Abstract
The prophage of coliphage N15 is not integrated into the chromosome but exists as a linear plasmid molecule with covalently closed hairpin ends (telomeres). Upon infection the injected phage DNA circularizes via its cohesive ends. Then, a phage-encoded enzyme, protelomerase, cuts the circle and forms the hairpin telomeres. N15 protelomerase acts as a telomere-resolving enzyme during prophage DNA replication. We characterized the N15 replicon and found that replication of circular N15 miniplasmids requires only the repA gene, which encodes a multidomain protein homologous to replication proteins of bacterial plasmids replicated by a theta-mechanism. Replication of a linear N15 miniplasmid also requires the protelomerase gene and telomere regions. N15 prophage replication is initiated at an internal ori site located within repA and proceeds bidirectionally. Electron microscopy data suggest that after duplication of the left telomere, protelomerase cuts this site generating Y-shaped molecules. Full replication of the molecule and subsequent resolution of the right telomere then results in two linear plasmid molecules. N15 prophage replication thus appears to follow a mechanism that is distinct from that employed by eukaryotic replicons with this type of telomere and suggests the possibility of evolutionarily independent appearances of prokaryotic and eukaryotic replicons with covalently closed telomeres.
INTRODUCTION
All cells with linear chromosomes must utilize special mechanisms to replicate the extreme termini of their DNA molecules, since DNA polymerases alone are unable to perform this function (1). Most eukaryotes have open-ended DNAs and employ special ‘telomerase’ enzymes for this purpose, but there are other solutions that ensure complete replication of linear DNA: protein priming, recombination and covalently closed terminal hairpins. Phage N15 belongs to the small group of systems known to replicate as linear DNA with hairpin ends. Such replicons are generally of eukaryotic origin, but a few from bacteria are known: for example the phage-plasmids φKO2 in Klebsiella oxytoca (2, S.R.Casjens and E.B.Gilcrease, unpublished observations) and PY54 in Yersinia enterocolytica (3), as well as the linear plasmids and chromosomes of Borrelia (4–6) and one of the two chromosomes of Agrobacterium tumefaciens (7,8).
The temperate coliphage N15 has a 46.4 kb double stranded DNA chromosome with 12 bp single stranded cohesive termini in its virion. N15 is similar to lambda in latent period, burst size, frequency of lysogenization, morphology of plaques and phage particles (9). However, unlike lambda, the N15 prophage is a linear plasmid with covalently closed hairpin ends (10–12), which, being telomeres, have been designated telL and telR. The gene order in the prophage plasmid DNA is a circular permutation of that in the virion DNA, and the telomere-forming site telRL in phage DNA is a 56 bp inverted repeat. After infection of Escherichia coli, the phage molecule becomes circularized via its cohesive ends. Then the phage-encoded enzyme ‘protelomerase’, the product of phage gene 29 (telN), cuts telRL sequence and joins the phosphodiester bonds making the covalently closed telR and telL ends. This cleavage-joining activity of purified TelN protein has been demonstrated in vitro (13).
Several models for the processing of replicative intermediates by this type of end-resolving enzyme have been proposed (14,15). In the first model bidirectional replication initiated at an internal ori site results in the formation of a circular dimer that is then processed by a telomere resolvase into two linear molecules. In a second model the linear molecule is first opened at the ends and converted into a monomeric circle, which then duplicates and is finally converted into linear molecules by protelomerase. In a third model replication starts at one of telomeres from nicking and end rearrangement. Subsequent strand-displacement replication results in the formation of head-to-head and tail-to-tail concatemers; the replicated telomeres in concatemers are subsequently resolved to generate monomeric molecules with covalently closed ends. These strategies are not the only possible ones and need not be mutually exclusive; for example, model 1 and 3 could combine to replicate the bulk and ends of the DNA, respectively [as is done for the protein-primed linear Streptomyces plasmid pSLA2 (16)]. Strategy 3 could be modified in such a way that end rearrangements initially occur at both telomeres. These models can be discriminated by several principal points: (i) location of the replication initiation site: internal or telomere-proximal; (ii) direction of replication: uni- or bi-directional; (iii) mode of replication: θ-mechanism or something else (e.g. rolling hairpin, see 17); (iv) structure of replicative intermediates: circular dimer, circular monomer or linear molecules.
Previously we demonstrated that N15 protelomerase, product of gene 29 (telN), is necessary for replication of the linear plasmid prophage through its action as a telomere-resolvase; a deficiency of protelomerase results in the accumulation of circular head-to-head dimer intermediates (18). These results are consistent with N15 replication following the first model mentioned above (for a review see 15), which is based on the Bateman’s model of replication of palindromic telomeres (19). At the same time little is known about N15 DNA replication itself. Which genes are required? Where is the replication initiation site located? Does replication proceed uni- or bidirectionally? It is not known whether a circular dimer is really formed in the course of the prophage replication or whether resolution of one of the duplicated telomeres precedes full replication of the molecule thus leading to the generation of Y-shaped structures.
The existing information about the N15 replicon is mainly based on computer analysis of the complete sequence (20), the properties of miniplasmids compared to full-length N15 DNA (21–23), and some data on the involvement of host genes in N15 replication (9 and references therein). Regions of the N15 genome important for plasmid maintenance were first identified by the construction of miniplasmids (21). The shortest known autonomously replicating linear N15 miniplasmid, pG59, contains N15 genes from 29 (telN) to 38 (cB) (24). The minimal plasmid replicon, contained within a 5.2 kb-long fragment (genes 33–37, plasmid map coordinates 4.1–9.3 kb), can drive the replication of circular N15 miniplasmids (21). Genes 33–37 might be co-translated since stop codons of these genes overlap or are adjacent to the initiation codon of the downstream genes. Transcription of this operon is predicted to be initiated at the promoter(s) located between genes 37 and 38 and controlled by the CB repressor whose binding sites overlap these promoters (25). Sequence analysis of the predicted 1324 amino acid product of gene 37 reveals that the protein is a homolog of primases of conjugative plasmids and the primase of phage P4. The latter enzyme, a multifunctional replication protein with primase, helicase and origin recognition activities, is the only phage-encoded protein essential for replication during the lytic cycle and for plasmid maintenance (reviewed in 26). The importance of gene 37 (also called repA) for replication of N15 DNA is further supported by the data that all replication mutations isolated so far have been mapped within this gene (9). Whether N15 genes 33–36 are necessary for replication was unknown. N15 replication was found to be independent of polA, recA, dnaJ, dnaK and grpE (27,28).
