Abstract
Emerging evidence suggests that the antimicrobial peptide, leucine leucine-37 (LL-37), could play a role in the progression of solid tumors. LL-37 is expressed as the COOH terminus of human cationic antimicrobial protein-18 (hCAP-18) in ovarian, breast, and lung cancers. Previous studies have shown that the addition of LL-37 to various cancer cell lines in vitro stimulates proliferation, migration, and invasion. Similarly, overexpression of hCAP-18/LL-37 in vivo accelerates tumor growth. However, the receptor or receptors through which these processes are mediated have not been thoroughly examined. In the present study, expression of formyl peptide receptor–like 1 (FPRL1) was confirmed on ovarian cancer cells. Proliferation assays indicated that LL-37 does not signal through a G protein–coupled receptor, such as FPRL1, to promote cancer cell growth. By contrast, FPRL1 was required for LL-37–induced invasion through Matrigel. The peptide stimulated mitogen-activated protein kinase and Janus-activated kinase/signal transducers and activators of transcription signaling cascades and led to the significant activation of several transcription factors, through both FPRL1-dependent and FPRL1-independent pathways. Likewise, expression of some LL-37–stimulated genes was attenuated by the inhibition of FPRL1. Increased expression of CXCL10, EGF, and PDGF-BB as well as other soluble factors was confirmed from conditioned medium of LL-37–treated cells. Taken together, these data suggest that LL-37 potentiates a more aggressive behavior from ovarian cancer cells through its interaction with FPRL1.
Introduction
Inflammatory molecules play a pivotal role in tumorigenesis and cancer progression (1). Recently, we have shown that one specific inflammatory molecule, called leucine leucine-37 (LL-37), is highly expressed in epithelial ovarian tumors, and reports from other laboratories indicate that breast and lung tumors also express elevated levels of LL-37 (2-4). However, little is known about the mechanism of action of LL-37 on tumor cells. LL-37 is the 37–amino acid peptide derivative of human cationic antimicrobial protein-18 (hCAP-18), originally identified as a product of various leukocytes (5-7). In recent years, expression of LL-37 has also been detected in other cell types such as epithelia, in which inflammatory stimuli can up-regulate peptide expression (8). Cleavage of hCAP-18 gives rise to two functionally distinct peptides, the importance of which in host defense against microorganisms has been clearly defined (5, 6, 9). In addition to its antimicrobial functions, LL-37 also plays a role in wound healing, angio-genesis, and leukocyte trafficking (10-19). LL-37 initiates these responses through multiple receptors including the Gαi protein–coupled receptor (GPCR), formyl peptide receptor–like 1 (FPRL1), the epidermal growth factor receptor (EGFR), the purinergic receptor P2X7, and another receptor (or receptors) that remains uncharacterized (12, 13, 15-17, 19-22). Unlike FPRL1 and P2X7 activation, EGFR activation does not occur through direct interaction with LL-37. Rather, EGFR is transactivated by LL-37–induced metalloproteinase cleavage of membrane-associated EGFR ligands, and this effect may be GPCR-dependent or GPCR-independent according to cell type (12, 13, 20).
Our previous study suggested that LL-37 facilitates tumor progression through multiple mechanisms, as treatment of ovarian cancer cells with recombinant peptide resulted in increased proliferation, migration, invasion, and matrixmetalloproteinase (MMP) activation (2). In agreement with these data, other laboratories have shown that LL-37 stimulates the proliferation of various cancer cell lines and that overexpression of hCAP-18/LL-37 in lung cancer xenografts increases tumor growth in nude mice (3, 4). More recently, hCAP-18/LL-37 has been shown to contribute to breast cancer metastases (23). Transactivation of EGFR and ErbB2, two members of the EGFR family, has been implicated in mediating the effects of LL-37 through the use of pharmacologic inhibitors. However, the specific involvement of FPRL1 has not been thoroughly examined, as only a general inhibitor of Gαi signaling (i.e., pertussis toxin) has been used to date. Therefore, we set out to investigate the role of FPRL1 in this, and other processes, as well as the mechanism through which LL-37 influences ovarian cancer cells.
