Abstract
Purpose
A young infant’s environment routinely consists of moving objects. The dynamics of the infant accommodative system are almost unknown and yet have a large impact on habitual retinal image quality and visual experience. The goal of this study was to record infants’ dynamic accommodative responses to stimuli moving at a range of velocities.
Methods
Binocular accommodative responses were recorded at 25 Hz. Data from infants 8 to 20 weeks of age and pre-presbyopic adults were analyzed. A high-contrast image of a clown was moved between 20- and 50-cm viewing distances at four velocities (a step, 50 cm/s, 20 cm/s, and 5 cm/s).
Results
Most infants who had clear responses were able to initiate their response within a second of stimulus onset. The infants were able to discriminate the different stimulus velocities and to adjust their response velocities and durations in an appropriate fashion.
Conclusions
The data indicate that by the third postnatal month infants are able to respond with latencies within a factor of two of adults’ and that there is little immaturity in the motor capabilities of the accommodative system compared with the sensory visual system at the same age.
Retinal image defocus results from the combination of an eye’s refractive error, the amount of accommodation exerted, and the distance of the object being viewed. The amount of defocus in an infant’s retinal image determines their visual experience and may affect experience-dependent processes such as the refinement of cortical synapses1 and emmetropization.2-4
The human neonate typically has a hyperopic refractive error5,6 and accommodates inaccurately to objects in their environment.7-13 Over time, emmetropization and increasing accommodative accuracy both act to improve the developing eye’s optical performance. The reduction in refractive error resulting from emmetropisation typically takes a number of months to years to occur, through a relatively slow process,3,6 and so it is the accommodative system that more routinely eliminates defocus in the infant eye by compensating for refractive error and viewing distance. A number of studies have documented that infants tend to overaccommodate to distant targets until approximately 2 months of age. They maintain their accommodation around approximately 30- to 50-cm viewing distances. During the next month, they start to adjust their accommodation to focus more accurately on nearer and farther targets.7-10,12
Most of the previous studies of infants’ accommodation have determined their steady state responses to stationary targets.7-10 The natural world, however, routinely consists of stimuli moving in depth, when objects are moved or the infant is carried through the environment. When tracking dynamic targets, the latencies and dynamics of infants’ accommodative responses have a large impact on their habitual retinal image defocus. Only one previous study has examined the dynamics of infants’ accommodation, to targets that underwent a step change from one distance to another.14 Howland et al.14 recorded responses at 2 Hz. A sample response from a 4.5-month-old infant was noted to be typical of the 21 responses they recorded from infants aged 4 to 9 months. This infant initiated an accommodative response within 1 second of the step stimulus and completed the response within another half second.
No studies have been published to date that have recorded response characteristics while infants are presented with gradually moving ramp rather than with step targets. Howland et al.14 suggest there are no dramatic immaturities in infants’ motor ability to change their accommodation at 4 months and older, but no studies have been conducted of younger infants or as a function of stimulus velocity. As noted, short response latencies and tracking of a moving target are critical for maintaining a focused retinal image in a dynamic environment.
The adult visual system is capable of tracking moving accommodative targets at relatively low stimulus velocities.15,16 As velocity increases, however, adults generate an increasing number of “catch-up” step responses and ultimately are not able to smoothly track at all.15 At high velocities, above approximately 4 D/s, the adult accommodative system merely generates a single step response related to the target’s final position.15 This combination of accommodative response strategies has been modeled as a dual-mode neural control system.15 Similar neural control models have been proposed to describe other oculomotor responses.17-20 The step responses are considered to be preprogrammed, whereas the slower, smooth tracking responses are under the influence of continuous feedback (see also Yamada and Ukai21). Young infants show evidence of immature step movements during a related form of tracking in that they exhibit a series of step saccades in pursuit eye movements at stimulus velocities that an adult can track smoothly.22,23
The goal of this study was to record infants’ responses to accommodative stimuli moving over a range of velocities to systematically determine their response latencies and whether they can adjust response velocity in a dynamic environment. We sought to determine whether young infants are able to discriminate and track different velocities and whether they demonstrate immaturities mimicking those found in their smooth pursuit responses.
Subjects and Methods
Subjects
Twenty-two full-term infants from 6 to 21 weeks of age were recruited from the local community to take part in the “ramp” stimulus protocol. Five of these infants were tested twice, with a 1-month interval between visits, yielding a total of 27 data collection sessions. A second protocol was used to collect responses to “step” stimuli. This step protocol was initially attempted with each infant completing the ramp stimulus protocol, but we found that the infants were not able to sustain their attention for both protocols. An additional group of 41 infants, from 6 to 23 weeks of age, was recruited to take part in the step protocol.
Six pre-presbyopic adults (age range, 24-32 years) served as a comparison group for the ramp protocol. Three of them were emmetropic and three had low myopia corrected with soft contact lenses. Four of these subjects also provided data that could be included in the analysis for the step protocol. The other two subjects’ pupils became too small for the equipment to function; therefore, two additional similar subjects were recruited for this protocol. The vision of both was corrected with soft contact lenses.
The infants’ parents and the adult subjects gave informed consent before taking part in the data collection. The study followed the tenets of the Declaration of Helsinki and was approved by the Indiana University Bloomington Campus Committee for the Protection of Human Subjects.