Rybchin and Svarchevsky (9), on the basis of sequence analysis, suggested that the plasmid replication initiation site (ori) is located between 30.1 and 30.2 kb on the N15 genome within the repA coding sequence. Another possible candidate sequence was located between genes 37 and 38. However, until the work presented here there was no experimental data mapping the ori site to any of the regions mentioned above or to determine how many ori sites exists in the N15 genome and whether the same site(s) are used for plasmid and lytic replication. Nothing was known about directionality of plasmid replication.
In the present manuscript we determined the minimal set of N15 genes necessary for replication of circular and linear miniplasmids, identified the ori site and investigated whether N15 replication proceeds uni- or bidirectionally. Finally, we employed electron microscopy to investigate the structure of replicative intermediates generated in the course of linear plasmid replication.
MATERIALS AND METHODS
Media, chemicals, enzymes and synthetic oligonucleotides
Cultures were grown with aeration at 37°C in LB broth sup plemented as appropriate with chloramphenicol (20 µg/ml), kanamycin (50 µg/ml), ampicillin (100 µg/ml) or tetracycline (20 µg/ml) and with 1.5% agar (Difco) for solid medium. Pfu polymerase (Promega), T4 DNA ligase (NBI Fermentas), T4 polynucleotide kinase (Sibenzyme) and restriction enzymes (Promega, New England Biolabs, Sibenzyme) were used in accordance with the recommendations of the manufacturers. All PCR amplifications were carried out using Pfu DNA polymerase (Promega). The synthetic oligonucleotides used are shown in Table 1.
Table 1. Oligonucleotides used in this work.
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| 33L | TCAGTTATTGGAGTCCGGTA |
| 35L | GATCATGCGCCACTATTCAC |
| 36L | GACGTCATAGTGGCTTCACC |
| 37L2 | TCCGGTTATATCGCCACG |
| 37L3 | GCTCTAGACCCGGGTTATATCGCCACGGCT |
| 37S1 | GGAATTCCCGGGTAGATGATACAATAAC |
| 37S2 | CATGCCATGGTAACCTTACAAGAATTCTAC |
| 38R | CGAGGCGAACGACTGAAAGC |
| 39R | TGTTATGCCCTTCCTTACCC |
| ORI-1 | ATTTAGACCTAAATATAGATATACATGGTAAC |
| ORI-2 | CGCGGTCAACTTCGATGGTGGTTTTTTCC |
| ORI-3 | TAGAGAAAACCACCATCGAAGTGG |
| ORI-5 | ATTTGGATCTAAATATAGATATACATGGTAAC |
| ORI-6 | CGCGATCCACTTCGATGGTGGTTTTTTCC |
| A-L | CACTTCACGGTGCGCAGATA |
| A-R | TAGTTACCGCCAGAACAGCC |
| B-L | CGCTGGACATTGTCGCAAGT |
| B-R | CATAATGCTGCCGCGTTGTA |
| C-L | CCTGTGGGCATTCAGTCTGGATCGC |
| C-R | CGCTGAAGAGATGCTCGACTGGGCA |
Bacterial strains, bacteriophages and plasmids
Escherichia coli strain MC1061 (29) was used as a host for phage propagation, DH10B (30) for all cloning experiments. Strain C2107 (polA12) was kindly provided by Gianni Dehò. Bacteriophage N15 was described by Ravin and Shulga (10). The coordinates of the N15 genome are from the N15 complete sequence (GenBank accession number AF064539). Cloning vector pBAD24 (31) was used for controlled expression of cloned N15 genes; pUC19 (32) was used for routine subcloning operations. Plasmids pKRP10 and pKRP11 (33) were used as sources of tetracycline and kanamycin resistance gene fragments, respectively.
Circular plasmids based on the N15 replicon
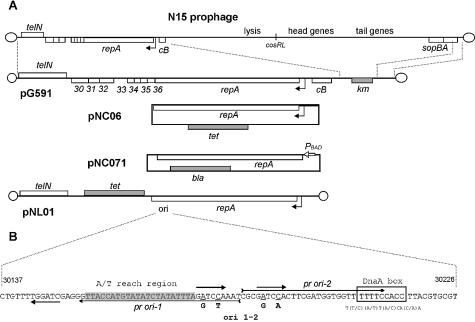
Circular plasmids whose replication is driven by N15 genes and target sequences were constructed by ligation of the N15 DNA fragment obtained by PCR amplification using appropriate oligonucleotides and the DNA fragment carrying tetracycline resistance gene excised from pKRP10 with SmaI; products of the ligation were transferred to E.coli DH10B by transformation according to standard procedures (34). In all plasmids the tetR and gene 37 (repA) are transcribed in the same direction, except for pNC06 where they are oriented in opposite directions. The following plasmids were constructed: pNC01 (N15 DNA fragment from base pair 28 610 to 34 696, primers 33L and 38R, includes genes 33-repA); pNC03 (from base pair 29 284 to 34 696, primers 35L and 38R, genes 35-repA); pNC04 (from base pair 29 622 to 34 696, primers 36L and 38R, genes 36-repA); pNC05 (from base pair 29 881 to 34 696, primers 37L2–38R, repA); pNC06 (from base pair 29 881 to 34 118, primers 37L2–37S1, repA). Plasmid pNC01–05 carries the repA-cB intergenic region and 588 bp of the 3′-terminal part of gene 38 (cB), while pNC06 carries only the last 10 bp of cB. Plasmid pNC07 was constructed by cloning the N15 repA gene (primers 37S2–37L3) in the expression vector pBAD24 between the NcoI and XbaI sites. Plasmid pNC071 was obtained from pNC07 by deleting the vector origin of replication (digestion with NaeI and self-ligation). Plasmid pNC11 consists of N15 DNA fragment carrying genes repA and cB (from base pair 29 881 to 35 000 obtained by PCR amplification with primers 37L2 and 39R) and kanamycin resistance gene (excised as SmaI fragment from pKRP11); KmR and repA are translated in the opposite directions. Plasmids pNC06ts52 and pNC11ts52 are equivalent to pNC06 and pNC11, respectively, except that they carry the temperature sensitive mutation ts52 in the plasmid repA gene; E.coli strains carrying these plasmids can grow at 30°C but not at 40°C under the selective conditions. We determined the nucleotide sequence of the mutant repA gene and found that the ts52 mutation is an A to G transition in position 33 019 that changes Phe 281 to Ser in the RepA protein. The N15 phage ts52 mutant which DNA was used as a template for relevant PCR amplifications was kindly provided by Valentin Rybchin.