Results
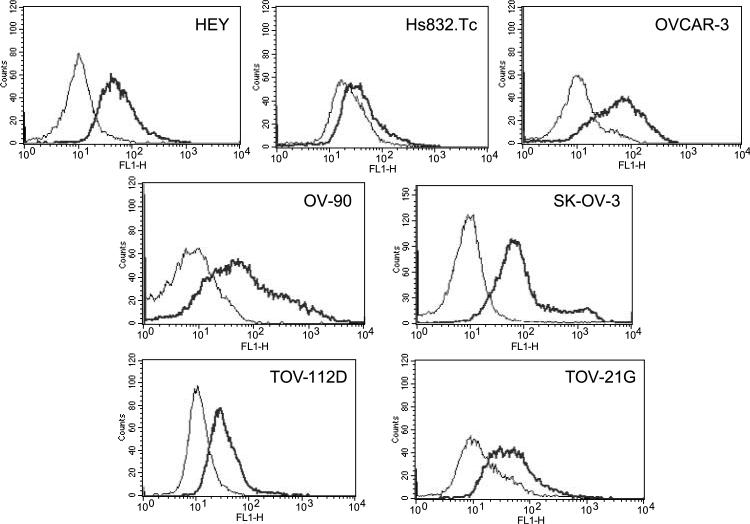
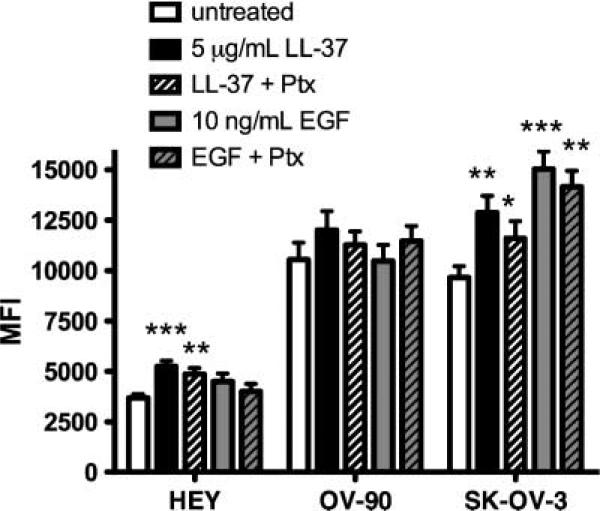
A panel of seven cell lines was examined by flow cytometry for expression of FPRL1. The ovarian cancer cell lines expressed FPRL1 to varying degrees and Hs832.Tc—a fibroblastic cell line derived from a benign ovarian cyst—expressed the lowest measured levels of FPRL1 (Fig. 1). We previously reported that LL-37 induces ovarian cancer cell proliferation (2). To determine if LL-37 signals through FPRL1 to mediate this effect, the same ovarian cancer cell lines used in our prior study were pretreated with pertussis toxin (Ptx) before exposure to the LL-37 peptide. Ptxtreatment reduced the proliferation rate of LL-37–treated HEY, OV-90, and SK-OV-3 cells after 48 hours; although this effect was not statistically significant (Fig. 2). These results indicate that LL-37 does not use FPRL1 (or any other Gαi-dependent GPCR) to stimulate ovarian cancer cell growth.
FIGURE 1.
FPRL1 is expressed on ovarian cancer cells. Ovarian cancer cell lines, representing different histologic subtypes, were analyzed for FPRL1 expression by flow cytometry. Primary antibodies were detected with Alexa-488–conjugated goat anti-rabbit antibodies. Black line, FPRL1 expression; gray line, isotype control (n = 3).
FIGURE 2.
LL-37 does not signal through a GPCR, such as FPRL1, to stimulate ovarian cancer cell proliferation. Graphic representation of ovarian cancer cell growth after exposure to LL-37 or EGF. Serum-starved cells were pretreated with or without 10 ng/mL of Ptx for 1 h, followed by LL-37 or EGF treatment. After 48 h, cellular DNA was measured using fluorescent probes. MFI, mean fluorescence intensity. Columns, mean of three or more independent experiments; bars, SE.
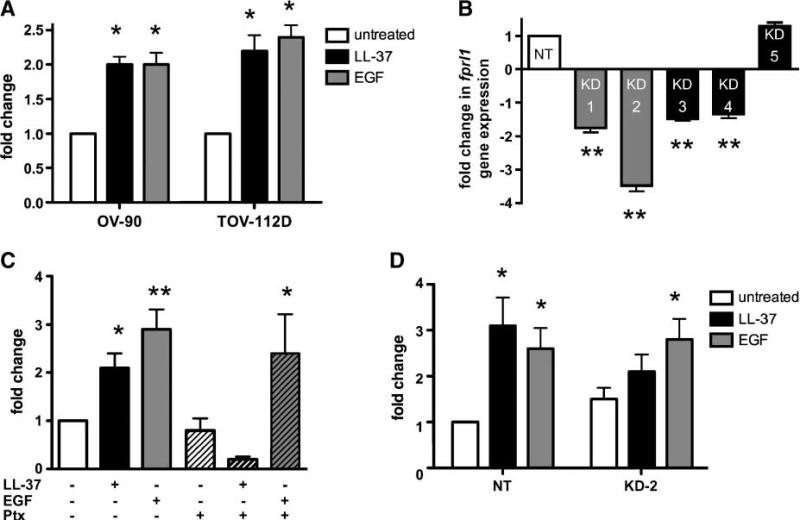
Previously, we also reported that LL-37 enhances the meta-static potential of SK-OV-3 ovarian cancer cells (2). Here, we confirmed the ability of LL-37 to induce the invasive behavior of two other ovarian cancer cell lines, OV-90 and TOV-112D (Fig. 3A). LL-37–mediated chemotaxis occurs through the FPRL1 receptor in some lymphoid and myeloid subsets; therefore, we hypothesized that LL-37 uses this receptor to initiate ovarian cancer cell invasion (16, 19). To address this question, we established FPRL1 knockdown cells by transduction of SK-OV-3 cells with lentiviruses containing FPRL1-specific shRNA constructs (termed FPRL1 KD-1 to KD-5). Another shRNA construct that does not recognize a specific mRNA target (called nontargets) was used as control. Fprl1 gene expression was measured using quantitative real-time PCR (qPCR) and it was observed that fprl1 expression was significantly diminished in SK-OV-3/FPRL1 KD-1, KD-2, KD-3, and KD-4 cells (Fig. 3B). SK-OV-3/FPRL1 KD-2 cells were chosen for use in subsequent experiments, as the level of fprl1 mRNA transcript was lowest in these cells. SK-OV-3 