Procedure
Accommodation responses to the dynamic stimuli were recorded with a commercially available video-based eccentric photorefractor (Power-Refractor; Multi Channel Systems, Reutlingen, Germany). Data were gathered remotely from 1 m, enabling the infant to be placed in a relatively natural setting while binocular refraction data were collected along the vertical meridian at 25 Hz.24,25 The manufacturer calibrated the commercial image analysis algorithm empirically using adult eyes, and this adult calibration has been tested by other groups.26-30 The validity of the calibration was recently assessed by Blade and Candy31 for infant eyes that did not undergo cycloplegia. This study found that a 0-D reading from this photorefractor typically corresponded to between 0 and 1 D of myopia for infants and adults, determined using simultaneous retinoscopy. Blade and Candy31 also recorded the photorefractor reading as a function of known defocus (induced anisometropia) for subjects. The mean slope of 13 infant defocus calibration functions was 1.06 (median, 1.07; range, 0.84–1.33) with no significant change with age between 4 and 24 weeks. The mean slope of the 13 adult functions was 0.90 (median, 0.90; range, 0.55–1.14). Thus, structural differences between infant and adult eyes resulted in relatively small mean inaccuracies in the refraction estimates provided by the photorefractor, though the inaccuracies did vary across subjects.
The adults were seated on a stool and the infants, who were given no optical correction, were seated in an infant car seat or on their parent’s lap with their heads gently supported. The axis of the photorefractor camera was aligned with the bridge of the subject’s nose, and the target was centered between the subject’s eyes in the real-time image from the photorefractor. The room was kept in dim illumination to attract the subject’s attention to the task.
Stimuli
A high-contrast colored picture of a clown was used as the stimulus target in both protocols. The image measured 3 cm × 2 cm and had a broad spatial frequency amplitude spectrum. It was mounted on a small internally illuminated box. The luminance of the target was 30 cd/m2 unless the subject’s pupils were very small, in which case it was reduced to cause pupil dilation above the required 3 mm minimum for the instrument to function.
Ramp Protocol
A stepper motor was used to move the target along a track between the viewing distances of 20 and 50 cm (Fig. 1). The target was immediately below the camera axis, at angles of 3° for the 50-cm viewing distance and 7° for the 20-cm viewing distance. Three stimulus velocities—50 cm/s, 20 cm/s, and 5 cm/s—were used. These velocities were selected to approximate 5-D/s, 2-D/s, and 0.5-D/s movements15 and the movement durations were 0.6, 1.5, and 6 seconds, respectively. Brief periods were required for the motor to accelerate and decelerate the target. Therefore, the exact stimulus position as a function of time was recorded using a linear potentiometer sampling at 5 kHz. This provided an accurate representation of the accommodative stimulus. Linear potentiometer and photorefractor recordings were synchronized using a trigger pulse at the start of the recording.
Figure 1.
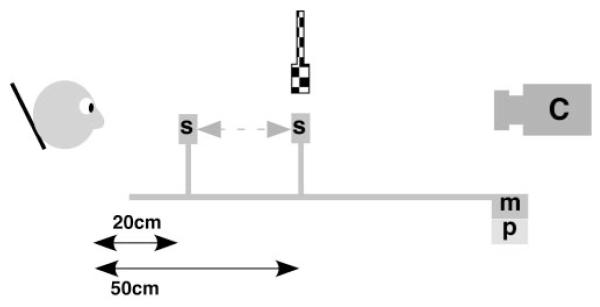
Schematic of the experimental apparatus. For the ramp protocol, the stimulus, S, was moved by a motor, m, along a track in front of the stabilized subject. The movement was recorded using a linear potentiometer, p, which was synchronized with the video recorded by the photorefractor camera, C. The target was moved from a viewing distance of 20 cm to a viewing distance of 50 cm from the subject (a 30-cm movement) to present the disaccommodative stimulus and in the opposite direction for the accommodative stimulus. For the step protocol, the stimulus, S, was fixed at 20 cm from the subject, and a second fixed stimulus was introduced above the camera axis at 50 cm, as represented by the checkered version. These stimuli were alternately illuminated using a toggle switch that was also synchronized with the output of the camera, C.
Each of the three velocities was presented as accommodative and disaccommodative stimuli three times in a pseudorandom, blocked design (see the stimulus function in Fig. 2). Thus, each of the 18 stimuli had an unpredictable velocity, though the continuous motion of the target necessitated an alternation between accommodative and disaccommodative stimuli. The target was held stationary for 5 seconds after each of the two fastest velocities and for 8 seconds after the slowest velocity to allow the subject to complete the response before the next stimulus was initiated. The full series took 157 seconds but could be paused as necessary during that time. Because the infants could not be instructed to maintain fixation on the stimulus, the adults were merely told to look at the clown with no further instruction.32
Figure 2.
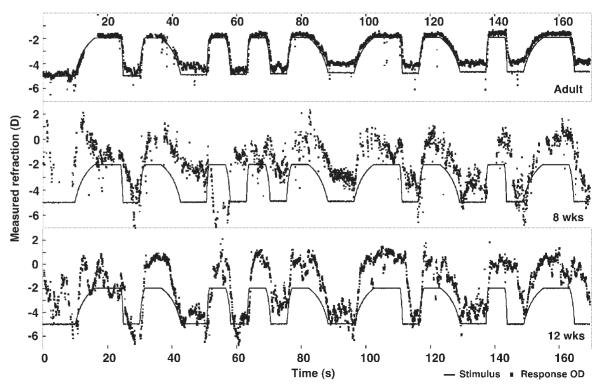
Raw data from the entire ramp session recorded from an adult (top), an 8 week-old infant (middle), and a 12 week-old infant (bottom). The black line represents the stimulus position as a function of time, which was the same for each subject. Data from the left and right eyes were so similar that only data from each right eye are plotted for clarity. Raw photorefractor data (collected at a viewing distance of 1 m) were shifted by 1 D to make the stimulus position of 0 in this figure equal to infinity. The 50-cm viewing distance therefore corresponds to a value of −2 D in the figure and the 20-cm distance to −5 D.
