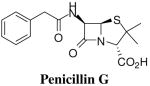
Abstract
In this study we report the first example of a direct diazo-transfer reaction on readily available 6-aminopenicillanates to give 6-azidopenicillanates in high yield. Subsequent Cu(I)-catalyzed Huisgen cycloaddition between these 6-azidopenicillanates and assorted terminal alkynes facilely furnished 6-triazolylpenicillanic acids was. Preliminary biological screening indicates that these triazolylpenicillanic acids possess low to moderate antibacterial activities.
Introduction
β-lactam antibiotics such as penicillin and cephalosporins are the most commonly prescribed antibacterial agents. Their antibacterial efficacy derives from ability to acylate a serine residue at the penicillin binding proteins (PBPs) active site.1, 2 Serine acylation inhibits the enzymatic activities of PBPs, thus interfering with the essential polymerization and cross-linking of the peptidoglycan components of the bacterial cell wall.1 However, the emergence of multi-drug resistant bacteria strains such as Staphylococcus aureus is threatening the clinical effectiveness of β-lactam antibiotics.3 The common resistance mechanism in gram-negative bacteria is the cellular expression of β-lactamases (or penicillinase), which hydrolyze the β-lactam ring and thus inactivate the antibiotics.4–7 Therapeutic agents targeting β-lactamase resistance include clavulanate, sulbactam, and tazobactam. These are generally administered in combination with β-lactamase susceptible β-lactams.8, 9 However, the emergence of multi-strains β-lactamase has reduced the efficacy of some of these β-lactamase inhibitors.10, 11
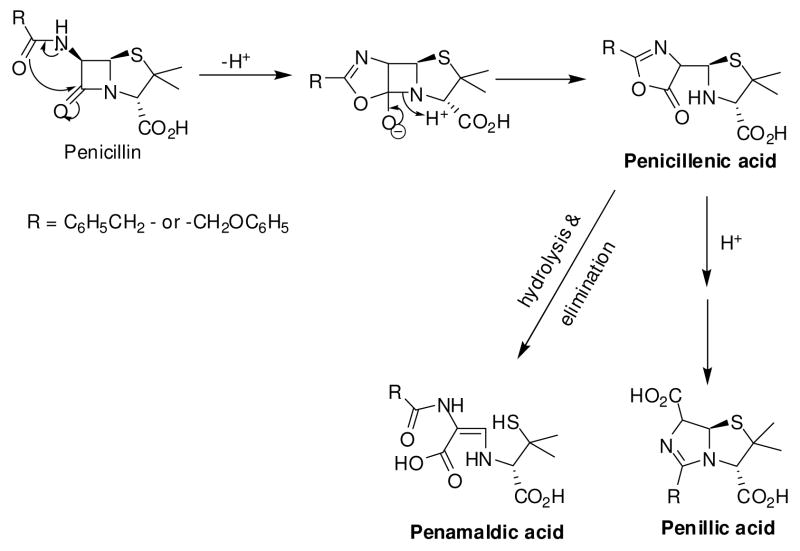
There are also other intracellular factors that attenuate the antibacterial activities of β-lactams. For example, the N-5 amide moiety in penicillins is highly reactive and has been proposed to facilitate the hydrolysis of the β-lactam ring in acidic media (Figure 1).12 Incorporation of electron-withdrawing groups and steric bulk at the N-acyl side chain has been shown to reduce the acid sensitivity of penicillins.13, 14
Figure 1.
Self-induced acid hydrolysis of penicillin.
Another shortcoming to the effectiveness of β-lactamase antibiotics is the bacterial outer membrane, which is a formidable barrier in gram negative organisms. In addition, the bacterial membrane could acquire efflux pumps that facilitate active elimination of drug from the bacterial cytosol.15, 16 All these changes could prevent the intracellular accumulation of pharmacologically relevant concentrations of the β-lactam antibiotics. One clever strategy to facilitate β-lactams diffusion across membrane is to covalently link them to membrane penetrating groups such as sideophores and peptides.1–3, 17 However, the synthesis of such β-lactam conjugates often involve long synthetic schemes and complicated compound purifications protocols.2b–e
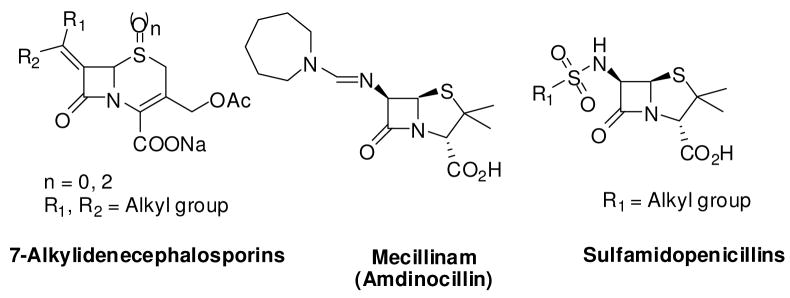
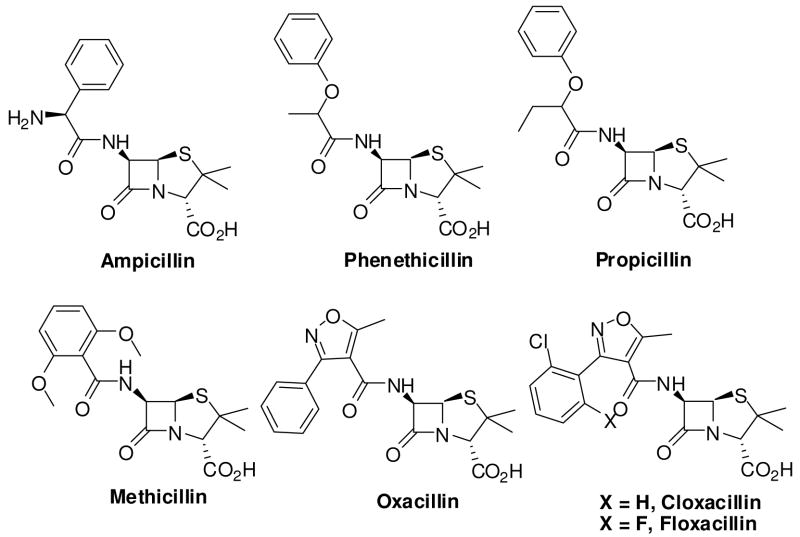
One of the most fruitful β-lactam SAR studies has been the modification of the N-acyl groups. These studies have led to the discovery of β-lactam analogs with superior antibacterial activity.16, 18–20 Much recent studies have shown that attachment of N-acyl groups incorporating the structural feature of peptidoglycan result in PBP specific β-lactams and cephalosporins.3, 17, 21 PBPs can also tolerate an assorted variety of non-amide groups at the C-6 and C-7 positions of penicillins and cephalosporins, respectively.20 Some of these non-amide β-lactam analogs, in addition to possessing potent antibacterial activity, have differential affinity for PBP isoforms (Figure 3). For example, mecillinam (amdinocillin), a β-lactam analog with an unusual 6-formylimido moiety, derives its antibacterial activity from its specific interaction with PBP-2.22 Very few β-lactams with traditional amide bond isosteres have been reported to date. One such example is the sulfonamide derivatives. A subset of these compounds have been synthesized and tested. However these compounds are still prone to chemical degradation and hydrolysis by β-lactamases.23
Figure 3.
Structure of amide isosteric β-lactams.
We proposed that 1,4-disubstituted-1,2,3-triazoles could serve as an isostere for the N-acyl group of penicillins and cephalosporins. Due to the similarities between some of its bond characteristics with an amide moeity, the triazole group is commonly used as an amide bond isostere.24, 25 Unlike amide bond, the triazole moiety is not susceptible to hydrolysis.24 Replacement of the reactive β-lactam acyl bond with a triazole ring may lead to β-lactams that are more stable in acidic environment. In addition, the triazole ring could in principle facilitate facile conjugation of β-lactams to assorted cell permeable peptides and peptoids for promoted uptake of β-lactams into bacterial cell. Here, we report our efforts on the design, synthesis, and preliminary biological activities of 6-triazolylpenicillanic acid.
Results and Discussion
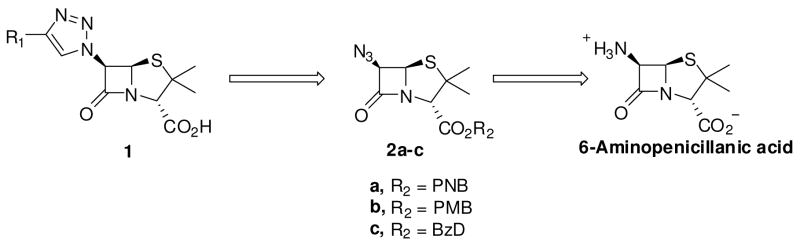
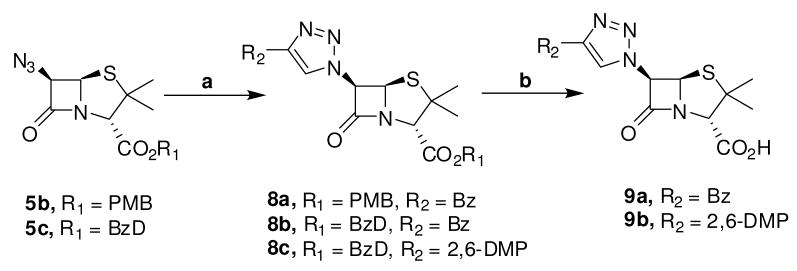
The key intermediate in the synthesis of the proposed 6-triazolylpenicillanic acids 1 is the 6-azidopenicillanates 2. It is anticipated that Cu(I)-catalyzed cycloaddition between 2 and appropriate alkynes (Sharpless Click Chemistry)26 followed by deprotection of the ester protection group will furnish the desired triazolylpenicillanic acid 1 (scheme 1). The synthesis of protected 6-azidopenicillanates similar to 2a–c has been described in the literature.27–30 These compounds are generally synthesized, in moderate yields, using multi-step reactions. For example, Barrett and Sakadarat described the synthesis of benzyl protected 6-azidopecillanate, the final intermediate in their total synthesis of 6-aminopenicillanic acid (6-APA), in a six-step reaction scheme with a total yield of about 15%.30 For the synthesis of 6-triazolylpenicillanates, we desired a more general and mild synthetic strategy to 6-azidopenicillanates.
