Abstract
Purpose
Eccentric photorefraction provides an opportunity to gather rapid and remote estimates of refraction and gaze position from infants. The technique has the potential for extensive use in vision screenings and studies of visual development. The goal of this study was to assess the refraction calibration of the PowerRefractor (Multichannel Systems) for use with uncyclopleged infants.
Methods
The defocus measurements from the instrument were compared with the results of simultaneous retinoscopy in one analysis and with known amounts of defocus induced with trial lenses in another. Data were collected from infants 1 to 6 months of age and adults.
Results
The PowerRefractor typically read <1 D of myopia when the retinoscopy reflex was judged to be neutral at the same working distance in both infants and adults. The slopes of both infant and adult validation functions (trial lens power vs. measurement of induced defocus) were close to 1 over a 4D range. The infant slopes were significantly greater than those of the adults, however.
Conclusions
The results suggest that the instrument is capable of detecting large amounts of defocus but needs individual calibration for detailed studies of accommodative accuracy and absolute levels of defocus, as has been recommended previously for adult subjects.
Keywords: eccentric photorefraction, infant, PowerRefractor, refraction, calibration
Measurements of infant refraction and gaze position are central to understanding the development of clear, single vision and to understanding clinically significant abnormality (e.g., accommodative esotropia, intermittent exotropia, and amblyogenic factors such as high hyperopia and anisometropia1,2).
The eccentric photorefraction technique can be used to make both of these measurements and is attractive for studying infants because it is fast, remote, and can record from both eyes simultaneously3-6 (see Fig. 1). When automated at video rates, its speed and potential to record data binocularly make it, in theory, preferable to conventional retinoscopy; infants’ short attention spans have less impact on data quality and anisometropia is easier to detect in a binocular measurement. Estimates of vergence, gaze position, and pupil size are made using a contrast detection algorithm to locate the pupils and first Purkinje images in each video frame, while the refraction estimate is made using the eccentric photorefraction principle.7
FIGURE 1.
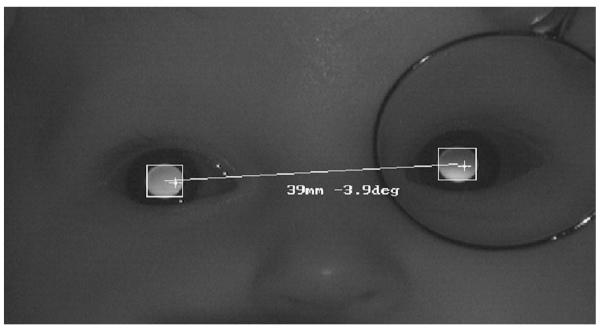
An example PowerRefractor image from an infant. The distribution of light in the pupils provides simultaneous estimates of defocus in the two eyes, and the positions of the Purkinje images relative to the center of the pupils provide an estimate of gaze position. The image also demonstrates the technique used in this study for the relative validation. A positive-powered lens was held before one eye, producing a myopic crescent in the pupil, and the induced anisometropia was determined as a function of lens power. (The camera aperture size was kept in the recommended range of 5.6–8 throughout data collection.)
The current study concerns calibration of the refraction estimate. The eccentric photorefraction principle is based on an analysis of light reflected from the retina. Light from a source in the plane of the front of the camera lens forms an image on the retina and is then reflected back through the pupil into the camera. The estimate of the eye’s defocus is derived from the distribution of reflected light across the subject’s pupil in the camera image5,6 (see Fig. 1). The standard analytical description of this light distribution includes the following parameters: the refractive error of the eye, the distance of the subject to the camera, the pupil size, and the eccentricity of the light source from the edge of the aperture at the front of the camera lens (the photorefraction aperture). Knowing each of the other parameters, therefore, should reveal the eye’s refractive error. Unfortunately, this analysis of the technique is not a complete description of the system. There are additional factors that affect the calibration of these instruments.7-9 These factors are mainly optical characteristics of the eye being measured and include: 1. Reflectance of the retina, which may affect the gradient of the light distribution in the pupil7,10,11, 2. The distance between the retinal structures that reflect the light and the photoreceptors that initiate the visual response, which may cause an absolute offset in the measurement. (Glickstein and Millodot12 suggest that light forming the reflex in retinoscopy is reflected from the inner limiting membrane. Mutti et al.13 propose reflection from the outer retina in rats, which would cause a smaller artifact. Also see Berendschot et al.14 for a review of reflectance in different retinal layers.), and 3. Higher-order monochromatic aberrations, which may disrupt the light distribution in the pupil.15,16
In a system with an extended light source, the slope of the light distribution in the pupil plane can be fit with a linear regression to estimate refractive error over a relatively wide operating range.7,16,17 The conversion of the slope of the linear regression into a refraction estimate has to be based on an empiric calibration rather than the theoretical analysis discussed previously, however, because of the additional factors.
