Abstract
Background
Adiponectin, an adipocyte-specific secretory protein, is known to circulate as different isoforms in the blood stream.
Methods
Using sucrose gradients and Western blotting on nondenaturing gels, adiponectin isoforms were examined in human serum, plasma, adipose tissue, and cells. The medium from human adipose tissue and human and mouse adipocytes were also examined for changes in isoform formation upon treatment with EGTA.
Results
Comparison of adiponectin complexes revealed distinct differences in distribution of high molecular weight (HMW) forms between human serum and plasma, with an apparent difference in molecular weight. Variation in molecular weight suggested a probable dissociation of the HMW isoforms in the presence of EDTA in the plasma. Examination of human serum samples treated with EDTA or EGTA showed a partial dissociation of the HMW isoform, while the addition of excess calcium, but not magnesium, to human plasma resulted in partial restoration of HMW adiponectin. When human adipose tissue–secreted adiponectin was treated with EGTA, there was a decrease in the HMW isoform by 61% (± 1.89%) and a corresponding increase in low molecular weight (LMW) and middle molecular weight (MMW) isoforms, compared to untreated samples. Analysis of mouse and human adipocytes also showed a reduction in HMW isoforms with a corresponding increase in MMW and LMW isoforms upon treatment with EGTA. The Simpson-Golabi-Behmel syndrome (SGBS) human adipocyte cell line, which primarily synthesizes LMW isoforms, produced increasing amounts of HMW adiponectin upon treatment with calcium in a dose-dependent manner.
Conclusion
These data indicate that calcium promotes the formation of HMW adiponectin, and calcium sequestration decreases HMW adiponectin. Because of the importance of HMW adiponectin in insulin sensitivity, these data demonstrate the importance of assay conditions and sample preparation in the measurement of adiponectin isoforms.
Introduction
Obesity, insulin resistance, and type 2 diabetes are increasingly prevalent worldwide1 and adipocyte secretory proteins (adipokines), are associated with the development of insulin resistance. Adiponectin is a major adipokine,2 and low levels of adiponectin are linked to diabetes, insulin resistance, coronary heart disease, and metabolic syndrome.3,4 A decrease in adiponectin expression is also associated with murine models of altered insulin sensitivity; administration of physiological relevant doses of adiponectin partially ameliorates insulin resistance.5
Adiponectin is initially synthesized as a 30 kDa monomer that is then assembled into more complex isoforms that are secreted and circulate in the blood. These isoforms have been identified as low molecular weight (LMW), middle molecular weight (MWM) and high molecular weight (HMW).6,7 The biological activity of adiponectin and the association with insulin sensitivity is largely coupled with formation of the higher order structure, and post- translational modifications such as glycosylation and hydroxylation have been shown to be critical for function.6,8 The higher order oligomeric complexes of adiponectin also require the formation of a disulfide bond at Cys-399 and in a recent study by Scherer and coworkers10 it is found that this cysteine residue is responsible for covalent bond formation with the ER chaperone ERp44 that plays a major role in the assembly of higher order forms of adiponectin. The HMW isoform varies among different species and Waki and coworkers7 speculated that the difference is due to low homology in the variable amino terminal region of adiponectin which contains the cysteine residue critical for complex formation. Mutations within human adiponectin collagen- like domain, G84R and G90S, have been shown to cause impairment of HMW formation which may contribute to the development of hypoadiponectinemia and diabetes.7 Treatment of patients with the PPARγ agonist thiazolidinedione (TZD) increased total adiponectin levels by 2-3 fold, primarily due to an increase in the HMW isoform, which correlated with increased insulin sensitivity.6,11,12
Calcium ions play a central role in regulation of various physiological processes, including muscle contraction, cell adhesion, cell division and growth, ion transport, protein folding, protein degradation, gene transcription, apoptosis, exocytosis and many others.13 In addition, a number of proteins, such as members of the S100 family of calcium binding proteins, are known to form higher order oligomers in a calcium dependent manner.14 MRP8 and MRP14 are members of the S100 family of calcium binding proteins, and post- translational modification of MRP8 and MRP14 results in (MRP/MRP14)2 tetramers with calcium binding increasing upon multimerization.15
In this study, we examined the adiponectin isoforms present in human serum and EDTA- plasma. EDTA is specifically known to chelate divalent cations such as magnesium, manganese, Zinc and calcium which act as cofactors for different enzymes. Based on the differences observed between serum and plasma adiponectin we compared adiponectin isoforms secreted from adipose tissue and adipocytes following calcium chelation or addition. In all cases, calcium chelators reduced the level of HMW isoforms, while the addition of calcium increased formation of HMW adiponectin.