All the accommodation cues (blur, retinal disparity, size, proximity) were consistent with each other for these stimuli. Subjects could use any of these cues to drive their responses. The movement of the stimulus was also correlated with audible noises from the motor (located at 1 m, adjacent to the camera). This helped attract the attention of the infants. Given that the subjects completed this protocol only once in a visit, time was minimal over which they might learn to incorporate these auditory cues into their accommodative responses.33 Occasionally, a toy was used to attract an infant’s attention, but only when the stimulus was stopped between movements and only at the current distance of the stimulus.
Step Protocol
A series of at least six step stimuli was presented to each of a different group of infants. Targets, as described, were fixed at 20 and 50 cm from the infant. The stimulus at 50 cm was at 3° eccentricity above the camera axis, as illustrated by the checked version in Figure 1, whereas the stimulus placed at 20 cm remained below the axis. An experimenter alternated the illumination of these targets from one to the other manually using a single toggle switch, with the timing of the alternation based on the subject’s behavior. The output of this switch was also synchronized with the photorefractor data for analysis of response dynamics. The goal of this condition was to define the characteristics of infants’ responses to the fastest possible stimulus.
After each infant visit, and before the data were examined, the experimenter noted a subjective rating of the session on a scale from 0 to 5 based on the infant’s behavior and cooperation. A score of 0 implied that the infant was sleepy or fussy, and a score of 5 indicated sustained calm, alert attention.
Data Analysis
All the infant sessions given a subjective score of 0 by the experimenter were excluded from the data analysis. Other individual data points were excluded if the subject’s pupils fell below the photorefractor minimum size of 3 mm, if the eye position was greater than 15° eccentricity (to avoid changes in refraction caused by peripheral optics34,35), or if the refraction estimate was outside the +4 D to −6 D working range of the instrument.25
Further analysis was performed only on responses that were clearly stimulus driven. A response was considered stimulus driven, or scorable, if it started after the beginning of the stimulus, the final accommodative position was in the expected direction of change, the accommodative position was stable before and after the response, and no data were missing because of blinks that made the response latency estimation ambiguous.
The stimulus position for each scorable disaccommodation response was fitted with the following exponential function using a least squares method. S(t) corresponds to the stimulus position as a function of time, t, in seconds. The function was fit over a time range from at least 1 second before the movement to at least 1 second after the end of the stimulus movement (part of a stimulus function is shown in Fig. 3):
| (1) |
where B is the average stimulus position before the movement (diopters), Tb is time at the beginning of the stimulus movement (seconds), Te is time at the end of the stimulus movement (seconds), A is amplitude of the stimulus movement (diopters), and Ta is the time constant (seconds).
Figure 3.
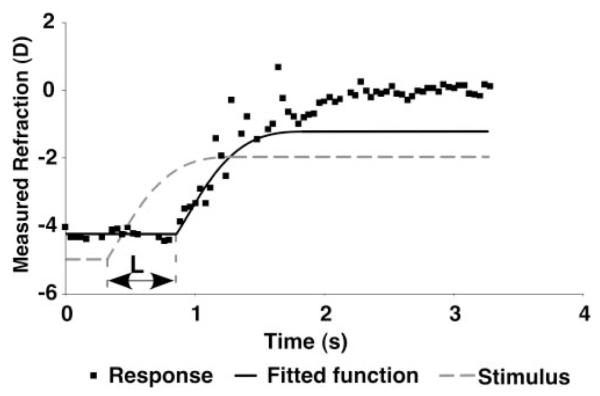
An example of the fit to the data used to calculate the latency of a response. The dashed line function represents the fit to the potentiometer stimulus data (equation 1). The solid line represents the fit of the first section of the stimulus function to the response data, with the rest of the stimulus function added after the fit. The amount of the stimulus function used was adjusted to achieve a visually acceptable fit. The latency (L) of the response was calculated by taking the difference between the beginning of the stimulus and the response, as shown.
B, Tb, Te, A, and Ta were all free parameters in the fit. This function was reversed for the fit to the accommodation stimuli that moved in the opposite direction. All fits to the stimuli had an R2 greater than 0.985.
No assumptions were made about the shape of each infant’s responses to the stimuli. Hung and Ciuffreda15 found that adult responses approximately follow the shape of ramp stimuli, but we did not want to assume this for infants and risk masking immaturities. Therefore, only the beginning of the stimulus function was used as the template for calculating latencies of the accommodative responses. This section was used to avoid later characteristics of the response shape influencing the latency fit and estimate. A function representing the flat portion before the stimulus and between 1 and 4 seconds of the actual stimulus was fit to the response to achieve a visually acceptable result (see Fig. 3). The fitted function, R(t), is described as follows and was used to estimate the time of the beginning of the response, Tbr:
| (2) |
where Br is the average accommodation before the beginning of the response (diopters) and Tbr is the time at the beginning of the response (seconds). The latency of the response could then be calculated from Tbr - Tb.
Again, this function was reversed for the fit to the accommodation responses in the opposite direction. All the fits were performed using software for technical computing (Matlab; MathWorks Inc., Natick, MA), and statistical analyses were completed using analytical software (SPSS for Windows; SPSS, Chicago, IL).
Results
Raw Data
Figure 2 shows examples of the whole ramp recording session from one adult and two infants aged 8 and 12 weeks. The graphs show the stimulus position and the refraction recorded from the right eye as a function of time. A negative refraction indicates that the subject was focused myopically and a positive value indicates that the subject was focused hyperopically. These data demonstrate that the adults and infants were able to respond to the stimuli, in the appropriate direction and with a velocity related to that of the stimulus.
