, as expressed by increased CD43+ cells by six hours and ED1+ and ED2+ cells by 24 hours.40, 41 Eighty-four rats in seven treatment or control groups were injected or observed (no-injection control group) at baseline and assessed at six, 24, and 72 hours post-injection using IHC and qPCR analysis (Figure 1). All seven groups were assessed at 24 hours: three treatment groups (15% dextrose, 5% sodium morrhuate, and P2G), three control groups (saline, no injection, and needlestick), and a positive control collagenase I.42 Four groups of rats were additionally assessed at six and 72 hours: one treatment group (15% dextrose) and two control groups (saline and no injection) and collagenase. Dextrose was chosen for time points six and 72 hours post-injection because clinically it is widely used15 and has been investigated in human studies.20–22 Saline injections were used to control for the volume associated with proliferant injection; saline has been used as a control in human studies.17–20
Figure 1. Study Design.
Rats were injected at time zero and euthanized at six, 24, and 72 hours post-injection. Rats euthanized at all time points were injected with the most commonly used PrT solution (dextrose) or saline (as a control) or collagenase (as a positive control). The same no-injection control rats were used at the six and 24 hour time points. Additional PrT solutions (sodium morrhuate and P2G) and control (needlestick) were investigated 24 hours post-injection. MCLs at all time points were analyzed using immunohistochemistry (IHC) for inflammation, and MCLs at 24 hours post-injection were analyzed using quantitative polymerase chain reaction (qPCR) for inflammatory genes.
For immunohistochemical (IHC) analysis, rats in the six and 24-hour post-injection groups were injected unilaterally (n=4), and one additional group of four rats were used as non-injected controls for both time points. Rats in the 72 hour post-injection groups were injected bilaterally (n=4), and an additional four rats were used as non-injected controls. For quantitative polymerase chain reaction (qPCR), additional rats were used (24 hours post-injection) (n=4). Rats euthanized at 72 hours were injected bilaterally so that one MCL could be used for IHC analysis and the other for qPCR. However, because the 72 hour post-injection IHC analysis showed no increase in inflammation, this time point was not analyzed using qPCR.
Injection Technique
Anesthesia was induced and maintained with 1–3% isoflurane in 100% oxygen. The stifles were shaved and aseptically prepared. To approximate clinical PrT as closely as possible, one injection was performed at each insertion site (tibial and femoral) with a total of 0.1 ml of PrT solution (0.05 ml at each insertion). To locate the ligament insertion sites, bony landmarks were used. During injection, the needle was in contact with bone, and the skin was lifted near the needle to minimize solution leakage. Confirmation that this technique delivered fluid to an appropriate location was demonstrated by injecting ink and then exposing the MCL in multiple postmortem rats. Fluid was consistently delivered to the targeted tibial and femoral insertion of the MCL in this manner and covered the entire ligament. Animals were euthanized six, 24, or 72 hours post-injection with an overdose of pentobarbital.
Immunohistochemistry (IHC)
Tissue was labeled with anti-CD43, anti-ED1, and anti-ED2 (mouse anti-rat primary antibodies from AbD Serotec, Raleigh, NC). CD43 has been previously used to identify neutrophils in rat muscle tissue.41 However, it is not specific to neutrophils and has also been used previously as a T lymphocyte label.43 CD43 is expressed by most leucocytes but not B lymphocytes. ED1 labels monocytes and most macrophage subpopulations.44 ED2 labels most tissue macrophages.44 IHC was performed as follows. At sacrifice, MCLs were carefully dissected, placed in optimal cutting temperature compound (OCT), flash frozen, and stored at −80°C. Specimens were cryosectioned onto microscope slides. Tissues were fixed in cold dry acetone. Slides were washed in PBST (1% Tween 20 in phosphate buffered saline, PBS). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide, and non-specific binding sites were blocked using 5% goat serum in PBS. Primary antibodies (diluted in PBS 1:50, 1:25, and 1:25 for CD43+, ED1+, ED2+, respectively) were incubated on the tissue for 2 hours in a moist chamber and washed with PBST. The rat adsorbed secondary linking antibody and horse radish peroxidase (HRP)-labeled strepavidin tertiary antibody (Innovex Biosciences, Richmond, CA) were incubated separately, with PBST wash between. Diaminobenzidine (DAB, Innovex Biosciences) was applied and washed with tap water. The slides were dehydrated with graded alcohols and xylenes and coverslipped.
Digital images were taken at 200x magnification at three locations along the MCL: near the tibial and femoral insertions and the midpoint between the two (midsubstance). Since most inflammatory cells were found near the epiligament, a short segment (0.5 mm long and 70 µm in thickness) of this region was analyzed at both edges of the section at each location along the ligament. ImageJ (NIH) was used to count the number of inflammatory cells labeled by IHC. Cell number was normalized to the area analyzed.
Quantitative Polymerase Chain Reaction (qPCR)
Quantitative polymerase chain reaction was performed 24 hours post-injection to determine gene expression of cytokines related to inflammation (platelet derived growth factor (PDGF), and interleukin-1β (IL-1β)). PDGF is produced by many cells including macrophages45 and fibroblasts46 and recruits neutrophils, macrophages, and fibroblasts to the site of injury.47 IL-1 is secreted by many cells including monocytes and macrophages, promotes cellular aggregation at the inflammatory site, and is involved in wound repair and fibrosis.48 PDGF and IL-1 gene expression are measured in rodent studies on inflammation and wound healing.49, 50 Briefly, MCLs were placed in trizol (400 ml, Invitrogen) and homogenized using a PowerGen 500 (Fisher Scientific, Waltham, MA). RNA extraction was performed using Phase Lock Gel (Eppendorf, Westbury, NY), RNeasy mini total RNA purification kit, and DNase treatment (Qiagen, Valencia, CA) following manufacturer instruction. An Ultrospec 3000 Spectrophotometer (Pharmacia Corporation) was used to quantify RNA concentration. Reverse transcription was performed per manufacturer instructions (Invitrogen, Carlsbad, CA). QPCR was performed using an iQ4 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Primers were purchased from Integrated DNA Technologies (Coralville, IA) and are 5′-GCAAGACGCGTACAGAGGTG-3′ (forward) and 5′-GAAGTTGGCATTGGTGCGA-3′ (reverse) for PDGF-B51 and 5′-TGTTTGGGATCCACACTCTC-3′ (forward) and 5′-TTCCCATTAGACAGCTGCAC-3′ (reverse) for IL-1β.52 Annealing temperatures used for PDGF-B and IL-1β were 60° C and 55° C, respectively. The PrimerDesign geNorm kit (PrimerDesign Ltd, Southampton, UK) was used to determine that ATP5B (an ATP synthase) was an appropriate normalizing gene for this experiment. ATP5B primers were purchased from PrimerDesign. PCR products were confirmed using gel electrophoresis.