Our goal was to test the validity of an adult empirical calibration for assessing infant subjects using one of the more commonly used instruments. The PowerRefractor (initially manufactured by Multichannel Systems but more recently by PlusOptix) is an example of a video-based eccentric photorefractor that is in principle well suited for use with pediatric populations. This instrument was calibrated using adult subjects.7,17 The results of the built-in calibration have been verified in uncyclopleged adults by other groups who have compared it with subjective refraction and autorefractors.18,19 It has also been found reliable, typically within 1Dof spherical equivalent, when compared with cycloplegic or noncycloplegic retinoscopy or autorefraction in populations extending down into childhood.20,21
The results of these studies suggest that the PowerRefractor, like the eccentric photorefraction technique in general, holds promise as a clinical and research tool for use with infants. The goal of the current study was to assess the built-in adult PowerRefractor refraction estimate for use with uncyclopleged infants under naturalistic conditions, because this younger population would benefit the most from the rapid assessment method and has eyes that differ the most from the adults for which the instrument was calibrated (e.g., in terms of fundus reflectance,22,23 optical power of the eye,24,25 the small eye artifact,12,13 and higher-order aberrations26,27). We wanted to determine whether the built-in adult calibration would be appropriate for use with free-viewing uncyclopleged infant subjects.
Other variants of the photorefraction technique and other eccentric photorefraction instruments have been calibrated directly for use as vision screening tools for infants,30-33 but to date the relatively widely used PowerRefractor system has not been validated for infants and we are not aware of an instance in which another adult defocus calibration has been validated for infants. Our study consisted of an absolute validation using the gold standard of retinoscopy and a relative validation using trial lenses to induce known amounts of defocus.
METHODS
Subjects
Infant subjects were recruited from public birth records and the local community. Adult subjects were students, staff, and faculty of Indiana University. Informed consent was gathered from adults and from parents for their infants after the study had been reviewed and approved by the Indiana University Institutional Review Board.
General Procedure
The infants included in the study were healthy, born at full term, and had no clinically significant ocular abnormalities. They were supported on a parent’s lap with their head stabilized against the parent. Some infants sucked on a pacifier or the parent’s finger, which provided further stabilization of their head. The adults sat on the same chair as the parents with no further head stabilization. The subjects were not cyclopleged to produce a validation for natural pupil sizes in dim lighting conditions (the adult pupil sizes ranged from 5–7 mm and the infant’s from 4–6 mm). These conditions are similar to those used in other experiments and vision screenings. The PowerRefractor camera was positioned 1 m from the subject with the subject’s face centered in the image in accordance with the recommended instrument protocol for this Multichannel Systems version (as shown in Fig. 1). None of the subjects wore an optical correction. Each adult’s and infant’s attention was attracted to the camera distance using an illuminated toy in a general attempt to stabilize their accommodation. The PowerRefractor was used in “binocular” mode (as named in the manual) in which pupil diameter, refraction along the vertical meridian, vergence, and horizontal, and vertical positions of both eyes are recorded at 25 Hz.
Specific Validation Procedures
The aim was to test the instrument’s built-in defocus calibration for naturalistic pupil sizes. The infants therefore were not cyclopleged and free to change their accommodation. Thus, the eye’s defocus could vary unpredictably and so a validation protocol would have to be performed while the accommodative state and the absolute refraction of the eye were unstable and unknown. Two protocols were designed to meet this requirement. One provided an absolute defocus comparison with retinoscopy, and the other provided a relative validation based on measurements of change in defocus.
Absolute Validation
Procedure
This protocol was designed to make a comparison between PowerRefractor readings and retinoscopy reflexes. The protocol was performed on the subject’s left eye. The focus of the eye was deliberately varied using trial lenses placed in front of the eye by an assistant and by allowing the subject to freely change their accommodation. The PowerRefractor measured the eye’s refraction continuously at 25 Hz while an experienced retinoscopist performed retinoscopy in the plane of the front of the camera lens at 5° eccentricity from the center of the photorefraction aperture (see Fig. 2). The retinoscopy was performed intermittently and as briefly as possible. The retinoscopist’s task was to judge the direction of motion of the reflex, which she described as “with,” “against,” or “neutral.” The procedure was recorded with an additional video camera that permitted synchronization of the retinoscopy judgments with the data recorded by the PowerRefractor. Thus, after the data collection, the retinoscopist’s judgments could be compared with the simultaneous measurements made by the PowerRefractor. At times when the eye was focused between the subject and the PowerRefractor, the retinoscopist should have seen an “against” reflex and the PowerRefractor should have read myopia (negative defocus). When the eye was focused further away than the PowerRefractor, the retinoscopist should have seen a “with” reflex and the PowerRefractor should have read hyperopia (positive defocus). The motion of the retinoscopy reflex would be neutral when the source of the retinoscopy reflection in the eye was conjugate with the retinoscope and the eye was “focused” at the retinoscope. The PowerRefractor reading can be compared with the retinoscope motion to determine whether the PowerRefactor reads zero when the retinoscope reflex is neutral in the plane of the PowerRefractor light source and aperture (as basic theory would predict).
FIGURE 2.
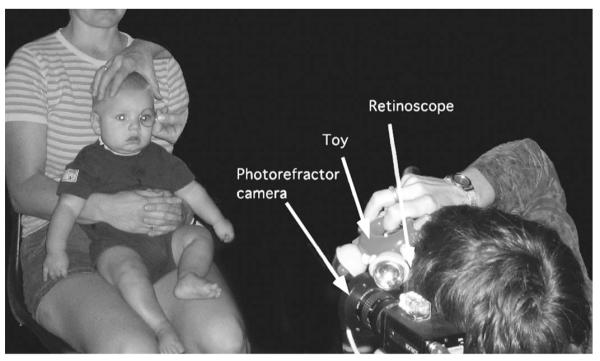
A demonstration of the absolute validation protocol. Retinoscopy was performed immediately adjacent to the PowerRefractor photorefraction aperture at a 1-m viewing distance.