Almost all responses from the subjects shown in Figure 2 were scorable, based on the criteria given. Infant sessions more typically included numerous instances in which the infant apparently did not respond. For example, the 8-week-old infant in Figure 2 did not appear to respond to the stimuli between approximately 60 and 70 seconds.
Infants with scorable data produced a range of 1 to 18 scorable responses per session (the full range possible). Eleven sessions were excluded from the ramp analysis as they had no scorable responses after the criteria described in the data analysis section had been applied. Ultimately, 16 sessions were analyzed from 14 infants of 8 to 20 weeks of age. We observed anecdotally that the youngest and oldest infants tended to have the least number of scorable responses and that those of approximately 12 weeks seemed to be able to sustain the number of responses and tolerate the repetitive task. Related observations of inconsistent behavior have been made in two other studies.12,36 The adult subjects produced a range of 9 to 18 scorable responses per session (their other responses were excluded because the pupil size became too small for the instrument to function).
Representative responses to individual stimuli are shown in Figures 4 to 7. Accommodative responses to all the ramp velocities from adults and infants are shown in Figures 4 and 5, respectively, and for disaccommodative and step stimuli in Figures 6 and 7, respectively.
Figure 4.
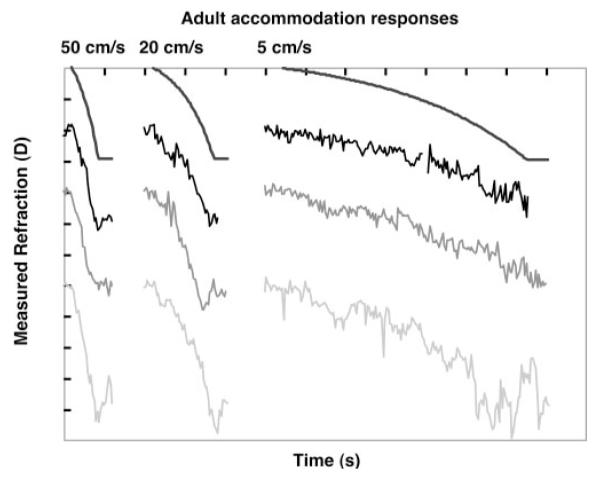
Adult responses to the ramp accommodative stimuli of different velocities. Each gray level represents a different adult, shifted vertically for clarity. The smooth black functions represent the stimuli for comparison (with the beginning of the stimulus and response aligned). Each unit on the y-axis represents 1 D, and on the x-axis it represents 1 second.
Figure 7.
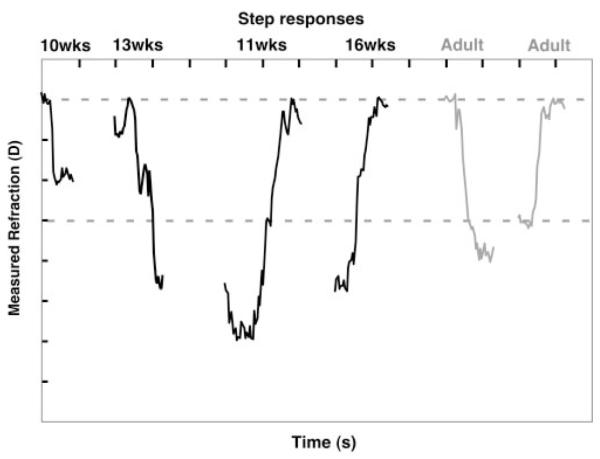
Responses to the step stimuli from one adult and a number of infants of different ages. The amplitude of the stimulus is represented by the dashed lines. Otherwise, the format is the same as for Figure 4. Successful step responses, as defined in the data analysis section, were collected from 10 infants of 10 to 19 weeks of age (from a total of 41 attempts).
Figure 5.
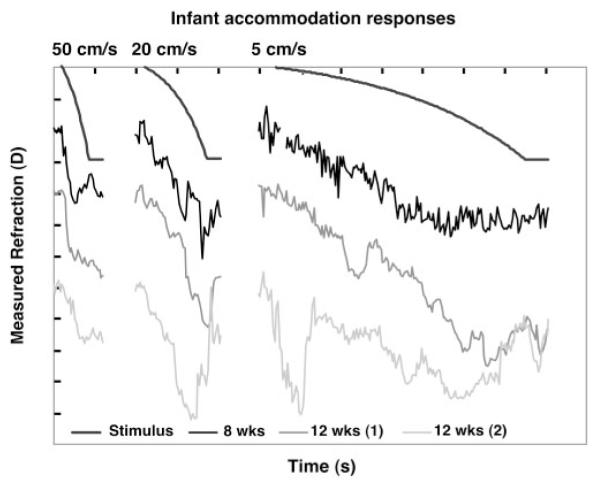
Infant responses to the ramp accommodative stimuli of different velocities. Each gray level represents a different infant whose age is shown at the bottom of the figure. The format is the same as for Figure 4.
Figure 6.
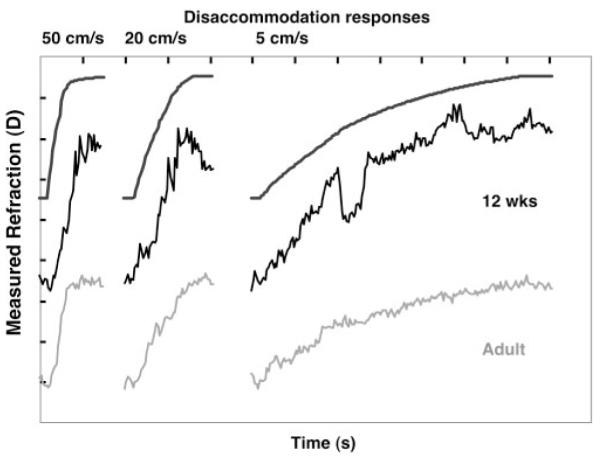
Responses to the ramp disaccommodative stimuli from one adult and one 12-week-old infant. The format is the same as for Figure 4.
