Abstract
The purpose of this review is to present an overview of the state-of-the-art imaging modalities used to track drug delivery from liposomal formulations into tumors during or after hyperthermia treatment. Liposomes are a drug delivery system comprised of a phospholipid bilayer surrounding an aqueous core and have been shown to accumulate following hyperthermia therapy. Use of contrast-containing liposomes in conjunction with hyperthermia therapy holds great promise to be able to directly measure drug dose concentrations as well as to non-invasively describe patterns of drug distribution with MR and PET/SPECT imaging modalities. We will review the rationale for using this approach and the potential advantages of having such information available during and after treatment.
Keywords: Hyperthermia, liposome, imaging, MRI, PET, chemodosimetry, dose painting, drug delivery, nanoparticle
Introduction
While radiation dosimetry has been successful in improving the efficacy of cancer therapy, accurate dosimetry for chemotherapy has been elusive. Efficacious chemotherapy regimens depend on doses that touch the limits of what is maximally tolerated by patients. Although improvements in imaging have greatly enhanced the diagnosis and real-time monitoring of cancer therapy, methods to image and quantify drug delivery to tumors have not been investigated thoroughly in any context.
With a modality such as hyperthermia (HT), where the physiologic effects of this treatment can be used to selectively deliver drugs to tumors, the ability to directly measure drug delivery is of emerging importance. Looking toward the future, one could envision using hyperthermia and other selective delivery methods to ‘paint’ drug delivery to tumors both in real time, while observing the delivery, and after treatment, where evaluation of drug dose concentrations will identify undertreated areas to be targeted on a subsequent treatment day. In this manner, the optimal drug dose concentration may be achievable in virtually every patient. The methods described represent a quantum leap forward beyond simple plasma pharmacodynamic models or limited data that could be obtained from direct tissue measurement following biopsies.
Nanoscopic drug delivery carriers provide a potential solution to the problem of systemic toxicity inherent to current chemotherapeutic drugs as well as a novel opportunity to image drug delivery. This review will focus primarily on liposomal drug delivery vehicles, as this is where most of the work on this concept has been done. However, these basic principles could be applied to other drug carriers, such as nanoparticles, or even macromolecur drugs like elastin-like polypeptide (ELP)-drug composites 1.
Following a brief review of liposomes as drug carriers, we will provide an overview of how liposomal drug delivery during hyperthermia can be non-invasively monitored using nuclear medicine and MRI methods (Figure 1). Recent advances in thermometry, chemodosimetry, and drug distribution patterning are presented. Finally, the rationale for using this approach and the potential advantages of having such information available during and after treatment will be discussed.
Figure 1.
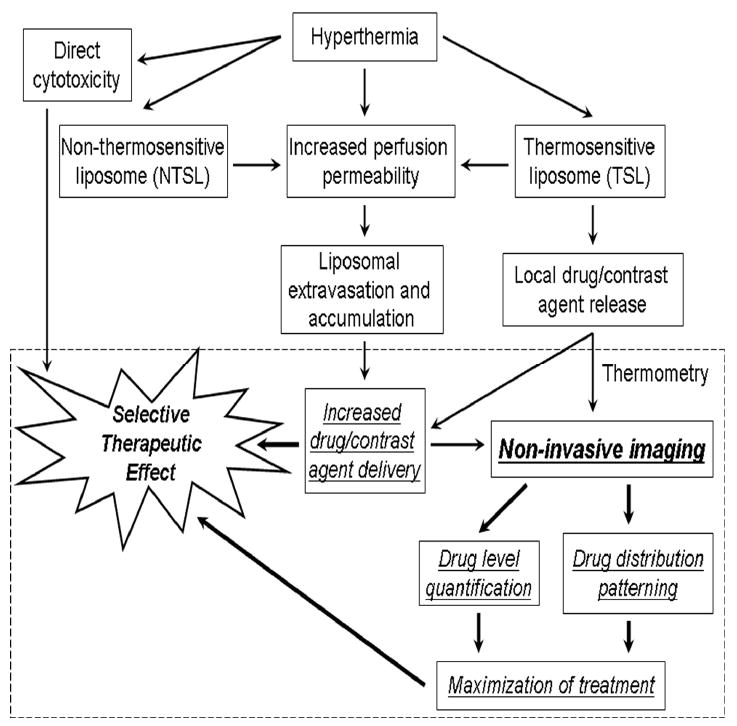
Flow chart for improving the selective therapeutic effect of combination hyperthermia and liposomal therapies through the use of non-invasive imaging. This review focuses on the section inside the dashed box. Liposomal thermometry is described in this review in the section of the same name 30, 31. Drug level quantification (chemodosimetry), drug distribution patterning, and maximization of treatment are described in this review in the section Liposomes, chemodosimetry, and drug distribution patterning 10, 11,. Other (background) block elements are described throughout this text and covered in depth in other reviews 4, 8, 17.
Liposomes as a drug carrier system
Drug carrier systems have the ability to increase the tolerability of conventional therapeutics through minimizing systemic side effects as well as increasing the amount of drug delivered to the treatment site. Liposomes have enjoyed increasing popularity as a drug-carrier system since the discovery of multilamellar vesicles in the 1960s 2. They consist of a self-assembling phospholipid bilayer enclosing an aqueous center. Typically they are only 100 nm in diameter, and act as drug carriers through the incorporation of hydrophobic molecules into the lipid bilayer or the entrapment of hydrophilic molecules in the aqueous core. Performance criteria of a successful drug-carrier system include retention of the drug within the carrier, evasion of host defenses, the ability to target the tumor site (through vessel pore-size exclusion or selective vehicle breakdown), and quick release of the drug at the target site 3. Multiple modifications have been made to liposomes to further exploit these four performance criteria, including the use of pH gradient and divalent metals for improved loading of aqueous soluble compounds, addition of polyethylene glycol (PEG) to decrease host immunorecognition, variations in particle size, antibodies and/or receptors for tissue targeting, and use of ‘triggers’ to allow quick release of drug at specific sites (reviewed extensively in 4). As discussed later, of particular importance here is the recent innovation in thermosensitive liposomes that are designed to respond to mild hyperthermic treatment in local tumors 5-8.
