Abstract
Recent evidence suggests that inflammatory molecules play critical roles in the development and progression of numerous tumors. However, one specific group of inflammatory molecules whose importance has been established in host immune responses, termed alarmins, has been largely overlooked in cancer biology. The function of several alarmins—including the defensins, LL-37, and HMGB1—in tumor development, progression, or suppression is discussed here. Taken together, these studies indicate that alarmins represent potential new targets for manipulation in a variety of tumors.
Introduction
In cancer biology research, exciting new evidence is emerging that focuses on a group of naturally occurring, self-molecules called alarmins. These molecules are the endogenous equivalent of pathogen-associated molecular patterns and they function to alert the host immune system of cell and tissue trauma (1). Although many cytokines and chemokines act in a similar manner, the term “alarmin” was coined to describe the unique characteristics of this group of “danger signals,” primarily based on their ability to bridge cellular and adaptive immunity. However, alarmins are also defined by other criteria. They are rapidly secreted from stimulated leukocytes and epithelia, passively released from necrotic cells but not apoptotic cells, and can activate receptor-mediated responses such as chemotaxis. Now, in addition to immunology studies, recent investigations have started addressing the role of alarmins in tumorigenesis and cancer progression. In this review, we will briefly describe their newly established functions in cancer with emphasis on the defensins, LL-37, and high-mobility group box 1 (HMGB1) protein. The S100 protein family and their functions in cancer has been recently reviewed elsewhere (2).
Defensins
Defensins are a family of cysteine-rich, cationic peptides produced by cells and tissues involved in host defense against microbial infections. The genes encoding defensins are clustered on the short arm of chromosome 8 and are translated into precursor proteins to maintain the bioactive peptides in an inactive state (3). In humans, the family is classified into two different categories, termed α- and β-defensins, based on the pattern of disulfide bonding and each subfamily consists of several members. Generally, α-defensins are expressed by neutrophils and other leukocytes where release of the COOH-terminal, 3 to 6 kDa, bioactive peptides occurs intracellularly for storage in specific granules. β-defensins are typically expressed by epithelial cells, and their posttranslational processing can be mediated by extracellular proteases. Expression of some defensins is constitutive, whereas expression of others is regulated by danger signals, developmental signals, cytokines, and growth factors in a tissue-specific manner.
One of the first studies to examine the effects of alarmins on tumor cells reported that the α-defensins, human neutrophil peptides (HNP) 1, 2, and 3, induce cell lysis (4). However, this effect is not specific to tumor cells and can be abrogated by serum factors.
Human β-defensin-1 (DEFB1) is constitutively expressed in prostate, kidney, and urogenital tract luminal epithelium. However, studies show a cancer-specific loss or suppression of DEFB1 expression in 82% of malignant prostate samples and 90% of renal clear cell carcinomas (5). Investigations into the mechanisms of decreased defb1 gene expression in tumor cells have found that the promoter region commonly contains point mutations, and these mutations dampen promoter activity. When DEFB1 is reintroduced to prostate, renal, and bladder cancer cell lines, proliferation is inhibited and apoptosis is induced (5, 6). These data indicate that the alarmin, DEFB1, is a potential tumor suppressor in some cancers (5, 6).
Similarly, studies involving human papillomavirus (HPV)-associated cervical cancer have shown that the expression of another β-defensin, DEFB4 (also called hBD-2), is significantly diminished in high-grade squamous intraepithelial lesions and squamous cell carcinomas when compared with low-grade squamous intraepithelial lesions and normal exocervical epithelium (7). The α-defensin, HNP-2, is only weakly expressed in these biopsies and no significant difference is present between normal, precancerous, and cancerous tissue for this alarmin. Addition of either alarmin, DEFB4 or HNP-2, to HPV-transformed keratinocyte models of cervical cancer in vitro stimulates the infiltration of CCR6+ and CCR6− myeloid-derived dendritic cells (mDC), respectively. HNP-2 also induces the migration of CCR6− mDC into HPV-infected xenografts in vivo. These observations suggest that the absence of alarmins contributes to the evasion of cervical tumors from host immune surveillance. Presumably, down-regulation of DEFB4 mediates this effect, whereas the reintroduction of alarmins could restore host antitumor immunity.