cells seeded onto Matrigel-coated inserts were then stimulated with LL-37 or EGF in an in vitro invasion assay. In contrast to the proliferation assay results, Ptx attenuated LL-37–induced SK-OV-3 cell invasion through Matrigel. EGF-stimulated invasion was unaffected by the inhibitor (Fig. 3C). SK-OV-3 cells transfected with control shRNA vectors (nontargets) responded to LL-37 and EGF stimulation in a similar manner as untransfected cells (Fig. 3D). EGF exposure significantly augmented the invasive behavior of SK-OV-3/FPRL1 KD-2, but LL-37 stimulation failed to significantly enhance their invasion when compared with untreated, knockdown cells. Taken together, these data suggest that LL-37 signals through FPRL1 to increase the metastatic potential of ovarian cancer cells.
FIGURE 3.
LL-37 mediates ovarian cancer cell migration and invasion through FPRL1. A. Graphic representation of ovarian cancer cell invasion. Serum-starved cells were seeded onto Matrigel-coated inserts in medium containing 10 μg/mL of LL-37 or 10 ng/mL of EGF. Columns, mean fold change of the mean fluorescence intensity of invaded cells compared with unstimulated controls; bars, SE (n = 3). B. Graph depicting fprl1 gene expression in knockdown (KD) cells. SK-OV-3 cells stably transduced with lentiviruses containing FPRL1-specific shRNA (KD-1 to KD-5) or nontarget (NT) sequences were analyzed by qPCR. Columns, mean of three independent experiments compared with nontarget cells; bars, SE. C. Graphic representation of SK-OV-3 ovarian cancer cell invasion through Matrigel-coated inserts. Untreated and Ptx-treated SK-OV-3 cells were stimulated with LL-37 or EGF as described above. P values for LL-37 or EGF groups were determined from their respective untreated or Ptx-treated alone controls. D. Graphic representation of SK-OV-3 nontarget cells and FPRL1 KD-2 cell invasion stimulated with LL-37 or EGF as above (*, P < 0.05; **, P < 0.01).
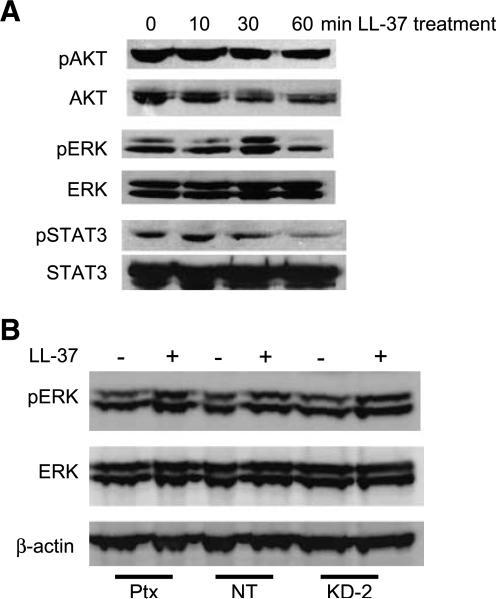
To better define the signaling pathways that are activated by LL-37, several of the established FPRL1-associated and EGFR-associated signaling cascades were studied (3, 4, 10, 12, 15, 20, 24). Western blot analysis of LL-37–treated SK-OV-3 cell lysates showed the robust phosphorylation of ERK1/2 and a slight activation of signal transducers and activators of transcription (STAT) 3, after the indicated time points (Fig. 4A). By contrast, AKT activation was constitutive for the time points measured. Similar results were observed in LL-37–treated OVCAR-3 cells (data not shown). SK-OV-3 cells were pretreated with Ptxbefore LL-37 stimulation, but ERK1/2 phosphorylation was maintained despite inhibition of Gαi/GPCR signaling (Fig. 4B). These observations were confirmed using SK-OV-3/FPRL1 KD-2 cells, indicating that LL-37–induced mitogen-activated protein kinase (MAPK) signaling does not occur through FPRL1 (or another GPCR).