Scheme 1.
Synthetic approach to 6-triazolylpenicillanic acids. Abbreviation: PNB = para-nitrobenzyl, PMB = para-methoxylbenzyl, BzD = benzhydryl.
Synthesis of 6-Azidopenicillanic Acid by Diazo-Transfer Reaction
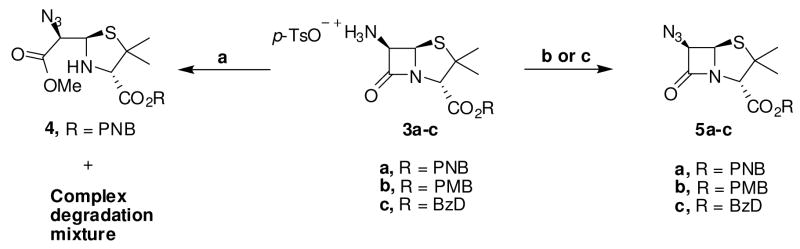
As a new general alternative, we envisioned that azidopenicillanic acid could be obtained from commercially available 6-aminopenicillanic acids through a diazo-transfer reaction. The diazo-transfer reaction is a mild, high yielding reaction that has been used to effect direct conversion of assorted amines to azides.31 In addition, a recent observation from our lab has extended the scope of this versatile reaction to include azide functionalization of amine-coated solid supports under heterogeneous reaction conditions.32 However, our initial efforts at initiating diazo-transfer reaction on unprotected 6-aminopenicillanic acid were unsuccessful. To thoroughly probe the feasibility of this reaction on β-lactam moiety, we decided to investigate various carboxyl protected 6-aminopenicillanates. The requisite carboxyl protected aminopenicillanates 3a–c were synthesized adapting literature protocols.21, 33–37 Using the PNB protected compound 3a, we initiated diazo-transfer reaction with freshly prepared triflyl azide under basic conditions in the classic CH2Cl2/MeOH/H2O solvent mixture.31b–e These conditions, however, gave a complex mixture from which we only isolated a minute amount of the ring-opened methyl ester azide 4. This product was presumably obtained from Et3N promoted methanolysis of compound 3a in addition to the desired diazo-transfer reaction. The reaction was repeated in anhydrous CH2Cl2 and Et3N. Gratifyingly, we obtained the desired azide 5a in yields of 50–68% within 2 to 2.5 h of reaction, though other uncharacterized degradation products persisted. Because of the potential hazard of handling triflyl azide in halogenated solvent, we investigated the compatibility of this reaction with non-halogenated solvents. The reaction worked equally well when CH2Cl2 was replaced with toluene, yielding azide 5a in 70% yields. Similarly, azides 5b and 5c were obtained from amines 2b and 2c in moderate to good yields (scheme 2). The β orientation of the azide group in 5a–c was authenticated by 1H NMR (J5,6 coupling constant = 4 Hz),29 thereby confirming that the reaction occurred with the characteristic retention of configuration.31b,e
Scheme 2.
Synthesis of protected 6-azidopenicillanic acid. Conditions: (a) TfN3, Et3N, CH2Cl2/MeOH/H2O, rt, (b) TfN3, Et3N, DMAP, rt, 2–2.5 hrs, (c) TfN3, Et3N, toluene, rt, 1.5–2.5 hrs.
Cu(I)-catalyzed Cycloaddition Reaction between 6-Azidopenicillanates and Terminal Alkynes
To identify optimum conditions for Cu(I)-catalyzed cycloaddition reaction, we investigated the reaction of azides 5b and 5c with 3-phenyl-1-propyne 6 and 2-ethynyl-1,3-dimethoxybenzene 7. Our choice of terminal alkynes 6 and 7 is partly informed by the possibility that cycloaddition between these alkynes and azides 5 will respectively furnish triazolyl isosteres of penicillin G and methicillin, two historically useful penicillin derivatives. Cu(I)-catalyzed reaction of azide 5b or 5c with alkyne 6 resulted in triazoles 8a and 8b in excellent yield. Subsequent TFA deprotection of the PMB and the BzD group furnished the desired penicillanic acid 9a in excellent yield. Similarly, the reaction of azide 5c with alkyne 7 followed by deprotection of the BzD group gave penicillanic acid 9 in 72% overall yield (scheme 3). Because of the rapidity of deprotection of the BzD group and the product quality, we focused much of our attention on the reactivity of azide 5c.
Scheme 3.
Cu(I)-catalyzed cycloadditions reaction between 6-azidopenicillanates and representative terminal alkynes. Conditions: (a) CuI, Hunig’s base, alkynes 6 or 7, THF, rt, (b) TFA, anisole, CH2Cl2, −5°C. Abbreviation: 2,6-DMP = 2,6-dimethoxylphenyl.
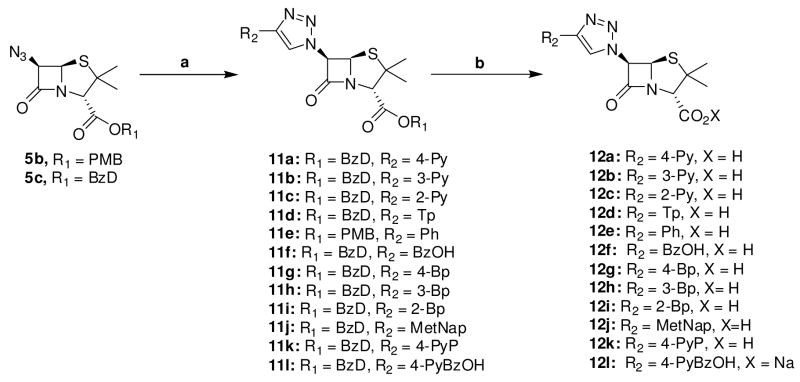
We turned to investigate the reactivity of azide 5c with assorted terminal alkynes in order to probe the scope of this reaction. We selected a subset of terminal alkynes whose closely related carboxylic acid analogs have been shown to support the antimicrobial activities of β-lactams.38–40 Alkynes such as 7, 10h, 10i, and 10k that we could not obtain from commercial sources were synthesized from the corresponding carboxylic acid, through the intermediacy of aldehyde, using the Bestmann-Ohira reagent.41–43 Hydroxyl alkyne 10f and 10l were obtained by a direct Grignard reaction of ethynylmagnesium bromide with the appropriate aldehydes. Detailed protocols for the synthesis of these alkynes are reported in the experimental section.
Cu(I)-catalyzed reaction of alkynes 10a–l with azidopenicillanate 5b or 5c proceeded smoothly at ambient temperature, leading to uneventful formation of triazole 11a–l in good to excellent yields. Subsequent carbonyl group deprotection furnished the desired 6-triazolylpenicillanic acid 12a–l (scheme 4).
Scheme 4.
Scope of Cu(I)-catalyzed cycloaddition between 6-azidopenicillanate and terminal alkynes. Conditions: a) CuI, Hunig’s base, alkynes 10, THF, rt, b) TFA, anisole CH2Cl2, −5°C.
Biological Evaluation
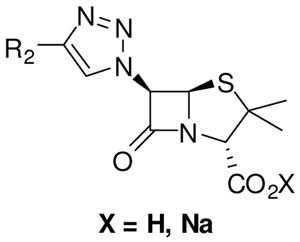
The synthesized 6-triazolylpenicillanic acids were screened at the National Institute of Allergy and Infectious Disease (NIAID) through the In vitro and Animal Models for Emerging Infectious Diseases and Biodefense Screening Program. Compounds were screened following the procedures recommended by the Clinical Laboratory Standard Institute (CLSI). Compound 9a, 12e, 12g, and 12l exhibited some antibacterial activity against the Gram-positive bacteria strain S. pneumoniae with MIC value of 4 to 8 μg/mL. Similarly, compound 12l showed a moderate activity (MIC ≈ 8 μg/mL) against B. anthracis. Other compounds listed in Table 1 displayed comparatively poor antibacterial activity (MIC > 8μg/mL).
Table 1.
Minimal Inhibitory Concentration (MIC) of 6-triazolylepenicillanic acids in μg/mL: Each MIC values were obtained from an average of three independent experiments.
 | |||||
|---|---|---|---|---|---|
| Compound | R2 | S. aureus ATCC 29213 (μg/mL) | E. coli ATCC 25922 (μg/mL) | S. pneumoniae ATCC 49619 (μg/mL) | B. anthracis AMES (μg/mL) |
| 9a | 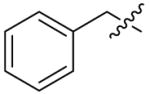 |
>8 | >8 | 4 | >8 |
| 9b | 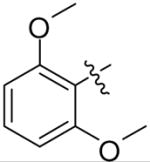 |
>8 | >8 | >8 | >8 |
| 12a | 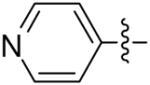 |
>8 | >8 | >8 | >8 |
| 12b | 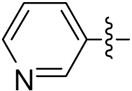 |
>8 | >8 | >8 | >8 |
| 12c | 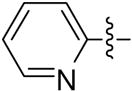 |
>8 | >8 | >8 | >8 |
| 12d | 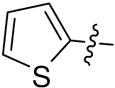 |
>8 | >8 | 8 | >8 |
| 12e | 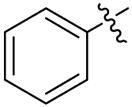 |
>8 | >8 | 4 | >8 |
| 12f | 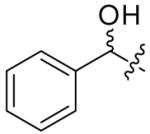 |
>8 | >8 | >8 | >8 |
| 12g | >8 | >8 | ≥8** | >8 | |
| 12h | 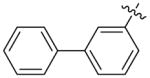 |
>8 | >8 | >8 | >8 |
| 12i | 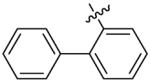 |
>8 | >8 | >8 | >8 |
| 12j | 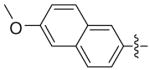 |
>8 | >8 | >8 | >8 |
| 12k | >8 | >8 | >8 | >8 | |
| 12l* | >8 | >8 | ≥8** | ≥8** | |
 |
1 | >2 | N. D.*** | ≤0.125 | |
 |
2 | 4 | N. D.*** | 1 | |
X = Na
Denotes that one of the 3 replicates had MIC value of 8 μg/mL.