Enhanced liposomal accumulation in tumors treated with hyperthermia has been observed in a variety of reports. A prior review published in this Journal in 1999 identified over 100 reports demonstrating that hyperthermia treatment can increase liposomal delivery and/or efficacy with a variety of drugs and tumor models 8. The review provided strong rationale for determining in a more systematic fashion the dosimetry of liposomal drug delivery as it is influenced by hyperthermia. Kong et al. identified a linear relationship between liposomal extravasation rate and increasing temperature (between 40°-42°C) in ovarian carcinoma xenografts in athymic nude mouse window chamber models, for a fixed heating time of 60 min 9. The paper also reported that enhanced extravasation was maintained for periods of up to 4 h after heating. The effect was subject to thermotolerance, suggesting that the enhanced extravasation was the result of increasing endothelial cell pore size. If heating was done in two fractions 24 h apart, with liposomes being given during the second heating period, little or no extravasation occurred. Further evidence for changes in pore size came from studies with variable sized liposomes. At 42°C, the rate of extravasation of 100 nm liposomes was equivalent to that of albumin (a 5 nm ×15 nm molecule) at the same temperature. Although hyperthermia caused enhanced extravasation of 200 and 400 nm liposomes, the rate of extravasation was significantly smaller than for the 100 nm liposomes. The temperature dependence of liposomal extravasation clearly establishes a rationale for developing methods to quantify and image liposomal drug delivery, even if the liposome is not thermally sensitive itself.
Matteucci et al. and Kleiter et al. have shown increased accumulation of liposomes after heat treatment in feline sarcomas and rat fibrosarcomas, respectively, using radio-labeled non-thermally sensitive liposomes after heating 10, 11.
Triggering liposomal release
The initial goal and success of liposomal encapsulation of drug was stable sequestration during the blood-borne delivery phase, thereby reducing drug toxicity. The drawback to this success is that traditional liposomes do not spontaneously release their contents when they reach the desired site of action (i.e., the perivascular space or tumor microvasculature). Thus one major challenge in liposomal drug delivery has been to engineer liposomes that can be ‘triggered’ to release their contents upon exposure to an environmental cue. The idea of using hyperthermia to trigger drug release was first suggested by Yatvin 12. Clinically applicable thermosensitive liposomes require a phase transition temperature above normal body temperature (>37°C) and within the range of tolerable local-regional hyperthermia (<42°C) and/or slightly above this range for use with thermal ablation therapy 8, 13, 14. Upon heating, the lipid bilayer of thermosensitive liposomes changes from a solid or crystalline impermeable membrane to a membrane with melting lipid domains whose edges (‘grain boundaries’) have sufficient mismatches in lipid molecule packing to result in an enhanced permeability to small molecules and ions 15, 16. When applied to drug delivery, this enhanced permeability can cause the release of encapsulated substances 17.
Despite this triggered enhancement of membrane permeability, the rate of drug release achieved with traditional thermosensitive liposomes was still far too slow to achieve significant drug accumulation in the tumor tissue. This mechanism of action relies on the conventional paradigm of enhanced permeability of tumor vasculature and retention of liposomes (and their encapsulated drug) in tumor tissue (EPR). It was not until Needham's invention of almost instantaneously releasing liposomes triggered by mild hyperthermia that true triggered drug release was realized for a clinical setting. By incorporating 10 mol% of a micelle-forming lysolipid in solid solution in the frozen bilayer, drug was found to release in 20 seconds after achieving the thermal transition temperature (around 41°C). This literally ‘opened up’ the drug delivery field to a brand new paradigm: drug release in the blood stream likely followed by endothelial cell kill 18. Upon application of mild hyperthermia (41°C), the liposomes are transformed from stable capsules in the bloodstream to a rapidly releasing material causing both vascular damage and an antineoplastic effect. Needham's novel low temperature thermosensitive liposomes encapsulated the anti-cancer drug doxorubicin, which has recently been shown to work via an anti-vascular mechanism 18.
Modification of phase transition temperature is achieved by varying the lipid composition of the bilayer, specifically the lysolipids monostearoylphosphatidylcholine (MSPC) and/or myristoylpalmitoylphosphatidylcholine (MPPC). Other commonly used lipids in liposome formulation include dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), or distearoyl phosphatidylcholine (DSPC; phase transition temperatures of pure lipids shown in Table I). Cholesterol is a common liposomal component used to increase liposomal stability but it dampens the enhanced permeability and broadens the phase transition temperature. As a broad transition temperature is undesirable for temperature-sensitive liposomes, cholesterol is not recommended for these formulations, although it has commonly been used in these formulations in the past 19. Other advantages of incorporating the lysolipid (MSPC) have been that the bilayer transition temperature (determined by the host bilayer lipid(s), such as DPPC) is not increased, as it is in other thermosensitive systems that include mixtures of DPPC with the higher melting lipid DSPC, and even the transition-eliminating cholesterol 20, 21. Thus, the new low temperature thermosensitive liposome is designed to be more compatible with the range of temperature attainable by mild hyperthermia, as well as having the fastest drug release ever measured for a temperature-triggered system.