Materials and Methods
Subject recruitment
To obtain adipose tissue for this study, subcutaneous fat biopsies were performed on 4 nondiabetic subjects (3 females and 1 male) who were recruited by local advertisement. All subjects provided written, informed consent under a protocol that was approved by the institutional Review Board of University of Arkansas for Medical Sciences (UAMS) and was conducted at the UAMS General Clinical Research Center. Subjects were generally healthy without any history of liver or kidney dysfunction. A history of cardiovascular disease and the use of aspirin or antiinflammatory medications were a contraindication for the study.
Treatment of serum and plasma samples
Subjects reported fasting to the General Clinical Research Center, and blood was drawn into standard red top vaccutainer tubes yielding either serum or purple top vaccutainer tubes containing 4.2 mM EDTA yielding plasma. To explore the differences in serum and plasma adiponectin isoforms, the serum samples were treated with 5 mM EDTA or EGTA (Sigma-Aldrich, St. Louis, MO) for 30 to 40 minutes in a 37°C water bath. Conversely, excess amounts (20 mM) of CaCl2 (Fisher Scientific, Pittsburgh, PA) and MgCl2 (20 mM) were added to plasma samples prior to analysis by polyacrylamide gel electrophoresis in 4% to 20% Criterion Precast Gels under nondenaturing and nonreducing conditions.
Adipose tissue adiponectin secretion and EGTA treatment
Adipose tissue (150 mg) collected from biopsy, was minced and incubated with 1 mL of serum-free DMEM with 20 mM HEPES (pH 7.4) at 37°C under sterile tissue culture conditions. Following a 24-hour incubation the sample was briefly spun in a microfuge to separate tissue and medium, and the tissue was homogenized in radioimmuno precipitation assay (RIP A) lysis buffer containing 1mM phenylmethylsulfonyl fluoride (PMSF). EGTA (5mM) was added to the medium as well as the tissue lysate and incubated for 30 to 40 minutes in a 37°C water bath. The EGTA-treated and untreated samples were then layered on top of a 5% to 20% sucrose gradient and centrifuged as described in “Sucrose gradient sedimentation analysis.”
Adipocyte isolation and EGTA treatment
To study adiponectin secretion from adipocytes in vitro, cells from either human or mouse adipose tissue were isolated as described previously.16 Equal volumes of adipocytes were incubated in 500 μL Dulbecco's modified Eagle's medium (DMEM) with 20 mM HEPES (pH 7.4,) at 37°C under sterile tissue culture conditions. After 24 hours the cells were spun at 800 rpm for 2 minutes and the medium was separated. The cells were then homogenized with RIPA lysis buffer containing 1mM PMSF. EGTA (5 mM) was added to the cell lysate and to the medium for 30 to 40 minutes in a 37°C water bath before layering over a sucrose gradient.
Cell culture
Human preadipocytes, originally derived from the stromal fraction of subcutaneous adipose tissue of an infant with Simpson-Golabi-Behmel syndrome (SGBS), were cultured as described previously.17,18 Briefly, SGBS cells were maintained in DMEM:F12 (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin. For experimental purposes, cells were plated and allowed to reach confluence before adding differentiation medium (DMEM:F12 with dexamethasone 25 nM (Sigma-Aldrich); IBMX 500 μM (Sigma-Aldrich); rosiglitazone 2 μM; human transferrin 0.01 mg/mL (Sigma-Aldrich); insulin 2 × 10−8 M (Novo Nordisk, Princeton, NJ); cortisol 10−7 M (Sigma-Aldrich); T3 0.2 nM (Sigma-Aldrich); biotin 33 mM (Sigma-Aldrich) and pantothenate 17 mM (Sigma-Aldrich)) for 4 days. Cell medium was then changed to an adipogenic medium (DMEM:F12 with human transferrin 0.01 mg/mL; insulin 2 × 10−8 M; cortisol 10−7 M; T3 0.2 nM; biotin 33 mM and pantothenate 17 mM) for 2 days or until the cells were ready for treatment. DMEM:F12 contains CaCl2 at a concentration of 1.05 mM. For experimental purposes, CaCl2 (2 mM, 5 mM, and 10 mM) was added to the cells in DMEM:F12 medium on day 6 of differentiation and incubated overnight before collection.