No Data
Conclusion
We have reported a convenient route for the synthesis of 6-triazolyl-penicillanic acid. Preliminary biological evaluations of compounds described in this report demonstrated that they possess low to moderate antibacterial activity. The triazole ring lacks the H-bonding donating N-H group which could act as an additional recognition feature in the binding of some β-lactams to the PBPs. This may partly explain the relatively low antimicrobial activities of these 6-triazolyl-penicillanic acids. Currently, we are investigating the SAR of the lead compounds in order to identify 6-triazolylpenicillanic acid with improved antibacterial activity against drug resistant bacterial strains.
Experimental Section
6-aminopenicillanoic acid (6-APA) was purchased from Sigma Aldrich. Anhydrous solvents and other reagents were purchased and used without further purification. Analtech silica gel plates (60 F254) were used for analytical TLC, and uv light was used to examine the spots. 200–400 Mesh silica gel was used in column chromatography. NMR spectra were recorded on a Varian-Gemini 400 magnetic resonance spectrometer. 1H NMR spectra are recorded in parts per million (ppm) relative to the peak of CDCl3, (7.24 ppm), acetone-d6 (2.09 ppm), or DMSO-d6 (2.49 ppm). 13C spectra were recorded relative to the central peak of the CDCl3 triplet (77.0 ppm), acetone-d6 (205.8 ppm), or the DMSO-d6 septet (39.7 ppm), and were recorded with complete hetero-decoupling. High-resolution mass spectra were recorded at the Georgia Institute of Technology mass spectrometry facility in Atlanta. Triflyl azide was prepared as described before and used without storage.31h, 31i The Bestmann-Ohira reagent was prepared as described by Ghosh et al42 while diphenyldiazomethane was prepared by using the procedure described by Ko et al.37 6-Aminopenicillanates 3a–c were synthesized adapting literature procedures.21, 33–37
Representative Procedure for the Alkyne Transformation Reaction. 2-Ethynyl-1,3-dimethoxybenzene (7)
2,6-dimethoxybenzaldehyde (0.45 g, 2.71 mmol) was first dissolved in anhydrous MeOH (25 mL) and stirred under argon at room temperature. Anhydrous K2CO3 (1.12 g, 8.12 mmol) and Bestmann-Ohira reagent (1.03 g, 5.42 mmol) were added to the reaction mixture and stirring continued for 24 h at room temperature. Solvent was evaporated off, and the remaining residue was dissolved in CH2Cl2 (25 mL) and washed with saturated NH4Cl (3 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash column chromatography (silica gel, 5:1 Hexane/EtOAc) to give 220 mg (50%) of 7 as a white solid. 1H-NMR (CDCl3, 400MHz) δ 3.55 (1H, s), 3.88 (6H, s), 6.53 (2H, d, J = 8.4 Hz), 7.24 (1H, t, 7.4 Hz); 13C-NMR (CDCl3, 100MHz) δ 56.0, 76.2, 85.7, 100.0, 103.5, 130.3, 162.0.
4-(4-Ethynylphenyl)pyridine (10k)
Reaction of 4-(4-formylphenyl)pyridine (0.3 g, 1.64 mmol) and Bestmann-Ohira reagent (0.627 g, 3.27 mmol) within 24 h as described for the synthesis of compound 7 followed by flash chromatography (silica gel, 2:1 Hexane/EtOAc) gave 261 mg (89%) of 10k as a white solid. 1H-NMR (CDCl3, 400MHz) δ 3.16 (1H, s), 7.47 (2H, d, J = 6.4 Hz), 7.59 (4H, s), 8.66 (2H, d, J = 6.4Hz). HRMS (EI) calc for [C13H9N] 179.0735, found 179.0746.
2-Ethnylbiphenyl (10i)
The required Biphenyl-2-carbaldehyde was synthesized from the corresponding carboxylic acid through NaBH4/BF3:OEt2 reduction to the primary alcohol.43 Subsequent PDC oxidation of the primary alcohol furnished Biphenyl-2-carbaldehyde. Reaction of biphenyl-2-carbaldehyde (0.66 g, 3.63 mmol) and Bestmann-Ohira reagent (1.72 g, 7.27 mmol) within 24 h followed by flash chromatography (silica gel, 5:1 Hexane/EtOAc) yielded 523 mg (81%) of 10i as a colorless oil. 1H-NMR (CDCl3, 400MHz) δ 3.03 (1H, s), 7.28–7.32 (1H, m), 7.36–7.45 (5H, m), 7.57–7.63 (3H, m).
3-Ethynylbiphenyl (10h)
The required Biphenyl-3-carbaldehyde was synthesized from the corresponding carboxylic acid through NaBH4/BF3:OEt2 reduction to the primary alcohol.43 Subsequent PDC oxidation of the primary alcohol furnished Biphenyl-3-carbaldehyde. Reaction of biphenyl-3-carbaldehyde (0.87 g, 4.78 mmol) and Bestmann-Ohira reagent (1.72 g, 9.56 mmol) within 24 h followed by flash chromatography (silica gel, 5:1 Hexane/EtOAc) gave 850 mg (100%) of 10h as a reddish oil. 1H-NMR (CDCl3, 400MHz) δ 3.03 (1H, s), 7.33–7.47 (5H, m), 7.55–7.57 (3H, m), 7.72 (1H, s). HRMS(EI) calc for [C14H10] 178.0782, found 178.0797.
Representative Procedure for the Alkyne Transformation via Grignard Reaction. 1-phenylprop-2-yn-1-ol (10f)
To a solution of benzaldehyde (0.5 g, 4.71 mmol) in anhydrous THF (2 mL) was added ethynylmagnesium bromide (14.0 mL, 0.5M in THF) at room temperature under argon. The reaction mixture was stirred for 1 h and quenched with distilled water. The reaction mixture was partitioned between CH2Cl2 (10 mL) and water (15 mL), and the two layers separated. The organic layer was washed in succession with distilled water (2 × 10 mL) and saturated brine (2 × 10 mL), and dried over Na2SO4. Solvent was evaporated off to give 0.626g (100%) of 10f as a brownish oil. 1H-NMR (CDCl3, 400MHz) δ 2.66 (1H, d, J = 2.4 Hz), 5.45 (1H, d, J = 4.0 Hz), 7.30–7.40 (3H, m), 7.54 (2H, d, J = 7.2 Hz); HRMS (FAB, thioglycerol) calc for [C9H8O] 132.0575, found 132.0566.
1-(4-(pyrdin-4-yl)prop-2-yn-1-ol (10l)
To a solution of benzaldehyde (0.5 g, 2.73 mmol) in anhydrous THF (2 mL) was added ethynylmagnesium bromide (8.2 mL, 0.5M in THF) at room temperature under argon. The reaction mixture was stirred for 1 h and quenched with distilled water. The reaction mixture was partitioned between CH2Cl2 (10 mL) and water (15 mL), and the two layers separated. The organic layer was washed in succession with distilled water (2 × 10 mL) and saturated brine (2 × 10 mL), and dried over Na2SO4. Solvent was evaporated off to give 0.491 (86%) of 10l as a brownish solid. 1H-NMR (CD3OD, 400MHz) δ 3.05 (1H, s), 5.46 (1H, s), 7.66–7.78 (6H, m), 8.56 (2H, d, J = 9.2 Hz); HRMS (FAB, thioglycerol) calc for [C14H11NO + H]+ 210.0918, found 210.0909.