Linear plasmids based on the N15 replicon
Plasmid pNL01 was constructed by cloning the N15 DNA fragment including the telRL site and telN gene (from base pair 24 493 to 26 930, see details in 18) into the SmaI site of pNC06. Restriction analysis showed that the resulting plasmid, pNL01, is linear in form, its structure is shown in Figure 1. Plasmid pG591 was obtained from pG59 (24) by deleting the 0.5 kb BglII fragment of the sopA gene. This linear plasmid, 13 165 bp long, carries all the N15 genes essential for replication and copy number control of the N15 prophage (from telN to cB) along with a KmR gene.
Figure 1.
(A) Map of N15 plasmid prophage and plasmids. Rectangles above and below the main line represent genes transcribed rightward and leftward, respectively. Empty rectangles show N15 genes while antibiotic resistance genes are shown as grey rectangles. The promoter-operator site of repA is shown as a bent arrow. Abbreviations are as follows: PBAD, arabinose inducible araPBAD promoter; ori, N15 replication initiation site; tet, tetracycline resistance gene; km, kanamycin resistance gene; bla, ampicillin resistance gene. (B) Nucleotide sequence of the replication origin. Thick arrows indicate GATCCA repeats, and the A/T-rich region is shaded. The DnaA binding site is boxed, and the consensus sequence is shown below. Positions of primers pr ori-1 and pr ori-2 are shown by arrows. Mutations ori 1–2, introduced using these primers are shown below the sequence and the corresponding nucleotides are underlined.
Site-directed mutagenesis of the ori site
Site-directed mutagenesis of the ori site in the plasmid pNC04 (contains N15 genes 36 and repA) was performed using a PCR-based approach. pNC04 DNA was used as a template for PCR amplification with a pair of primers (ORI-1 and ORI-2) designed to amplify the whole plasmid and carrying the desired mutations (see Fig. 1). In the control reactions we used a similar pair of primers, ORI-5 and ORI-6, without mutations (positive control) or primers ORI-3 and 36L (negative control, ori site is absent in the PCR product). The PCR fragment obtained was phosphorylated by T4 polynucleotide kinase and circularized by self-ligation. Products of the ligation reaction (0.1 µg per transformation in each case) were used to transform DH10B cells; transformants were plated on LB agar supplemented with antibiotic. Absence of the transformant colonies indicates the lethality of the designed mutation (see Table 2) for pNC04.
Table 2. Site directed mutagenesis of N15 ori site.
| ori mutations | Positive control | Negative control | |
|---|---|---|---|
| pNC04 | 4a | 1520 | 2b |
| pG591 | 3c | 290 | 1b |
| Phage N15 | 2b | 220 | 0 |
Site-directed mutagenesis was performed as described in Materials and Methods. The values in the table indicate the number of colonies (for pNC04 and pG591) or plaques (for phage N15) obtained in relevant experiments. The positive control represents successful reconstruction of the plasmid or phage with DNA that contains no ori mutation; in the negative control no ori site was present in the PCR product.
aSequencing of ori regions of these clones showed that in three clones mutations were successfully introduced in one of GATCCA repeats (the right one shown in Fig. 1) while the other repeats contain the wild type sequence. One clone contains wild type sequence.
bSequencing of ori regions of these clones showed no mutations.
cColonies grow slowly in the presence of kanamycin, sequencing of ori regions of these clones showed that in all three clones mutations were successfully introduced in one of GATCCA repeats (the right one shown in Fig. 1) while the other repeats contain wild type sequence.
To test the effect of ori mutations on the viability of the linear plasmid, pG591, we first cloned the N15 HindIII fragment (from base pair 29 326 to 31 329) carrying the predicted ori site, into the pUC19 vector (plasmid pUC 2kb). Then site-directed mutagenesis of the ori site was performed similar to that described above for pNC04, giving plasmid pUC 2kb1/2. The pG591 DNA was digested with ScaI whose two target sites flank the ori; left and right telomere-proximal fragments were isolated from an agarose gel, purified and ligated to the ori-containing ScaI fragment excised from either pUC 2kb1/2 or pUC 2kb (positive control). In the negative control reaction no ori-containing ScaI fragment was included. All ligation reactions included equal amounts of telomere-proximal ScaI fragments and 3-fold molar excess of ori-containing fragment. Products of the ligation reaction (0.1 µg per transformation in each case) were used to transform DH10B cells, transformants were plated on LB agar supplemented with antibiotic. The absence of the transformant colonies indicated the lethality of the designed mutation (see Table 2) for pG591.
The effect of the ori mutations on the phage replication was tested as for pG591 with the following modification. The N15 phage DNA was digested with ScaI (N15 genome contains only two ScaI sites that flank the ori site), the ori-containing fragment was removed using agarose gel electrophoresis. The resulting N15 DNA fragments were ligated to ori-containing ScaI fragments, as described for pG591. Products of the reaction (1 µg per transformation in each case) were used to transform DH10B cells; plaques produced by N15 phages were obtained by plating the transformed cells into the MC1061 lawn. The absence of the plaques indicates the lethality of the designed mutations (see Table 2) for lytic development of N15.
Southern blot analysis of directionality of N15 replication
Total bacterial DNA was isolated at different times post activation of an integrated N15 replicon, digested with XhoI, PstI and NcoI and used for Southern blot hybridization with three radiolabeled probes simultaneously: A, B and C. Probes were prepared using Prime-a-Gene labeling Kit (Promega) using equal amounts of template PCR fragment in each case; they could reveal the following fragments: 6283 bp (probe A), 3120 bp (probe B) and 2213 bp (probe C). The following oligonucleotide pairs were used to amplify template E.coli DNA fragments: A-L and A-R for probe A, B-L and B-R for probe B, C-L and C-R for probe C. For quantitative determination of signals each band was cut out from the membrane and radioactivity levels were determined using an X-ray counter.
Isolation of plasmid DNA for electron microscopy analysis
Replicating plasmid DNA was isolated and purified using method described by Strutowska et al. (35) with some modifications. DH10B/pG591 cells from a fresh culture were grown in 200 ml LB medium at 37°C until the OD590 reached 0.4–0.6, quickly chilled, centrifuged and the pellet was resuspended in 4.5 ml of 50 mM Tris–HCl pH 8.0, 10 mM EDTA, 100 µg/ml RNase and the suspension was maintained on ice for for 5 min. Afterwards, 0.5 ml of 0.25 M EDTA was added and incubation on ice was continued for another 10 min. Cell lysis was achieved by adding 0.5 ml of 20% SDS solution and incubating in 65°C water bath for 5 min. Then 0.6 ml of preheated 5 M KCl was added and the tubes were placed on ice for 20–30 min. Plasmid DNA was recovered from the supernatant after centrifugation at 26 000 g for 30 min at 4°C, and precipitated by adding an equal volume of absolute ethanol. The precipitated DNA was pelleted by centrifugation at 14 000 g for 10 min at 4°C and the pellet was washed by 70% ethanol and resuspended in 1 ml of TE buffer (0.01 M Tris–HCl, pH 7.4, 0.001 M EDTA) with 100 µg/ml of RNase. Tubes were incubated at 37°C for 15 min. Afterwards, proteinase K was added to a concentration of 100 µg/ml and incubation was continued at 65°C for 30 min. Proteins were extracted twice with phenol:chloroform (1:1) and once with chloroform. The DNA was precipitated overnight at –20°C with 2.5 vol of absolute ethanol and resuspended in TE. Finally, plasmid DNA was purified using Qiagen Plasmid Midi Kit following the instructions of the manufacturer. A typical yield was 2–5 µg of pG591 DNA per preparation.