Sucrose gradient sedimentation analysis
To study adiponectin isoforms, plasma, serum and cell lysate or secreted protein fractions were separated using a sucrose gradient, followed by Western blotting with antiadiponectin antibody (R&D Systems, Minneapolis, MN) after nondenaturing and non-reducing gel electrophoresis. Sucrose gradients (5%–20%) were formed in 5 mL centrifuge tubes as described previously (Beckman, Palo Alto, CA).12 To reduce the amount of IgG in the sample, serum and plasma samples were precleared with Protein G and Protein A (Roche, Indianapolis, IN) prior to analyses. Each mL of serum or plasma samples were precleared with 50 μL each of Protein A and Protein G and placed on a rotator for an hour. Precleared serum or plasma samples (100 μL) and culture medium or lysate (1 mL) were then layered on top of the gradient and spun at 50,000 rpm for 6 hours at 4°C in an SW50 rotor in a Beckman L8-80 ultracentrifuge. Equal fractions of 250 μL were collected successively from the top of the gradients. Each of the sucrose gradient fractions was then again treated with Protein G for an hour to remove any remaining IgG.
Western blot and densitometry
Samples were loaded and run on 4% to 20% Criterion Precast Gels (Bio-Rad, Hercules, CA) and transferred onto Trans-Blot Transfer Medium (Bio-Rad). One lane on each gel contained molecular weight markers (Benchmark prestained protein ladder, Invitrogen, or Himark Prestained HMW Protein Standard) varying between 181 and 5.7 kDa and 500 and 121 kDa on Tris glycine gels, respectively. Anti-human or anti-mouse adiponectin (R & D System) and donkey anti-goat IgG horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA) were used for Western Blot detection. Adiponectin isoforms detected in Figures 1, 3, 4 and 5 were quantified with ImageQuant TL (Amersham Biosciences, Piscataway, NJ) software and expressed as a percentage of total control adiponectin levels. A secondary antibody reaction with donkey anti-goat IgG HRP was done separately on each of the blots to see the presence of any nonspecific binding (data not shown).
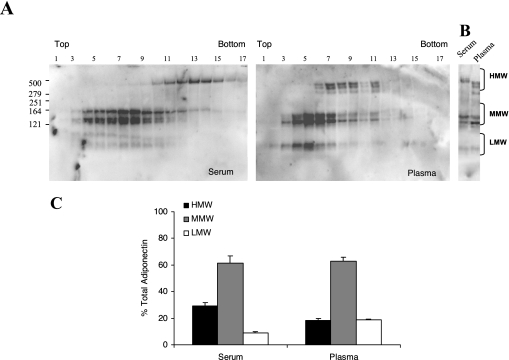
FIG. 1.
Human serum and plasma differ in distribution of HMW adiponectin in a sucrose density gradient. (A) Representative gradient gel of human serum and plasma from the same subject. The different adiponectin isoforms were identified by a 5% to 20% sucrose density gradient centrifugation and analyzed by nonreducing and nondenaturing gel SDS-PAGE followed by Western blotting. The migration of the HMW (above 279 kDa), MMW (between 164 and 121 kDa) and LMW (below 121 kDa) isoforms of adiponectin are indicated, along with the top and bottom of the sucrose gradient. (B) Sucrose gradient fraction (# 11) of serum and plasma run adjacent to each other in two consecutive lanes. (C) Densitometric analysis of the different complexes of adiponectin in serum and plasma expressed as percentage of total adiponectin. Results are the mean ± SEM of 4 independent experiments.
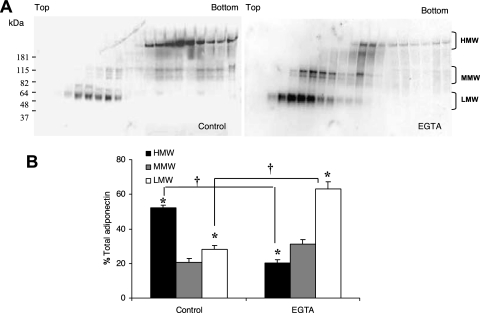
FIG. 3.
Decreased HMW adiponectin secretion with EGTA treatment in vitro. (A). Conditioned medium from control and EGTA (5 mM)-treated human adipose tissue was separated in a 5% to 20% sucrose density gradient and adiponectin isoforms were identified by Western blotting under nondenaturing and nonreducing conditions. The top and bottom of the sucrose gradient is noted along with identification of the adiponectin bands referred to as HMW (above 181 kDa), MMW (between 181 and 115 kDa) and LMW (below 82 kDa). (B) Densitometric analysis of the different complexes of adiponectin expressed as a percentage of total adiponectin with no EGTA added. Results are the mean ± SEM of 2 independent experiments. Significant differences are indicated by *, compared to control, and + for comparisons between before and after EGTA treatments (P < 0.05).