The series of retinoscopy calls were recorded with different lenses over the eye. In addition to judging the direction of motion of the reflex, the retinoscopist also requested the power of the following trial lens in an attempt to reach a neutral judgment (the nulling technique is somewhat similar to the ones used by Angi et al.,8 Bobier31 and Seidemann and Schaeffel34). The “neutral” point was not always reached because the subjects were free to change their accommodation and the infants had a limited attention span.
Data Analysis
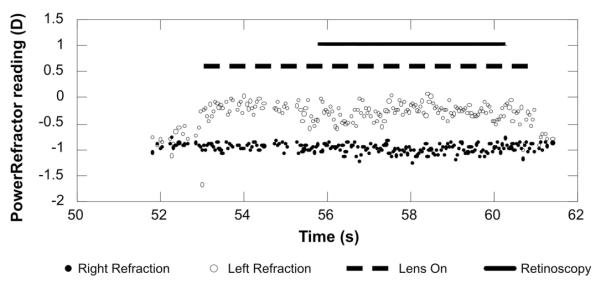
The direction of motion of the retinoscopy judgment was compared with the simultaneous mean PowerRefractor reading preferably over 2 seconds, but not less than 1 second, of data (25–50 measurements). The longest period of data (up to 2 seconds) that fulfilled the following two criteria was used: 1) the gaze position of either eye could be no more than 15° from the pupillary axis, because the optics of the infant eye are likely to change with eccentricity as has been shown in adults.7,11,35-38 2) No more than one consecutive data point was missing (the instrument does not collect data if its built-in software criteria are not met). The identified periods of data were also confirmed to contain no data more than 2 D from the mean (both missing data and extreme outliers could be caused by blinks for example18). If possible, PowerRefractor data collected during the retinoscopy was used; otherwise, data immediately preceding the retinoscopy was used (occasionally the use of the retinoscope interrupted the PowerRefractor recording typically by causing the pupil to constrict below the diameter required for the recording). It was not possible to use the data immediately after the retinoscopy because the lens placed over the eye was typically removed at that time. An example dataset for one retinoscopy call is shown in Figure 3.
FIGURE 3.
Example of the absolute validation data. The PowerRefractor data from the right and left eyes are represented as a function of time by the filled and open symbols, respectively. An apparent anisometropia develops at 53 seconds when a lens was placed over the left eye. The dashed line illustrates the time that the lens was over the eye. The retinoscopy was performed on that eye between 56 and 60 seconds as shown by the solid line. The retinoscopy call was “against” and the mean PowerRefractor reading was −0.27 D.
Subjects were included in the absolute analysis if they provided PowerRefractor data for at least two retinoscopy calls. These may or may not have included a neutral call. The data were then combined across subjects to examine the consistency with which a hyperopic PowerRefractor reading resulted in a “with” judgment and a myopic PowerRefractor reading resulted in an “against” judgment. The PowerRefractor reading corresponding to the transition between “with” and “against” judgments corresponds to the reading when the eye is focused at the retinoscope and photorefractor aperture. This estimate from the data pooled across the population could then be compared with the individual subjects for whom a true “neutral” judgment was made.
Relative Validation
Procedure
After determining the PowerRefractor reading for an eye focused at the light source and photorefraction aperture, the second protocol was designed to determine whether the PowerRefractor estimates of changes in defocus are appropriate (e.g., for studies of dynamic accommodative responses). This was achieved by recording the PowerRefractor measurement as a function of defocus of one eye (in a similar manner to the calibrations described by Schaeffel et al.7 and Choi et al.17). The defocus was achieved using a trial lens in front of one eye again. The total defocus of the eye had to be known to plot the PowerRefractor measurement against total defocus, however, and, although the infants were encouraged to maintain their accommodation at the camera distance, it was not fully stable. The simultaneously recorded data from the two eyes were therefore used to calculate the amount of anisometropia induced by the lens of known power to eliminate the effect of changes in accommodation (Fig. 1). The difference in readings between the two eyes was calculated while positive lenses of 1, 2, 3, and 4 D were held in front of one eye (the data were corrected for the subject’s true refractive anisometropia by subtracting the difference between the eyes with no lens present).
Data Analysis
Data in which the gaze position exceeded 15° from the pupillary axis were excluded. The difference between the measurements from the two eyes was calculated for each of the remaining 25-Hz samples. These differences were then averaged over a second of data to generate an estimate of the total anisometropia for each lens power. The final induced anisometropia estimate was derived by subtracting the subject’s ocular anisometropia with no lenses (also averaged over 1 second) (Fig. 4). We were concerned that the induced anisometropia might be highly variable if the infant made dramatic changes in accommodation. Therefore, we only included babies in the analysis if the uncovered eye (right eye in Fig. 1) had a mean reading that fell between +1.50 and −2.00 D for every lens power.
FIGURE 4.