Electron microscopy of DNA
DNA samples were prepared for microscopy by the aqueous drop spreading method (without uranyl acetate staining) of Thresher and Griffith (36). Parlodion (Electron Microscope Sciences, Fort Washington, PA) films were placed on 400 mesh copper grids and dried for 1 h under a heat lamp. A DNA solution (∼0.5 µg/ml DNA, 0.25 M ammonium acetate, 7 µg/ml cytochrome C) was freshly mixed, and a 50 µl drop placed on a sheet of parafilm. After 90 s, a grid (parlodion-coated surface down) was briefly touched to the surface and then dehydrated by successive submersion washes with 75 and 90% ethanol for 45 and 3 s, respectively, followed by air drying. DNA attached to the grids was then visualized by rotary shadow casting at a low angle with Pt:Pd (80:20) wire in a vacuum evaporator (Earnest F. Fullam Model No. 12510:EFFA), and micrographs were captured electronically in a Hitachi H70100 electron microscope.
RESULTS
Identification of genes necessary for replication of the N15 prophage
In the order to identify the minimal set of genes necessary and sufficient to drive replication of N15-based plasmid (mini-replicon) we first constructed a set of circular miniplasmids consisting of different fragments of N15 DNA obtained as PCR fragments and a tetracycline resistance gene (Fig. 1). The shortest miniplasmid, pNC06, contained the N15 DNA fragment from base pair 29 881 to 34 118, which includes the repA gene, a region between repA and cB genes predicted to contain promoter(s) of repA and overlapping CB-repressor binding sites, and the last 10 bp of cB. The repA gene is thus necessary and sufficient to drive replication of a circular N15 based plasmid.
We then investigated whether other N15 genes might be required for replication of a linear miniplasmid. Maintenance of the linear plasmid requires the presence of the telomere resolution site and protelomerase (18). We inserted the N15 fragment including telRL and the telN gene (24 493–26 930 on the phage map, 20) into the SmaI site of pNC06, and resulting plasmids were selected by transformation of DH10B. Restriction analysis showed that the plasmid, pNL01 (Fig. 1), is linear in form. Thus, only repA and telN are required for replication of a linear N15 based miniplasmid. It should be mentioned that the pNL01 copy number is much higher than that of N15 prophage or pG591. This is likely due to the absence of the cB repressor gene that is believed to control N15 copy number through binding to the promoter of repA (23,25,37).
The N15 repA gene encodes a 1324 amino acid protein. The deduced amino acid sequence of RepA contains motifs characteristics for bacterial primases and helicases (9). In particular, RepA, shows some homology to the phage P4 α replication protein which has been shown to have primase, helicase and origin recognition activity. These observations suggest that RepA may also be a multi-domain protein similar to the replication proteins of bacterial plasmids that utilize a θ-replication mechanism.
Identification of the N15 prophage replication initiation site
Since the shortest circular miniplasmid based on the N15 replicon, pNC06, contains the repA gene and repA-cB intergenic region, we tested whether the replication initiation site, ori, is located within the coding sequence of repA or upstream of it. The N15 repA gene, with no adjacent N15 sequence, was cloned in the expression vector pBAD24 (31) under control of arabinose-inducible araPBAD promoter (plasmid pNC07). Then the vector origin of replication was deleted (digestion with NaeI and self-ligation), and resulting plasmids (pNC071, Fig. 1) were selected by transformation of DH10B. Transformation readily gave colonies when transformants were plated on ampicillin-selective agar supplemented with arabinose (for induction of the repA gene), while no colonies were obtained on selective agar with glucose (no induction of repA gene). The same result was obtained when the ligation products were used for transformation of E.coli strain C-2107 (polA12), which cannot maintain replication of pBAD24 since it is based on the pBR322 replicon. Both these results show that at least one N15 ori site, capable of initiating replication of this circular N15 based plasmid, is located within the repA gene.
Inspection of the nucleotide sequence of repA revealed a region (Fig. 1) that could represent the ori site. This region contains three GATCCA repeats arranged in an iteron-like manner, a sequence TTTTCCACC that is very similar to the E.coli DnaA-binding site consensus [T(T/C)(A/T)T(A/C)CA(C/A)A (38)] and a 22-bp A/T-rich region (18.2% G+C) between two of the repeats. These are all characteristics of ori sites of θ-replicating bacterial plasmids. It should be specially mentioned that N15 replication is strongly dam-dependent (9 and our unpublished observations) and GATCCA sequences contain the GATC Dam-methylase recognition site.
In order to test the role of the predicted ori site in replication of N15, we performed site-directed mutagenesis of this region. The reason for using such a complicated approach rather than direct tests, such as deletion analysis of plasmids replicating in a strain that expresses RepA in trans, is that N15 RepA protein acts only in cis (to be published elsewhere). We tested the effect of mutations (ori 1–2) that change the sequence of two GATCCA repeats to eliminate the Dam recognition sites (see Fig. 1) but do not change the amino acid sequence of the RepA protein (see Materials and Methods). In the first experiment we attempted to introduce these mutations simultaneously into the circular plasmid pNC04 by PCR-based approach (see Materials and Methods); we found that we were unable to construct this mutant and conclude that it is lethal for this plasmid (Table 2). Two control experiments were performed in a similar way: the first one involved an equivalent pair of primers without mutations, where the wild type plasmid was successfully reconstituted. In the second control experiment we used a primer pair ORI-3 and 36L; in this case the ori site is totally absent in the PCR product and the corresponding mutant was not obtained (Table 2). Similar experiments using this strategy to place other mutations (not relevant to this study) in the repA gene were routinely successful (our unpublished results). Moreover, in the experiment with ori 1–2 mutations, we obtained a few slowly growing colonies carrying pNC04 with the desired G to A and C to A mutations in only one of the GATCCA ori-repeats (the rightmost one shown in Fig. 1) while the second repeat contains the wild type sequence. These plasmids could result from partial reversion of mutations that we introduced to wild type in vivo. These data further support our conclusion that ori 1–2 mutations are lethal for pNC04, when present in multiple GATCCAs.