In contrast to the potential tumor suppressive roles of the defensins briefly described thus far, a proangiogenic, protumorigenic function of murine β-defensin 29 (Defb29), the homologue of human DEFB4, has been reported (8). These results suggest tissue-specific effects of DEFB4 and its homologues. Coexpression of Defb29 and VEGFA in a mouse ovarian cancer cell line accelerates tumor growth through the accumulation of CD11c+CCR6+ mDC. Importantly, these leukocyte precursor cells incorporate into tumor vasculature and acquire endothelial-like characteristics. In these tumors, Defb29 secretion mediates the recruitment of these cells; whereas VEGFA secretion induces their integration into preexisting vessels via activation of VEGFR-2. Human ovarian tumor epithelium and some stromal leukocytes express DEFB4, indicating that the human homologue of murine Defb29 may function in a similar manner. Although DEFB4 mRNA transcripts are not found in benign tumors or normal postmenopausal ovaries, stage III tumors exhibit 7-fold more DEFB4 mRNA than stage I or borderline tumors. Moreover, in human tumors coexpressing DEFB4 and VEGFA, a CD11c+CCR6+ mDC population expressing denditric cells and endothelial cell markers can be identified in ovarian ascites and in the vasculature of primary tumors.
The role of α-defensins in neovascularization has been examined as well. These molecules, namely HNP-1 and HNP-3, inhibit angiogenesis by disrupting fibronectin signaling through a5h1 integrin (9). However, the role of α-defensins in the context of tumor angiogenesis has not been investigated. From these data, it is tempting to speculate that the balance between the antiangiogenic properties of α-defensins and proangiogenic properties of β-defensins can be skewed to favor tumor survival of some cancers.
hCAP-18/LL-37
Human cationic antimicrobial protein 18 (hCAP-18) is the only member of the cathelicidin family identified in humans thus far.
Like defensins, hCAP-18 comprises three distinct domains including an NH2-terminal signal peptide, a highly conserved cathelin-like domain, and a COOH-terminal peptide termed LL-37 (10). As its name implies, LL-37 is made up of 37 amino acids beginning with 2 leucine residues. Proteolytic processing releases LL-37 from its inactive, precursor hCAP-18 to initiate its antimicrobial activity as well as its other effects on host cells. Some of these effects include stimulation of angiogenesis, proliferation, and chemotaxis, often mediated by the G protein–coupled receptor, formyl peptide receptor–like 1 (FPRL1) or epidermal growth factor receptor (EGFR; refs. 11, 12). Like other alarmins, hCAP-18/LL-37 is constitutively secreted by a variety of immune and epithelial cells and is elevated in response to inflammation and tissue injury (13). LL-37 and its proform, hCAP-18, are overexpressed in breast, lung, and ovarian tumors when compared with their normal tissue counterparts in both the tumor epithelium and stromal cells (14-16). Cumulatively, our data and that of others indicate that LL-37 is an autocrine survival factor released by tumor epithelial cells. In vitro, LL-37–induced proliferation is observed only in the presence of serum, raising the question: does LL-37 need a cofactor or adaptor molecule to initiate its protumorigenic events? Recent studies from other model disease systems support this notion. In psoriatic skin lesions, LL-37 is elevated in comparison with normal healthy skin, and the excessive proliferation and high turnover of keratinocytes results in an aberrantly high concentration of human DNA in the extracellular environment (17). LL-37 binds the DNA and converts the free nucleic acids into a danger signal that triggers IFN production from plasmacytoid dendritic cells through interaction with Toll-like receptor (TLR)9. Because the tumor microenvironment commonly contains necrotic and hypoxic sites, it is feasible that similar mechanisms may occur and contribute to the chronic inflammatory-like state often seen in tumors. It is also possible that tumor-derived LL-37 binds DNA within the necrotic debris then interacts with tumor epithelium to stimulate proliferation and release of additional cytokines and growth factors. In support of this notion, we have observed hCAP-18/LL-37 in necrotic regions of ovarian tumors.1 However, LL-37 may also promote the growth of solid tumors via alternative mechanisms in addition to its direct effects on tumor epithelium because our data suggest its participation in angiogenesis and recruitment of CD45+ leukocytes in ovarian cancers. However, these concepts have yet to be tested in vivo.