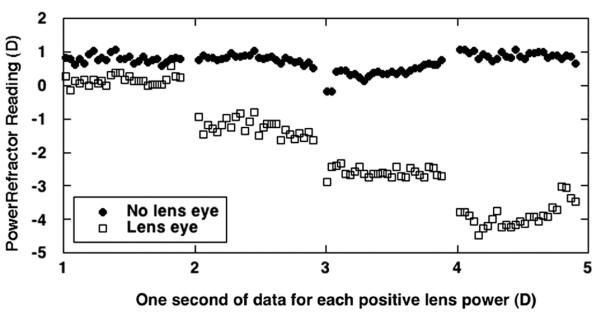
Example of relative validation data from a 22-week-old infant. One second of PowerRefractor data is plotted for each of the four lens powers. The eye with the lenses placed over it is represented by the open squares, and the uncovered eye is represented by the filled circles. The data demonstrate the increasing induced anisometropia with increasing lens power.
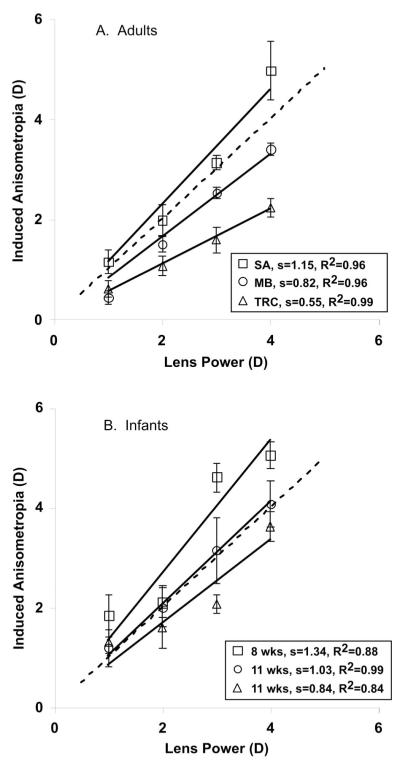
The data were summarized by plotting induced anisometropia against trial lens power (Fig. 6). An ordinary least-squares regression was then performed for each subject to estimate the slope of this function. The y intercept was set to zero for the regression (a prediction resulting from the baseline anisometropia subtraction). Only infants who provided data for all four lenses were included in the analysis.
FIGURE 6.
Results of the relative validation protocol. Induced anisometropia, as measured by the PowerRefractor, is plotted as a function of lens power for individual subjects. Examples of adult and infant data are given in A and B, respectively. The y intercept was constrained to be zero for each of these fits. The slope and R2 of each regression is provided.
RESULTS
Absolute Validation
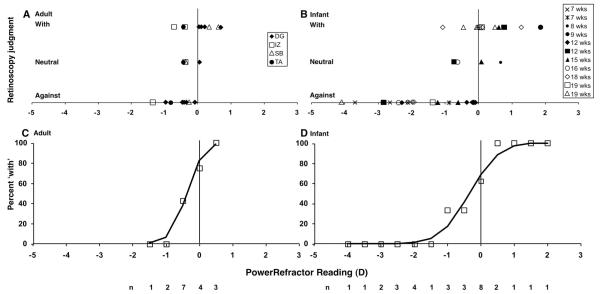
The absolute validation data are shown in Figure 5A and B, in which the motion of the retinoscopy reflex (the retinoscopist’s judgment) is plotted as a function of the simultaneous PowerRefractor reading. The adult data are shown in Figure 5A and the infant data in Figure 5B. Data were successfully recorded from 11 infants ages 7 to 19 weeks. The protocol was attempted with 39 infants from 2 to 24 weeks of age, indicating a success rate of 28%. This success rate is relatively low and reflects the demands made on the infant. They had to repeatedly and stably look in the direction of the retinoscopist for more than 2 seconds while a lens was placed over one of their eyes. From our experience, this success rate also indicates the likely success in performing this validation for individual infants in future studies. Data were also collected from four adults from 22 to 35 years of age (of five attempted, the only presbyopic adult attempted had pupils that were too small for the PowerRefractor to make measurements).
FIGURE 5.
Results of the absolute validation protocol. A and C show adult data and B and D show infant data. (A and B) The retinoscopy judgments are plotted as a function of PowerRefractor reading for each subject. (C and D) The individual data points are combined to form psychometric functions. The percentage of “with” judgments is plotted as a function of PowerRefractor reading in 0.5-D bins centered on the values shown. The solid functions show the fits to the data using Probit analysis. The total number of judgments (n) from which each percentage was calculated is shown beneath the panels.
The goal of this analysis was to find the PowerRefractor reading that corresponds to a neutral judgment for retinoscopy at the camera distance. This is equivalent to the measurement at which the retinoscopy reflex transitions between “with” and “against” motions. The data collected from a number of infants did not include a neutral call and therefore we took two approaches to this analysis. In one, we summarized the PowerRefractor readings for which neutral judgments were made. In the other, we generated a psychometric function using the retinoscopist’s “with” and “against” judgments for all of the subjects. The 50% “with” threshold on this function is equivalent to 50% “against” and represents the transition between the two judgments.