We then studied whether the same ori site is used for replication of the linear N15 based plasmids and the whole N15 prophage. The N15 DNA fragment (HindIII, from nucleotide 29 326 to 31 329) has been cloned in pUC19, mutations ori 1–2 were introduced by PCR-based site directed mutagenesis (verified by sequencing), as for pNC04. In one experiment the mutated fragment (ScaI, from nucleotide 29 575 to 30 554) was used to substitute the corresponding wild type fragment in a linear N15 based plasmid, pG591. Again, we found that mutation of the GATCCA repeats (ori) is apparently lethal (Table 2). In the second experiment the same ScaI fragment was used to substitute for the corresponding unique fragment of N15. Products of ligation were used to transform DH10B cells; plaques produced by recombinant N15 phages were obtained by plating the transformed cells into the MC1061 lawn. Again, ori 1–2 mutations were found to be detrimental for phage development (Table 2). It should be mentioned that absence of plaques does not necessary mean that ori 1–2 mutations are lethal for prophage replication in the lysogen, since lysogens could not be identified in this experiment, but this seems likely taking into account the data for pG591.
Bidirectional replication of the N15 prophage
The next principle point for development of a model of N15 prophage replication is the directionality of replication–is it uni- or bidirectional? To determine the direction of plasmid replication we integrated the N15 replication region into the E.coli chromosome, activated N15 replication and measured the rates of amplification of chromosomal markers flanking the integration site.
Integration of N15 DNA fragments into the chromosome was carried out following the method and using vectors and strains described by Platt et al. (39). Briefly, an N15 replicon that included only gene repAts52 (XbaI fragment from pNC06ts52) was cloned in the plasmid pCD11PSK and integrated into the host chromosome (strain DH10B) at the lambda attachment site giving strain VK-1. The second variant of the N15 replicon containing both genes repAts52 and cB (XbaI fragment from pNC11ts52) gave strain VK-2. Plasmid pCA12 (40) carrying the N15 antirepressor gene (antA) under control of the arabinose-inducible araPBAD promoter was then introduced into VK-2. N15 antirepressor is known to counteract CB repression (40) that in turn is believed to control the expression of repA and prophage copy number (25), thus leading to activation of N15 replication. In all cases the integrated N15 replicon is inactive at 40°C due to the presence of the ts52 mutation in the repA.
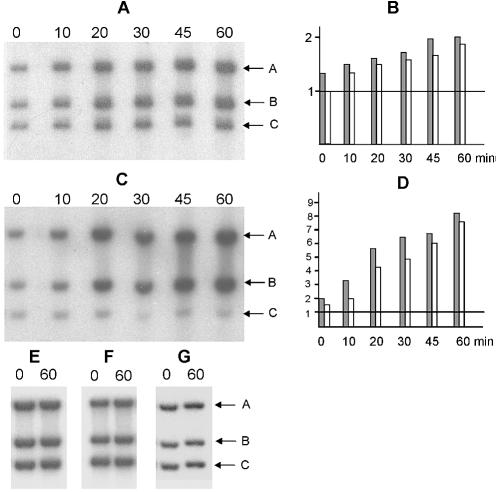
Cultures of VK-1 and VK-2/pCA12 were grown at 40°C to OD600 = 0.5, at which time the integrated replicon was activated either by shifting the temperature from 40 to 30°C (for VK-1) or by simultaneous temperature shift and induction of antirepressor by adding arabinose to a final concentration of 0.01% (for VK-2/pCA12). Total cellular DNA was isolated at different times after induction, digested with appropriate restriction enzymes and used for Southern blot analysis. Hybridization patterns of DNA samples with three probes are shown for VK1 and VK2/pCA12 (Fig. 2). In a control experiments the same strains were tested at non-permissive temperature, 40°C (Fig. 2E and F). The second control experiment involved a strain, VK-3, with integrated pCD11PSK vector plasmid that has no N15 origin of replication (Fig. 2G). One probe, A, was used for hybridization to E.coli DNA between positions –3888 and –2697 relative to the center of the λ integration site (gene ybhJ). The second probe, B, was used for hybridization to the bioB gene, located on the opposite side of the λ att site (+2066 to +3270). We employed a third probe, C, for hybridization to a marker (uidA) that lies far from the integration site (∼900 kb) and which served to normalize the amount of probe A- and B-specific DNA at different times after induction. Comparison of the band intensities of probe A- and probe B-specific DNA in induced cultures of both VK-1 and VK-2/pCA12 strains (Fig. 2) showed that DNA on both sides of the integration site are coordinately amplified relative to DNA far from the integration site. The amplification in both directions relative to the distant uidA location on the chromosome is ∼2-fold for VK-1 and up to 8-fold for VK-2. The reason for this difference could be that in the second strain temperature shift and inactivation of CB repressor should lead both to the activation of existing RepA and new RepA synthesis due to repA gene derepression, while only RepA activation occurs in VK-1 after the temperature shift. We conclude that replication initiated from the N15 origin proceeds bidirectionally. A less plausible interpretation of these results that cannot be ruled out is that replication is in fact unidirectional, initiating independently in either direction.
Figure 2.
Southern blot analysis of directionality of N15 replication. (A) Total cellular DNA was isolated from strains VK-1 (A and E), VK-2/pCA12 (C and F) and VK-3 (G) at times indicated above the blots (in min) post-activation of replication, fractionated by 0.5% (w/v) agarose electrophoresis and hybridized to radiolabeled probes A, B and C (see text). Positions of the fragments that hybridize to these probes are shown by arrows. Replication was activated by shifting the temperature from 40 to 30°C (A) or by simultaneous temperature shift and induction of anti- repressor by adding arabinose to the medium up to 0.01% (C and G). Control experiments shown in (E and F) were performed exactly as experiments shown in (A and C) except that cultures were kept constantly at 40°C. (B and D) show the band intensities of probe A (gray rectangles) and B (white rectangles)-specific signals normalized to probe C-specific signal taken as 1.0. To obtain these quantitative data, each band was cut from the membrane after hybridization and exposure to X-ray film, and the radioactive signals were counted in an X-ray counter.