HMGB1
The HMGB1 protein is a DNA-binding protein present in almost all mammalian nuclei where it functions in many nuclear processes including gene transcription (18). However, HMGB1 is also found extracellularly after passive release by necrotic cells or through active secretion from stimulated inflammatory cells (19). In the extracellular environment, this alarmin initiates proinflammatory responses to coordinate repair of damaged tissue through recruitment of leukocytes and stimulation of angiogenesis (19-22). HMGB1 signals through receptor for advanced glycation end products (RAGE) to activate these processes. The role of HMGB1 in cancer has been recently reviewed; therefore, only the most recent and relevant reports involving the extracellular alarmin activity of HMGB1 will be discussed here (23). HMGB1 is overexpressed in a number of cancers where the protein contributes to the development of tumors and their metastases with lung carcinomas being the exception. In addition to tumor epithelial cells, tumor-associated macrophages within necrotic regions of breast tumors also express the protein (21). It has been proposed that macrophages contribute HMGB1 in these hypoxic areas to activate endothelial cells and induce neovascularization. Interestingly, HMGB1-induced angiogenesis may also occur through recruitment of endothelial progenitor cells (EPC) promoting tumor progression (24).
Although many studies show a protumorigenic role for this alarmin, a recent study by Apetoh and colleagues (25) revealed that HMGB1 triggers an antitumor immune response after chemotherapy or radiotherapy. HMGB1, released from dying tumor cells, stimulates mDC tumor antigen processing through its interaction with TLR4. However, a common TLR4 mutation (Asp299Gly)— found in 8% to 10% of Caucasians—reduces the binding of HMGB1 to TLR4 and prevents mDC antigen presentation to CTLs. This TLR4 mutation is associated with an increased frequency of metastasis and worsened survival in breast cancer patients, suggesting that clinical outcome is affected by HMGB1-activated TLR4 signaling. Taken together, this study indicates that antitumor immunity can be reprogrammed by necrotic tumor cell release of HMGB1.
Concluding Remarks
The studies outlined above describe new mechanisms by which alarmins promote tumor progression, facilitate host immune response evasion, or serve to trigger antitumor immunity. These functions are summarized in Fig. 1. Whereas some tumors acquire the ability to down-regulate alarmins able to induce apoptosis or recruit antigen-presenting cells, other tumors hijack normal alarmin function to send out fraudulent S.O.S signals as a means to promote their survival. However, the role of many alarmins in cancer development and the mechanisms by which tumor progression is potentiated remain poorly understood.
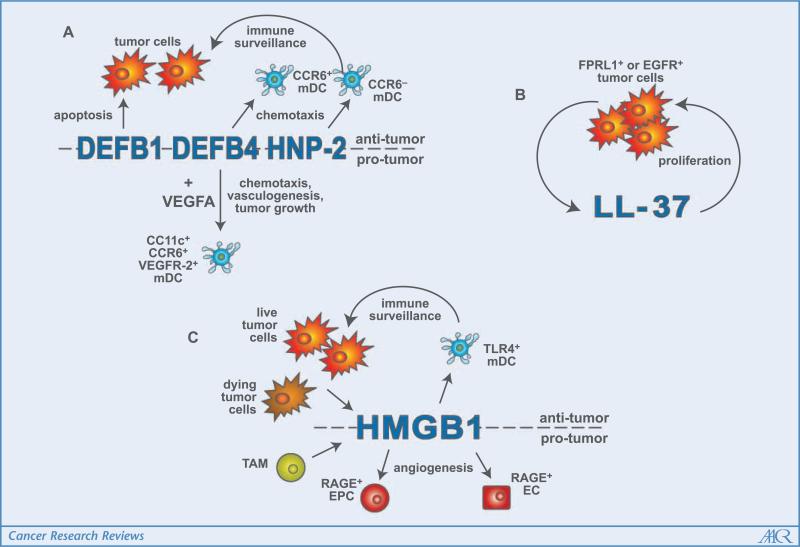
Figure 1.
Summary of alarmin function in the tumor microenvironment. A, in various tumor types, the β-defensins, DEFB1 and DEFB4, as well as the α-defensin, HNP-2, are down-regulated. However, when these molecules are reintroduced, they can induce apoptosis or function as chemoattractants for mDC, reinstating immunoediting. Other tumor types use DEFB4 and VEGFA to recruit mDC and initiate their incorporation into tumor vasculature, which results in tumor growth. B, breast, lung, and ovarian tumor epithelium secrete increased levels of LL-37 when compared with normal epithelial cells. In an autocrine manner, LL-37 feeds back on the tumor cells to stimulate proliferation, mediating these effects through transactivation of the EGFR or potentially, the G protein–coupled receptor, FPRL1. C, stimulated secretion of HMGB1 from tumor cells and tumor-associated macrophages or passive release by necrotic, dying tumor cells into the extracellular milieu has both protumorigenic and antitumorigenic effects. HMGB1 recruits EPCs and activates resident endothelial cells through RAGE signaling to induce angiogenesis. On the other hand, HMGB1 interacts with TLR4 on mDC to stimulate tumor antigen processing and presentation to CTLs, resulting in tumor elimination.