The example adults and infants all generated stimulus velocity-dependent responses in the directions of accommodation and disaccommodation, demonstrating that coarsely appropriate and adult-like capabilities are present for these velocities at approximately 8 weeks of age. The durations of the responses are also clearly related to the stimulus durations. Although the infants were not seen to initiate their responses in the incorrect direction, they demonstrated adjustments to stimulus velocity during the response, even to the point of changes in response direction. This is consistent with the use of a feedback system during the slowest responses, at least when rapid velocity corrections occurred after a relatively long delay, in a pattern not characteristic of microfluctuations of accommodation.37 Inspection of repeated responses to the same stimulus velocities in Figure 2 shows that these adjustments are not characteristically present in each response and, therefore, that the infant may be correcting for errors in any reflex or cognitively driven individual response.
Response Latencies
Latencies derived from the fits to the stimuli and responses are shown in Figure 8. Figure 8A shows latencies for the accommodative responses, and Figure 8B shows data for the disaccommodative responses. Each point represents the latency for an individual subject (responses were averaged within a subject if there were two or more scorable responses for that direction and velocity). Adult latencies were typically less than 500 ms, and at least some infants in each age group were capable of responding in this time frame. Fifteen of the 28 infant accommodative and 24 of the 31 infant disaccommodative latencies were shorter than 500 ms. Accommodative latencies tended to be longer than disaccommodative ones, even for the slowest velocity adult responses. This could at least partially be attributed to the stimulus design. The stimulus was moved in centimeters per second. Therefore, the further it was from the subject, the longer it took to move a specific dioptric distance. For the accommodative stimuli, the stimulus started moving toward the subject from the farthest distance; hence, it took longer to reach a subject’s dioptric threshold for an accommodative response than for the disaccommodative case. This would make the latency appear longer for the accommodative direction and the slowest velocity.
Figure 8.
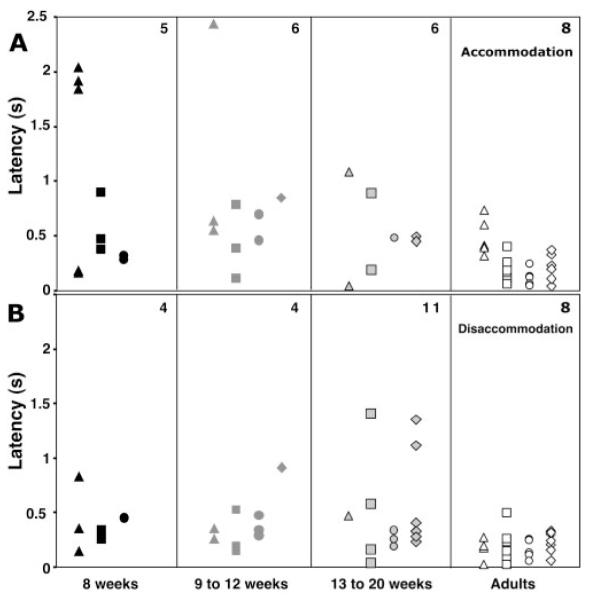
Latencies of individual responses as a function of stimulus direction, age, and stimulus velocity. (A) Latencies for the accommodative responses. (B) Latencies for the disaccommodative responses. Data are grouped as a function of age as shown on the x-axis. Each data point represents the latency for one subject. Top right: number of subjects contributing points; triangles: data for the 5-cm/s condition; squares: data for the 20-cm/s condition; circles: data for the 50-cm/s condition; diamonds: data for the step condition. With this apparatus it was easier, based on anecdotal evidence, to elicit responses from infants in the ramp protocol than in the step protocol.
Univariate ANOVA was performed with stimulus direction, age group, and stimulus velocity as factors and latency as the dependent variable. Some subjects provided multiple data points across direction and velocity while others provided only one data point. Therefore, this analysis was not conducted in a repeated-measures design. The only significant main effect or interaction was the main effect of age group (P = 0.001). Post hoc analysis indicated that latencies in each infant group were significantly longer than found in adults (P < 0.03) but that none of the infant groups was significantly different from the others (P > 0.69). This result is consistent with the observation from Figure 8 that only some of the infants performed with an adultlike latency and that a number of them took longer to respond than did adults. Mean latencies and standard deviations for the combined directions and velocities were 0.676 seconds (±0.66) in the 8-week group, 0.580 seconds (±0.51) in the 9- to 12-week group, 0.483 seconds (±0.39) in the 13- to 20-week group, and 0.209 seconds (±0.15) in the adult group.
Discussion
Velocity-Dependent Stimulus Tracking
Hung and Ciuffreda15 demonstrated that the adult accommodative system can generate tracking responses related to ramp stimulus velocity up to approximately 5 D/s. They suggested that adults demonstrate a dual-mode behavior consisting of smooth tracking at slower velocities with a progressive inclusion of rapid catch-up step responses as stimulus velocity increases. At the fastest velocities, the responses consist of only steps. The adult data collected in the present study also demonstrate velocity-dependent responses. The adults produced relatively smooth tracking of the slowest velocity, with a faster response and some evidence of step behavior for the middle velocity and half-second, steplike responses for the fastest stimulus (Figs. 4, 6).