Electron microscopy analysis of replicative intermediates
The most difficult step in any electron microscopy investigation of plasmid replication is isolation of partly replicated intermediates. Taking into account that the N15 DNA is approximately one-hundredth the size of the E.coli chromosome and the fact that both the prophage and the chromosome replicate once per cell division, one would expect (if elongation rates are equal) that not more than 1% of the N15 DNA in an asynchronously dividing culture will be present as partly replicated molecules at any given time. Since the N15 copy number is about 3–4 per chromosome, the N15 replicative intermediates will constitute ∼0.04% of total cellular DNA. Thus, specific isolation of intermediates is required. To facilitate such isolation we employed for this analysis the short N15-based linear plasmid pG591, which is 13.2-kb long. This plasmid contains all the phage genes that are essential for replication of the plasmid prophage (genes 29–38), and it is maintained at the same low copy number as N15 itself. Two enrichments were used for specific capture of intermediates. First, plasmid DNA was isolated by gentle cell lysis and DNA purification on a Qiagen column as described in Materials and Methods. The resulting DNA sample contained ∼20–50% N15-specific DNA. In a second step, plasmid and chromosomal bands were separated by electrophoresis in low melting temperature agarose gel. DNA was isolated from the gel region between the full-length linear plasmid and high-molecular-weight chromosomal bands using β-agarase. This second enrichment step allowed us to find about one intermediate per 500 molecules according to electron microscopy observations.
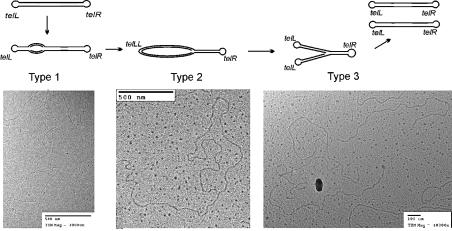
Electron microscopic analysis allowed us to identify three types of replicating molecules (Fig. 3). Type 1 molecules, which are linear and the length of pG591, contain an internal ‘bubble’ located near the position predicted for the ori site (defined by its distance from the ends of pG591). These molecules likely represent an early step of internally initiated bidirectional replication. We found only two molecules of this type, presumably because they are rare and migrate near the non-replicating plasmid monomer in agarose. Type 2 molecules (two examples) are circles located at the end of a linear DNA; these could result from replication to one end of the molecule without protelomerase resolution of the ends (most likely the left end, which is closer to the ori site than the right end). Type 3 molecules, the more abundant ones (10 examples), are Y-shaped molecules with two equal-length arms whose lengths are consistent with a single fork on a pG591 molecule; these could result from TelN cleavage of the circle in a type 2 molecule. No circular molecules, either dimers or monomers were found. These findings, although not conclusive, are consistent with a model in which bidirectional replication initiates bidirectional fork movement out from the ori within the repA gene and TelN rapidly resolves the daughter telomeres so that dimer circles do not accumulate.
Figure 3.
Electron microscopy analysis of replication of linear plasmid pG591. Schemes of three types of replicating molecules and are shown above examples of electron microscope images.
DISCUSSION
Location of the replication initiation site is one of the principle points in determining the mode of replication of linear DNA. For instance, poxvirus DNA replication is thought to start from nicking in the telomere-proximal region and hairpin rearrangement at the telomeres (41 and references therein). On the other hand, the Borrelia linear plasmids may replicate from an internally located ori site (42,43), and the protein-primed linear plasmids of Streptomyces appear to have both terminal and internal ‘origins’ in the same molecule (16). Circular autonomously replicating plasmids containing non-telomere-proximal fragments of N15 DNA were described some time ago (see 9 and references therein) suggesting that at least one N15 ori site is internally located. However, it was not known whether this internal ori site is actually used in the replication of the N15 prophage or whether plasmid replication might be driven from another site. Coexistence of several origins is not unusual. For example, two origins are known in phage-plasmid P4 (44), and in phage P1 one origin is used for its circular prophage plasmid replication, while the second is used for lytic replication (45).
Here we identified a region resembling bacterial replication initiation sites: it carries three GATCCA repeats, an A/T-rich region and a putative DnaA binding site. Since the N15 RepA protein acts only in cis (our unpublished observations), we were unable to test the functional role of this ori site in standard ways, such as deletion analysis of plasmids replicating from this site in a strain that exogenously expresses RepA. We note that phage P2 and φX-174 replication proteins have been studied in some detail and have been shown to be cis acting, so this is not a particularly unusual property for a phage replication protein (summarized in 46). Instead, keeping in mind that N15 replication is dam-dependent, we hypothesized that GATCCA repeats are particularly important for initiation and performed site-directed mutagenesis of this feature without changing the amino acid sequence of repA. In fact we found that mutations introduced in two of the repeats are almost certainly lethal for both circular and linear plasmid replication. Also, this mutation prevents productive lytic infection by phage N15. Precisely, our result means that this particular site is required for replication in cis, but not necessarily that this is the origin of replication. However, inspection of the remaining sequence of repA revealed no other candidate ori sites, and our electron microscopy data are consistent with replication initiation at the site we found (±0.5 kb). Thus, we believe that the site we identified is in fact the origin of replication, and that this site is utilized in the RepA-driven replication of both circular and linear N15 replicon based plasmids.
Directionality of replication is an important point for development of any model of replication. For instance, unidirectional replication suggests circularization before replication. Here we show that replication of N15 proceeds bidirectionally. Internally initiated bidirectional replication of linear molecules would result in the formation of circular head-to-head dimers if telomeres were not resolved. In fact, Ravin et al. (18) showed that deficiency of protelomerase results in accumulation of such intermediates. The electron microscopy analysis of intermediates generated in course of replication of an N15-based linear plasmid, pG591 did not reveal circular dimers, instead, we observed Y-shaped molecules. The asymmetric location of the ori site (5 kb from telL versus 8 kb from telR in pG591) and/or different times for fork escape from the origin in the two directions results in duplication and subsequent resolution of one telomere before the other is replicated.
Other prokaryotic linear replicons with covalently closed ends presumably follow a similar strategy of replication. Comparison of the TelN protein sequence with sequences deposited in GenBank revealed regions of homology shared by TelN and proteins of other prokaryotic ‘hairpin’ replicons: BBB03 gene of Borrelia burgdorferi (20,47), gene AgrC4584 of A.tumefaciens (our unpublished analysis), protelomerase gene of the phage-plasmid PY54 (3). A gene highly similar to telN is also found in phage-related linear plasmid φKO2 (S.R.Casjens and R.W.Hendrix, unpublished observations).
Replication of linear plasmids and chromosomes of B.burgdorferi is probably initiated at an internal ori site and proceeds bidirectionally (42,43). Kobryn and Chaconas (48) demonstrated that BBB03 protein cleaves in vitro a synthetic unresolved telomere site, and that the same site functions as a substrate for telomere resolution in vivo (49). The authors suppose that the final step in the replication of linear Borrelia plasmids and chromosomes is a breakage and reunion of the duplicated telomere on the circular dimer (reviewed in 50). The cleavage-joining activity of PY54 and φKO2 protelomerases has also been demonstrated in vitro (3, W.M.Huang and S.R.Casjens, unpublished).