FIGURE 4.
Activation of MAPK signaling pathways by LL-37 does not occur through FPRL1. A. The influence of recombinant LL-37 (5 μg/mL) on phosphorylation of AKT, ERK, and STAT3 in SK-OV-3 cells. Images are representative of three or more independent experiments. B. Cellular protein was isolated from Ptx-treated SK-OV-3 cells, SK-OV-3 nontarget cells, and SK-OV-3/FPRL1 KD-2 cells that were treated with LL-37 for 30 min. Phosphorylation and expression of ERK was measured by Western blot analysis. β-actin levels were assessed to ensure equal loading.
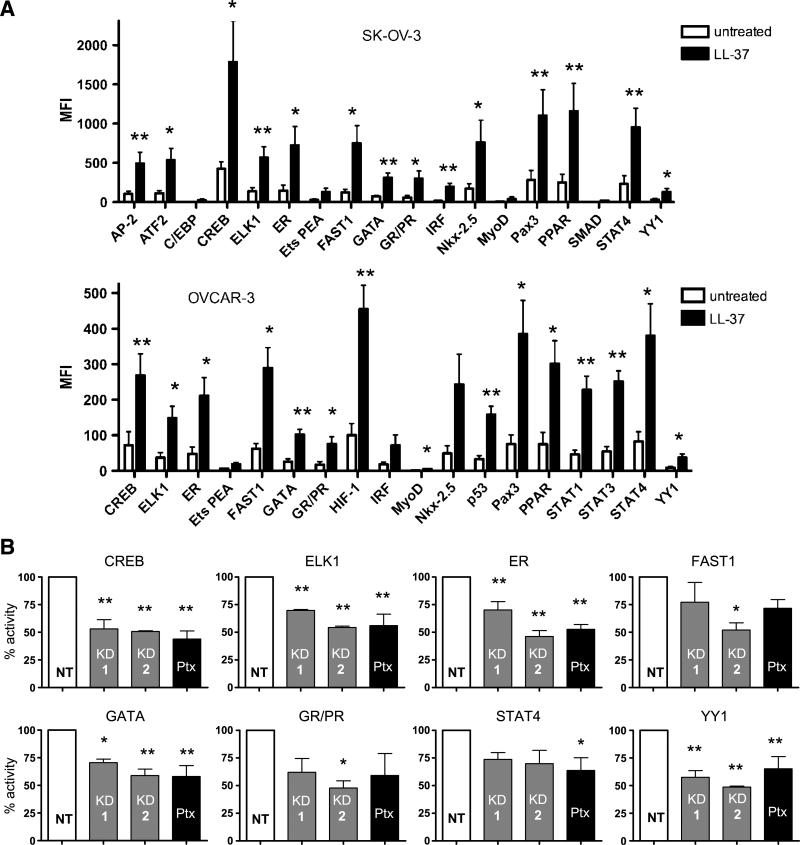
Nuclear protein extracts isolated from LL-37–treated ovarian cancer cells were then analyzed using a Luminex-based assay, as a quantitative measurement of LL-37–activated signaling pathways at the transcription factor level. This method allowed us to measure not only nuclear accumulation of the transcription factors, but also activation, because fluorescence intensity is based on DNA binding activity to specific oligonucleotide probes. LL-37 induced a number of transcription factors in SK-OV-3 and OVCAR-3 cells; however, only those transcription factors that were increased more than 4-fold were analyzed for statistical significance. The transcription factors that were significantly increased in both cell lines when compared with untreated cells included cAMP-responsive element binding protein (CREB), ELK1, estrogen receptor, FAST1, GATA, glucocorticoid receptor/progesterone receptor, PPAR, PAX3, STAT4, and YY1 (Fig. 5A). In SK-OV-3 cells, AP-2, ATF2, IRF, and Nkx-2.5 were also significantly induced, whereas C/EBP, Ets PEA, MyoD, and SMAD were not. LL-37 treatment of OVCAR-3 cells resulted in significant activation of HIF-1, MyoD, p53, STAT1, and STAT3, but not Ets PEA, IRF, and Nkx-2.5. The transcription factors that were not expressed in either cell line or not increased more than 4-fold by LL-37 included activator protein-1, androgen receptor, Brn3, c-myb, E2F1, FKHR, HNF1, ISRE, MEF2, NF-1, NFAT, NF-E2, NF-κB, NF-Y, Oct, PAX5, RUNX AML, and STAT5 (data not shown).
FIGURE 5.
Inhibition of FPRL1 negatively affects LL-37–induced nuclear accumulation and activity of multiple transcription factors. A. Graphic representation of transcription factor-DNA binding. Nuclear extracts from serum-starved ovarian cancer cells, treated or untreated with 5 μg/mL of LL-37 for 1 h, were analyzed for transcription factors bound to specific fluorescently labeled oligonucleotide probes. MFI, mean fluorescence intensity. Columns, mean; bars, SE. B. Analysis of transcription factor activity in SK-OV-3/FPRL1 KD and Ptx-treated cells after LL-37 treatment as described above. Columns, mean of four independent experiments; bars, SE (*, P < 0.05; **, P < 0.01).