Statistical Methods
The number of CD43+, ED1+, and ED2+ cells at the tibial insertion, midsubstance, and femoral insertion were compared between groups of animals injected with dextrose, saline, collagenase, and no injection at each time point. For the 24 hour experiments, sodium morrhuate, P2G, and needlestick groups were also included. IHC results were analyzed by an analysis of variance of rank transformed data, followed by Fisher’s protected least significant difference tests to determine if differences existed between treatment groups. QPCR data were analyzed similarly to IHC except that the data were not rank transformed. A significance level of α = 0.1 was used for overall F-tests and a significance level of α = 0.05 was used for pairwise tests. All analyses were performed using SAS statistical software version 9.1, SAS Institute Inc., Cary, NC.
Results
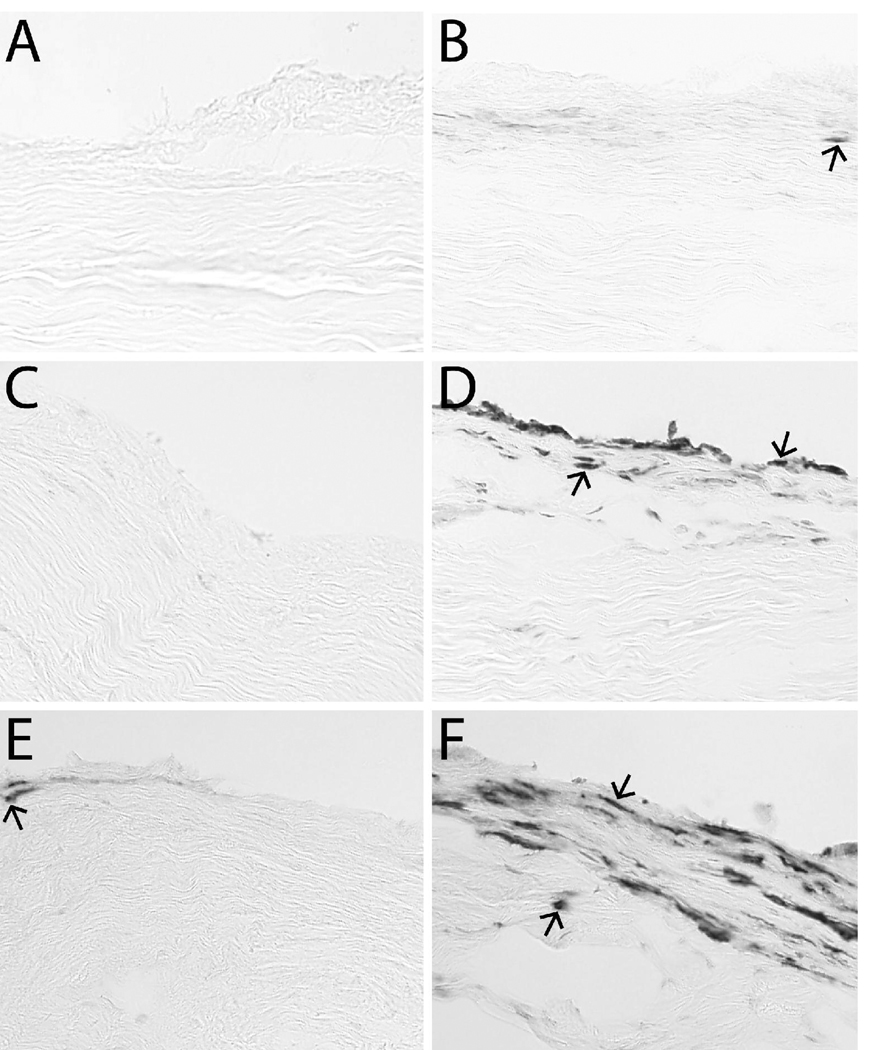
The eighty-four rats used in this study weighed 342 ± 20 g (mean ± SD) at injection. Inflammatory cells (neutrophils and macrophages) were found primarily in the epiligament. Figure 2 shows dextrose-injected ligaments compared to no-injection ligaments labeled for CD43-positive leukocytes, ED1-positive macrophages, and ED2-positive macrophages. Few CD43+ leukocytes were seen at the femoral insertion six hours after dextrose injection (Figure 2, part B). This is similar to no-injection ligaments (part A). An increase in ED1+ macrophages was seen at the femoral insertion six hours after dextrose injection compared to no injection (Figure 2, part D and C, respectively). An increase in ED2+ macrophages was seen at the femoral insertion six hours after dextrose injection compared to no injection (Figure 2, part F and E, respectively). The location of inflammatory cells in the epiligament was consistent between injection treatment groups.
Figure 2. Immunohistochemistry Images.
No-injection controls (left column: A, C, E) and dextrose-injected ligaments six hours post-injection (right column: B, D, F). Dextrose-injected ligaments had similar numbers of CD43-positive lymphocytes (arrow) (B) compared to no injection (A). Dextrose-injected ligaments had more ED1-positive macrophages (arrows) (D) compared to no injection (C). Dextrose-injected ligaments had more ED2-positive macrophages (arrows) (F) compared to no injection (E).