Table I. Chemical properties of contrast-containing liposomes.
| Lipid | Ratio | Phase transition temp (°C) | Contrast agent | Reference |
|---|---|---|---|---|
| NTSL, non-thermosensitive liposomes. | ||||
| DSPC | Pure | 54 | - | 8 |
| DPPC | Pure | 41.5-41.9 - | 47 | |
| DPPG | Pure | 41 | - | 6 |
| DSPC : chol : DSPE-PEG | 75 : 50 : 3 | NTSL | 99mTc | 10 |
| DSPC : chol : DSPE-PEG | 75 : 50 : 3 | NTSL | 99mTc | 11 |
| DSPC : chol | 55 : 45 | NTSL | MnSO4 | 35 |
| DPPC : DSPE-PEG | 95 : 5 | NTSL | MnSO4 | 34 |
| DPPC : DPPG | 95 : 5 | 56.7 GdDTPA-BMA | 31 | |
| DPPC : MSPC : DSPE-PEG | 90 : 10 : 4 | 39.5-41.3 MnSO4 | 35, 36 | |
| DOPC : chol : PEG-DSG | 3 : 2 : 0.3 | Unreported | Gadoteridol® | 32 |
It should be noted that other triggers have been utilized for liposomal drug release, including pH. These liposomes have been used in the context of imaging; however, their uses and mechanisms of release are outside the scope of this review 4, 22, 23.
Clinical applications
Multiple potential clinical applications exist for the use of thermosensitive liposomes loaded with chemotherapeutic agents in combination with local hyperthermia. These include improving local control of tumors in combination with radiation therapy (RT), downstaging advanced tumors to allow for surgical intervention, and effectively treating tumors that are drug resistant when given free drug. Use of thermosensitive liposomes allows for increased drug accumulation specifically at the heated site, and increased drug accumulation has been shown to correlate with enhanced growth delay efficacy 6, 17, 24. Specifically, thermosensitive doxorubicin-containing liposomes have been shown to result in intratumoral drug levels 30 times higher than that achieved with free drug and corresponding significantly enhanced therapeutic effects 6, 24. Six of nine mice treated with doxorubicin-containing thermosensitive liposomes and hyperthermia had no visible tumor 60 days after treatment 6.
With thermally targeted drug delivery, it is imperative that we be able to detect where drug is delivered, whether it is effectively released from the liposomal carrier, and what the concentration of delivered drug is. Incorporation of both a chemotherapeutic agent and a contrast agent into a liposomal delivery vehicle may allow for drug delivery and patterns of distribution to be monitored in real-time. However, there are inherent challenges to this concept, including potential differences in accumulation and clearance of both drugs from tissue and overall limited retention of agents in tissue, which must be taken into account. Magnetic resonance imaging (MRI) and nuclear imaging (PET/SPECT) systems have both been tested with liposomal delivery systems, and each system has unique strengths and weaknesses.
Imaging methods
Multiple imaging modalities exist for use in conjunction with imageable liposomes and hyperthermia. These include magnetic resonance imaging (MRI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Liposomes have also been used as contrast agents with computed tomography (CT) and ultrasound imaging; however these imaging modalities will not be reviewed here 25, 26.
Magnetic resonance imaging
MRI is a technique that uses radio frequency energy to excite atomic nuclei, which release this energy through relaxation and reliagnment with the bulk magnetic field of the MRI unit 27. The ‘T1′ signal describes the time constant for the exponential dependent signal recovery that occurs when the proton's magnetic moment realigns with the bulk magnetic field. Contrast agents used in MRI are paramagnetic metals (i.e., gadolinium (Gd), chelates, manganese) that primarily shorten the T1 within a tissue by increasing energy transfer from the water proton. The proportionality constant that relates the change in concentration to the change in 1/T1 is termed ‘relaxivity’, or R1.
Liposomal contrast agents used in MRI take advantage of the necessary interaction between contrast agent and water in creating T1 shortening. When such agents are encapsulated within the liposome's aqueous core, they can only cause signal enhancement upon membrane disruption allowing interaction with bulk water or with the limited amount of water that can traverse the membrane and enter the liposomal core. Membrane disruption or a controlled rapid increase in membrane permeability, as occurs for the thermosensitive liposomes, allows bulk water molecules to enter the aqueous core or the contrast agent to be released into the bulk water 28. The resulting interaction yields significant signal enhancement 29.
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT)
PET and SPECT modalities both use radioactive isotope tracers as contrast agents. As these agents undergo decay they either emit a positron (PET) or a gamma ray (SPECT). The signal is unaffected by the position of the isotope within or surrounding the liposome.
Applications of imageable liposomes with hyperthermia
Imageable liposomes administered in conjunction with hyperthermia therapy can provide information about thermometry (including absolute temperatures), liposomal extravasation and accumulation in the perivascular space or deeper into the interstitial tissue, and drug distribution and concentrations (Figure 1). All of these applications will be discussed below, with a special emphasis on chemodosimetry.
Liposomal thermometry
The use of contrast-containing liposomes for magnetic resonance (MR) thermometry during thermal ablation or regional hyperthermia has been reviewed recently 30. Briefly, release of contrast agent is achieved when the liposomal phase transition temperature (Tm) has been reached; therefore, enhancement is seen as the absolute temperature rises to at least liposomal Tm. The authors review liposomal thermometry and make comparisons to intrinsic MR thermometry, discuss the sensitivity of paramagnetic thermosensitive liposomes, and detail recent studies 30. Shortly after this review was published, Salomir et al. described feasibility studies utilizing Gd-containing thermosensitive liposomes in conjunction with local hyperthermia in the rabbit kidney 31. The liposome composition in these studies was a more traditional formulation that did not contain lysolipid (Table II). These studies monitored release of contrast agent upon application of hyperthermia from an in situ kidney catheter. In four of six animals tested with these traditional thermosensitive liposomes and hyperthermia, a strong T1 signal was seen immediately following hyperthermia. No signal was seen in the other two animals, which was thought to be due to an increased length of time between liposomal delivery and hyperthermia treatment. The authors proposed that a delay in hyperthermia treatment may result in clearance of circulating liposomes from the bloodstream, either due to intratumoral extravasation or uptake by the reticuloendothelial system. Once liposomes have been cleared from the bloodstream, there would be no enhancement upon hyperthermic treatment.