Nuclear accumulation and activity of the transcription factors significantly enhanced in both ovarian cancer cell lines were also analyzed in SK-OV-3/FPRL1 KD-1, KD-2 cells, and Ptx-treated cells after LL-37 stimulation. CREB, ELK1, estrogen receptor, GATA, and YY1 were inhibited in cells lacking FPRL1 as well as Ptx-treated cells, suggesting that LL-37 induces these transcription factors through FPRL1 signaling (Fig. 5B). For each transcription factor examined, SK-OV-3/FPRL1 KD-1 cells exhibited more activity than FPRL1 KD-2 cells, which was consistent with qPCR data indicating that SK-OV-3/FPRL1 KD-1 cells express higher levels of the FPRL1 receptor. The activity of FAST1 and glucocorticoid receptor/progesterone receptor were significantly reduced in FPRL1 KD-2 cells, but not in FPRL1 KD-1 cells or Ptx-treated cells, whereas STAT4 activity was significantly diminished only in Ptx-treated cells. These data suggest that LL-37 signals through both FPRL1-dependent and FPRL1-independent pathways.
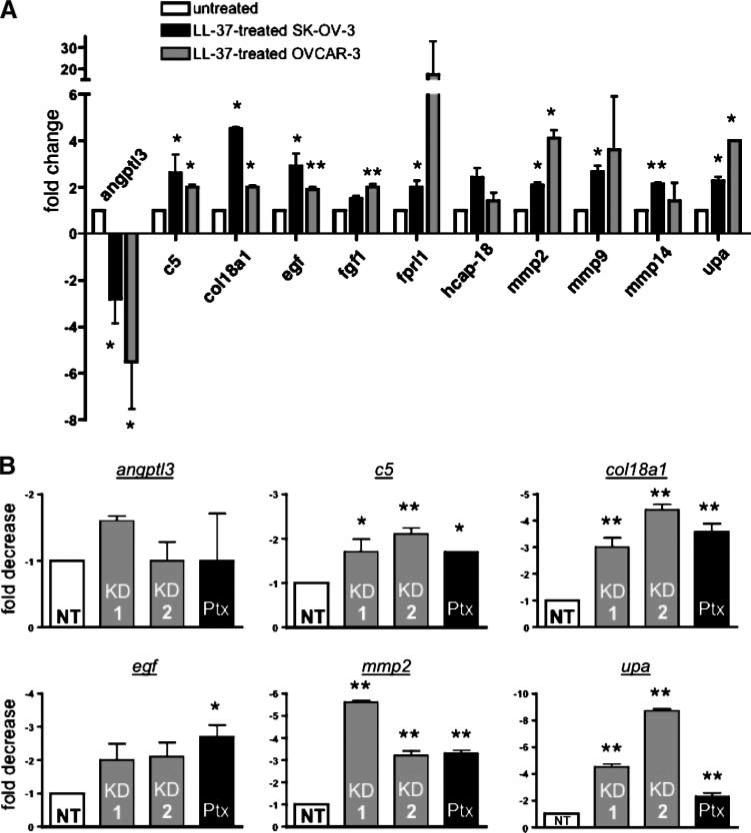
To determine which genes are regulated by the LL-37–FPRL1 interaction in ovarian cancer cells, RNA was isolated from SK-OV-3 and OVCAR-3 cells after treatment with the peptide and analyzed by focused gene microarray. The expression profiles of 168 angiogenic and inflammatory genes were monitored after LL-37 treatment (Supplementary Table S1). Several of these genes were chosen for further validation by qPCR based on the magnitude of change in gene expression. These genes included angiopoietin-like 3 (angptl3), complement 5 (c5), collagen type XVIII (col18a1), epidermal growth factor (egf), fibroblast growth factor 1 (fgf1), fprl1, and hcap-18/ll-37. The matrixmetalloproteinases, mmp2, mmp9, and mmp14, as well as urokinase plasminogen activator (upa), were also analyzed in OVCAR-3 cells because we have previously shown that LL-37 increases their expression in SK-OV-3 cells. As shown in Fig. 6A, LL-37 treatment resulted in the significant induction of the following genes in both cell lines: c5, col18a1, egf, mmp2, and upa. Gene expression of angptl3 was significantly decreased in both cell lines. In SK-OV-3 cells, fgf1 and hcap-18/ll-37 expression was not affected by LL-37, whereas fprl1 transcript was significantly more abundant. The peptide increased expression of fgf1, fprl1, hcap-18/ll-37, mmp9, and mmp14 in OVCAR-3 cells; although, only the fgf1 induction was significant.