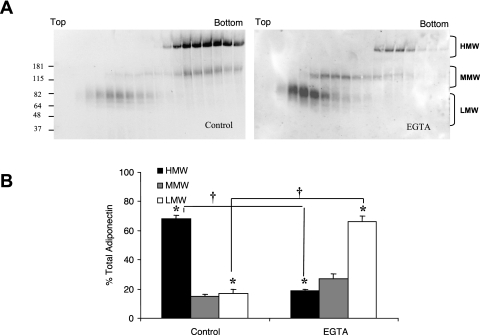
FIG. 4.
Effect of EGTA treatment on adiponectin isoforms in mouse adipocytes. (A). Adiponectin isoforms from mouse adipocytes were separated by 5% to 20% sucrose density gradient centrifugation and analyzed by nondenaturing and nonreducing gradient gel SDS-PAGE followed by Western blotting. The top and bottom of the sucrose gradient is noted, along with identification of the adiponectin bands referred to as HMW (above 181 kDa), MMW (between 181 and 115 kDa) and LMW (below 82 kDa). (B). Quantitative analysis by densitometry of the HMW, MMW, and LMW isoforms expressed as a percentage of total adiponectin with no EGTA added. Results are the mean ± SEM of 2 independent experiments. Significant differences are indicated by *, compared to control, and + for comparisons between before and after EGTA treatments (P < 0.05).
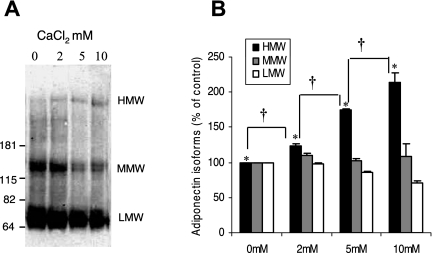
FIG. 5.
Effect of calcium treatment on adiponectin HMW isoform in SGBS cells. (A) Representative blot of SGBS cell lysate from day 6 differentiated cells. As described in Methods, cells were cultured in medium containing 1.05 mM CaCl2, and additional CaCl2, in the concentration indicated, was then added to the medium for 16 hours and analyzed by nondenaturing and nonreducing gel SDS-PAGE and Western blotting. (B) Densitometric analysis of the HMW (above 181 kDa), MMW (181 to 115k Da), and LMW (82 to 62k Da) adiponectin for each treatment of calcium expressed as percentage of control HMW, MMW, and LMW. Results are the means ± SEM of 3 independent experiments. Significant differences are indicated by *, compared to control, and + for comparisons between different concentration of CaCl2 added (P < 0.05).
Statistics
All data were expressed as the means (±SEM), and the Student t test was used for statistical analysis, with the level of statistical significance set at P less than 0.05.
Results
Human serum and plasma differ in distribution of HMW adiponectin
To identify subtle differences in isoforms, serum and plasma from the same subjects were examined using sucrose density gradient separation followed by SDS-PAGE and Western blotting. As shown in Figure 1A, serum and plasma display a difference in HMW distribution when separated on a sucrose density gradient. The HMW isoforms of adiponectin in serum sedimented mainly in the bottom (denser) fractions of the gradient (fractions 11–16 while in plasma, the HMW complexes demonstrated a shift to the middle portion of the gradient (fractions 6–11). In addition, the migration of the adiponectin isoform on the gels was different. HMW adiponectin from plasma migrated as a doublet or triplet. To better compare the migration on the gel, a single fraction from the sucrose gradient (fraction #11) from both serum and plasma was run adjacent to each other. As shown in Figure 1B, the HMW form of adiponectin in serum migrated as a single band of about 485 kDa, consistent with the previous demonstration of HMW adiponectin as an 18-mer.19 From plasma, however, HMW adiponectin was represented by 2-3 bands, all of which migrated faster, consistent with the progressive loss of 30 kDa subunits.
From the sucrose gradients, we quantitated the adiponectin isoforms using densitometry, dividing the fractions into HMW, MMW, and LMW. As shown in Figure 1C, quantitation of the different adiponectin isoforms in serum showed 30% ± 1.06 HMW, 60% ± 1.29 MMW, and 10% ± 1.56 of isoforms were in the LMW range. From EDTA- plasma, the percentage from the HMW fraction was slightly less (18% ± 1.67 of total adiponectin), and the fraction from LMW was slightly higher 20% ± 1.11, however these changes were not statistically significant.
Because of these differences in adiponectin isoforms between serum and plasma, we examined the effects of EDTA and calcium sequestration on adiponectin isoform formation.