Table II. Chemodosimetry with liposomally-encapsulated contrast agents.
| Therapies | Imaging modalities | Groups | Timepoints (post-injection) | Results | Reference |
|---|---|---|---|---|---|
| TSL, thermosensitive liposomes; NTSL, non-thermosensitive liposomes. | |||||
| Gd-TSL, HT | MRI | TSL + HT | 20 min | Strong T1 effect visible after liposomal heating in 4/6 animals | 31 |
| 99mTc-lip, HT | Planar scintigraphy | 1. 99mTc-lip + HT | 120, 240, 480 min | 2-13-fold increase in liposome accumulation w/ HT (p = 0.001) | 10 |
| 2. 99mTc-lip-HT | |||||
| Gd-NTSL, rhodamine-NTSL | MRI | 1. Gd-NTSL vs. rhodamine-NTSL into primate brain | ∼ 20 min (?) | Imaging of Gd-NTSL was ‘highly accurate at determining tissue distribution’ compared to histologic analysis of rhodamine-NTSL distribution | 32 |
| 2. Real-time Gd-NTSL into primate brain | |||||
| 99mTc-lip, Doxil (NTSL), HT | PET | 1. Doxil + HT | 5 h, 18 h | HT increased intratumoral 99mTc (p = 0.0006); positive correlation between [99mTc] and [doxorubicin] in both HT and unheated groups (Pearson's = 0.92) | 11 |
| 2. Doxil-HT | |||||
| 3. 99mTc-lip + HT | |||||
| 4. 99mTc-lip-HT | |||||
| 5. 99mTc-lip + Doxil + HT | |||||
| 6. 99mTc-lip + Doxil - HT | |||||
| MnSo4-Dox liposomes, HT | MRI | 1. NSTL + HT | 0, 5 min, 30 min, 90 min | 1. Rapid + stable peripheral enhancement | 34 |
| 2. NTSL - HT | 2. More uniform enhancement | ||||
| 3. TSL + HT | 3. Intermediate between 1 and 2 | ||||
| 4. TSL - HT | 4. Minimal enhancement | ||||
| MnSo4-Dox liposomes, HT | MRI | 1. NSTL + HT | 45 min | Positive correlation between HPLC and histologic fluorescence estimate of [DOX] and T1-based estimate | 35 |
| 2. NTSL - HT | |||||
| 3. TSL + HT | |||||
| 4. TSL - HT | |||||
| MnSo4-Dox liposomes, HT | MRI | 1. TSL prior to HT | During treatment, 60 days | 1. Central drug distribution | 36 |
| 2. TSL during HT | 2. Peripheral drug distribution; highest/fastest intratumoral concentration and longest growth delay | ||||
| 3. TSL prior to and during HT | 3. Uniform drug distribution | ||||
Liposomes, chemodosimetry, and drug distribution patterning
Imageable liposomes (both non-thermally sensitive and thermally sensitive) have been used as surrogates to monitor drug distribution and concentrations as well as investigational tools to further understand extravasation, accumulation, and content release of liposomes delivered with hyperthermia treatment.
PET/SPECT imaging
To examine the accumulation of liposomes administered with hyperthermia treatment in feline sarcomas, Matteucci et al. used technetium-99m (99mTc)-labeled non-thermosensitive liposomes with planar scintigraphy 10 (Tables 1, 2). Planar scintigraphy is similar to SPECT imaging in that it captures emitted gamma photons in a single plane. The authors established that hyperthermia therapy increased liposomal accumulation between 2- to 13-fold when compared with accumulation achieved at normothermic temperatures in the same animals (p = 0.001).
Kleiter et al. investigated Doxil® liposomes (doxorubicin-containing non-thermosensitive liposomes) administered concomitantly with 99mTc-labeled liposomes (acting as a tracer) in order to non-invasively estimate the effect of hyperthermia on intratumoral accumulation of doxorubicin in rat fibrosarcomas using SPECT 11 (Tables 1, 2). The authors determined that hyperthermia increased intratumoral accumulation of 99mTc liposomes at 18 h post-treatment as compared to unheated controls (p = 0.0006) validating the 99mTc liposome as a tracer (Figure 2A-B). Thermal enhancement ratios ranged from 3 to 4.1 at 5 h post-treatment and from 3.5 to 4.4 at 18 h post-treatment. The authors also found a significant positive correlation between 99mTc liposome uptake and doxorubicin concentration in tumor tissue for both the hyperthermic and unheated groups (Pearson correlation coefficient of 0.92 for both groups combined; Figure 2C). Consequently, the labeled non-thermally sensitive liposome could be used as a surrogate marker for Doxil® delivery. As a secondary effect, the authors found that co-administration of 99mTc-labeled liposomes with Doxil® did not have adverse effects on the intratumoral accumulation of doxorubicin.
Figure 2.