FIGURE 6.
LL-37 modulates target gene expression through FPRL1 signaling. A. Graphic representation of genes regulated by LL-37. Serum-starved SK-OV-3 and OVCAR-3 cells were treated with 5 μg/mL of LL-37 for 6 h. RNA was isolated and analyzed by qPCR using the δCt method. B. Analysis of target gene expression in SK-OV-3/FPRL1 KD and Ptx-treated cells after LL-37 treatment. Columns, mean of three or more independent experiments; bars, SE (*, P < 0.05; **, P < 0.01).
The involvement of FPRL1 in LL-37–stimulated gene expression was then assessed in knockdown and Ptx-treated cells. Expression of c5, col18a1, mmp2 and upa were significantly repressed by the absence of FPRL1 signaling (Fig. 6B). In contrast, angptl3 expression was unaffected in SK-OV-3/FPRL1 KD cells or Ptx-treated cells. Ptx pretreatment significantly decreased egf expression by LL-37, but this effect was not observed in FPRL1 KD cells.
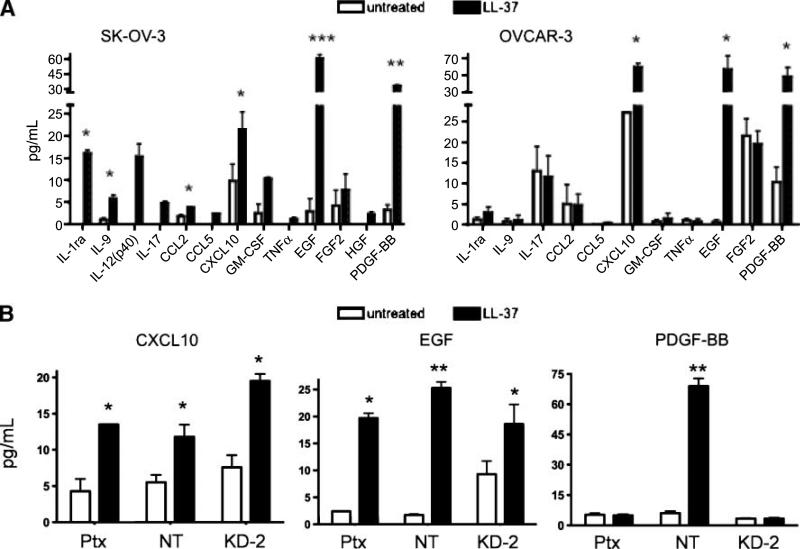
The soluble factors released from ovarian cancer cells after LL-37 treatment were analyzed by a quantitative Luminex-based assay. Both LL-37–treated SK-OV-3 and OVCAR-3 cells produced significantly more CXCL10 (IP-10), EGF, and PDGF-BB when compared with untreated cells (Fig. 7A). In addition, treatment of SK-OV-3 cells led to the increased secretion of IL-1ra, IL-9, and CCL2. Exposure of LL-37 to Ptx-treated SK-OV-3 cells, stably transfected nontarget cells, or SK-OV-3/FPRL1 KD cells significantly enhanced the release of CXCL10 and EGF when compared with untreated cells (Fig. 7B). However, LL-37 failed to stimulate PDGF-BB secretion in Ptx-treated and FPRL1 KD-2 cells. These data suggest that LL-37 uses FPRL1 for PDGF-BB release, but signals through another receptor(s) to increase CXCL10 and EGF release from ovarian cancer cells.
FIGURE 7.
LL-37 increases the release of protumorigenic molecules from ovarian cancer cells. A. Serum-starved ovarian cancer cells were treated with 5 μg/mL of LL-37 for 48 h, then conditioned medium was analyzed by Luminex-based cytokine arrays. The amount of cytokines and growth factors in conditioned medium is represented graphically. B. Ptx-treated SK-OV-3 cells, SK-OV-3 nontarget cells, and SK-OV-3/FPRL1 KD-2 cells were treated with LL-37 as above. The levels of EGF, CXCL10, and PDGF-BB were measured in conditioned medium after 48 h. Columns, mean of three or more independent experiments; bars, SE (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Discussion
Recent reports suggest an autocrine role for LL-37 in tumor cell proliferation, survival, and metastasis (1-4, 23). However, little is known about the receptors through which the promiscuous peptide, LL-37, influences these processes. Here, we present data indicating that FPRL1 is not involved in LL-37–stimulated cell growth, but is involved in promoting a more aggressive phenotype in ovarian cancer cells. We also show that the peptide uses FPRL1 to induce transcription factor activation, gene expression, and soluble factor release from ovarian cancer cells. At this time, the extent to which other receptors, such as EGFR,ErbB2, or P2X7, play a role in mediating responses to LL-37 is unclear because FPRL1 was the only receptor examined in this study.