Infant data in Figures 2, 5, and 6 demonstrate similar velocity- and duration-dependent responses. They also show some evidence of steps during responses to the middle velocity though the infant responses contained more variability overall. Of interest, the infant responses to step stimuli—particularly the 13-week-old accommodative response—also show some evidence of an interrupted response, the double step (Fig. 7). Thus, the data show limited evidence of dual-mode behavior in infancy. During the third postnatal month, this evidence is not as clear as the mixture of saccadic and pursuit eye movements found in young infants when they track objects moving in a fronto-parallel plane.23,38-40 Saccadic interruptions to smooth pursuit eye movements are typically seen at younger ages than tested here. The responses tend to include fewer saccades and more smooth pursuit with increasing age over the first 4 months. It would be necessary to elicit tracking accommodative responses in infants younger than 2 months to systematically investigate the parallels between the smooth pursuit and accommodative systems. This may not be simple to accomplish given infants’ low accommodative gain at the younger ages.9
Overall, the data indicate that infants have a dynamic accommodative system by 8 weeks of age, in agreement with the observation of Howland et al.14 for older infants. If anything, the infant data include more variability and response corrections than the adult data. Green et al.41 suggest that infants’ limited accommodative responses and accuracy in the first 2 postnatal months are a result of their sensory immaturities, particularly immature acuity. Somewhat in agreement with this, the current data suggest, at least by 8 weeks, there is no dramatic immaturity in motor control limiting the response velocities infants can generate and that infants can complete rapid responses within approximately 0.5 second. They can also slow their responses to track a slower moving stimulus, which extends the duration of response.
Calculating the accuracy and velocity of the infants’ responses depends on the dioptric calibration of the photorefractor (PowerRefractor; Multi Channel Systems). Blade and Candy31 recently tested the calibration of this instrument for infant subjects from 1 to 6 months of age, as described. That study demonstrated that, on average, the photorefractor tends to slightly overestimate a change in defocus of an infant eye and that the correction factor varies across infants but not with age. Based on the results of that validation study, calibration factors for infants’ accommodative response amplitudes may vary by a factor of two, although the mean value is likely to be close to the readings provided by the photorefractor.
Variability in subjects’ calibration factors might have contributed to the range of apparent response amplitudes seen across subjects (e.g., Figs. 4 -7). This range could also have resulted from two other factors that may contribute to withinsubject variability. The first is the response starting position. For example, if the subject’s motor response overshot the final stimulus position in one response, a larger amplitude response would have to be generated to reach the final position of the next stimulus. The second is the location of the range of best vision (depth of focus) relative to the target before and after the target movement. In theory, the accommodative response only has to move the range of best vision from wherever it starts to include the target at the end of the response. Working under the assumption that infants have a larger depth of focus than adults, it is feasible that they would have more variability in their response amplitudes. Despite these factors, the withinsubject infant data in Figures 2, 5, and 6 clearly demonstrate stimulus velocity-dependent response velocities and durations.
The responses shown in the figures suggest that infants can produce different response velocities close to those produced by adults. This is made more compelling by the fact that the average infant calibration factor is close (within a factor of 1.2) to the average adult value. Interestingly, the infants had, on average, the ability to produce accommodative velocities as high as the faster ones produced by the adults. This seemed especially true when the step responses and apparent corrective responses were considered (e.g., in the response to the slowest stimulus from the infant at the bottom of Fig. 5). Other infants also demonstrated a fast catch-up step response after a long latency, such as the infant shown at the bottom of Figure 2, at 145 seconds.
It is important to note that the photorefractor only measured refraction along the vertical meridian and that an absolute offset in response could be the result of a real accommodative lead or lag relative to the stimulus, the instrument’s calibration, or astigmatism leading to the infant focusing another meridian on the target. These possibilities cannot be distinguished in our data, though Blade and Candy31 found calibration offsets of typically less than 1 D in adults and infants.
Latency
Adult latencies shorter than 500 ms are consistent with previous studies of step responses, in which mean values are typically between 300 and 400 ms.42-48 These studies found small differences between accommodative and disaccommodative latencies, typically around 50 ms, but the ordering differed between data sets with no clear trend (e.g., Table 3 in Tucker and Charman44). Hung and Ciuffreda15 found mean response latencies for their ramp stimuli that showed the same pattern and approximate values as for adults in the present study (Fig. 8A). Although Hung and Ciuffreda15 found this similar pattern using D/s stimuli, the tendency for the accommodative latencies to be longer in the current data, especially at the slowest velocity, is consistent with the motion of the stimulus in cm/s, as discussed.
Infant latencies compared well with those of the adults. A number of infants demonstrated the ability to respond with adultlike latency. These data were collected with all the accommodative cues present, and the response could be initiated through the processing of blur, retinal disparity, size, or proximity information. Further data collection is required to determine which of these cues drove the response latency. The chief result in the ANOVA was that overall the infants responded more slowly than the adults. There were also many other unscorable instances when infant subjects did not respond to the stimulus. It is not possible to determine whether the slower responses or the lack of responses were the result of individual subjects’ capabilities (e.g., poorer sensory pro- cessing of the stimulus or stage of neural myelination49,50) or of the motivation to respond.9,51,52
In a number of instances (Fig. 8), latencies for the step stimuli were longer than for the fastest ramp stimulus, even though the step stimulus was the fastest stimulus presentation. This is likely to be related to the stimulus design. First, the targets used to present the step stimulus were not on the same visual axis. Second, the stimulus involved a clear discontinuity. In other words, the subject was obliged to “find” the second stimulus when the illumination was switched from the first one. The fastest ramp stimulus moved, with auditory noise, along a continuous trajectory permitting the subject to track the target.