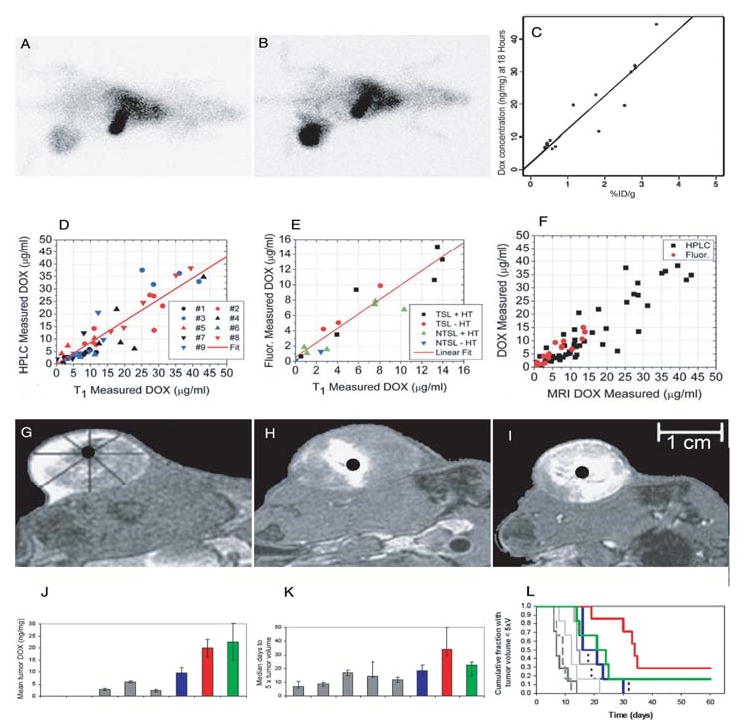
Scientigraphic images of rats treated without hyperthermia (A) or with local HT (B) 18 hours after injection of radiolabeled liposomes. Increased intratumoral accumulation of liposomes can be seen following HT. Intratumoral doxorubicin concentration 18 hours postinjection as a function of uptake of a 99mTc liposomal tracer (C). Adapted and reprinted with permission from 11. The results for HPLC validated [DOX] measurements (D), fluorescence validated [DOX] measurements (E), and an overlay of both experiments (F) from individual animals. Adapted and reprinted with permission from 35. Tumor drug distribution after administration of DOX- and Mn-containing thermosensitive liposomes and HT with 3 different schedules. Thermosensitive liposomes administered during steady-state HT result in peripheral enhancement (G); thermosensitive liposomes administered before HT result in central enhancement (H); thermosensitive liposomes administered in split doses (half before HT and half during steady-state HT) result in uniform concentrations (I). Liposome content release shows white. Adapted and reprinted with permission from 36. Tumor doxorubicin concentration and antitumor effect for therapeutic protocols described in Figures 2G-I. Overall tumor doxorubicin concentration (ng/mg) as measured by HPLC (J); rat fibrosarcoma growth time for each group (n = 6,7) measured as median days to five times the original tumor volume (K); Kaplan-Meier plot showing cumulative fraction of animals with tumor volume less than five times the treatment volume for each group over time (L). In all panels, gray = control groups (control, HT alone, free Dox, free Dox + HT, Dox/Mn-LTSL alone); blue = Dox/Mn-LTSL before HT; red = Dox/Mn-LTSL during HT; green = Dox/Mn-LTSL split dose. Adapted and reprinted with permission from 36.
MR imaging
Saito et al. compared distribution of Gd-containing non-thermosensitive liposomes imaged with MRI to histologic analysis of rhodamine-containing non-thermosensitive liposomes distribution to determine the feasibility of imaging to assess drug distributions 32 (Tables 1 and 2). These studies were done in vivo in non-human primate brains and included convection-enhanced drug delivery for the treatment of brain tumors. Convection-enhanced drug delivery involves the injection of therapeutic agent directly into the brain under continuous positive pressure in order to bypass blood-brain barrier effects and elevated interstitial fluid pressure found in tumors 33. The authors found in vivo MRI imaging with Gd-containing non-thermosensitive liposomes to be ‘highly accurate at determining tissue distribution’ when compared to histologic analysis of rhodamine-containing non-thermosensitive liposome distribution. Unfortunately, this study was hampered by lack of statistical analysis and small sample size (n = 6) and further experiments would be necessary to confirm the results.
Viglianti et al. tested both non-thermosensitive and low temperature thermosensitive liposomes containing a novel manganese sulfate (MnSO4)-doxorubicin formulation in rat fibrosarcomas using MRI 34 (Tables 1 and 2). MnSO4 was used both as the paramagnetic contrast agent (Mn2+) and as a method for loading doxorubicin into the liposome. Qualitative analysis was performed using in vivo images (Figure 3). Quantitative analysis utilized signal intensity ratios (signal of tissue of interest/signal of unheated muscle 34) calculated as an average from three experimental animals from selected tumor regions of interest normalized to muscle regions of interest. Vessel signal intensity ratios are used as a representative measure of blood pool liposomal concentration. In these experiments, tumors that were heated reached a steady state temperature prior to administration of liposomes.
Figure 3.
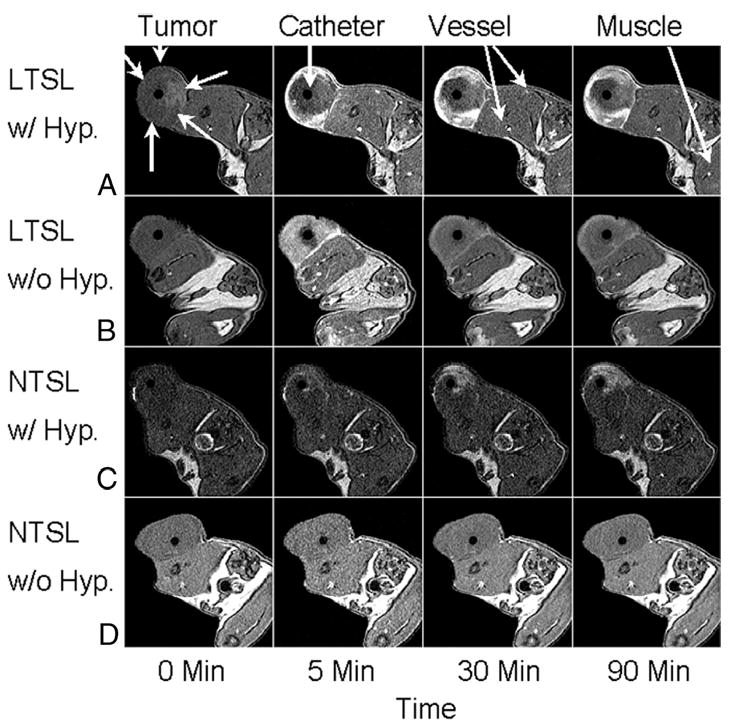
MRI axial image results of rats with transplanted flank fibrosarcomas treated with (A) low temperature thermosensitive liposomes with HT, (B) unheated low temperature thermosensitive liposomes, (C) non-thermosensitive liposomes with HT, and (D) unheated non-thermosensitive liposomes from 0-90 min. The flank tumor is indicated along with the heating catheter, venous vessels, and unheated muscle. Reprinted with permission from 34.