From the literature reviewed here, one particular question prevails: in what context are alarmins protumorigenic or antitumorigenic? For example, DEFB4 and HMGB1 maintain properties that both inhibit and advance tumor growth. We would propose that due to the overall charge and “sticky” nature of alarmins, the molecular makeup of the tumor microenvironment influences their activity, as serum factors do (4, 14). Fragments of extracellular matrix, dying tumor cells, or other soluble factors may dictate which receptor or cell type is activated by alarmins, altering the balance between tumor evasion and tumor eradication. Elucidating these binding partners will be essential to designing efficacious cancer therapies. Admittedly, alarmins will be difficult targets of cancer treatments because of their importance in innate and adaptive immune responses. Systemically inhibiting their functions could leave patients vulnerable to opportunistic pathogens, whereas increasing the efficacy of alarmins might provoke chronic inflammation or autoimmune diseases. Despite these challenges, the unique, tissue-specific role of individual alarmins in tumorigenesis offers exciting prospects for the design and study of new, cancer-specific initiatives. Recent studies also suggest that the immunosuppressive tumor microenvironment can be reset to allow appropriate antitumor responses from the host, bypassing the need for specific targeting of individual alarmin molecules. Additionally, alarmins may be used as biomarkers of cancer progression as lung carcinoma patients exhibit increased LL-37 serum levels (16). In conclusion, an increasing body of evidence now exists to indicate that dysregulation of tumor-associated alarmins promotes cancer progression; yet, further investigation is warranted in this overlooked area of cancer research.
Acknowledgments
Grant support: NIH (1P20RR20152-01).
We thank the other members of the Scandurro laboratory, Suzanne Tomchuck and Kevin Zwezdaryk, Dr. Cindy A. Morris, Dr. Lisa Morici, and Dr. Heather L. LaMarca for their constructive criticism of the manuscript, and special thanks to Dr. Jeff Rosen's laboratory for providing desk space to write the review. We apologize to our colleagues whose work was not included due to space limitations.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
S.B. Coffelt, F.C. Marini, K. Watson, et. al. The pro-inflammatory peptide, LL-37, promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells, submitted for publication.
References
- 1.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–64. doi: 10.1016/j.ejso.2007.04.009. Epub 2007 Jun 13. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein A, Ganz T, Selsted ME, Lehrer RI. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68:1407–10. [PubMed] [Google Scholar]
- 5.Sun CQ, Arnold R, Fernandez-Golarz C, et al. Human β-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66:8542–9. doi: 10.1158/0008-5472.CAN-06-0294. [DOI] [PubMed] [Google Scholar]
- 6.Bullard RS, Gibson W, Bose SK, et al. Functional analysis of the host defense peptide Human β Defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45:839–48. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubert P, Herman L, Maillard C, et al. Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J. 2007;21:2765–75. doi: 10.1096/fj.06-7646com. [DOI] [PubMed] [Google Scholar]
- 8.Conejo-Garcia JR, Benencia F, Courreges MC, et al. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–8. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 9.Economopoulou M, Bdeir K, Cines DB, et al. Inhibition of pathologic retinal neovascularization by α-defensins. Blood. 2005;106:3831–8. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agerberth B, Gunne H, Odeberg J, et al. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 14.Coffelt SB, Waterman RS, Florez L, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–9. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 15.Heilborn JD, Nilsson MF, Jimenez CI, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–9. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 16.von Haussen J, Koczulla R, Shaykhiev R, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 18.Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–9. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 19.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 20.Mitola S, Belleri M, Urbinati C, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Schlueter C, Weber H, Meyer B, et al. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166:1259–63. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Beijnum JR, Dings RP, van der Linden E, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–48. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- 23.Ellerman JE, Brown CK, de Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–48. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 24.Chavakis E, Hain A, Vinci M, et al. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–12. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 25.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]