Representative Procedures for Diazo-transfer Reaction. Method A: p-Nitrobenzyl 6-azidopenicillanate (5a)
4-Nitrobenzyl 6-aminopenicillanate salt 3a (5.0 g, 9.5 mmol) was suspended in EtOAc (40 mL) and washed with saturated NaHCO3 (2 × 30 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to give pure 4-nitrobenzyl 6-aminopenicillanate. To a solution of 4-nitrobenzyl 6-aminopenicillanate in anhydrous CH2Cl2 (10 mL) was added triflyl azide solution (25 mmol) in CH2Cl2 (25 mL), and Et3N (2.1 mL, 15.0 mmol). The reaction mixture was stirred at room temperature for 2 h. Solvent was evaporated off and the residue was directly purified by flash chromatography (silica, gradient 4:1; 3:1 Hexane/EtOAc) to give 2.35 g (68%) of 5a as a colorless gel. 1H-NMR (CDCl3, 400MHz) δ 1.38 (3H, s), 1.61 (3H, s), 4.49 (1H, s), 4.92 (1H, d, J = 4.0 Hz), 5.23 (2H, q, J =24.0, 12.0 Hz), 5.43 (1H, d, J = 4.0 Hz), 7.50 (2H, d, J = 8.8 Hz), 8.17 (2H, d, J = 8.8 Hz); 13C-NMR (CDCl3, 100MHz) δ 26.4, 31.5, 64.3, 65.7, 66.5, 67.6, 70.0, 123.7, 128.0, 141.5, 147.9, 167.0, 169.8;
Method B: Benzhydryl 6-azidopenicillanate (5c)
Benzhydryl 6-aminopenicillanate salt 3c (3.5 g, 6.32 mmol) was suspended in EtOAc (60 mL) and washed with saturated NaHCO3 (3 × 40 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to give 2.4 g of pure benzhydryl 6-aminopenicillanate. To a solution of benzhydryl 6-aminopenicillanate in anhydrous CH2Cl2 (15 mL) was added triflyl azide solution (14 mmol) in CH2Cl2 (35 mL), DMAP (1.15 g, 9.24 mmol), and Et3N (0.1 mL). The reaction mixture was stirred at room temperature for 1.5 h and solvent was evaporated off. The residue was directly purified by flash chromatography (silica, gradient 1:0; 5:1 Hexane/EtOAc) to give 1.38 g (54%) of 5c as a colorless gel. 1H-NMR (CDCl3, 400MHz) δ 1.26 (3H, s), 1.64 (3H, s), 4.57 (1H, s), 4.89 (1H, d, J = 4.0 Hz), 5.47 (1H, d, J = 4.0 Hz), 6.93 (1H, s), 7.28–7.35 (10H, m); 13C-NMR (CDCl3, 100MHz) δ 26.2, 31.9, 64.7, 66.6, 67.8, 70.2, 78.4, 126.8, 127.5, 128.1, 128.4, 128.5, 138.8, 166.5, 169.8; HRMS (FAB, mnba) calc for [C21H20N4O3S + H]+ 409.1334, found 409.1357.
Method C: p-Nitrobenzyl 6-azidopenicillanate (5a)
4-Nitrobenzyl 6-aminopenicillanate salt 3a (0.75 g, 1.4 mmol) was suspended in EtOAc (30 mL) and washed with saturated NaHCO3 (2 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to give pure 4-nitrobenzyl 6-aminopenicillanate. To a solution of pure 4-nitrobenzyl 6-aminopenicillanate in anhydrous toluene (5 mL) was added triflyl azide solution (2.97 mmol) in toluene (25 mL), and Et3N (0.3 mL, 2.1 mmol). The reaction mixture was stirred at room temperature for 2 h. The reaction was diluted with EtOAc (10 mL) and washed with 1N HCl (20 mL), distilled water (2 × 20 mL), and saturated brine (20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to give 361 mg (70%) of 5a as a yellowish solid. 1H-NMR (CDCl3, 400MHz) δ 1.42 (3H, s), 1.66 (3H, s), 4.52 (1H, s), 4.92 (1H, d, J = 4.0 Hz), 5.26 (2H, q, J = 24.0, 12.0 Hz), 5.46 (1H, d, J = 4.0 Hz), 7.53 (2H, d, J = 8.8 Hz), 8.24 (2H, d, J = 8.8 Hz).
p-Methoxybenzyl 6-azidopenicillanate (5b)
6-azidopenicillanate 5b was prepared from p-Methoxybenzyl 6-aminopenicillanate 3b (4.0 g, 7.9 mmol) and triflyl azide (25 mmol) in CH2Cl2 (total volume = 25 mL), and Et3N (1.44 mL, 10.3 mmol) using method A. The reaction mixture was stirred at room temperature for 2 h. Purification of the crude product by flash chromatography (silica, gradient 4:1; 3:1 Hexane/EtOAc) afforded 1.40 g (49%) of 5b as a colorless gel. 1H-NMR (CDCl3, 100MHz) δ 1.36 (3H, s), 1.61 (3H, s), 3.79 (3H, s), 4.45 (1H, s), 4.88 (1H, d, J = 4.0 Hz), 5.10 (2H, app. q, J = 24.0, 12.0 Hz), 5.44 (1H, d, J = 4.0 Hz), 6.87 (2H, d, J = 8.8 Hz), 7.27 (2H, d, J = 8.8 Hz); 13C-NMR (CDCl3, 400MHz) δ 26.6, 31.7, 55.3, 64.6, 66.5, 67.4, 67.7, 70.2, 113.9, 126.5, 130.4, 159.7, 167.2, 169, 7; HRMS (FAB, mnba) calc for [C16H18N4O4S + H]+ 363.1127, found 363.1151.
Representative Procedure for Cu(I)-Catalyzed Cycloaddition Reaction. p-Methoxybenzyl benzyl-6-triazolylpenicillanate (8a)
p-Methoxybenzyl 6-azidopenicillanate 5b (0.27g, 0.635 mmol) and 3-phenyl-1-propyne 6 (0.17 mL, 1.39 mmol) were dissolved in anhydrous THF (8 mL) and stirred under argon at room temperature. Copper (I) iodide (0.011 g, 0.06 mmol), and Hunig’s base (0.1 mL) were then added to the reaction mixture, and stirring was continued for 2 h. The reaction mixture was diluted with CH2Cl2 (40 mL) and washed with 1:4 NH4OH/saturated NH4Cl (3 × 30 mL) and again with saturated NH4Cl (30 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash chromatography (silica, gradient 3:1; 2:1; 3:2 Hexane/EtOAc) to give 246 mg (71%) of 8a as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.35 (3H, s), 1.57 (3H, s), 3.79 (3H, s), 4.08 (2H, s), 4.47 (1H, s), 5.12 (2H, app. q, J = 24.0, 12.0 Hz), 5.69 (1H, d, J = 4.0 Hz), 6.27 (1H, d, J = 4.4 Hz), 6.88 (2H, d, J = 8.8 Hz), 7.20–7.30 (7H, m), 7.42 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.9, 30.9, 32.0, 55.2, 65.3, 66.2, 67.3, 67.5, 70.5, 114.0, 122.4, 126.5, 128.5, 128.6, 130.6, 138.5, 147.2, 160.0, 167.1, 168.1; HRMS (FAB, thioglycerol) calc for [C25H26N4O4S + H]+ 479.1753, found 479.1756.
Benzhydryl benzyl-6-triazolylpenicillanate (8b)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.270 g, 0.661 mmol) and 3-phenyl-1-propyne 6 (0.17 mL, 1.39 mmol) within 2.5 h followed by flash chromatography (silica, gradient 3:1; 2:1; 3:2 Hexane/EtOAc) gave 246 mg (71%) of 8b as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.61 (3H, s), 1.57 (3H, s), 4.09 (2H, s), 4.59 (1H, s), 5.73 (1H, d, J = 4.0 Hz), 6.29 (1H, d, J = 4.4 Hz), 6.94 (1H, s), 7.21–7.35 (15H, m), 7.43 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 31.2, 32.0, 65.4, 66.3, 67.4, 70.6, 78.6, 122.4, 126.5, 126.9, 127.6, 128.3, 128.5, 128.6, 128.7, 138.5, 138.7, 138.8, 147.3, 166.3, 168.1; HRMS (FAB, mnba) calc for [C30H28N4O3S + H]+ 525.1956, found 525.1960.
Benzhydryl 2,6-dimethoxyphenyl-6-triazolylpenicillanate (8c)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.227 g, 0.555 mmol) and 2-Ethynyl-1,3-dimethoxybenzene 7 (0.06 g, 0.37 mmol) within 4 h followed by flash chromatography (silica gel, 1:1 Hexane/EtOAc) gave 120 mg (57%) of 8c as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.26 (3H, s), 1.69 (3H, s), 3.78 (6H, s), 4.64 (1H, s), 5.80 (1H, d, J = 4.8 Hz), 6.42 (1H, d, J = 4.4 Hz), 6.62 (2H, d, J = 8.4 Hz), 6.96 (1H, s), 7.26–7.35 (11H, m), 7.95 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 31.4, 56.0, 65.2, 66.4, 67.7, 70.6, 78.6, 104.1, 125.2, 126.9, 127.6, 128.3, 128.5, 128.6, 128.7, 129.9, 138.9, 139.9, 158.3, 166.4, 168.6; HRMS (FAB, thioglycerol) calcd for [C31H30N4O5S + H]+ 571.2015, found 571.2049.
Benzhydryl 4-pyridyl-6-triazolylpenicillanate (11a)
4-Ethynylpyridyl hydrochloride (0.06 g, 0.414 mmol) was suspended in EtOAc (30 mL) and washed with saturated NaHCO3 (1 × 30 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to give 4-ethynylpyridine 10a as white brown solid. The reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 10a (0.076 g, 0.734 mmol) within 3 h followed by flash chromatography (silica gel, gradient 1:1; 1:4 Hexane/EtOAc) gave 166 mg (74%) of 11a as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.26 (3H, s), 1.69 (3H, s), 4.65 (1H, s), 5.80 (1H, d, J = 4.0 Hz), 6.39 (1H, d, J = 4.0 Hz), 6.96 (1H, s), 7.32–7.36 (10H, m), 7.77 (2H, d, J = 5.20 Hz), 8.19 (1H, s), 9.03(2H, d, J = 5.20 Hz); 13C-NMR (CDCl3, 100MHz) δ 26.8, 31.1, 65.7, 66.2, 67.2, 70.8, 78.8, 120.1, 122.1, 126.7, 127.5, 128.2, 128.4, 128.5, 138.6, 138.6, 144.5, 149.2, 165.9, 167.5; HRMS (FAB, thioglycerol) calc for [C28H25N5O3S + H]+ 512.1756, found 512.1763.