Low temperature thermosensitive liposomes were injected once the tumor had reached thermal steady state (LTSL w/Hyp) and tumors showed rapid, sustained peripheral enhancement as quickly as 5 min post-treatment (Figure 3A). Vessel signal intensity ratios for low temperature thermosensitive liposomes with and without hyperthermia both peaked 5 min post-treatment and then decreased, reflecting vasculature clearance of liposomes. This clearance was due to the reticuloendothelial system since these liposomes were not fully covered with PEG (a similar condition for all formulations tested). Low temperature thermosensitive liposomes with hyperthermia signal intensity ratios had ‘immediate and substantial increases’ in tumor enhancing regions and slow signal intensity ratios increase in tumor non-enhancing regions. This slow increase in tumor non-enhancing regions was due to diffusion of free Mn2+ ions from tumor enhancing to tumor non-enhancing areas post-liposomal content release.
For unheated tumors, injection of low temperature thermosensitive liposomes showed more uniform signal enhancement (tumor enhancing region) resulting in a lack of a tumor non-enhancing region. The tumor enhancing signal peaked at 5 min, but then faded as thermosensitive liposomes were cleared from the vasculature (Figure 3B). The remaining enhancement seen with unheated thermosensitive liposomes at the end of the experiment was more uniform in the tumor but less intense than the enhancement seen with thermosensitive liposomes and hyperthermia. This was indicative of extravasation and/or accumulation of the liposomes into the perivascular space without leakage of contents due to intrinsic water exchange across the lipid membrane rather than temperature-mediated content release.
The non-thermosensitive liposomes with hyperthermia images (NTSL w/Hyp) showed increasing heterogeneous enhancement throughout the experiment at a level greater than that seen with unheated thermosensitive liposomes, but less than thermosensitive liposomes with hyperthermia (Figure 3C). Vessel signal intensity ratios initially increased similarly to thermosensitive liposomes with and without hyperthermia; tumor-enhancing signal intensity ratios increased over the course of the experiment, indicating extravasation and accumulation of liposomes into the perivascular space. However, the heterogeneity of this enhancement, compared to unheated thermosensitive liposomes, resulted from hyperthermia's effect on the vascular permeability 9.
For unheated non-thermosensitive liposomes, marginal enhancement was seen in the tumor and normal tissue at 5 min post-treatment with all other time points being equivalent to background (Figure 3D). Qualitatively, the vessel signal had a similar profile to other liposome groups, peaking around 5 min and subsequently decreasing thereafter. The tumor minimally enhanced, however, quantitative analysis was not performed using the signal intensity ratio method since only one animal was imaged using these experimental parameters. Additionally, the low relaxivity of non-thermosensitive liposomes limits quantification of image enhancement without the use of hyperthermia to increase accumulation of the liposomes in the tumor 34.
Viglianti et al. continued this work with the same experimental groups (low temperature thermosensitive or non-thermosensitive liposomes ± hyperthermia) to determine whether MRI imaging can accurately describe doxorubicin concentrations in tissue using T1 shortening 35 (Table II). T1-based liposomal doxorubicin concentrations were calculated and compared to direct tissue measurements with high-performance liquid chromatography (HPLC) and independently in a second set of experiments with histologic slices using doxorubicin (DOX) fluorescence for measurement of DOX intratumoral concentration (Figure 2D-E). The relationship between both HPLC and histologic fluorescence and T1-based estimation of doxorubicin concentration had an intercept not significantly different from 0 and slope not significantly different than 1 (Figure 2F). Therefore T1-based MRI measurements could be used to estimate intratumoral DOX level non-invasively and accurately.
Ponce et al. utilized the DOX/Mn-low temperature thermosensitive liposomes and imaging methodology previously used by Viglianti et al. 35 to quantitate the variations in liposome drug distribution, caused by different hyperthermia treatment sequence, on treatment efficacy 36 (Tables 1 and 2). Again, experiments were carried out in fibrosarcoma-bearing rats. Three experimental groups were explored: (a) animals were administered low temperature thermosensitive liposomes during hyperthermia (HT) therapy, (b) 15 min prior to HT therapy, (c) or both prior to and during HT with the equivalent dose amount split 36. While administering low temperature thermosensitive liposomes during HT (a) yielded peripheral drug distributions, administration of low temperature thermosensitive liposomes prior to HT (b) yielded more central distributions (Figure 2G-H). Administration of low temperature thermosensitive liposomes both prior to and during HT (c) yielded a more uniform distribution (Figure 2I). The sequence of low temperature thermosensitive liposomes during HT resulting in peripheral drug distribution (a) resulted in maximal intratumoral accumulation and overall concentration drug (accumulation, p = 0.003; concentration, p = 0.028; Figure 2J) as well as the greatest tumor growth delay (34 days (a) versus 18.5 (b) and 22 days (c); Figure 2K,L). Although the scheduled delivery yielding a homogeneous pattern (c) had almost as much delivered drug as the central distribution (b), it failed to achieve as substantial a growth delay. This indicates that drug distribution, in addition to concentration, plays an important role in determining anti-tumor efficacy.
Discussion
The first attempt to characterize the ability of paramagnetic metal-containing liposomes to function as MRI contrast agents was almost 20 years ago 37. Today, use of drug delivery systems, such as liposomes, with inherent properties for noninvasive imaging allows for real-time monitoring of intratumoral drug concentrations and patterns of distribution. Extensive PubMed and Web of Science searches using keywords ‘liposome’, ‘temperature’, ‘hyperthermia’, ‘MRI/CT/PET/SPECT’ and ‘image/ing’ have returned few research groups that have worked in this area. Most groups used non-thermosensitive liposomes to image the reticuloendothelial system (lung, liver, and spleen) 38-41. We were the initial group to demonstrate imaging outside the reticuloendothial system using two novel tools: hyperthermia and low temperature thermosensitive liposomes.
Quantification of drug concentration with MRI during heating requires correction for temperature effects on T1. This could be obtained using non-invasive MR thermometry 42, 43. If local temperature is not known, drug dose concentrations can still be evaluated after hyperthermia treatment with the high resolution of MRI to identify undertreated areas that can be the target of subsequent treatments.