CD43+ Leukocytes
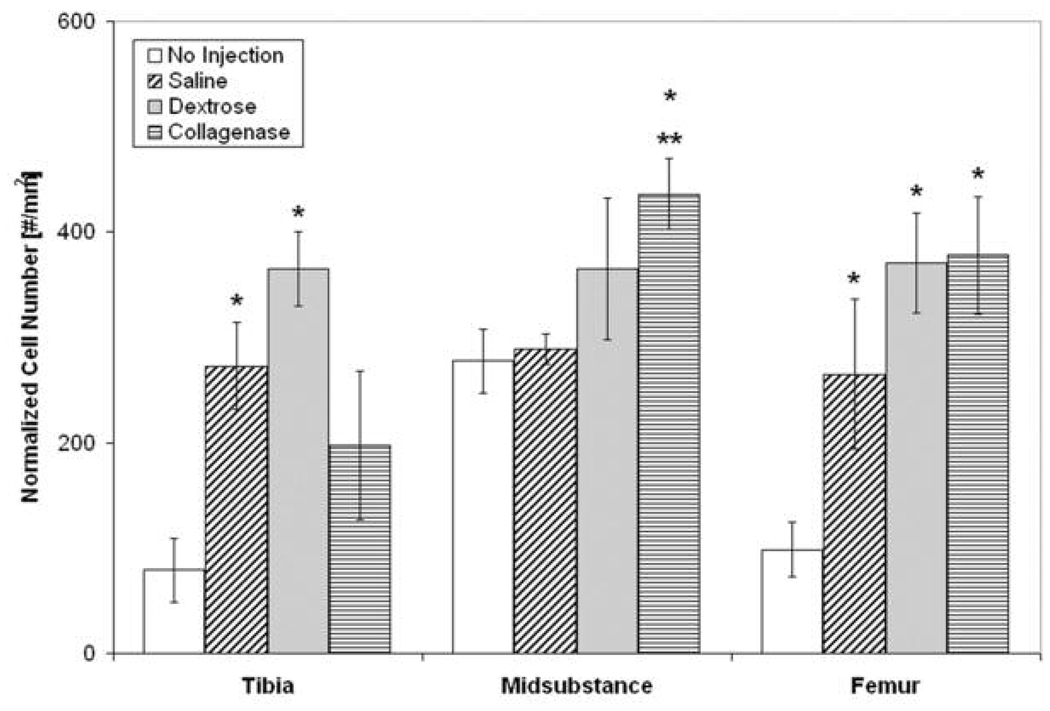
The number of CD43+ leukocytes was mainly increased at the tibal insertion and midsubstance 24 hours post-injection, Figure 3. At the tibial insertion, there was a significant increase in the number of CD43+ leukocytes with saline, sodium morrhuate, P2G, and collagenase (positive control) injection compared to no injection and needlestick (p < 0.05). At the midsubstance, there was a significant increase in the number of CD43+ leukocytes in needlestick-, sodium morrhuate-, P2G- and collagenase-injected ligaments compared to no injection (p < 0.05). At the femoral insertion, collagenase-injected ligaments (positive control) had more CD43+ leukocytes compared to no injection and needlestick (p < 0.05). In addition, at six hours post-injection, there was a significant increase in the number of CD43+ leukocytes in collagenase-injected ligaments (positive control) compared to no injection and saline injection at the tibial insertion (236.9 ± 83.9 vs. 4.5 ± 3.9 and 17.4 ± 12.9, respectively, mean ± SE, p < 0.05) and compared to no injection at the midsubstance (453.1 ± 194.5 vs. 0.0 ± 0.0, mean ± SE, p < 0.05). No change in CD43+ leukocyte number was found 72 hours post-injection.
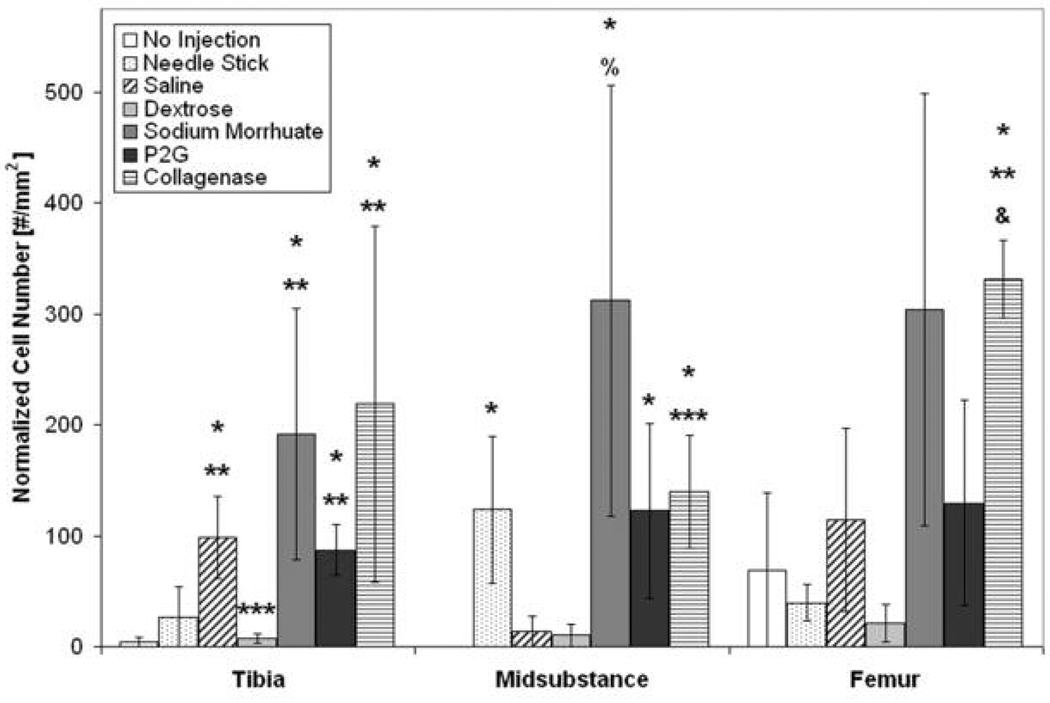
Figure 3. Normalized Number of CD43+ Leukocytes 24 hours Post-Injection at the Tibial Insertion, Midsubstance, and Femoral Insertion.