In the neutral judgment analysis, the four adult neutral calls lie between PowerRefractor readings of −0.43 and 0.05 D (with a mean of −0.28 D (standard deviation [SD] ± 0.22 D), whereas the neutral calls from the four infant sessions that included them lie between −0.73 and 0.67 D (with a mean of −0.15 D [SD ± 0.66 D] for these infants 8, 12, 15, and 16 weeks of age). In the psychometric function analysis, the “with” and “against” judgments from Figure 5A and B were combined into 0.5-D bins to form the psychometric functions shown in Figure 5C and D. In Figure 5A and B, the “with” calls extend from positive PowerRefractor readings to approach a reading of −1 D. The “against” calls extend from negative PowerRefractor readings to a reading of zero. This overlap in the judgments defines the slope of the psychometric functions in Figure 5C and D. The 50% points on the psychometric functions were derived using probit analysis (SPSS, Finney, 1952). The adult value was −0.37 D (95% confidence interval [CI]: 0.32 D to −1.22 D) and the infant value was −0.34 (95% CI: 0.31 D to −1.11 D). A t-test indicated no significant difference between these values (p > 0.25). The infant and adult data do show a similar amount of overlap of “with” and “against” judgments, possibly as a result of the angular offset between the photorefraction aperture and retinoscope causing variability if the subject switches fixation between the two instruments, characteristics of individual eyes that affect the linearity of the light distribution in the pupil, or the assessment of the direction of the retinoscopy call (most studies have shown that an interval of ± 0.50 D contains 80% of the variance in repeated retinoscopy estimates39). Overall, however, the derived transition points and neutral judgments are comparable in the two groups. The data indicate that the PowerRefractor typically reads between zero and −1 D when the retinoscope reflex is judged to be neutral in the photorefractor aperture plane.
Relative Validation
Induced anisometropia is plotted as a function of lens power in Figure 6. Data from three representative adults and infants are shown in Figures 6A and B, respectively. A perfect validation would produce data that lie along the 1:1 function. The data in each panel represent the range of function slopes found.
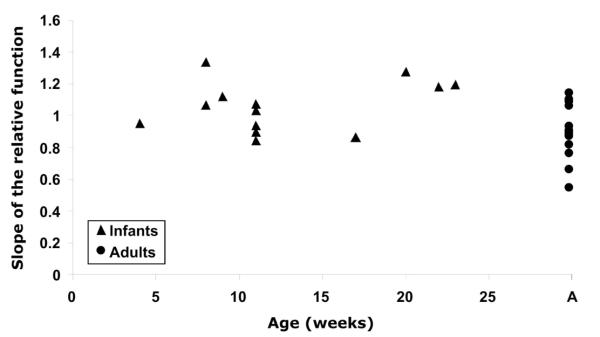
The slopes of the validation functions are plotted as a function of age in Figure 7. Slopes are shown from the 13 infants whose data met the inclusion criteria. The protocol was attempted on 26 infants from 4 to 23 weeks of age, indicating a 45% success rate. Data are also shown from 13 adults from 22 to 35 years of age (this protocol was also attempted on the one presbyopic adult whose pupils were too small for the PowerRefractor to make measurements). Although there is a difference between the infant and adult slopes, there is no significant effect of age over the infant age range tested here (ordinary least-squares regression: y = 0.0086x + 0.9497: R2 = 0.1009, p > 0.10). The infant slopes ranged from 0.84 to 1.33 with a mean of 1.06 (SD ± 0.16), and the adults had a range of 0.55 to 1.14 with a mean of 0.90 (SD ± 0.18). The difference between these distributions was statistically significant at the α = 0.05 level (t = 2.42, df = 24, p = 0.023, in a two-tailed t test assuming unequal variance). The infant regressions had a mean R2 of 0.88 with a range of 0.652 to 0.993 and the adults had a mean R2 of 0.98 with a range of 0.896 to 0.998. The data imply that despite structural differences between adult and infant eyes, the commercial Multichannel Systems PowerRefractor calibration is fairly accurate in measuring changes in defocus in both populations, although a small but significant difference in slope between the groups was demonstrated.
FIGURE 7.
The slopes of the individual relative validation functions are plotted as a function of age in weeks. A linear regression fit to the infant data has a slope that does not differ significantly from zero.
The linear regressions performed on each individual’s data were constrained so that the y intercept was equal to zero as predicted by the subtraction of the subject’s true refractive anisometropia with no lens. A second analysis was performed to determine whether the adult and infant slopes were matched if the intercept of the regression was permitted to vary. In this analysis, the mean infant slope was 1.11 (SD ± 0.24) (range, 0.74–1.40) and the mean adult slope was 0.92 (SD ± 0.20) (range, 0.53–1.25). Again, a t test revealed that these slope distributions were statistically significantly different at the α = 0.05 level (t = 2.17, df = 23, p = 0.04 in a two-tailed t test assuming unequal variance), and also that the adult and infant distributions of fitted intercepts were not significantly different. The mean adult intercept was −0.06 D (SD ± 0.22 D) and the mean infant intercept was −0.15 D (SD ± 0.60 D) (t = −0.44, df = 15, p = 0.66, in a two-tailed t test assuming unequal variance). Thus, the outcome was the same for this second analysis.
DISCUSSION
We have performed an empirical analysis of photorefraction measurements provided by a commercial instrument. We have tested the validity of the calibration provided in the PowerRefractor software for infant subjects. We used two protocols, one that compared the measurements with simultaneous retinoscopy judgments, an absolute comparison, and another that compared the readings with known changes in defocus, a relative comparison. We performed these analyses empirically because the current theoretical descriptions of the eccentric photorefraction technique are not complete for predicting defocus.