Alternatively, radionuclide tracers used with PET/SPECT systems may provide quantification of drug concentrations independent of local temperature. A linear relationship between SPECT enhancement and drug concentration has been described but needs to be validated in other models and with contrast-containing thermosensitive liposomes. The low resolution of PET/SPECT limits the clinician's ability to define undertreated areas.
To date chemodosimetry and distribution studies have only reported on one tumor line, a rat fibrosarcoma model (FSA) with peripheral feeding vessels 34-36. Different tumor types have been shown to have different vascular patterns, which may yield differing drug distributions patterns. Craciunescu et al. analyzed dynamic contrast-enhanced MR images (DCE-MRI) in patients with locally-advanced breast cancer and noted tumor vasculature to demonstrate either ring enhancement on the tumor periphery or more homogenous enhancement throughout the tumor1. Studies of the vascular patterns of rodent carcinomas also yielded highly variable findings, with two related fibrosarcomas showing both central and peripheral vascular patterns and mammary carcinomas showing vascular structure similar to the normal mammary gland 44, 45. Variability in vascular anatomy could be the basis for determining the sequence of therapy. For example, it may be advantageous to heat tumors with central feeding vessels before dosing with drug to ensure drug distribution concentrated at the tumor center.
Additionally, different contrast agents (Mn2+, Gd chelates) may co-localize with encapsulated drugs to varying extents. Ponce et al. used both a positively charged contrast agent (Mn2+) and chemotherapeutic (doxorubicin) which co-localized well because both tended to be retained in tissue 36. Chelated contrast agents, such as GdDTPA, are known to have rapid wash-out and may not co-localize well with drugs 46. Any assumptions made regarding co-localization of encapsulated drug and contrast agent must be validated against true tissue drug concentrations in order to draw accurate conclusions from imaging results 35.
Conclusion
Imageable liposomes, especially thermosensitive liposomes, have great potential to be used in combination with hyperthermia therapy for non-invasive chemodosimetry. Knowledge of these parameters during treatment will allow for individualization of treatments based on these patterns, similar to what is achieved with IMRT (intensity-modulated radiation therapy), and allow for immediate modifications during treatment resulting in optimized drug delivery. Use of thermosensitive imageable liposomes represents one of the most promising options to provide real-time optimization of intratumoral drug concentration and distribution during administration of concomitant hyperthermia and chemotherapy.
Acknowledgments
Our thanks to Drs Vikas Prabhakar and Hong Yuan for thoughtful scientific discussions and advice. Work supported by a grant from the NIH/NCI CA42745 and the Medical Student Research Scholarship of the Duke Center for Hyperbaric Medicine and Environmental Physiology.
Footnotes
Notes: 1. Craciunescu OI, Blackwell KL, Jones EL, Rosen EL, Yu D, Vujaskovic Z, Wong TZ, MacFall JR, Liotcheva V, Prosnitz LR, Samulski TV, Dewhirst MW. DCE-MRI parameters have predictive value in estimating response of locally advanced breast cancer to neoadjuvant chemotherapy and hyperthermia. Breast Cancer Res Treat. In review.
References
- 1.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Current Opin Chem Biol. 2006;10:652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Molecular Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe M, Easthope SE, Keating GM, Lamb HM. Polyethylene glycol-liposomal doxorubicin: A review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi's sarcoma. Drugs. 2002;62:2089–2126. doi: 10.2165/00003495-200262140-00012. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Rev. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Advanced Drug Delivery Rev. 2001;53:285–305. doi: 10.1016/s0169-409x(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 6.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 7.Mills JK, Needham D. The materials engineering of temperature-sensitive liposomes. Methods Enzymol. 2004;387:82–113. doi: 10.1016/S0076-6879(04)87006-X. [DOI] [PubMed] [Google Scholar]
- 8.Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia. 1999;15:345–370. doi: 10.1080/026567399285558. [DOI] [PubMed] [Google Scholar]
- 9.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- 10.Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, Needham D, Thrall DE. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6:3748–3755. [PubMed] [Google Scholar]
- 11.Kleiter MM, Yu D, Mohammadian LA, Niehaus N, Spasojevic I, Sanders L, Viglianti BL, Yarmolenko PS, Hauck M, Petry NA, Wong TZ, Dewhirst MW, Thrall DE. A tracer dose of technetium-99m-labeled liposomes can estimate the effect of hyperthermia on intratumoral doxil extravasation. Clin Cancer Res. 2006;12:6800–6807. doi: 10.1158/1078-0432.CCR-06-0839. [DOI] [PubMed] [Google Scholar]
- 12.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20:781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- 14.Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. Int J Hyperthermia. 2004;20:671–677. doi: 10.1080/02656730400007220. [DOI] [PubMed] [Google Scholar]
- 15.Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973;311:330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 16.Mouritsen OG, Zuckermann MJ. Model of interfacial melting. Phys Rev Lett. 1987;58:389–392. doi: 10.1103/PhysRevLett.58.389. [DOI] [PubMed] [Google Scholar]
- 17.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia. 2006;22(3):205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Tong S, Dewhirst MW, Yuan F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol Cancer Ther. 2004;3:1311–1317. [PubMed] [Google Scholar]
- 19.Gaber MH. Effect of bovine serum on the phase transition temperature of cholesterol-containing liposomes. J Microencapsul. 1998;15:207–214. doi: 10.3109/02652049809006850. [DOI] [PubMed] [Google Scholar]