At the tibial insertion, there was a significant increase in the number of CD43+ leukocytes with saline, sodium morrhuate, P2G, and collagenase (positive control) injection compared to no injection (*p < 0.05) and needlestick (**p < 0.05). In addition, there was a decrease in CD43+ leukocytes in ligaments after dextrose injections compared to saline injection (***p < 0.05). At the midsubstance, there was a significant increase in the number of CD43+ leukocytes with needlestick, sodium morrhuate, P2G and collagenase injection compared to no injection (* p < 0.05). Sodium morrhuate- and collagenase-injected ligaments also had more CD43+ leukocytes 24 hours post-injection compared to saline-injected ligaments (% p = 0.052) and *** p < 0.05, respectively). At the femoral insertion, collagenase-injected ligaments (positive control) had more CD43+ leukocytes compared to no injection (* p < 0.05) and needlestick (** p < 0.05) and had a trend toward an increase in CD43+ leukocytes compared to saline-injected ligaments (& p = 0.07). *p < 0.05 vs. no injection, **p < 0.05 vs. needlestick, ***p < 0.05 vs. saline injection, % p = 0.052 vs. saline injection, and & p = 0.07 vs. saline injection at the same location, mean ± SE.
ED1+ Macrophages
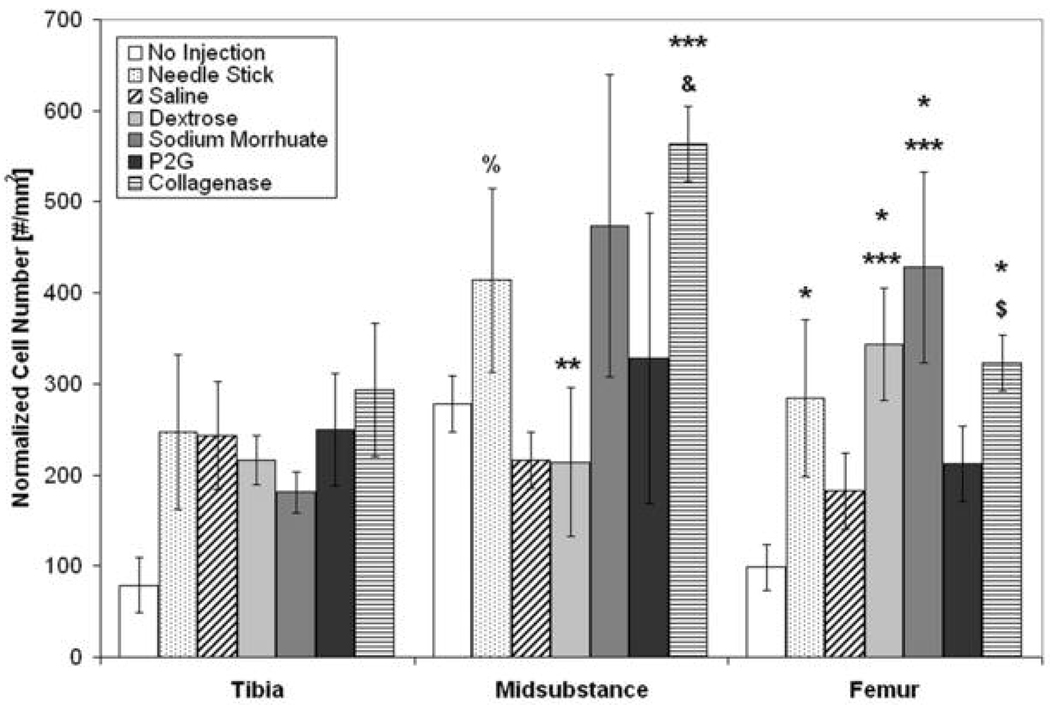
Increased ED1+ macrophages were found in some treatment groups at both insertions and at the midsubstance six and 24 hours post-injection. Six hours post-injection at the tibial insertion, there was an increase in the number of ED1+ macrophages of saline- and collagenase-injected (positive control) ligaments compared to no injection (p < 0.05), Figure 4. At the femoral insertion, there was an increase in the number of ED1+ macrophages in dextrose-injected ligaments compared to no injection (p < 0.05). There was also an increase in ED1+ macrophages with collagenase injection compared to no injection and saline injection six hours post-injection (p < 0.05). Twenty-four hours post-injection, there was an increase in the number of ED1+ macrophages with P2G injection compared to no injection at the tibia, with needlestick compared to saline at the midsubstance, and with dextrose compared to no injection at the femur (p < 0.05), Table 1. There was also a trend toward a decrease in ED1+ macrophages with sodium morrhuate injection compared to needlestick (p = 0.053). In addition, there was an increase with collagenase injection (positive control) at all three locations. No change in ED1+ macrophage number was found 72 hours post-injection.
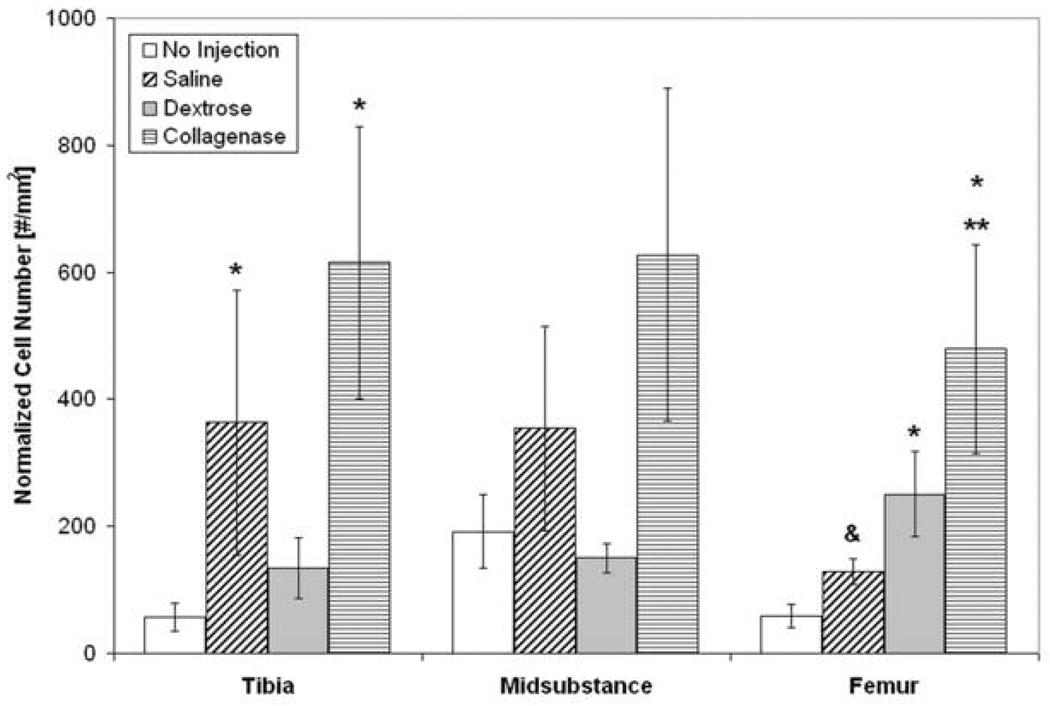
Figure 4. Normalized Number of ED1+ Macrophages six hours Post-Injection at the Tibial Insertion, Midsubstance, and Femoral Insertion.