Interestingly, the N15-Borrelia model of replication differs from that used by eukaryotic replicons with covalently closed telomeres (e.g. poxviruses, African Swine Fever Virus and Chlorella viruses). Particularly, poxvirus replication is thought to be initiated near the telomeres, resulting in the formation of head-to-head and tail-to-tail concatemers through strand-displacement replication (41 and references therein); the replicated telomeres in these concatemers are subsequently resolved by an as yet unknown enzyme to generate monomeric molecules with covalently closed ends. In addition, poxvirus genomes contain no recognizable homologs of protelomerase genes (our unpublished observation). These observations combine to suggest the possibility of evolutionarily independent appearances of prokaryotic and eukaryotic replicons with covalently closed telomeres rather than transfer of protelomerase genes from eukaryotes (poxviruses) to prokaryotes (Borrelia) or vice versa, as has been suggested previously (51,52).
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Taisia Strakhova for expert technical advice. We thank Boris Kuznetsov for assistance in sequencing the recombinant plasmids, Gregory Phillips for providing strains and plasmids and Jack Griffith for electron microscopy advice. This work was supported by grant 01-0786 from the International Association for the Promotion of Cooperation with Scientists from the New Independent States of the former Soviet Union (INTAS) and by grant RB1-1043 from the US Civilian Research and Development Foundation (CRDF).
REFERENCES
- 1.Watson J.D. (1972) Origin of concatemeric T7 DNA. Nature New Biol., 239, 197–201. [DOI] [PubMed] [Google Scholar]
- 2.Stoppel R.D., Meyer,M. and Schlegel,H.G. (1995) The nickel resistance determinant cloned from the enterobacterium Klebsiella oxytoca: conjugational transfer, expression, regulation and DNA homologies to various nickel-resistant bacteria. Biometals, 8, 70–79. [DOI] [PubMed] [Google Scholar]
- 3.Hertwig S., Klein,I., Lurz,R., Lanka,E. and Appel,B. (2003) PY54, a linear plasmid prophage of Yersinia enterocolitica with covalently closed ends. Mol. Microbiol., 48, 989–1003. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A.G. and Garon,C.F. (1987) Linear plasmids of the Borrelia burgdorferi have covalently closed ends. Science, 237, 409–411. [DOI] [PubMed] [Google Scholar]
- 5.Fraser C.M., Casjens,S., Huang,W.M., Sutton,G.G., Clayton,R., Lathigra,R., White,O., Ketchum,K.A., Dodson,R., Hickey,E.K. et al (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, 390, 580–586. [DOI] [PubMed] [Google Scholar]
- 6.Casjens S., Murphy,M., van Vugt,R., Sampson,L., DeLange,M. and Huang,W. (1997) Telomeres of the linear chromosomes of the Lyme disease spirochetes: nucleotide sequence and possible exchange with linear plasmid telomeres. Mol. Microbiol., 26, 581–596. [DOI] [PubMed] [Google Scholar]
- 7.Allardet-Servent A., Michaux-Charachon,S., Jumas-Bilak,E., Karayan,L. and Ramuz,M. (1993) Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J. Bacteriol., 175, 7869–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodner B., Hinkle,G., Gattung,S., Miller,N., Blanchard,M., Qurollo,B., Goldman,B., Cao,Y., Askenazi,M., Halling,C., Mullin,L., Houmiel,K., Gordon,J., Vaudin,M., Iartchouk,O., Epp,A., Liu,F., Wollam,C., Allinger,M., Doughty,D., Scott,C., Lappas,C., Markelz,B., Flanagan,C., Crowell,C., Gurson,J., Lomo,C., Sear,C., Strub,G., Cielo,C. and Slater,S. (2001) Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science, 294, 2323–2328. [DOI] [PubMed] [Google Scholar]
- 9.Rybchin V.N. and Svarchevsky,A.N. (1999) The plasmid prophage N15, a linear DNA with covalently closed ends. Mol. Microbiol., 33, 895–903. [DOI] [PubMed] [Google Scholar]
- 10.Ravin V.K. and Shulga,M.G. (1970) Evidence for extrachromosomal location of prophage N15. Virology, 40, 800–807. [DOI] [PubMed] [Google Scholar]
- 11.Svarchevsky A.N. and Rybchin,V.N. (1984) Physical mapping of plasmid N15 DNA. Mol. Gen. Mikrobiol. Virusol., 10, 16–22. [Google Scholar]
- 12.Malinin A.Y., Vostrov,A.A., Rybchin,V.N. and Svarchevsky,A.N. (1992) Structure of the linear plasmid N15 ends. Mol. Gen. Mikrobiol.Virusol., 5–6, 19–22. [PubMed] [Google Scholar]
- 13.Deneke J., Ziegelin,G., Lurz,R. and Lanka,E. (2000) The protelomerase of temperate Escherichia coli phage N15 has cleaving-joining activity. Proc. Natl Acad. Sci. USA, 97, 7721–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casjens S. (1999) Evolution of the linear DNA replicons of the Borrelia spirochetes. Curr. Opin. Microbiol., 2, 529–534. [DOI] [PubMed] [Google Scholar]
- 15.Ravin N.V. (2003) Mechanisms of replication and telomere resolution of the linear plasmid prophage N15. FEMS Microbiol. Lett., 221, 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Bao K. and Cohen,S.N. (2003) Recruitment of terminal protein to the ends of Streptomyces linear plasmids and chromosomes by a novel telomere-binding protein essential for linear DNA replication. Genes Dev., 17, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotmore S.F., Christensen,J. and Tattersall,P. (2000) Two widely spaced initiator binding sites create an HMG1-dependent parvovirus rolling-hairpin replication origin. J. Virol., 74, 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravin N.V., Strakhova,T.S. and Kuprianov,V.V. (2001) The protelomerase of the phage-plasmid N15 is responsible for its maintenance in linear form. J. Mol. Biol., 312, 899–906. [DOI] [PubMed] [Google Scholar]
- 19.Bateman A. (1975) Simplification of palindromic telomere theory. Nature, 253, 379. [DOI] [PubMed] [Google Scholar]
- 20.Ravin V., Ravin,N., Casjens,S., Ford,M., Hatfull,G. and Hendrix,R. (2000) Genomic sequence and analysis of the atypical bacteriophage N15. J. Mol. Biol., 299, 53–73. [DOI] [PubMed] [Google Scholar]
- 21.Svarchevsky A.N. (1986) Plasmid N15: The Particularities of Genetics and DNA Structure. PhD Thesis. The Leningrad State University, Leningrad, Russia. [Google Scholar]