Absolute Validation
The point at which an eccentric photorefractor reads zero should not depend on an assumed conversion factor from slope of the light intensity distribution to diopters of defocus. The “slope” for zero defocus is zero and so a multiplicative factor has no effect on the result. The PowerRefractor should therefore read zero when the retinoscope is held in the same plane as the photorefraction aperture and the retinoscopy reflex is neutral. A nonzero reading from the photorefractor in this situation would imply a dioptric offset. Possible reasons for this might include any remaining longitudinal chromatic aberration resulting from the wavelength difference between the PowerRefractor and retinoscope light sources40 (with no correction, the PowerRefractor would read more hyperopia than the retinoscopy) or a compensation built into the PowerRefractor software for either tonic accommodation or the distance of the camera relative to infinity.34 Our results show that the adults and infants actually had similar offsets between the PowerRefractor and retinoscopy results. The PowerRefractor typically read a small amount of myopia when the retinoscopy reflex was judged to be neutral. The most simple explanation for this difference would be a compensation in the software for the viewing distance of the camera.
Two other studies have made simultaneous comparisons between the photorefractor reading and the focus of the eye. Allen et al.18 plotted PowerRefractor reading as a function of target fixation distance for five adults. For a fixation distance of −1 D, equivalent to the camera distance of 1 m, they found a PowerRefractor reading of approximately −1 D (p. 246, Fig. 1). Seidemann and Schaeffel34 performed retinoscopy on adults while data were collected with the PowerRefractor. Their Figure 1 (p. 422) demonstrates that for a retinoscopy neutralization at −1 D (the 1-m camera distance), the PowerRefractor readings were between approximately0Dand −1 D. The results of these two other studies are in good agreement with the data shown here in Figure 5.
Relative Validation
The slopes of the relative validation functions are close to one for the infants and adults. The data suggest that even the slopes at the extremes of each population lie within a factor of two of the ideal 1:1 line. The mean values also suggest that the infants had higher slopes than found for adults with approximately the same variability in the two groups. Variability in adult slopes is noted by Schaeffel et al.7 and Seidemann and Schaeffel.34 The increased slope of the infants’ induced anisometropia function must come from a factor that causes a multiplicative increase in calculated slope of the light distribution in the pupil. This could be caused by an increase in fundus reflectance, for example, the light intensity at each point in the pupil would be multiplied by a common factor. Whatever the factor causing this, it does not appear to be compensated for in a correction for mean image intensity such as that described by Schaeffel et al.7
Using this induced anisometropia protocol to control for a change in infants’ accommodation depends on the assumption that infants’ accommodation is fully consensual. If this were not true, the apparent anisometropia would change with changes in accommodation; the anisometropia would either increase or decrease depending on the eye driving the response and the direction of the response. We saw no clear evidence of this behavior in the data and limited the range of mean values from the eye with no lens to prevent excessive changes in accommodation. We were therefore comfortable making this assumption and are not able to explain the difference in mean infant and adult slopes by assuming that infants’ accommodation is not consensual.
Clinical Estimation of Refractive Error
One of the most promising uses of the eccentric photorefraction technique is to examine children for the presence of high refractive errors, strabismus, or media abnormalities. A number of studies have compared screening data from other eccentric photorefractors with a full clinical examination of refractive error.8,30-32,41-45 Abrahamsson et al.,20 Suryakumar and Bobier,46 and Schmidt et al.47 have all used the PowerRefractor to screen for refractive error. Abrahamsson et al. found that over 90% of their children from 6 months to 5 years of age had differences between PowerRefractor and autorefractor readings of <1 D, and Suryakumar and Bobier found that the PowerRefractor underestimated hyperopia less than a number of other similar screening instruments in uncyclopleged preschool children. Schmidt et al. found the PowerRefractor did not perform as well as a number of their other screening tools in the Vision in Preschoolers study.
We do not examine the ability of the instrument to detect refractive error, but look at its accuracy at measuring defocus in one meridian (the combination of refractive error and accommodative performance). The children that participated in the other studies were typically older than the infants tested here and so, under the assumption that children’s eyes are more mature than infants’, the children should only have more adult-like defocus calibrations.
An interesting observation has been made in a number of studies, that cycloplegia can actually make screening for refractive error less effective, particularly for assessing astigmatism and its axis.20,32,41,44,46 The aberrations introduced into the periphery of the image of a dilated pupil are thought to disrupt the gradient of the light-intensity profile. It is feasible that our defocus measurements would have suggested poorer performance of the instrument if we had conducted our protocols in less naturalistic conditions using cycloplegia.
CONCLUSION
The data collected here suggest that the PowerRefractor (Multichannel Systems version) is able to consistently detect large amounts of defocus in infants, as required in a vision screening environment. For more detailed analyses of absolute accommodative accuracy and small amounts of defocus, however, the absolute validation indicated an offset between the photorefraction and retinoscopy defocus estimates, and the relative protocol demonstrated different slopes for adults and infants with clear individual differences. It is therefore necessary to consider these factors when making detailed analyses using this instrument. Although this is true, the same requirement has also been noted by the developers of the instrument for work with adults.7,34
Our experience in collecting these data suggests it will not be simple to do individual calibrations for infant subjects or to replicate the validation easily for individual instruments in different laboratories. These data were collected on different groups of infants, and the combined success rates imply that successful completion of both protocols on an individual would require excluding a large number of infants. The option of using cycloplegia would depend on the goals and design of the analysis.