Six hours post-injection at the tibial insertion, there was an increase in the number of ED1+ macrophages with saline and collagenase (positive control) injection compared to no injection (* p < 0.05). At the femoral insertion, there was an increase in the number of ED1+ macrophages in dextrose-injected ligaments and a trend toward an increase in saline-injected ligaments compared to no injection (* p < 0.05 and & p = 0.07, respectively). There was also an increase in ED1+ macrophages with collagenase injection compared to no injection and saline injection six hours post-injection (*, ** p < 0.05). *p < 0.05 vs. no injection, **p < 0.05 vs. saline injection, and & p = 0.07 vs. no injection at the same location, mean ± SE.
Table 1. Normalized Number of ED1+ Macrophages 24 hours Post-Injection at the Tibial Insertion, Midsubstance, and Femoral Insertion.
Twenty-four hours post-injection, there was an increase in the number of ED1+ macrophages with P2G injection compared to no injection at the tibia, with needlestick compared to saline at the midsubstance, and with dextrose compared to no injection at the femur (p < 0.05). There was also a trend toward a decrease in ED1+ macrophages with sodium morrhuate injection compared to needlestick (& p = 0.053). In addition, there was an increase with collagenase injection (positive control) compared to no injection and saline injection (p < 0.05) at all three locations, a trend toward an increase compared to needlestick (p = 0.053) at the tibial insertion, and an increase compared to needlestick at the femoral insertion (p < 0.05).
| Tibia | Midsubstance | Femur | |
|---|---|---|---|
| No Injection | 57.2 ± 21.4 | 191.7 ± 57.5 | 59.1 ± 18.3 |
| Needle Stick | 174.1 ± 56.7 | 447.3 ± 174.5 *** | 291.7 ± 235.0 |
| Saline | 135.7 ± 98.4 | 120.9 ± 45.0 | 227.7 ± 141.1 |
| Dextrose | 145.1 ± 81.0 | 220.3 ± 48.0 | 359.8 ± 99.0 * |
| Sodium Morrhuate | 213.9 ± 110.6 | 364.9 ± 346.8 & | 286.8 ± 183.2 |
| P2G | 512.2 ± 291.9* | 219.3 ± 116.5 | 191.1 ± 121.7 |
| Collagenase | 969.4 ± 141.5 * *** & | 717.1 ± 167.7 * *** | 801.1 ± 71.7 * ** *** |
p < 0.05 vs. no injection
p < 0.05 vs. needlestick
p < 0.05 vs. saline, & p = 0.053 vs. needlestick, mean ± SE.
ED2+ Macrophages
The number of ED2+ macrophages was increased at the tibial and femoral insertion six hours post-injection and at the midsubstance and femoral insertion 24 hours post-injection in many treatment groups. Six hours post-injection, there was an increase in the number of ED2+ macrophages in saline- and dextrose-injected ligaments compared to no injection at the tibial and femoral insertions (p < 0.05), Figure 5. There was also an increase in the number of ED2+ macrophages in collagenase-injected ligaments. Twenty-four hours post-injection at the femoral insertion, there was an increase in the number of ED2+ macrophages with needlestick, dextrose, sodium morrhuate, and collagenase injection compared to no injection (p < 0.05), Figure 6. Similarly, there was an increase with dextrose and sodium morrhuate injection compared to saline injection (p < 0.05). There were no changes in ED2+ macrophage numbers 72 hours post-injection.
Figure 5. Normalized Number of ED2+ Macrophages Six hours Post-Injection at the Tibial Insertion, Midsubstance, and Femoral Insertion.
Six hours post-injection, there was an increase in the number of ED2+ macrophages in saline- and dextrose-injected ligaments compared to no injection at the tibial and femoral insertions (*p < 0.05). In addition, there was an increase in the number of ED2+ macrophages in collagenase-injected (positive control) ligaments compared to no injection and saline injection at the midsubstance and compared to no injection at the femoral insertion. *p < 0.05 vs. no-injection, **p < 0.05 vs. saline injection at the same location, mean ± SE.
Figure 6. Normalized Number of ED2+ Macrophages 24 hours Post-Injection at the Tibial Insertion, Midsubstance, and Femoral Insertion.
Twenty-four hours post-injection at the femoral insertion, there was an increase in the number of ED2+ macrophages with needlestick, dextrose, sodium morrhuate, and collagenase injection compared to no injection (* p < 0.05). There was also an increase with dextrose and sodium morrhuate injection compared to saline injection (*** p < 0.05) and a trend toward an increase with collagenase compared to saline injection ($ p = 0.055). At the midsubstance, there was a trend towards an increase in ED2+ macrophages in needlestick compared to saline-injected ligaments (% p = 0.062) and a decrease in dextrose-injected ligaments compared to needlestick (** p < 0.05). There was also a significant increase in ED2+ macrophages in collagenase-injected ligaments compared to saline (***p < 0.05) and a trend toward an increase compared to no injection (& p = 0.056). *p < 0.05 vs. no-injection, **p < 0.05 vs. needlestick, ***p < 0.05 vs. saline injection, & p = 0.056 vs. no injection, % p = 0.062 vs. saline injection, and $ p = 0.055 vs. saline injection at the same location, mean ± SE.