Implications
This study has demonstrated that infants as young as 8 weeks of age are able to generate binocular accommodative responses within half a second of object motion. They are also able to interpret incoming visual information to generate response velocities and durations that are related to those of the stimulus (at least for the four velocities tested here). The temporal dynamics of their responses are relatively adultlike compared with the significant immaturities in spatial acuity and contrast sensitivity documented in the literature for these ages.51,53 Performing dioptric instrument calibrations for subjects would permit more specific analysis of the spatial accuracy of their motor responses.
The results of this study imply that infants, when motivated to fixate and focus on targets, are capable of accommodating rapidly to moving objects in their environment or on stationary objects as they are carried through their environment. Thus, for the typically hyperopic infant, habitual retinal image quality acting as the input to neural and refractive development depends largely on their accommodative accuracy rather than immaturities in the dynamics of the motor component of their accommodative system. Infants appear to transition quickly from the documented period of low gain in their steady state accommodative response function before 2 months of age (see, for example, Banks 9) to short latency tracking over a range of velocities.
Acknowledgments
The authors thank Bill Monette for developing the experimental equipment, Diane Goss for recruiting the infant subjects, the infants and parents for their participation, and Jingyun Wang and Diane Goss for help with the data collection. They also thank Shrikant Bharadwaj and Pamela Blade for helpful discussions.
Supported by National Eye Institute Grant R01 EY014460 (TRC).
Footnotes
Disclosure: G.M. Tondel, None; T.R. Candy, None
References
- 1.Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- 2.Troilo D. Neonatal eye growth and emmetropization—a literature review. Eye. 1992;6:154–160. doi: 10.1038/eye.1992.31. [DOI] [PubMed] [Google Scholar]
- 3.Saunders KJ, Woodhouse JM, Westall CA. Emmetropisation in human infancy: rate of change is related to initial refractive error. Vision Res. 1995;35:1325–1328. doi: 10.1016/0042-6989(94)00222-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 5.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 6.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 7.Haynes H, White B, Held R. Visual accommodation in human infants. Science. 1965;148:528–530. doi: 10.1126/science.148.3669.528. [DOI] [PubMed] [Google Scholar]
- 8.Braddick O, Atkinson J, French J, Howland HC. A photorefractive study of infant accommodation. Vision Res. 1979;19:1319–1330. doi: 10.1016/0042-6989(79)90204-9. [DOI] [PubMed] [Google Scholar]
- 9.Banks MS. The development of visual accommodation during early infancy. Child Dev. 1980;51:646–666. [PubMed] [Google Scholar]
- 10.Brookman KE. Ocular accommodation in human infants. Am J Optom Physiol Opt. 1983;60:91–99. doi: 10.1097/00006324-198302000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Aslin RN, Shea SL, Metz HS. Use of the Canon R-1 autorefractor to measure refractive errors and accommodative responses in infants. Clin Vis Sci. 1990;5:61–70. [Google Scholar]
- 12.Hainline L, Riddell P, Grose-Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behav Brain Res. 1992;49:33–50. doi: 10.1016/s0166-4328(05)80192-5. [DOI] [PubMed] [Google Scholar]
- 13.Currie DC, Manny RE. The development of accommodation. Vision Res. 1997;37:1525–1533. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- 14.Howland HC, Dobson V, Sayles N. Accommodation in infants as measured by photorefraction. Vision Res. 1987;27:2141–2152. doi: 10.1016/0042-6989(87)90128-3. [DOI] [PubMed] [Google Scholar]
- 15.Hung GK, Ciuffreda KJ. Dual-mode behaviour in the human accommodation system. Ophthalmic Physiol Opt. 1988;8:327–332. [PubMed] [Google Scholar]
- 16.Sun FC, Stark L. Switching control of accommodation: experimental and simulation responses to ramp inputs. IEEE Trans Biomed Eng. 1990;37:73–79. doi: 10.1109/10.43618. [DOI] [PubMed] [Google Scholar]
- 17.Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collewijn H, Tamminga EP. Human smooth and saccadic eye movements during voluntary pursuit of different target motions on different backgrounds. J Physiol. 1984;351:217–250. doi: 10.1113/jphysiol.1984.sp015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung GK, Semmlow JL, Ciuffreda KJ. A dual-mode dynamic model of the vergence eye movement system. IEEE Trans Biomed Eng. 1986;33:1021–1028. doi: 10.1109/TBME.1986.325868. [DOI] [PubMed] [Google Scholar]
- 20.Semmlow JL, Hung GK, Ciuffreda KJ. Quantitative assessment of disparity vergence components. Invest Ophthalmol Vis Sci. 1986;27:558–564. [PubMed] [Google Scholar]
- 21.Yamada T, Ukai K. Amount of defocus is not used as an error signal in the control system of accommodation dynamics. Ophthalmic Physiol Opt. 1997;17:55–60. [PubMed] [Google Scholar]
- 22.Salapatek P, Aslin RN, Simonson J, Pulos E. Infant saccadic eye movements to visible and previously visible targets. Child Dev. 1980;51:1090–1094. [PubMed] [Google Scholar]
- 23.Roucoux A, Culee C, Roucoux M. Development of fixation and pursuit eye movements in human infants. Behav Brain Res. 1983;10:133–139. doi: 10.1016/0166-4328(83)90159-6. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol. 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi M, Weiss S, Schaeffel F, et al. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optom Vis Sci. 2000;77:537–548. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsson M, Ohlsson J, Bjorndahl M, Abrahamsson H. Clinical evaluation of an eccentric infrared photorefractor: the PowerRefractor. Acta Ophthalmol Scand. 2003;81:605–610. doi: 10.1046/j.1600-0420.2003.0149.x. [DOI] [PubMed] [Google Scholar]