- 20.Needham D, McIntosh TJ, Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochem. 1988;27:4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- 21.Marsh D. CRC handbook of lipid bilayers. CRC Press; Boca Raton: 1990. [Google Scholar]
- 22.Karanth H, Murthy RS. pH-sensitive liposomes-Principle and application in cancer therapy. J Pharm Pharmacol. 2007;59:469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- 23.Lokling KE, Skurtveit R, Bjornerud A, Fossheim SL. Novel pH-sensitive paramagnetic liposomes with improved MR properties. Magn Reson Med. 2004;51:688–696. doi: 10.1002/mrm.20009. [DOI] [PubMed] [Google Scholar]
- 24.Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I, Pruitt AF, Klein A, Case B, Thrall DE, Needham D, Dewhirst MW. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin Cancer Res. 2006;12:4004–4010. doi: 10.1158/1078-0432.CCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 25.Myhr G. Multimodal ultrasound mediated drug release model in local cancer therapy. Med Hypotheses. 2007;69:1325–1333. doi: 10.1016/j.mehy.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haacke EM, Brown RW, Thompson ML, Venkatesan R. Magnetic resonance imaging: Physical principles and sequence design. Wiley; New York: 1999. [Google Scholar]
- 28.Fossheim SL, Il’yasov KA, Hennig J, Bjornerud A. Thermosensitive paramagnetic liposomes for temperature control during MR imaging-guided hyperthermia: In vitro feasibility studies. Acad Radiol. 2000;7:1107–1115. doi: 10.1016/s1076-6332(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 29.Unger EC, Winokur T, MacDougall P, Rosenblum J, Clair M, Gatenby R, Tilcock C. Hepatic metastases: Liposomal Gd-DTPA-enhanced MR imaging. Radiol. 1989;171:81–85. doi: 10.1148/radiology.171.1.2928550. [DOI] [PubMed] [Google Scholar]
- 30.Lindner LH, Reinl HM, Schlemmer M, Stahl R, Peller M. Paramagnetic thermosensitive liposomes for MR-thermometry. Int J Hyperthermia. 2005;21:575–588. doi: 10.1080/02656730500158410. [DOI] [PubMed] [Google Scholar]
- 31.Salomir R, Palussiere J, Fossheim SL, Rogstad A, Wiggen UN, Grenier N, Moonen CT. Local delivery of magnetic resonance (MR) contrast agent in kidney using thermosensitive liposomes and MR imaging-guided local hyperthermia: A feasibility study in vivo. J Magn Reson Imaging. 2005;22:534–540. doi: 10.1002/jmri.20416. [DOI] [PubMed] [Google Scholar]
- 32.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Hong K, Berger MS, Park JW, Bankiewicz KS. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv. 2007;4:169–180. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 34.Viglianti BL, Abraham SA, Michelich CR, Yarmolenko PS, MacFall JR, Bally MB, Dewhirst MW. In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: Illustration of targeted delivery. Magn Reson Med. 2004;51:1153–1162. doi: 10.1002/mrm.20074. [DOI] [PubMed] [Google Scholar]
- 35.Viglianti BL, Ponce AM, Michelich CR, Yu D, Abraham SA, Sanders L, Yarmolenko PS, Schroeder T, MacFall JR, Barboriak DP, Colvin OM, Bally MB, Dewhirst MW. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn Reson Med. 2006;56:1011–1018. doi: 10.1002/mrm.21032. [DOI] [PubMed] [Google Scholar]
- 36.Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. J Nat Cancer Inst. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 37.Bacic G, Niesman MR, Bennett HF, Magin RL, Swartz HM. Modulation of water proton relaxation rates by liposomes containing paramagnetic materials. Magn Reson Med. 1988;6:445–458. doi: 10.1002/mrm.1910060410. [DOI] [PubMed] [Google Scholar]
- 38.Niesman MR, Bacic GG, Wright SM, Swartz HJ, Magin RL. Liposome encapsulated MnCl2 as a liver specific contrast agent for magnetic resonance imaging. Invest Radiol. 1990;25:545–551. doi: 10.1097/00004424-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Schwendener RA, Wuthrich R, Duewell S, Wehrli E, von Schulthess GK. A pharmacokinetic and MRI study of unilamellar gadolinium-, manganese-, and iron-DTPA-stearate liposomes as organ-specific contrast agents. Invest Radiol. 1990;25:922–932. doi: 10.1097/00004424-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Suga K, Mikawa M, Ogasawara N, Okazaki H, Matsunaga N. Potential of Gd-DTPA-mannan liposome particles as a pulmonary perfusion MRI contrast agent: An initial animal study. Invest Radiol. 2001;36:136–145. doi: 10.1097/00004424-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Unger E, Fritz T, Shen DK, Wu G. Manganese-based liposomes. Comparative approaches. Invest Radiol. 1993;28:933–938. doi: 10.1097/00004424-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Gellermann J, Hildebrandt B, Issels R, Ganter H, Wlodarczyk W, Budach V, Felix R, Tunn PU, Reichardt P, Wust P. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer. 2006;107:1373–1382. doi: 10.1002/cncr.22114. [DOI] [PubMed] [Google Scholar]
- 43.Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia. 2006;22:255–262. doi: 10.1080/02656730600661149. [DOI] [PubMed] [Google Scholar]
- 44.Falk P. Patterns of vasculature in two pairs of related fibrosarcomas in the rat and their relation to tumour responses to single large doses of radiation. Eur J Cancer. 1978;14:237–250. doi: 10.1016/0014-2964(78)90187-1. [DOI] [PubMed] [Google Scholar]
- 45.Falk P. The vascular pattern of the spontaneous C3H mouse mammary carcinoma and its significance in radiation response and in hyperthermia. Eur J Cancer. 1980;16:203–217. doi: 10.1016/0014-2964(80)90152-8. [DOI] [PubMed] [Google Scholar]
- 46.Mussurakis S, Buckley DL, Bowsley SJ, Carleton PJ, Fox JN, Turnbull LW, Horsman A. Dynamic contrast-enhanced magnetic resonance imaging of the breast combined with pharmacokinetic analysis of gadolinium-DTPA uptake in the diagnosis of local recurrence of early stage breast carcinoma. Invest Radiol. 1995;30:650–662. doi: 10.1097/00004424-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Mills JK, Needham D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim Biophys Acta. 2005;1716:77–96. doi: 10.1016/j.bbamem.2005.08.007. [DOI] [PubMed] [Google Scholar]