Quantitative Polymerase Chain Reaction (qPCR)
The entire ligament was analyzed with qPCR to investigate gene expression related to inflammation. Despite differences observed above in the number of inflammatory cells, few differences were noted between injection groups 24-hours post-injection. The only difference was in saline-injected ligaments, which had higher platelet derived growth factor (PDGF) expression levels compared to dextrose, sodium morrhuate, P2G, needlestick, and no-injection control. IL-1β levels were extremely low (threshold reached after more than 33 cycles) and were not analyzed further.
Discussion
PrT is hypothesized to work by creating a host inflammatory reaction.24 While prior studies have reported limited results suggesting an inflammatory response associated with PrT solutions,32, 37 this is the first study to rigorously assess whether the most commonly used PrT solutions cause inflammation when controlling for volume (saline control), injection (needlestick control), and procedural effects (no-injection control). Animals in all injection and needlestick groups showed increases in inflammatory cells compared to animals that were not injected. To our knowledge, no study has reported an increase in inflammatory cells in ligaments treated with PrT. However, PrT solutions did not cause an overall inflammatory response that was consistently different from that caused by saline and needlestick controls. The inflammatory response found using IHC varied between the three locations analyzed (tibial and femoral insertions and midsubstance) and some groups only showed an increase in two of the three cell types, at one or two of the three locations analyzed.
However, some consistent differences in inflammatory cell number were noted. One consistent change was in CD43+ leukocytes, which were increased with sodium morrhuate and P2G injection at the tibial insertion and midsubstance at 24 hours. ED1+ macrophages were increased with dextrose injection at the femoral insertion at both six and 24 hours. ED2+ macrophages were increased six hours post-injection in dextrose- and saline-injected ligaments (only groups analyzed at this time point) at the tibial and femoral insertions and were increased 24 hours post-injection in dextrose-, sodium morrhuate-, and needlestick-injected ligaments at the femoral insertion.
PDGF and IL-1β were used as examples of pro-inflammatory cytokines and were investigated with qPCR. Few differences in their expression were observed however. The inconsistency between IHC and qPCR results may arise from the different part of the ligament used in each assay. With IHC, only the epiligament was analyzed because the majority of inflammatory cells were located in this small area. However, qPCR was performed on the entire ligament because the small size of the rat MCL prevented dissecting the epiligament from the ligament proper. Perhaps if only the epiligament had been analyzed with qPCR, an increase in inflammatory cytokines would have been found.
The significance of the location of inflammatory cells (within the epiligament) is unclear. One possibility is that proliferant injections produced an early inflammatory response, and cells had not yet migrated into the tissue. An alternative explanation is that a small inflammatory response was produced by the single injection that did not cause cells to migrate further into the tissue. Both of these are unlikely because after the administration of a known inflammatory agent (collagenase I), the muscle and fascia surrounding the MCL showed gross signs of strong inflammation (edema and redness) after six and 24 hours, but the majority of inflammatory cells in the MCL were still located within the epiligament. In addition, the increase in inflammatory cells was similar between the known inflammatory agent and the proliferants. Perhaps inflammation within uninjured ligaments is mainly present in the epiligament and surrounding tissue.
Our results differ from those reported in the only other study on the in vivo cellular effects of PrT. Maynard et al. investigated the biological effects of sodium morrhuate injections on rabbit tendons and reported a trend toward increased cell number with this agent compared to saline injections.37 Our study found an increase in macrophages in many treatment groups (including sodium morrhuate at 24 hours), but this effect was diminished by 72 hours. Differences in study design may account for differences in results between the two studies. 1) Our study investigated a much shorter-term effect (six to 72 hours) than the Maynard study (one week to nine weeks). 2) The Maynard study noted that minimal differences were found with only a single injection. Therefore, the trend toward an increase in cell number found in the Maynard study might be a longer-term effect of three or more injections. 3) We used quantitative immunohistochemistry rather than hematoxylin and eosin stains to more specifically identify cell types.
While our results differ from those of Maynard et al., they may reflect clinical effects of PrT solutions and saline injections. Five randomized controlled trials in patients with low back pain or arthritis18–22 have reported improvement in clinical outcomes (pain, function or disability scores) in subjects receiving prolotherapy injections compared to baseline scores. However, the control groups in these studies also showed substantial clinical improvement.18–22
These studies used saline18–20 or bacteriostatic water21, 22 injections as a control for the PrT injections of either dextrose20–22 or P2G.18, 19 Clinical improvement in subjects receiving control injections suggests that the effect of control injections themselves may also have a beneficial effect, either from volume effect or from needle trauma. Dermal puncture, dry needling,39, 53 real and sham acupuncture,54 and the injection of autologous blood at tendon insertions55, 56 all have been reported to improve clinical outcomes in human clinical studies involving chronic musculoskeletal pain. The mechanisms for such effects are unknown.
Limitations in our study may include the use of a rat model to study PrT and its effects on tissue inflammation. Rat models, however, have been widely employed for studying ligament and tendon healing57–59 and inflammation.44, 57, 60 In addition, PrT injections are typically performed on chronically painful and loose joints. Chronic overuse and long-term injury animal models are not easily developed or typically used. Therefore, in order to simplify short-term effects, we chose to inject healthy normal ligaments. It is unclear whether the inflammatory response to injections would differ after a chronic injury. Additionally, we cannot rule out the possibility of a type II error due to insufficient sample size. Larger studies may better clarify the degree to which each injectant produces a host inflammatory response. Also, the relationship between inflammation and a clinically meaningful outcome (i.e. reduced pain, increased strength) is unknown and requires investigation using larger animal models or human trials.
The current study tested a commonly stated but poorly investigated mechanism of action of three solutions used in PrT.24 Our results suggest that injection with one of three PrT solutions produces an inflammatory response compared to no injection, but that proliferants themselves may not be exclusively responsible. Future animal and clinical trials should attempt to control for the effects of the injection itself.
Conclusions
In this model, PrT injections created an inflammatory response that was not consistently different from that caused by saline injections or needlestick. This inflammatory response varied between the three locations (tibial and femoral insertions and midsubstance) and time points (six, 24, and 72 hours) assessed. Our results suggest that clinical PrT may likewise produce an inflammatory response that may or may not be dependent on the proliferant. Future studies should use a chronic injury animal model to determine if PrT has effects on such tissue different from that of healthy tissue.