- 27.Allen PM, Radhakrishnan H, O’Leary DJ. Repeatability and validity of the PowerRefractor and the Nidek AR600-A in an adult population with healthy eyes. Optom Vis Sci. 2003;80:245–251. doi: 10.1097/00006324-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Hunt OA, Wolffsohn JS, Gilmartin B. Evaluation of the measurement of refractive error by the PowerRefractor: a remote, continuous and binocular measurement system of oculomotor function. Br J Ophthalmol. 2003;87:1504–1508. doi: 10.1136/bjo.87.12.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schimitzek T, Haase W. Efficiency of a video-autorefractometer used as a screening device for amblyogenic factors. Graefes Arch Clin Exp Ophthalmol. 2002;240:710–716. doi: 10.1007/s00417-002-0524-5. [DOI] [PubMed] [Google Scholar]
- 30.Wolffsohn JS, Hunt OA, Gilmartin B. Continuous measurement of accommodation in human factor applications. Ophthalmic Physiol Opt. 2002;22:380–384. doi: 10.1046/j.1475-1313.2002.00050.x. [DOI] [PubMed] [Google Scholar]
- 31.Blade PJ, Candy TR. Validation of the PowerRefractor for measuring human infant refraction. Optom Vis Sci. 2006;83:346–353. doi: 10.1097/01.opx.0000221402.35099.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwood AM, Turner JE, Houston SM, Riddell PM. Variations in accommodation and convergence responses in a minimally controlled photorefractive setting. Optom Vis Sci. 2001;78:791–804. doi: 10.1097/00006324-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Cornsweet TN, Crane HD. Training the visual accommodation system. Vision Res. 1973;13:713–715. doi: 10.1016/0042-6989(73)90034-5. [DOI] [PubMed] [Google Scholar]
- 34.Navarro R, Artal P, Williams DR. Modulation transfer of the human eye as a function of retinal eccentricity. J Opt Soc Am A Opt Image Sci Vis. 1993;10:201–212. doi: 10.1364/josaa.10.000201. [DOI] [PubMed] [Google Scholar]
- 35.Seidemann A, Schaeffel F, Guirao A, Lopez-Gill N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A. 2002;19:2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 36.Braddick O, Atkinson J. Accommodation and acuity in the human infant. In: Freeman RD, editor. Developmental Neurobiology of Vision. Plenum Press; New York: 1979. pp. 289–300. [Google Scholar]
- 37.Charman WN, Heron G. Fluctuations in accommodation: a review. Ophthalmic Physiol Optics. 1988;8:153–164. doi: 10.1111/j.1475-1313.1988.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 38.Shea SL, Aslin RN. Oculomotor responses to step-ramp targets by young human infants. Vision Res. 1990;30:1077–1092. doi: 10.1016/0042-6989(90)90116-3. [DOI] [PubMed] [Google Scholar]
- 39.Phillips JO, Finocchio DV, Ong L, Fuchs AF. Smooth pursuit in 1-to 4-month-old human infants. Vision Res. 1997;37:3009–3020. doi: 10.1016/s0042-6989(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 40.Rosander K, von Hofsten C. Development of gaze tracking of small and large objects. Exp Brain Res. 2002;146:257–264. doi: 10.1007/s00221-002-1161-2. [DOI] [PubMed] [Google Scholar]
- 41.Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Res. 1980;20:827–835. doi: 10.1016/0042-6989(80)90063-2. [DOI] [PubMed] [Google Scholar]
- 42.Campbell FW, Westheimer G. Dynamics of accommodation responses of the human eye. J Physiol. 1960;151:301–320. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirachi D, Liu J, Lee M, Jang J, Wang J, Stark L. Accommodation dynamics, I: range nonlinearity. Am J Optom Physiol Opt. 1978;55:631–641. doi: 10.1097/00006324-197809000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Tucker J, Charman WN. Reaction and response times for accommodation. Am J Optom Physiol Opt. 1979;56:490–503. doi: 10.1097/00006324-197908000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Heron G, Winn B. Binocular accommodation reaction and response times for normal observers. Ophthalmic Physiol Opt. 1989;9:176–183. doi: 10.1111/j.1475-1313.1989.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 46.Beers AP, van der Heijde GL. Age-related changes in the accommodation mechanism. Optom Vis Sci. 1996;73:235–242. doi: 10.1097/00006324-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Kasthurirangan S, Vilupuru AS, Glasser A. Amplitude dependent accommodative dynamics in humans. Vision Res. 2003;43:2945–2956. doi: 10.1016/j.visres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Mordi JA, Ciuffreda KJ. Dynamic aspects of accommodation: age and presbyopia. Vision Res. 2004;44:591–601. doi: 10.1016/j.visres.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Ballesteros MC, Hansen PE, Soila K. MR imaging of the developing human brain, 2: postnatal development. Radiographics. 1993;13:611–622. doi: 10.1148/radiographics.13.3.8316668. [DOI] [PubMed] [Google Scholar]
- 50.Tsuneishi S, Casaer P. Stepwise decrease in VEP latencies and the process of myelination in the human visual pathway. Brain Dev. 1997;19:547–551. doi: 10.1016/s0387-7604(97)00076-4. [DOI] [PubMed] [Google Scholar]
- 51.Hainline L. The development of basic visual abilities. In: Slater A, editor. Perceptual Development: Visual, Auditory, and Speech Perception in Infancy. Psychology Press; East Sussex: 1998. pp. 5–50. [Google Scholar]
- 52.McKenzie BE, Day RH. Infants’ attention to stationary and moving objects at different distances. Aust J Psychol. 1976;28:45–51. [Google Scholar]
- 53.Teller DY. First glances: the vision of infants: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 1997;38:2183–2203. [PubMed] [Google Scholar]