Several lines of evidence support the notion that LL-37 stimulates a more aggressive phenotype in ovarian cancer cells. We show here, and in previous studies, that LL-37 increases ovarian cancer cell migration, invasion, and MMP secretion and conclude from the present study that this effect occurs, in part, through FPRL1 signaling (2). Our data corroborate observations from other labs, which indicate that LL-37 promotes breast cancer metastases (23). Interestingly, overexpression of hCAP-18/LL-37 in breast cancer cells implanted into severe combined immunodeficiency mice does not result in more proliferative tumors, as in the case of in vitro experiments and lung tumor xenografts (4, 23). These observations suggest that LL-37 may function differently in hormone-dependent malignancies (i.e., ovarian and breast) than in other hormone-independent tumor types (i.e., lung), by promoting metastasis in one and proliferation in another. In keratinocytes, LL-37 promotes protease expression and induces the expression of molecules involved in the epithelial-to-mesenchymal transition, such as SNAIL and SLUG transcription factors (15). Thus, it is tempting to speculate that LL-37 is involved in the epithelial-to-mesenchymal transition of hormone-dependent tumors as well.
We found that LL-37 activates the MAPK signaling pathway in ovarian cancer cells and that ERK phosphorylation is maintained despite the inhibition or lack of FPRL1. This same response has now been shown for breast cancer cells, but contrasts with observations made in fibroblasts in which LL-37–induced ERK activation requires a Ptx-sensitive GPCR (23, 25). These findings highlight the promiscuous interactions of LL-37 with various receptors and indicate that its mechanism of action is cell type–specific.
A number of transcription factors were found to be down-stream of LL-37–FPRL1 signaling. Activation of some of these transcription factors, such as CREB, may contribute to the invasive behavior of ovarian cancer cells by up-regulating prometastatic genes (i.e., mmp2), as similar findings have been shown for other cancer cell types (26). Moreover, stimulation of some transcription factors, such as STAT4, occurred in spite of the inhibition of FPRL1, whereas stimulation of others was partially abrogated in FPRL1 knockdown cells. These data suggest that not only are multiple receptors involved in LL-37 signaling, but they may also indicate that crosstalk between FPRL1 and other receptors is important for LL-37–mediated events.
LL-37 also regulated gene expression through FPRL1-dependent and FPRL1-independent pathways. Several of these genes, such as egf, mmp2, and upa have established roles in promoting tumor progression (27). The function of other genes in this process, including angptl3, c5, and col18a1, are not as well defined; although increased expression of c5 by the LL-37–FPRL1 interaction may have important relevance to ovarian tumor progression given recently published observations in murine tumor models (28). LL-37–induced gene expression was confirmed at the protein level for some gene products and it seemed that LL-37 regulates protein expression at the posttranslational level as well. For example, LL-37 increased EGF mRNA and protein expression, but the effect of the peptide on CXCL10 expression was seen only at the protein level because mRNA transcript levels did not change in the focused gene array analysis (Supplementary Table S1).
Taken together, these data provide further evidence in support of a protumorigenic role for LL-37 in epithelial ovarian cancers. In addition to its stimulatory effects on proliferation and the invasive behavior of ovarian cancer cells, LL-37 can also induce the release of proangiogenic factors such as EGF, PDGF-BB, and MMPs. Thus, it is likely that ovarian tumor–derived LL-37 influences both tumor epithelial cells and their microenvironment through autocrine and paracrine means—a notion supported by our recent findings indicating that LL-37 recruits progenitor cell populations to ovarian tumors (29). Although further investigations are required, the LL-37–FPRL1 interaction on tumor epithelial cells provides an attractive, potential target for cancer therapeutics.
Materials and Methods
Cell Culture
Ovarian cancer cell lines Hs832(C).T (benign ovarian cyst), OV-90 (papillary serous adenocarcinoma), SK-OV-3 (adenocarcinoma), TOV-112D (endometrioid adenocarcinoma), and TOV-21G (clear cell adenocarcinoma) were obtained from American Type Culture Collection and propagated according to their recommendations. HEY (xenograft HX-62, papillary cystadenocarcinoma) and OVCAR-3 (adenocarcinoma) cell lines were kind gifts from Frank Marini (M.D. Anderson Cancer Center, Houston, TX). These cells were maintained in RPMI 1640 (Life Technologies) containing 10% fetal bovine serum (Atlanta Biologicals) and 100 units/mL of penicillin and 100 mg/mL of streptomycin (Life Technologies).
Flow Cytometry
Ovarian cancer cells were stained with an anti–FPRL-1 antibody (1:100; LifeSpan Biosciences) or rabbit IgG isotype controls (Dako) for 30 min at 4°C after blocking in 10% goat serum. Cells were washed; then, primary antibodies were detected with Alexa-488–conjugated goat anti-rabbit secondary antibodies (1:250; Molecular Probes) for 30 min at 4°C. After washing again, analysis was done on a BD FACSCAlibur (BD Biosciences) using BD CellQuest Pro software.