Acknowledgements
This publication was made possible by Grant Numbers F31AT002453 and K23AT001879 from the National Center for Complementary and Alternative Medicine (NCCAM) and by a Grant from The Hackett Hemwall Foundation.
References
- 1.Woodwell DA, Cherry DK. National Ambulatory Medical Care Survey: 2002 summary. Adv Data. 2004;346:1–44. [PubMed] [Google Scholar]
- 2.McCaig LF, Nawar EN. Advance data from vital and health statistics; no. 372. Hyattsville, MD: National Center for Health Statistics; 2006. National Hospital Ambulatory Medical Care Survey: 2004 Emergency Department Summary. [PubMed] [Google Scholar]
- 3.Palesy PD. Tendon and ligament insertions--a possible source of musculoskeletal pain. Cranio. 1997;15:194–202. doi: 10.1080/08869634.1997.11746012. [DOI] [PubMed] [Google Scholar]
- 4.Scavenius M, Bak K, Hansen S, et al. Isolated total ruptures of the anterior cruciate ligament--a clinical study with long-term follow-up of 7 years. Scand J Med Sci Sports. 1999;9:114–119. doi: 10.1111/j.1600-0838.1999.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 6.Andriachi T, Sabiston P, De Haven K. Injury and Repair of the Musculoskeletal Soft Tissues. In: Woo S-Y, Buckwalter JA, et al., editors. Ligament: Injury and Repair. Park Ridge, IL: AAOS; 1987. pp. 103–128. [Google Scholar]
- 7.Wilson JJ, Best TM. Common overuse tendon problems: A review and recommendations for treatment. Am Fam Physician. 2005;72:811–818. [PubMed] [Google Scholar]
- 8.Stovitz SD, Johnson RJ. NSAIDS and musculoskeletal treatment: What is the evidence? Physician and Sports Medicine. 2003;31:35–52. doi: 10.3810/psm.2003.01.160. [DOI] [PubMed] [Google Scholar]
- 9.Assendelft WJ, Hay EM, Adshead R, Bouter LM. Corticosteroid injections for lateral epicondylitis: a systematic overview. Br J Gen Pract. 1996;46:209–216. [PMC free article] [PubMed] [Google Scholar]
- 10.Bisset L, Beller E, Jull G, et al. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ. 2006;333:939. doi: 10.1136/bmj.38961.584653.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews JH. Nonsurgical treatment of pain in lumbar spine stenosis. Am Fam Physician. 1999;59:280. 283–4. [PubMed] [Google Scholar]
- 12.Schnirring L. Are Your Patients Asking About Prolotherapy? The Physician and Sports Medicine. 2000;28:15–17. [Google Scholar]
- 13.Rabago D, Best T, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376–380. doi: 10.1097/01.jsm.0000173268.05318.a4. [DOI] [PubMed] [Google Scholar]
- 14.Dorman TA. Prolotherapy: a survey. J Orthop Med. 1993;15:49–50. [Google Scholar]
- 15.Dagenais S, Ogunseitan O, Haldeman S, et al. Side effects and adverse events related to intraligamentous injection of sclerosing solutions (prolotherapy) for back and neck pain: A survey of practitioners. Arch Phys Med Rehabil. 2006;87:909–913. doi: 10.1016/j.apmr.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Linetsky FS, Rafael M, Saberski L. Pain management with regenerative injection therapy (RIT) In: Weiner RS, editor. Pain Management. pp. 381–402. [Google Scholar]
- 17.Dechow E, Davies RK, Carr AJ, Thompson PW. A randomized, double-blind, placebo-controlled trial of sclerosing injections in patients with chronic low back pain. Rheumatology. 1999;38:1255–1259. doi: 10.1093/rheumatology/38.12.1255. [DOI] [PubMed] [Google Scholar]
- 18.Klein RG, Eek BC, DeLong WB, Mooney V. A randomized double-blind trial of dextrose-glycerinephenol injections for chronic, low back pain. J Spinal Disord. 1993;6:23–33. [PubMed] [Google Scholar]
- 19.Ongley MJ, Klein RG, Dorman TA, et al. A new approach to the treatment of chronic low back pain. Lancet. 1987;2:143–146. doi: 10.1016/s0140-6736(87)92340-3. [DOI] [PubMed] [Google Scholar]
- 20.Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine. 2004;29:9–16. doi: 10.1097/01.BRS.0000105529.07222.5B. [DOI] [PubMed] [Google Scholar]
- 21.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000;6:311–320. doi: 10.1089/10755530050120673. [DOI] [PubMed] [Google Scholar]
- 22.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74. 77–80. [PubMed] [Google Scholar]
- 23.Scarpone M, Rabago D, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2007 doi: 10.1097/JSM.0b013e318170fc87. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks A. A rationale for prolotherapy. Journal of Orthopaedic Medicine. 1991;13:54–59. [Google Scholar]
- 25.Natarajan R, Gonzales N, Xu L, Nadler JL. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem Biophys Res Commun. 1992;187:552–560. doi: 10.1016/s0006-291x(05)81529-3. [DOI] [PubMed] [Google Scholar]
- 26.McGinn S, Poronnik P, King M, et al. High glucose and endothelial cell growth: novel effects independent of autocrine TGF-beta 1 and hyperosmolarity. Am J Physiol Cell Physiol. 2003;284:C1374–C1386. doi: 10.1152/ajpcell.00466.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kondo S, Beissert S, Wang B, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen G, Brynskov J, Saermark T. Phenol toxicity and conjugation in human colonic epithelial cells. Scand J Gastroenterol. 2002;37:74–79. doi: 10.1080/003655202753387392. [DOI] [PubMed] [Google Scholar]
- 29.Gunduz S, Kalyon TA, Dursun H, et al. Peripheral nerve block with phenol to treat spasticity in spinal cord injured patients. Paraplegia. 1992;30:808–811. doi: 10.1038/sc.1992.156. [DOI] [PubMed] [Google Scholar]
- 30.Westerlund T, Vuorinen V, Kirvela O, Roytta M. The endoneurial response to neurolytic agents is highly dependent on the mode of application. Reg Anesth Pain Med. 1999;24:294–302. [PubMed] [Google Scholar]
- 31.Dagenais S, Mayer J, Haldeman S, Wooley J. Dose-Escalation, Placebo-Controlled Acute Toxicity Evaluation of a Drug Commonly Used in Prolotherapy Following Spinal Injection in Rats; NASS 21st Annual Meeting: The Spine Journal; 2006. 76S. [Google Scholar]