Benzhydryl 3-pyridyl-6-triazolylpenicillanate (11b)
The reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.441 mmol) and 3-ethynylpyridine 10b (0.068 g, 0.661 mmol) within 3 h followed by flash chromatography (silica gel, gradient 1:1; 1:3 Hexane/EtOAc) gave 170 mg (76%) of 11b as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.26 (3H, s), 1.70 (3H, s), 4.66 (1H, s), 5.80 (1H, d, J = 4.0 Hz), 6.40 (1H, d, J = 4.0 Hz), 6.96 (1H, s), 7.32–7.36 (10H, m), 8.13 (1H, s), 8.26 (1H, d, J = 8.24 Hz), 8.58 (1H, br s), 9.03(1H, br s); 13C-NMR (CDCl3, 100MHz) δ 26.9, 31.1, 65.7, 66.3, 67.3, 70.7, 78.7, 120.8, 126.7, 127.5, 128, 2, 128, 4, 128, 5, 133.8, 138.5, 138.6, 138.6, 144.0, 146.1, 148.3, 166.0, 167.6; HRMS (FAB, thioglycerol) calc for [C28H25N5O3S + H]+ 512.1756, found 512.1773.
Benzhydryl 2-pyridyl-6-triazolylpenicillanate (11c)
The reaction of benzhyldryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 2-ethynylpyridine 10c (0.06 mL, 0.55 mmol) within 6 h followed by flash chromatography (silica gel, gradient 4:1; 2:1; 1:2; Hexane/EtOAc) gave 145 mg (64%) of 11c as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.25 (3H, s), 1.71 (3H, s), 4.64 (1H, s), 5.78 (1H, d, J = 4.0 Hz), 6.39 (1H, d, J = 4.0 Hz), 6.96 (1H, s), 7.32–7.36 (10H, m), 7.78 (1H, m), 8.18 (1H, d, J = 7.69 Hz), 8.45 (1H, s), 8.58 (1H, d, J = 4.39 Hz); 13C-NMR (CDCl3, 100MHz) δ 26.9, 31.3, 65.7, 66.5, 67.4, 70.8, 78.7, 120.5, 123.0, 126.8, 127.5, 128.2, 128.4, 128.5, 128.6, 138.6, 138.7, 166.1, 167.8; HRMS (FAB, thioglycerol) calc for [C28H25N5O3S + H]+ 512.1756, found 512.1765.
Benzhydryl 2-thiopyl-6-triazolylpenicillanate (11d)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 3-ethynylthiopene 10d (0.06 g, 0.55 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:0; 5:1; 3:1 Hexane/EtOAc) gave 150 mg (79%) of 11d as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.25 (3H, s), 1.69 (3H, s), 4.64 (1H, s), 5.78 (1H, d, J = 4.4 Hz), 6.36 (1H, d, J = 4.0 Hz), 6.96 (1H, s), 7.30–7.38 (11H, m), 7.44 (1H, dd, J =4.0, 1.2 Hz), 7.70 (1H, dd, J = 2.0, 1.2 Hz), 7.90 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 30.9, 66.1, 67.3, 70.8, 78.7, 120.1, 121.6, 125.7, 126.4, 126.9, 127.6, 128.3, 128.6, 128.7, 131.1, 138.7, 138.8, 143.8, 166.3, 168.2; HRMS (FAB, thioglycerol) calc for [C27H24N4O3S2 + H]+ 517.1368, found 517.1374.
p-Methoxybenzyl phenyl-6-triazolylpenicillanate (11e)
Reaction of p-methoxybenzyl-6-azidopenicillanate 5b (0.15 g, 0.414 mmol) and phenylacetylene 10e (0.14 mL, 1.24 mmol) within 2 h followed by flash chromatography (silica gel, gradient 3:1; 3:2 Hexane/EtOAc) gave 165 mg (86%) of 11e as a white foam. 1H-NMR (CDCl3, 400MHz) δ 1.38 (3H, s), 1.66 (3H, s), 3.80 (3H, s), 4.54 (1H, s), 5.14 (2H, app. q, J = 24.0, 12.0 Hz), 5.75 (1H, d, J = 4.0 Hz), 6.37 (1H, d, J = 4.0 Hz), 6.89 (2H, d, J = 8.8 Hz), 7.29–7.35 (3H, m), 7.39–7.42 (2H, m), 7.82 (2H, m), 7.99 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 27.0, 30.8, 55.2, 65.4, 66.1, 67.2, 67.6, 70.7, 114.0, 120.3, 125.8, 126.5, 128.4, 128.8, 129.9, 130.6, 147.5, 160.0, 167.1, 168.2; HRMS (FAB, thioglycerol) calc for [C24H24N4O4S + H]+ 465.1596, found 465.1571.
Benzhydryl α-hydroxylbenzyl-6-triazolylpenicillanate (11f)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.2 g, 0.49 mmol) and 1-phenylprop-2-yn-1-ol 10f (0.13 g, 0.985 mmol) within 2 h followed by flash chromatography (silica gel, 2:1 Hexane/EtOAc) gave 191 mg (72%) of 11f as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.23 (3H, d, J = 12 Hz), 1.59 (3H, d, J = 8.0 Hz), 4.61 (1H, s), 5.71 (1H, d, J = 4.4 Hz), 5.99 (1H, d, J = 5.2 Hz), 6.23 (1H, dd, J = 4.0, 1.6 Hz), 6.95 (1H, s), 7.24–7.42 (15H, m), 7.57 (1H, s). 13C-NMR (CDCl3, 100MHz) δ 26.6, 31.2, 31.3, 65.3, 65.3, 66.3, 66.4, 67.3, 67.4, 68.7, 68.9, 70.4, 70.5, 78.5, 122.0, 122.1, 126.1, 126.2, 126.3, 126.4, 126.6, 126.7, 127.1, 127.3, 127.6, 127.7, 128.0, 128.2, 128.3, 128.4, 138.5, 138.6, 141.5, 150.9, 166.0, 167.4, 167.5; HRMS (FAB, thioglycerol) calc for [C30H28N4O4S + H]+ 541.1909, found 541.1936.
Benzhydryl 4-biphenyl-6-triazolylpenicillanate (11g)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 4-ethynylbiphenyl 10g (0.098 g, 0.55 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:0; 5:1; 3:1 Hexane/EtOAc) gave 158 mg (73%) of 11g as a white solid. 1H-NMR (DMSO-d6, 400MHz) δ 1.26 (3H, s), 1.69 (3H, s), 4.92 (1H, s), 5.88 (1H, d, J = 2.0 Hz), 6.81 (1H, d, J = 2.0 Hz), 6.96 (1H, s), 7.32 (2H, d, J = 8.0 Hz), 7.38 (5H, t, J = 7.2 Hz), 7.47 (5H, t, J = 6.8 Hz), 7.52 (2H, d, J = 8.0 Hz), 7.77 (2H, d, J = 8.0 Hz), 7.90 (2H, d, J = 8.0 Hz), 8.66 (1H, s)
Benzhydryl 3-biphenyl-6-triazolylpenicillanate (11h)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 3-ethynylbiphenyl 10h (0.098 g, 0.55 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:0; 5:1; 3:1 Hexane/EtOAc) gave 215 mg (100%) of 11h as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.14 (3H, s), 1.58 (3H, s), 4.54 (1H, s), 5.68 (1H, d, J = 4.4 Hz), 6.28 (1H, d, J = 4.0 Hz), 6.86 (1H, s), 7.12 (1H, s), 7.19–7.24 (10H, m), 7.31–7.39 (3H, m), 7.45 (1H, d, J = 8.0 Hz), 7.52 (2H, d, J = 8.0 Hz), 7.69 (2H, d, J = 8.0 Hz), 7.95 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 30.9, 65.5, 66.2, 67.3, 70.7, 78.7, 120.5, 124.6, 124.7, 126.9, 127.2, 127.5, 127.6, 128.3, 128.5, 128.6, 128.7, 129.3, 130.4, 138.7, 138.8, 140.6, 141.9, 147.5, 166.3, 168.2; HRMS (FAB, thioglycerol) calc for [C35H30N4O3S + H]+ 587.2116, found 587.2143.
Benzhydryl 2-biphenyl-6-triazolylpenicillanate (11i)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 2-ethynylbiphenyl 10i (0.098 g, 0.55 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:0; 5:1; 3:1 Hexane/EtOAc) gave 150 mg (70%) of 11i as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.20 (3H, s), 1.47 (3H, s), 4.47 (1H, s), 5.63 (1H, d, J = 4.0 Hz), 6.26 (1H, d, J = 4.4 Hz), 6.80 (1H, s), 6.93 (1H, s), 7.22–7.45 (18H, m), 8.15 (1H, d, J = 8.0 Hz); 13C-NMR (CDCl3, 100MHz) δ 26.7, 31.3, 65.4, 66.3, 67.4, 70.4, 78.6, 123.0, 126.9, 127.2, 127.5, 127.8, 128.1, 128.2, 128.5, 128.6, 128.6, 128.7, 128.8, 129.2, 130.3, 138.8, 140.2, 141.5, 166.3, 167.0; HRMS (FAB, thioglycerol) calc for [C35H30N4O3S + H]+ 587.2116, found 587.2143.
Benzhydryl 6-methoxynapthalyl-6-triazolylpenicillanate (11j)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 2-ethynyl-6-methoxylnapthalene (0.067 g, 0.37 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:0; 5:1; 3:1 Hexane/EtOAc) gave 175 mg (81%) of 11j as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.27 (3H, s), 1.72 (3H, s), 3.91 (3H, s), 4.67 (1H, s), 5.80 (1H, d, J = 4.4 Hz), 6.41 (1H, d, J = 4.4 Hz), 6.97 (1H, s), 7.13–7.16 (2H, m), 7.31–7.35 (10H, m), 7.77 (2H, dd, J = 6.4, 2.8 Hz), 7.88 (1H, dd, J = 6.4, 1.6 Hz), 8.08 (1H, s), 8.26 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 31.0, 55.3, 65.5, 66.2, 67.3, 70.8, 78.7, 119.3, 120.2, 124.3, 124.6, 125.1, 126.9, 127.4, 127.6, 128.3, 128.6, 128.7, 128.9, 129.7, 134.4, 138.7, 138.8, 147.7, 158.0, 166.3, 168.2; HRMS (FAB, mnba) calc for [C34H30N4O4S + H]+ 591.2066, found 591.2061.