Proliferation Assay
Ovarian cancer cells were seeded in 96-well plates and allowed to adhere overnight. The next day, cells were washed with PBS and serum-free medium was added. After 24 h, cells were pretreated with 10 ng/mL of Ptxfor 1 h before the addition of 5 μg/mL of recombinant LL-37 (Innovagen) or 10 ng/mL of EGF (R&D Systems) in medium containing 0.5% fetal bovine serum. Cells were allowed to incubate for 48 h and the remainder of the experiment was done as previously described using Invitrogen's CyQuant NF Cell Proliferation Kit (2).
Invasion Assays
Serum-starved ovarian cancer cells were assessed in invasion assays as described previously (2). Cells were pretreated with 10 ng/mL of Ptxfor 1 h prior to seeding on growth factor–reduced Matrigel (BD Biosciences). LL-37 was added to a final concentration of 10 μg/mL. EGF was used at a concentration of 10 ng/mL. Both chemoattractants were added in combination with 0.5% fetal bovine serum.
Western Blot Analysis
Protein extracts were generated from LL-37–treated ovarian cancer cells, then analyzed by the same method as previously described (2, 30). All antibodies were purchased from Cell Signaling Technology and used at a concentration of 1:1,000. Horseradish peroxidase–conjugated secondary antibodies were purchased from Amersham Biosciences (1:5,000).
Transcription Factor Assay
Serum-starved ovarian cancer cells were treated with 5 μg/mL of LL-37 for 1 h. Nuclear extracts were isolated and analyzed using Panomics’ Transcription Factor Assay kit following the instructions of the manufacturer on a Bio-Plex 200 (Bio-Rad).
Production of FPRL1 Knockdown Cells
SK-OV-3 cells were transduced with lentiviruses (Mission Transduction Particles; Sigma-Aldrich) containing FPRL1-specific shRNA (called KD-1 to KD-5) or nontarget sequences. Cells were treated with puromycin for 2 weeks for selection. Stable cell pools were analyzed for fprl1 expression using real-time qPCR.
Focused Gene Array Analysis and Real-time qPCR
Total RNA was isolated from OVCAR-3 and SK-OV-3 cells following LL-37 treatment (5 μg/mL)for 6 h using the RNeasy Mini Kit (Qiagen). Purified RNA was treated with TURBO DNA-free (Ambion) before reverse transcription using SuperArray reagents or iScript (Bio-Rad) for gene array analysis or qPCR, respectively. Gene array and qPCR were done as previously described (2, 30).
Analysis of Soluble Factors Secreted by Ovarian Cancer Cells
Ovarian cancer cells were plated at a density of 1.5 × 105 in 24-well plates, allowed to adhere overnight, serum-starved for 24 h, then treated with 5 μg/mL of LL-37 in the presence of 0.5% fetal bovine serum for 48 h. Conditioned medium was collected and analyzed with Human Angiogenesis Antibody Arrays (Panomics) and Bio-Plex Cytokine Assays (Human Group I and II; Bio-Rad) on a Bio-Plex 200 (Bio-Rad) following the instructions of the manufacturer.
Statistical Analyses
Student's t test or one-way ANOVA followed by Newman-Keuls post hoc test was used to determine P values using GraphPad Prism software. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Heather LaMarca for her critical review of the manuscript.
Grant support: NIH 1P20RR20152-01.
Footnotes
Current address for S.B. Coffelt: Tumour Targeting Group, Academic Unit of Pathology, University of Sheffield, Sheffield, United Kingdom.
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s). Cancer Res. 2008;68:6482–5. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffelt SB, Waterman RS, Florez L, et al. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–9. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 3.Heilborn JD, Nilsson MF, Jimenez CI, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–9. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 4.von Haussen J, Koczulla R, Shaykhiev R, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Agerberth B, Gunne H, Odeberg J, et al. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrick JW, Hirata M, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 8.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 9.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–6. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 10.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 12.Tokumaru S, Sayama K, Shirakata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 13.Shaykhiev R, Beisswenger C, Kandler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–8. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen F, Mittler D, Hirsch T, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12:1494–502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 15.Carretero M, Escamez MJ, Garcia M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–36. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niyonsaba F, Iwabuchi K, Someya A, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 19.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 20.Tjabringa GS, Aarbiou J, Ninaber DK, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 21.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 β processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 22.Lau YE, Rozek A, Scott MG, et al. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun. 2005;73:583–91. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber G, Chamorro CI, Granath F, et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–65. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 25.Iaccio A, Cattaneo F, Mauro M, Ammendola R. FPRL1-mediated induction of superoxide in LL-37-stimulated IMR90 human fibroblast. Arch Biochem Biophys. 2009;481:94–100. doi: 10.1016/j.abb.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–22. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- 27.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Markiewski MM, DeAngelis RA, Benencia F, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffelt SB, Marini FC, Watson K, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009;106:3806–11. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.