- 32.Dagenais S, Ogunseitan O, Haldeman S, et al. Acute Toxicity Pilot Evaluation of Proliferol in Rats and Swine. Int J Toxicol. 2006;25:171–181. doi: 10.1080/10915810600683218. [DOI] [PubMed] [Google Scholar]
- 33.de Lorimier AA. Sclerotherapy for venous malformations. J Pediatr Surg. 1995;30:188–193. doi: 10.1016/0022-3468(95)90558-8. discussion 194. [DOI] [PubMed] [Google Scholar]
- 34.Stroncek DF, Hutton SW, Silvis SE, et al. Sodium morrhuate stimulates granulocytes and damages erythrocytes and endothelial cells: probable mechanism of an adverse reaction during sclerotherapy. J Lab Clin Med. 1985;106:498–504. [PubMed] [Google Scholar]
- 35.Aneja A, Karas SG, Weinhold PS, et al. Suture plication, thermal shrinkage, and sclerosing agents: effects on rat patellar tendon length and biomechanical strength. Am J Sports Med. 2005;33:1729–1734. doi: 10.1177/0363546505275492. [DOI] [PubMed] [Google Scholar]
- 36.Liu YK, Tipton CM, Matthes RD, et al. An in situ study of the influence of a sclerosing solution in rabbit medial collateral ligaments and its junction strength. Connect Tissue Res. 1983;11:95–102. doi: 10.3109/03008208309004846. [DOI] [PubMed] [Google Scholar]
- 37.Maynard JA, Pedrini VA, Pedrini-Mille A, et al. Morphological and biochemical effects of sodium morrhuate on tendons. J Orthop Res. 1985;3:236–248. doi: 10.1002/jor.1100030214. [DOI] [PubMed] [Google Scholar]
- 38.Harrison MEG. Department of Physical Education. Provo, Utah: Brigham Young University; 1995. The Biomechanical Effects of Prolotherapy on Traumatized Achilles Tendons of Male Rats. [Google Scholar]
- 39.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. doi: 10.1016/0304-3959(79)90142-8. [DOI] [PubMed] [Google Scholar]
- 40.Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol. 1999;65:492–498. [PubMed] [Google Scholar]
- 41.Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156:2103–2110. doi: 10.1016/S0002-9440(10)65081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wetzel BJ, Nindl G, Swez JA, Johnson MT. Quantitative characterization of rat tendinitis to evaluate the efficacy of therapeutic interventions. Biomed Sci Instrum. 2002;38:157–162. [PubMed] [Google Scholar]
- 43.Sun D, Tani M, Newman TA, et al. Role of chemokines, neuronal projections, and the blood-brain barrier in the enhancement of cerebral EAE following focal brain damage. J Neuropathol Exp Neurol. 2000;59:1031–1043. doi: 10.1093/jnen/59.12.1031. [DOI] [PubMed] [Google Scholar]
- 44.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- 45.Shimokado K, Raines EW, Madtes DK, et al. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 46.Paulsson Y, Hammacher A, Heldin CH, Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987;328:715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- 47.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiological Reviews. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 48.Miller KM, Anderson JM. In vitro stimulation of fibroblast activity by factors generated from human monocytes activated by biomedical polymers. Journal of Biomedical Materials Research. 1989;23:911–930. doi: 10.1002/jbm.820230808. [DOI] [PubMed] [Google Scholar]
- 49.Head CC, Farrow MJ, Sheridan JF, Padgett DA. Androstenediol reduces the anti-inflammatory effects of restraint stress during wound healing. Brain Behav Immun. 2006;20:590–596. doi: 10.1016/j.bbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhou FH, Foster BK, Sander G, Xian CJ. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35:1307–1315. doi: 10.1016/j.bone.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Ostendorf T, van Roeyen CR, Peterson JD, et al. A fully human monoclonal antibody (CR002) identifies PDGF-D as a novel mediator of mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2003;14:2237–2247. doi: 10.1097/01.asn.0000083393.00959.02. [DOI] [PubMed] [Google Scholar]
- 52.Andrieu-Soler C, Berdugo M, Doat M, et al. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2005;46:4072–4078. doi: 10.1167/iovs.05-0105. [DOI] [PubMed] [Google Scholar]
- 53.Testa V, Capasso G, Benazzo F, Maffulli N. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34:573–580. doi: 10.1097/00005768-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Haake M, Müller H, Schade-Brittinger C, et al. German Acupuncture Trials (GERAC) for chronic low back pain. Randomized, multicenter, blinded, parallel-group trial with 3 groups. Arch Intern Med. 2007;167:1892–1898. doi: 10.1001/archinte.167.17.1892. [DOI] [PubMed] [Google Scholar]
- 55.Connell DA, Ali KE, Ahmad M, et al. Ultrasound-guided autologous blood injection for tennis elbow. Skeletal Radiol. 2006;35:371–377. doi: 10.1007/s00256-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 56.Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg [Am] 2003;28:272–278. doi: 10.1053/jhsu.2003.50041. [DOI] [PubMed] [Google Scholar]
- 57.Kawamura S, Ying L, Kim HJ, et al. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425–1432. doi: 10.1016/j.orthres.2005.01.014.1100230627. [DOI] [PubMed] [Google Scholar]
- 58.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech. 2005;38:69–75. doi: 10.1016/j.jbiomech.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 59.Provenzano PP, Alejandro-Osorio AL, Valhmu WB, et al. Intrinsic fibroblast-mediated remodeling of damaged collagenous matrices in vivo. Matrix Biol. 2005;23:543–555. doi: 10.1016/j.matbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Hannawa KK, Eliason JL, Woodrum DT, et al. L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112:241–247. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]