ACKNOWLEDGMENTS
The authors thank Diane Goss for recruiting the infant subjects for this study, Grazyna Tondel and Jingyun Wang for help with data collection, and Sarah Needham and Stacy Hufnagel for help developing the retinoscopy protocol.
The research was supported by National Institutes of Health grant EY-014460 (TRC).
REFERENCES
- 1.Ingram RM, Walker C, Wilson JM, Arnold PE, Dally S. Prediction of amblyopia and squint by means of refraction at age 1 year. Br J Ophthalmol. 1986;70:12–5. doi: 10.1136/bjo.70.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, Watson P, Moore A. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10:189–98. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- 3.Rosengren B. A method of skiascopy with the electric ophthalmoscope. Acta Ophthalmol (Copenh) 1937;15:501–6. [PubMed] [Google Scholar]
- 4.Kaakinen K. A simple method for screening of children with strabismus, anisometropia or ametropia by simultaneous photography of the corneal and the fundus reflexes. Acta Ophthalmol (Copenh) 1979;57:161–71. doi: 10.1111/j.1755-3768.1979.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 5.Bobier WR, Braddick OJ. Eccentric photorefraction: optical analysis and empirical measures. Am J Optom Physiol Opt. 1985;62:614–20. [PubMed] [Google Scholar]
- 6.Howland HC. Optics of photoretinoscopy: results from ray tracing. Am J Optom Physiol Opt. 1985;62:621–5. [PubMed] [Google Scholar]
- 7.Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol (Lond) 1993;461:301–20. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angi MR, Bergamo L, Bisantis C. The binocular videorefractoscope for visual screening in infancy. Ger J Ophthalmol. 1993;2:182–8. [PubMed] [Google Scholar]
- 9.Gekeler F, Schaeffel F, Howland HC, Wattam-Bell J. Measurement of astigmatism by automated infrared photoretinoscopy. Optom Vis Sci. 1997;74:472–82. doi: 10.1097/00006324-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkinson IJ, Chong KM, Molteno AC. Characterization of the fundal reflectance of infants. Optom Vis Sci. 1991;68:513–21. doi: 10.1097/00006324-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Wolffsohn JS, Hunt A, Gilmartin B. Continuous measurement of accommodation in human factor applications. Ophthal Physiol Opt. 2002;22:380–4. doi: 10.1046/j.1475-1313.2002.00050.x. [DOI] [PubMed] [Google Scholar]
- 12.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 13.Mutti DO, Ver Hoeve JN, Zadnik K, Murphy CJ. The artifact of retinoscopy revisited: comparison of refractive error measured by retinoscopy and visual evoked potential in the rat. Optom Vis Sci. 1997;74:483–8. doi: 10.1097/00006324-199707000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Berendschot TT, DeLint PJ, van Norren D. Fundus reflectance—historical and present ideas. Prog Retin Eye Res. 2003;22:171–200. doi: 10.1016/s1350-9462(02)00060-5. [DOI] [PubMed] [Google Scholar]
- 15.Roorda A, Campbell MC, Bobier WR. Geometrical theory to predict eccentric photorefraction intensity profiles in the human eye. J Opt Soc Am (A) 1995;12:1647–56. doi: 10.1364/josaa.12.001647. [DOI] [PubMed] [Google Scholar]
- 16.Roorda A, Campbell MC, Bobier WR. Slope-based eccentric photorefraction: theoretical analysis of different light source configurations and effects of ocular aberrations. J Opt Soc Am (A) 1997;14:2547–56. doi: 10.1364/josaa.14.002547. [DOI] [PubMed] [Google Scholar]
- 17.Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B, Wilhelm H. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optom Vis Sci. 2000;77:537–48. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Allen PM, Radhakrishnan H, O’Leary DJ. Repeatability and validity of the PowerRefractor and the Nidek AR600-A in an adult population with healthy eyes. Optom Vis Sci. 2003;80:245–51. doi: 10.1097/00006324-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Hunt OA, Wolffsohn S, Gilmartin B. Evaluation of the measurement of refractive error by the PowerRefractor: a remote, continuous and binocular measurement system of oculomotor function. Br J Ophthalmol. 2003;87:1504–8. doi: 10.1136/bjo.87.12.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrahamsson M, Ohlsson J, Bjorndahl M, Abrahamsson H. Clinical evaluation of an eccentric infrared photorefractor: the PowerRefractor. Acta Ophthalmol Scand. 2003;81:605–10. doi: 10.1046/j.1600-0420.2003.0149.x. [DOI] [PubMed] [Google Scholar]
- 21.Schimitzek T, Lagreze WA. Accuracy of a new photo-refractometer in young and adult patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:637–45. doi: 10.1007/s00417-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 22.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–9. [PubMed] [Google Scholar]
- 23.Streeten BW. Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol. 1969;81:383–94. doi: 10.1001/archopht.1969.00990010385017. [DOI] [PubMed] [Google Scholar]