- 22.Vostrov A.A., Malinin,A.Y., Rybchin,V.N. and Svarchevsky,A.N. (1992) Construction of linear plasmid vectors for cloning in Escherichia coli cells. Genetika, 28, 186–188. [PubMed] [Google Scholar]
- 23.Ravin N.V. and Ravin,V.K. (1994) An ultrahigh-copy plasmid based on the mini-replicon of the temperate phage N15. Mol. Gen. Mikrobiol. Virusol., 1, 37–39. [PubMed] [Google Scholar]
- 24.Dorokhov B.D., Lane,D. and Ravin,N.V. (2003) Partition operon expression in the linear plasmid prophage N15 is controlled both by Sop proteins and by protelomerase. Mol. Microbiol., 50, 713–721. [DOI] [PubMed] [Google Scholar]
- 25.Łobocka M.B., Svarchevsky,A.N., Rybchin,V.N. and Yarmolinsky,M.B. (1996) Characterization of the primary immunity region of the Escherichia coli linear plasmid prophage N15. J. Bacteriol., 178, 2902–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegelin G. and Lanka,E. (1995) Bacteriophage P4 DNA replication. FEMS Microbiol. Rev., 17, 99–107. [DOI] [PubMed] [Google Scholar]
- 27.Svarchevsky A.N. and Rybchin,V.N. (1984) Characteristics of plasmid properties of bacteriophage N15. Mol. Gen. Mikrobiol. Virusol., 10, 34–39. [Google Scholar]
- 28.Tilly K. (1991) Independence of bacteriophage N15 lytic and linear plasmid replication from the heat shock proteins DnaJ, DnaK and GrpE. J. Bacteriol., 173, 6639–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadaban M.J. and Cohen,S.N. (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol., 138, 179–207. [DOI] [PubMed] [Google Scholar]
- 30.Grant S., Jessee,J., Bloom,F. and Hanahan,D. (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl Acad. Sci. USA, 87, 4645–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzman L.-M., Belin,D., Carson,M.,J. and Beckwith,J. (1995) Tight regulation, modulation and high-level expression by vectors containing arabinose PBAD promoter. J. Bacteriol., 177, 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanish-Perron C., Vieira,J. and Messing,J. (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene, 33, 103–119. [DOI] [PubMed] [Google Scholar]
- 33.Reece K.S. and Phillips,G.J. (1995) New plasmids carrying antibiotic-resistance cassettes. Gene, 165, 141–142. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 35.Srutkowska S., Konopa,G. and Wegrzyn,G. (1998) A method for isolation of plasmid DNA replication intermediates from unsynchronized bacterial cultures for electron microscopy analysis. Acta Biochim Pol., 45, 233–240. [PubMed] [Google Scholar]
- 36.Thresher R. and Griffith,J. (1992) Electron microscopic visualization of DNA and DNA–protein complexes as adjunct to biochemical studies. Methods Enzymol., 211, 481–490. [DOI] [PubMed] [Google Scholar]
- 37.Ravin N.V. and Ravin,V.K. (1999) Use of a linear multicopy vector based on the mini-replicon of temperate coliphage N15 for cloning DNA with abnormal secondary structures. Nucleic Acids Res., 27, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuller R.S., Funnell,B.E. and Kornberg,A. (1984) The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell, 38, 889–900. [DOI] [PubMed] [Google Scholar]
- 39.Platt R., Drescher,C., Park,S.-K. and Phillips,G. (2000) Genetic system for reversible integration of DNA constructs and lacZ gene fusions into the Escherichia coli chromosome. Plasmid, 43, 12–23. [DOI] [PubMed] [Google Scholar]
- 40.Ravin N.V., Svarchevsky,A.N. and Dehò,G. (1999) The antiimunity system of phage-plasmid N15: identification of the antirepressor gene and its control by a small processed RNA. Mol. Microbiol., 34, 980–994. [DOI] [PubMed] [Google Scholar]
- 41.DeMasi J., Du,S., Lennon,D. and Traktman,P. (2001) Vaccinia virus telomeres: interaction with the viral I1, I6 and K4 proteins. J. Virol., 75, 10090–10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picardeau M., Lobry,J.R. and Hinnebusch,B.J. (1999) Physical mapping of an origin of bidirectional replication at the centre of the Borrelia burgdorferi linear chromosome. Mol. Microbiol., 32, 437–445. [DOI] [PubMed] [Google Scholar]
- 43.Stewart P., Chaconas,G. and Rosa,P. (2003) Conservation of plasmid maintenance functions between linear and circular plasmids in Borrelia burgdorferi. J. Bacteriol., 185, 3202–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tocchetti A., Serina,S., Terzano,S., Dehò,G. and Ghisotti,D. (1998) Identification of two replicons in phageplasmid P4. Virology, 245, 344–352. [DOI] [PubMed] [Google Scholar]
- 45.Cohen G. and Sternberg,N.L. (1989) Genetic analysis of the lytic replicon of bacteriophage P1. I. Isolation and partial characterization. J. Mol. Biol., 206, 99–109. [DOI] [PubMed] [Google Scholar]
- 46.Bertani L.E. and Six,E.W. (1988) The P2-like phages and their parasite P4. In: Calendar,R. (ed.), The Bacteriophages, vol. 2. Plenum Press, New York, NY, pp. 73–143. [Google Scholar]
- 47.Casjens S., Palmer,N., van Vugt,R., Huang,W.M., Stevenson,B., Rosa,P., Lathigra,R.,W., Sutton,G., Peterson,J., Dodson,R., Haft,D., Hickey, E, Gwinn,M., White,O. and Fraser,C. (2000) A genome in flux: The twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol., 35, 490–516. [DOI] [PubMed] [Google Scholar]
- 48.Kobryn K. and Chaconas,G. (2002) ResT, a telomere resolvase encoded by the Lyme disease spirochete. Mol. Cell, 9, 195–201. [DOI] [PubMed] [Google Scholar]
- 49.Chaconas G., Stewart,P.E., Tilly,K., Bono,J.L. and Rosa,P. (2001) Telomere resolution in the Lyme disease spirochete. EMBO J., 20, 3229–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobryn K. and Chaconas,G. (2001) The circle is broken: telomere resolution in linear replicons. Curr. Opin. Microbiol., 4, 558–564. [DOI] [PubMed] [Google Scholar]
- 51.Hinnebusch J. and Barbour,A.G. (1991) Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J. Bacteriol., 173, 7233–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinnebusch J. and Tilly,K. (1993) Linear plasmids and chromosomes in bacteria. Mol. Microbiol., 10, 917–922. [DOI] [PubMed] [Google Scholar]