Benzhydryl 4-pyridylphenyl-6-triazolylpenicillanate (11k)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 4-(4-ethynylphenyl)pyridine 10k (0.098 g, 0.55 mmol) within 5 h followed by flash chromatography (silica gel, gradient 1:1; 1:2; 1:4 Hexane/EtOAc) gave 202 mg (94%) of 11k as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.27 (3H, s), 1.71 (3H, s), 4.66 (1H, s), 5.80 (1H, d, J = 4.0 Hz), 6.40 (1H, d, J = 4.0 Hz), 6.97 (1H, s), 7.31–7.35 (12H, m), 7.61 (2H, br s), 7.72 (2H, d, J = 8.0 Hz), 7.97 (2H, d, J = 8.0 Hz), 8.09 (1H, s); 13C-NMR (CDCl3, 100MHz) δ 26.7, 30.9, 65.6, 66.2, 67.3, 70.7, 78.7, 120.8, 126.5, 126.9, 127.5, 127.6, 128.3, 128.6, 128.7, 131.2, 137.3, 138.7, 138.8, 146.6, 148.8, 148.9166.3, 168.1; HRMS (FAB, thioglycerol) calc for [C34H29N5O3S + H]+ 588.2069, found 588.2100.
Benzhydryl 4-pyridylbenzylhydroxyl-6-triazolylpenicillanate (11l)
Reaction of benzhydryl 6-azidopenicillanate 5c (0.15 g, 0.367 mmol) and 1-(4-(pyrdin-4-yl)prop-2-yn-1-ol 10l (0.115 g, 0.55 mmol) within 2 h followed by flash chromatography (silica gel, gradient 1:2; 1:3 Hexane/EtOAc) gave 190 mg (84%) of 11l as a white solid. 1H-NMR (CDCl3, 400MHz) δ 1.22 (3H, d, J = 12 Hz), 1.60 (3H, d, J = 5.6 Hz), 4.58 (1H, s), 5.72 (1H, d, J = 4.4 Hz), 6.08 (1H, s), 6.28 (1H, d, J = 4.4 Hz), 6.92 (1H, s), 7.24–7.32 (10H, m), 7.44 (2H, dd, J = 6.0, 1.6 Hz) 7.52–7.58 (4H, m), 7.67 (1H, s), 8.53 (2H, dd, J = 6.4, 1.6 Hz). 13C-NMR (CDCl3, 100MHz) δ26.7, 30.6, 31.1, 31.2, 64.3, 65.4, 66.2, 66.3, 67.3, 68.4, 68.5, 70.5, 78.6, 121.4, 122.0, 126.6, 126.9, 127.1, 127.4, 128.1, 128.3, 128.4, 128.5, 137.1, 137.2, 138.5, 142.9, 147.8, 149.6, 150.8, 150.9, 166.0, 167.5, 167.6; HRMS (FAB, thioglycerol) calc for [C35H31N5O4S + H]+ 618.2175, found 618.2180
Representative Procedure for Deprotection of Carboxyl Protecting Group. Benzyl-6-triazolylpenicillanic acid (9a)
p-Methoxybenzyl benzyl-6-triazolylpenicillanate 8a (0.13 g, 0.26 mmol) was dissolved and stirred in anhydrous CH2Cl2 (1 mL) at −5°C. Anhydrous anisole (0.2 mL, 1.83 mmol) and trifluoroacetic acid (0.5 mL, 6.49 mmol) were added, and the reaction mixture stirred for 2 h. The reaction mixture was diluted with cold Et2O (10 mL), and the solvent was evaporated off in an ice bath. The residue was concentrated in vacuo on an ice bath for 15 minutes, and redissolved in THF (5 mL) and ½ saturated NaHCO3 (15 mL) at 0°C. The resulting mixture was stirred at 0°C for 15 minutes and partitioned between deionized water (5 mL) and EtOAc (20 mL). The two layers were separated and the aqueous layer was extracted with EtOAc (2 × 20 mL). The aqueous layer was acidified to pH 3 in an ice bath with 1N HCl and extracted with EtOAc (3 × 20 mL). The combined organic layer was dried over Na2SO4 and concentrated in vacuo to give 89 mg (100%) of 9a as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.55 (3H, s), 1.70 (3H, s), 4.07 (2H, s), 4.55 (1H, s), 5.85 (1H, d, J = 4.4 Hz), 6.58 (1H, d, J = 4.4 Hz), 7.26–7.28 (5H, m), 7.79 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 26.8, 31.0, 31.8, 65.1, 67.0, 67.8, 70.7, 123.2, 126.7, 128.8, 128.9, 129.0, 139.9, 168.4, 168.5; HRMS (FAB, mnba) calc for [C17H18N4O3S + H]+ 359.1177, found 359.1177.
2,6-Dimethoxyphenyl-6-triazolylpenicillanic acid (9b)
The reaction of a CH2Cl2 solution of 8c with anisole and TFA within 2 h, as described for the synthesis of 9a from 8a, afforded 85 mg (100%) of 9b as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.59 (3H, s), 1.77 (3H, s), 3.77 (6H, s), 4.62 (1H, s), 5.93 (1H, d, J = 4.4 Hz), 6.73 (1H, d, J = 4.4 Hz), 6.74 (2H, d, J = 8.4 Hz), 7.35 (1H, t, J = 8.4 Hz), 8.15 (1H, s); 13C-NMR (acetone-d6, 400MHz) δ 26.9, 31.2, 55.9, 65.2, 67.2, 68.0, 70.7, 104.6, 126.2, 131.0, 158.7, 168.6; HRMS (FAB, mnba) calc for [C18H20N4O5S + H]+ 405.1232, found 405.1214.
4-pyridyl-6-triazolylpenicillanic acid (12a)
The reaction of a CH2Cl2 solution of 11a with anisole and TFA within 0.5 h, as described for the synthesis of 9a from 8a, afforded 80 mg (100%) of 12a as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.56 (3H, s), 1.73 (3H, s), 4.60 (1H, s), 5.95 (1H, d, J = 4.0 Hz), 6.78 (1H, d, J = 4.0 Hz), 8.57 (2H, br s), 9.01 (2H, br s), 9.07 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 27.1, 31.6, 65.7, 67.7, 68.0, 71.4, 122.8, 126.8, 142.8, 143.6, 146.6, 167.5, 170.7; HRMS (FAB, mnba) calc for C15H15N5O3S + H]+ 346.0973, found 346.0972.
3-pyridyl-6-triazolylpenicillanic acid (12b)
The reaction of a CH2Cl2 solution of 11b with anisole and TFA within 0.5 h, as described for the synthesis of 9a from 8a, afforded 80 mg (100%) of 12b as a reddish white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.57 (3H, s), 1.74 (3H, s), 4.60 (1H, s), 5.95 (1H, d, J = 4.0 Hz), 6.76 (1H, d, J = 4.0 Hz), 8.15 (1H, br s), 8.84 (1H, s), 8.92 (2H, m), 9.41 (1H, br s); HRMS (FAB, mnba) calc for [C15H15N5O3S + H]+ 346.0973, found 346.0972.
2-pyridyl-6-triazolylpenicillanic acid (12c)
The reaction of a CH2Cl2 solution of 11c with anisole and TFA within 0.5 h, as described for the synthesis of 9a from 8a, afforded 80 mg (100%) of 12c as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.57 (3H, s), 1.76 (3H, s), 4.63 (1H, s), 5.95 (1H, d, J = 4.0 Hz), 6.79 (1H, d, J = 4.0 Hz), 7.79 (1H, m), 8.39 (1H, m), 8.48 (1H, m), 8.90 (1H, d, J = 5.1 Hz), 8.95 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 27.1, 31.5, 65.7, 67.6, 68.0, 71.2, 122.8, 125.3, 125.7, 143.2, 145.9, 146.7, 160.2, 167.8, 168.6; HRMS (FAB, mnba) calc for [C15H15N5O3S + H]+ 346.0973, found 346.0974.
2-Thiopyl-6-triazolylpenicillanic acid (12d)
The reaction of a CH2Cl2 solution of 11d with anisole and TFA within 1 h, as described for the synthesis of 9a from 8a, afforded 81 mg (100%) of 12d as a yellowish solid. 1H-NMR (acetone-d6, 400MHz) δ 1.57 (3H, s), 1.74 (3H, s), 4.59 (1H, s), 5.90 (1H, d, J = 4.0 Hz), 6.65 (1H, d, J = 4.0 Hz), 7.54 (1H, m), 7.57 (1H, dd, J = 3.0, 1.5 Hz), 7.87 (1H, dd, J = 3.0, 1.5 Hz), 8.34 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 27.1, 31.3, 65.4, 67.3, 68.0, 71.2, 121.4, 121.6, 126.3, 127.1, 132.4, 143.7, 168.3, 168.9; HRMS (FAB, mnba) calc for [C14H14N4O3S2 +H]+ 351.0585, found 351.0577.