- 24.Bennett AG, Francis JL. Ametropia and its correction. In: Davson H, editor. The Eye. Vol 4. Visual Optics and the Optical Space Sense. Academic Press Inc; New York: 1962. pp. 133–80. [Google Scholar]
- 25.Mandell RB. Corneal contour of the human infant. Arch Ophthalmol. 1967;77:345–8. doi: 10.1001/archopht.1967.00980020347010. [DOI] [PubMed] [Google Scholar]
- 26.Molteno AC, Sanderson GF. Spherical aberration in human infant eyes. Trans Ophthalmol Soc N Z. 1984;36:69–71. [PubMed] [Google Scholar]
- 27.Wang J, Candy TR. Higher order monochromatic aberrations of the human infant eye. J Vis. 2005;5:543–55. doi: 10.1167/5.6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riddell PM, Hainline L, Abramov I. Calibration of the Hirschberg test in human infants. Invest Ophthalmol Vis Sci. 1994;35:538–43. [PubMed] [Google Scholar]
- 29.Hasebe S, Ohtsuki H, Kono R, Nakahira Y. Biometric confirmation of the Hirschberg ratio in strabismic children. Invest Ophthalmol Vis Sci. 1998;39:2782–5. [PubMed] [Google Scholar]
- 30.Atkinson J, Braddick OJ, Durden K, Watson PG, Atkinson S. Screening for refractive errors in 6–9 month old infants by photorefraction. Br J Ophthalmol. 1984;68:105–12. doi: 10.1136/bjo.68.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobier WR. Quantitative photorefraction using an off-center flash source. Am J Optom Physiol Opt. 1988;65:962–71. doi: 10.1097/00006324-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Hamer RD, Norcia AM, Day SH, Haegerstrom-Portnoy G, Lewis D, Hsu-Winges C. Comparison of on- and off-axis photorefraction with cycloplegic retinoscopy in infants. J Pediatr Ophthalmol Strabismus. 1992;29:232–9. doi: 10.3928/0191-3913-19920701-11. [DOI] [PubMed] [Google Scholar]
- 33.Molteno AC, Hoare-Nairne J, Parr JC, Simpson A, Hodgkinson IJ, O’Brien NE, Watts SD. The Otago photoscreener, a method for the mass screening of infants to detect squint and refractive errors. Trans Ophthalmol Soc N Z. 1983;35:43–9. [PubMed] [Google Scholar]
- 34.Seidemann A, Schaeffel F. An evaluation of the lag of accommodation using photorefraction. Vision Res. 2003;43:419–30. doi: 10.1016/s0042-6989(02)00571-0. [DOI] [PubMed] [Google Scholar]
- 35.Jennings JA, Charman WN. Off-axis image quality in the human eye. Vision Res. 1981;21:445–55. doi: 10.1016/0042-6989(81)90091-2. [DOI] [PubMed] [Google Scholar]
- 36.Navarro R, Artal P, Williams DR. Modulation transfer of the human eye as a function of retinal eccentricity. J Opt Soc Am (A) 1993;10:201–12. doi: 10.1364/josaa.10.000201. [DOI] [PubMed] [Google Scholar]
- 37.Navarro R, Moreno E, Dorronsoro C. Monochromatic aberrations and point-spread functions of the human eye across the visual field. J Opt Soc Am (A) 1998;15:2522–9. doi: 10.1364/josaa.15.002522. [DOI] [PubMed] [Google Scholar]
- 38.Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am (A) 2002;19:2363–73. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 39.Goss DA, Grosvenor T. Reliability of refraction—a literature review. J Am Optom Assoc. 1996;67:619–30. [PubMed] [Google Scholar]
- 40.Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42:2409–17. doi: 10.1016/s0042-6989(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 41.Schimitzek T, Haase W. Efficiency of a video-autorefractometer used as a screening device for amblyogenic factors. Graefes Arch Clin Exp Ophthalmol. 2002;240:710–6. doi: 10.1007/s00417-002-0524-5. [DOI] [PubMed] [Google Scholar]
- 42.Kovtoun TA, Arnold RW. Calibration of photoscreeners for singlesubject, contact-induced hyperopic anisometropia. J Pediatr Ophthalmol Strabismus. 2004;41:150–8. doi: 10.3928/0191-3913-20040501-07. [DOI] [PubMed] [Google Scholar]
- 43.Donahue SP, Johnson TM, Leonard-Martin TC. Screening for amblyogenic factors using a volunteer lay network and the MTI photoscreener. Initial results from 15,000 preschool children in a statewide effort. Ophthalmology. 2000;107:1637–46. doi: 10.1016/s0161-6420(00)00298-0. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy RA, Sheps SB. A comparison of photoscreening techniques for amblyogenic factors in children. Can J Ophthalmol. 1989;24:259–64. [PubMed] [Google Scholar]
- 45.Tong PY, Enke-Miyazaki E, Bassin RE, Tielsch JM, Stager DR, Sr, Beauchamp GR, Parks MM. National Children’s Eye Care Foundation Vision Screening Study Group. Screening for amblyopia in preverbal children with photoscreening photographs. Ophthalmology. 1998;105:856–63. doi: 10.1016/s0161-6420(98)95026-6. [DOI] [PubMed] [Google Scholar]
- 46.Suryakumar R, Bobier WR. The manifestation of noncycloplegic refractive state in pre-school children is dependent on autorefractor design. Optom Vis Sci. 2003;80:578–86. doi: 10.1097/00006324-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt P, Maguire M, Dobson V, Quinn G, Ciner E, Cyert L, Kulp MT, Moore B, Orel-Bixler D, Redford M, Ying GS. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision In Preschoolers Study. Ophthalmology. 2004;111:637–50. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]