Phenyl-6-triazolylpenicillanic acid (12e)
The reaction of a CH2Cl2 solution of 11e with anisole and TFA within 2 h, as described for the synthesis of 9a from 8a, afforded 68.5 mg (93%) of 12e as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.47 (3H, s), 1.67 (3H, s), 4.54 (1H, s), 5.80 (1H, d, J = 4.0 Hz), 6.76 (1H, d, J = 4.4 Hz), 7.34 (1H, m), 7.44 (2H, m), 7.89 (2H, m), 8.60 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 27.2, 31.2, 65.4, 67.3, 68.0, 71.2, 121.6, 126.1, 126.1, 128.6, 129.4, 131.3, 147.3, 168.4; HRMS (FAB, mnba) calc for [C16H16N4O3S + H]+ 345.1021, found 345.1015.
α-Hydroxylbenzyyl-6-triazolylpenicillanic acid (12f)
The reaction of a CH2Cl2 solution of 11f with thioanisole and TFA within 0.5 h, as described for the synthesis of 9a from 8a, afforded 76 mg (100%) of 12f as a pale yellowish solid. 1H-NMR (acetone-d6, 400MHz) δ 1.53 (3H, s), 1.67 (3H, s), 3.37 (1H, s), 4.55 (1H, s), 5.84 (1H, bs), 5.97 (1H, s), 6.55 (1H, bs), 7.22–7.43 (4H, m), 7.80 (1H, s); HRMS (FAB, thioglycerol) calc for [C17H18N4O4S + H]+ 375.1127, found 375.1192.
4-Biphenyl-6-triazolylpenicillanic acid (12g)
The reaction of a CH2Cl2 solution of 11g with anisole and TFA within 2 h, as described for the synthesis of 9a from 8a, afforded 65 mg (100%) of 12g as a yellowish solid. 1H-NMR (DMSO-d6, 400MHz) δ 1.48 (3H, s), 1.65 (3H, s), 4.38 (1H, s), 5.79 (1H, d, J = 4.0 Hz), 6.72 (1H, d, J = 4.0 Hz), 7.35–7.40 (1H, m), 7.47 (2H, t, J = 8.0 Hz), 7.74 (4H, dd, J = 11.2, 8.0 Hz), 7.99 (2H, d, J = 8.0 Hz), 8.63 (1H, s); HRMS (FAB, mnba) calc for [C22H20N4O3S + H]+ 421.1334, found 421.1333.
3-Biphenyl-6-triazolylpenicillanic acid (12h)
The reaction of a CH2Cl2 solution of 11h with anisole and TFA within 2 h, as described for the synthesis of 9a from 8a, afforded 54 mg (83%) of 12h as a white solid. 1H-NMR (acetone-d6, 400MHz) δ 1.59 (3H, s), 1.77 (3H, s), 4.61 (1H, s), 5.93 (1H, d, J = 4.0 Hz), 6.71 (1H, d, J = 4.0 Hz), 7.38 (1H, m), δ 7.48 (2H, m), 7.54 (1H, t, J = 8.0 Hz), 7.64 (1H, m), 7.72 (2H, m), 7.96 (1H, dt, J = 8.0, 1.5 Hz), 8.23 (1H, t, 1.5 Hz), 8.58 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 27.2, 31.2, 65.5, 67.3, 68.0, 71.3, 122.0, 124.6, 125.1, 127.2, 127.5, 128.1, 129.4, 130.0, 131.9, 141.1, 142.2, 147.2, 168.4, 168.9; HRMS (ESI, mnba) calc for [C22H20N4O3S + H]+ 421.1329, found 421.1312.
2-Biphenyl-6-triazolylpenicillanic acid (12i)
The reaction of a CH2Cl2 solution of 11i with anisole and TFA within 1 h, as described for the synthesis of 9a from 8a, afforded 65 mg (100%) of 12i as a white solid. H-NMR (acetone-d6, 400MHz) δ 1.53 (3H, s), 1.59 (3H, s), 4.41 (1H, s), 5.78 (1H, d, J = 4.0 Hz), 6.55 (1H, d, J = 4.0 Hz), 7.00 (1H, s), 7.22–7.25 (2H, m), 7.32–7.52 (6H, m), 8.07 (1H, dd, J = 5.8, 1.5 Hz); HRMS (ESI, mnba) calc for [C22H20N4O3S + H}+ 421.1329, found 421.1359.
6-Methoxynaphthalyl-6-triazolylpenicillanic acid (12j)
The reaction of a CH2Cl2 solution of 11j with anisole and TFA within 1 h, as described for the synthesis of 9a from 8a, afforded 56 mg (89%) of 12j as a white foam. 1H-NMR (DMSO-d6, 400MHz) δ 1.58 (3H, s), 1.78 (3H, s), 3.91 (3H, s), 4.17 (1H, s), 5.80 (1H, d, J = 4.0 Hz), 6.62 (1H, d, J = 4.0 Hz), 7.16 (1H, dd, J = 6.5, 2.2 Hz), 7.30 (1H, m), 7.85 (2H, dd, J = 3.0, 5.0 Hz), 7.98 (1H, m), 8.39 (1H, m), 8.55 (1H, s); 13C-NMR (acetone-d6, 100MHz) δ 26.7, 30.7, 55.1, 65.0, 67.0, 67.6, 70.8, 106.1, 119.5, 121.3, 124.4, 124.5, 126.2, 127.7, 129.3, 129.9, 134.9, 147.4, 158.4, 168.3, 168.4; HRMS (FAB, thioglycerol) calc for [C21H20N4O4S + H]+ 425.1283, found 425.1264.
4-Pyridylphenyl-6-triazolylpenicillanic acid (12k)
The reaction of a CH2Cl2 solution of 11k with anisole and TFA within 1 h, as described for the synthesis of 9a from 8a, afforded 37 mg (65%) of 12k as a white foam. 1H-NMR (acetone-d6, 400MHz) δ 1.58 (3H, s), 1.77 (3H, s), 4.61 (1H, s), 5.94 (1H, d, J = 4.0 Hz), 6.74 (1H, d, J = 4.0 Hz), 8.17 (4H, m), 8.47 (2H, br s), 8.64 (1H, s), 9.04 (2H, br s); HRMS (FAB, mnba) calc for [C21H19N5O3S + H]+ 422.1287, found 422.1301.
4-Pyridylbenzylhydroxyl-6-triazolylpenicillanic acid salt (12l)
Benzhydryl 4-pyridylphenyl-6-triazolylpenicillanate 11l (0.09 g, 0.14 mmol) was dissolved and stirred in anhydrous CH2Cl2 (1 mL) at −5°C. Anhydrous thioanisole (0.2 mL, 1.83 mmol) and TFA (0.5 mL, 6.49 mmol) were added, and the reaction mixture stirred for 0.5 h. The reaction mixture was diluted with cold Et2O (10 mL), and the solvent was evaporated off in an ice bath. The residue was concentrated in vacuo on an ice bath for 15 minutes, and redissolved in aqueous NaHCO3 (0.018 g, 0.21 mmol) at 0°C. The resulting mixture was stirred at 0°C for 15 minutes and partitioned between deionized water (5 mL) and EtOAc (20 mL). The two layers were separated and the aqueous layer was extracted with EtOAc (2 × 20 mL). The aqueous layer was frozen in acetone-dry ice bath and concentrated in vacuo to give 35 mg (52%) of 12l as a white solid. 1H-NMR (DMSO-d6, 400MHz) δ 1.46 (3H, s), 1.61 (3H, m), 4.51 (1H, s), 5.75 (1H, d, J = 4.4 Hz), 5.94 (1H, s), 6.67 (1H, d, J = 4.0 Hz), 7.57 (2H, d, J = 8.0 Hz), 7.86 (4H, m), 8.75 (2H, m); HRMS (FAB, mnba) calc for [C22H20N5NaO4S + H]+ 474.1212, found 474.1234.
Supplementary Material
Figure 2.
The structure of some acid- and β-lactamase-resistant penicillins.
Acknowledgments
We thank Professor James Power and Josh Canzoneri for a helpful discussion on the manuscript. This work was financially supported by Georgia Institute of Technology. Compound screening was supported, in part, by the National Institute of Allergy and Infectious Diseases, under contract number N01-AI-30061. C. P. C. is a recipient of a GAANN predoctoral fellowship from the Gatech Center for Drug Design, Development and Delivery. P. A. P. is a Gatech PURA fellow.
Footnotes
Supporting Information Available: Proton and carbon NMR spectra for all compounds described in the experimental section. This material is available free of charge via the Internet at http://pubs.acs.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Lee M, Hesek D, Suvorov M, Lee W, Vakulenko S, Mobashery S. J Am Chem Soc. 2003;125:16322–16326. doi: 10.1021/ja038445l. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hwu JR, Tsay S-C, Hakimelahi S. J Med Chem. 1998;41:4681–4685. doi: 10.1021/jm980476h. [DOI] [PubMed] [Google Scholar]; (b) Wittmann S, Schnabelrauch M, Scherlitz-Hofmann I, Mollmann U, Ankel-Fuchs D, Heinisch L. Bioorg Med Chem. 2002;10:1659–1670. doi: 10.1016/s0968-0896(02)00044-5. [DOI] [PubMed] [Google Scholar]; (c) Kline T, Fromhold M, Mckennon TE, Cai S, Treiberg J, Ihle N, Sherman D, Schwan W, Hickey MJ, Warrener P. Bioorg Med Chem. 2000;8:73–93. doi: 10.1016/s0968-0896(99)00261-8. [DOI] [PubMed] [Google Scholar]; (d) Ghosh A, et al. J Chem Biol. 1996;3:1011. doi: 10.1016/s1074-5521(96)90167-2. [DOI] [PubMed] [Google Scholar]; (e) Heinisch L, Wittmann S, Stoiber T, Berg A, Ankel-Fuchs D, Mollmann U. J Med Chem. 2002;45:3032–3040. doi: 10.1021/jm010546b. [DOI] [PubMed] [Google Scholar]
- 3.Fuda C, Hesek D, Lee M, Morio K, Nowak T, Mobashery S. J Am Chem Soc. 2005;127:2056–2057. doi: 10.1021/ja0434376. [DOI] [PubMed] [Google Scholar]
- 4.Mourey L, Kotra LP, Bellettini J, Bulychev A, O’Brien M, Miller MJ, Mobashery S, Samana JP. J Bio Chem. 1999;274:25260–25265. doi: 10.1074/jbc.274.36.25260. [DOI] [PubMed] [Google Scholar]
- 5.Hermann JC, Hense C, Ridder L, Mulholland AJ, Holtje HD. J Am Chem Soc. 2005;127:4454–4465. doi: 10.1021/ja044210d. [DOI] [PubMed] [Google Scholar]
- 6.Padayatti PS, Sheri A, Totir MA, Helfand MS, Carey MP, Anderson VE, Carey PR, Bethel CR, Bonomo RA, Buynak JD, van dan Akker F. J Am Chem Soc. 2006;128:13235–13242. doi: 10.1021/ja063715w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meroueh SO, Fisher JF, Schlegel HB, Mobashery S. J Am Chem Soc. 2005;127:15397–15407. doi: 10.1021/ja051592u. [DOI] [PubMed] [Google Scholar]
- 8.Heinze-Krauss I, Angehrn P, Charnas RL, Gubernator K, Gutknecht EM, Hubschwerlen C, Kania M, Oefner C, Page MGP, Sogabe S, Specklin JL, Winkler F. J Med Chem. 1998;41:3961–3971. doi: 10.1021/jm980023c. [DOI] [PubMed] [Google Scholar]
- 9.Buynak JD, Vogeti L, Doppalapudi VR, Solomon GM, Chen H. Bioorg Med Chem Lett. 2002;12:1663–1666. doi: 10.1016/s0960-894x(02)00205-6. [DOI] [PubMed] [Google Scholar]
- 10.Badarau A, Llinas A, Laws AP, Damblon C, Page MI. Biochem. 2005;44:8578–8589. doi: 10.1021/bi050302j. [DOI] [PubMed] [Google Scholar]
- 11.Kollman PA, Massova I. J Comp Chem. 2002;23:1559–1576. doi: 10.1002/jcc.10129. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande AD, Baheti KG, Chatterjee NR. Curr Sci. 2004;87:1684–1695. [Google Scholar]
- 13.Leopold IH. Investigative Ophthalmology. 1964;5:504–511. [PubMed] [Google Scholar]
- 14.Zygmunt WA, Harrison EF, Browder HP. Applied Microbiology. 1965;13:491–493. doi: 10.1128/am.13.3.491-493.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal S, Bredin J, Pages JM, Barbe J. J Med Chem. 2005;48:1395–1400. doi: 10.1021/jm049652e. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JF, Meroueh SO, Mobashery S. Chem Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 17.Josephine HR, Kumar I, Pratt RF. J Am Chem Soc. 2004;126:8122–8123. doi: 10.1021/ja048850s. [DOI] [PubMed] [Google Scholar]
- 18.Bush K, Macielag M, Weidner-Wells M. Curr Opin Microbiol. 2004;7:466–476. doi: 10.1016/j.mib.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Dalhoff A, Thomson CJ. Chemotherapy. 2003;49:105–120. doi: 10.1159/000070616. [DOI] [PubMed] [Google Scholar]
- 20.Buynak JD. Curr Med Chem. 2004;1:1951–1964. doi: 10.2174/0929867043364847. [DOI] [PubMed] [Google Scholar]
- 21.Josephine HR, Charlier P, Davies C, Nicholas RA, Pratt RF. Biochem. 2006;45:15873–15883. doi: 10.1021/bi061804f. [DOI] [PubMed] [Google Scholar]
- 22.Neu HC. Pharmacotherapy. 1985;5:1–10. doi: 10.1002/j.1875-9114.1985.tb04448.x. [DOI] [PubMed] [Google Scholar]
- 23.Davern P, Sheehy J, Smyth T. J Chem Soc Perkin Tans. 1994;2:381–387. [Google Scholar]
- 24.(a) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Horne WS, Stout CD, Ghadiri MR. J Am Chem Soc. 2003;125:9372–9376. doi: 10.1021/ja034358h. [DOI] [PubMed] [Google Scholar]; (c) Billing JH, Nilsson UJ. J Org Chem. 2005;70:4847–4850. doi: 10.1021/jo050585l. [DOI] [PubMed] [Google Scholar]; (d) Brik A, Alexandratos J, Lin YC, Elder JH, Olson AJ, Wlodawer A, Goodsell DS, Wong CH. ChemBioChem. 2005;6:1167–1169. doi: 10.1002/cbic.200500101. [DOI] [PubMed] [Google Scholar]; (e) Whiting M, Muldoon J, Lin YC, Silverman SM, Lindstom W, Olson AJ, Kolb HC, Finn MG, Sharpless KB, Elder JH, Fokin VV. Angew Chem, Int Ed. 2006;45:1435–1439. doi: 10.1002/anie.200502161. [DOI] [PubMed] [Google Scholar]; (f) Bock VD, Speijer D, Hiemstra H, van Maarseveen JH. Org Biomol Chem. 2007;5:971–975. doi: 10.1039/b616751a. [DOI] [PubMed] [Google Scholar]
- 25.Kolb HC, Sharpless KB. DDT. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 26.Numerous examples of click chemistry have appeared in the literature. Cited here are two pioneering examples: Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j.
- 27.Firestone RA, Maciejewicz NS, Ratcliffe RW, Christensen BG. J Org Chem. 1974;39:437–440. doi: 10.1021/jo00918a003. [DOI] [PubMed] [Google Scholar]
- 28.Bachi MD, Breiman RJ. J Chem Soc Perkin Trans. 1980;1:11–13. [Google Scholar]
- 29.Kemp JEG, Closier MD, Narayanaswami S, Stefaniak MH. Tetrahedron Lett. 1980;21:2991–2994. [Google Scholar]
- 30.Barrett AGM, Sakadarat S. J Org Chem. 1990;55:5110–5117. [Google Scholar]
- 31.For examples, see: Ruff JK. Inorg Chem. 1965;4:567–570.Cavender CJ, Shiner VJ. J Org Chem. 1972;37:3567–3569.Zaloom JR, David CJ. J Org Chem. 1981;46:5173–5176.Eaton PE, Fisher AM, Hormann RE. Synlett. 1990:737.Alper PB, Hung SC, Wong CH. Tetrahedron Lett. 1996;37:6029–6032.Ding Y, Swayze EE, Hofstadler SA, Griffey RH. Tetrahedron Lett. 2000;41:4049–4052.Greenberg WA, Priestley ES, Sears PS, Alper PB, Rosenbohm C, Hendrix M, Hung SC, Wong CH. J Am Chem Soc. 1999;121:6527–6541.Liu Q, Tor Y. Org Lett. 2003;5:2571–2572. doi: 10.1021/ol034919+.Titz A, Radic Z, Schwardt O, Ernst B. Tetrahedron Lett. 2006;47:2383–2385.
- 32.Oyelere AK, Chen PC, Yao LP, Boguslavsky N. J Org Chem. 2006;71:9791–9796. doi: 10.1021/jo0618122. [DOI] [PubMed] [Google Scholar]
- 33.Buynak JD, Wu K, Bachmann B, Khasnis D, Hua L, Nguyen HK, Carver CL. J Med Chem. 1995;38:1022–1034. doi: 10.1021/jm00006a022. [DOI] [PubMed] [Google Scholar]
- 34.Venkatesan AM, Gu Y, Santos OD, Abe T, Agarwal A, Yang Y, Peterson PJ, Weiss WJ, Mansour TS, Nukaga M, Hujer AM, Bonomo RA, Knox JR. J Med Chem. 2004;47:6556–6568. doi: 10.1021/jm049680x. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan JC, Commons TJ. J Org Chem. 1978;43:2203–2208. [Google Scholar]
- 36.Lee M, Hesek D, Mobashery S. J Org Chem. 2005;70:367–369. doi: 10.1021/jo0487395. [DOI] [PubMed] [Google Scholar]
- 37.Ko KY, Kim JY. Bull Korean Chem Soc. 1999;20:771–772. [Google Scholar]
- 38.Stedman RJ, Hoover JRE, Chow AW, Dolan MM, Hall NM, Ferlauto RJ. J Med Chem. 1964;7:251–255. doi: 10.1021/jm00333a002. [DOI] [PubMed] [Google Scholar]
- 39.Hoover JRE, Chow AW, Stedman RJ, Hall NM, Greenberg HS, Dolan MM, Ferlauto RJ. J Med Chem. 1964;7:245–251. doi: 10.1021/jm00333a001. [DOI] [PubMed] [Google Scholar]
- 40.Venkatesan AM, Agarwal A, Abe T, Ushirogochi H, Yamamura I, Ado M, Tsuyoshi T, Santos OD, Gu Y, Sum FW, Li Z, Francisco G, Lin YI, Petersen P, Yang Y, Kumagai T, Weiss WJ, Shlaes DM, Know JR, Mansour TS. J Med Chem. 2006;49:4623–4637. doi: 10.1021/jm060021p. [DOI] [PubMed] [Google Scholar]
- 41.Quesada E, Taylor RJK. Tetrahedron Lett. 2005;46:6473–6476. [Google Scholar]
- 42.Ghosh AK, Bischoff A, Cappiello J. J Eur Org Chem. 2003:821–832. doi: 10.1002/ejoc.200390125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.(a) Anhoury ML, Arickx M, Crooy P, De Neys R, Eliaers J. J Chem Soc Perkin I. 1974:191–192. [Google Scholar]; (b) Callant P, D’Haenens L, Vandewalle M. Synth Commun. 1984;14:155–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.