Calcium but not magnesium is involved in HMW formation of adiponectin
To distinguish the roles of calcium and magnesium in HMW isoform formation, we examined the effects of two metal ion chelators EDTA and EGTA, as well as CaCl2 or MgCl2, on human serum and plasma. Serum samples were treated with either EDTA or EGTA, the latter of which specifically chelates only calcium ions. In serum, the major band is the HMW band at about 485 kDa, and a minor band at about 460 kDA is often seen. Compared to untreated control serum, both chelators resulted in a loss of the 485 kDa band, and an increase in the appearance of faster- migrating bands (Figure 2A lanes 2 and 3) suggesting that calcium, and not magnesium, is required for formation of HMW structures of adiponectin.
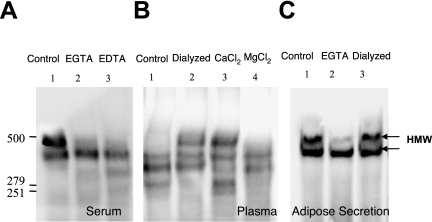
FIG. 2.
Formation of HMW adiponectin in human serum, plasma and adipose tissue secretions in presence and absence of calcium. (A) Representative blot of human serum treated with 5 mM EGTA (Lane 2) or 5 mM EDTA (Lane 3) compared to untreated (Lane 1) when analyzed by nonreducing and nondentauring gel SDS-PAGE and Western blotting. (B) Representative blot of human plasma untreated (lane 1) followed by dialysis of plasma in DMEM medium, which contains CaCl2 at 1.8 mM (lane 2), addition of excess of CaCl2 (20 mM) or MgCl2 (20 mM) to plasma are shown in lane 3 and lane 4, analyzed by nonreducing and nondentauring gel SDS-PAGE and Western blotting. (C) Adipose tissue secretion of adiponectin in DMEM medium (lane 1). EGTA treatment (5 mM) of adipose tissue secretion is shown in lane 2, followed by EGTA- treated adipose tissue secretion dialyzed in DMEM medium in lane 3. The HMW isoforms (above 279 kDa) are indicated with the arrows (←). The blots are representative of 4 independent experiments.
To further assess the specific roles of calcium or magnesium in adiponectin oligomer stability, CaCl2 and MgCl2 were added in molar excess to EDTA in the plasma samples. Addition of CaCl2 (20 mM, Figure 2B lane 3) led to the formation of HMW isoforms of adiponectin, compared with control (Figure 2B, lane 1), whereas, addition of MgCl2 (20 mM, Figure 2B, lane 4) did not increase the HMW isoform. Dialysis of the plasma in DMEM tissue culture medium, which contains calcium at a concentration of 1.8 mM, also supported the formation of HMW form of adiponectin (Figure 2B, lane 2). Human adipose tissue explants secrete predominantly HMW adiponectin into the medium (Figure 2C), and two bands are usually noted when this material is examined on a non-denaturing gel. The addition of EGTA (5 mM, Figure 2C, lane 2) to culture medium following secretion from human adipose tissue explants disrupted the 485 kDa HMW isoform into a slightly faster migrating isoform, and subsequent dialysis of EGTA-treated medium against normal culture medium was found to restore the level of HMW isoform to that of untreated controls (lane 3 of Figure 2C).
Adiponectin secreted from human adipose tissue explants is also sensitive to EGTA
To further characterize the effect of EGTA on HMW adiponectin isoforms, conditioned media resulting from incubation of human adipose tissue explants were analyzed by sucrose gradient sedimentation, polyacrylamide gel electrophoresis, and Western blot. In a manner similar to that described above, the adiponectin in adipose tissue conditioned media was quite sensitive to calcium sequestration. When adipose tissue conditioned medium was treated with EGTA, there was a substantial shift in isoforms, with a 61% ± 1.89 reduction in the HMW isoform of adiponectin compared to controls (P < 0.05; Figures 3A and 3B), with a corresponding increase in MMW and LMW isoforms. Table 1 shows the percentages of the different isoforms of adiponectin before and after EGTA treatment in the human adipose tissue conditioned medium.
Table 1.
Summary of the Percentages of Different Isoforms of Adiponectin in Presence and Absence of EGTA Treatment with Respect to Total Adiponectin
| Treatment | HMW (%) | MMW (%) | LMW (%) | |
|---|---|---|---|---|
| Human Adipose tissue | Control | 52.3 ± 2.05 | 20.6 ± 1.75 | 28.3 ± 3.03 |
| EGTA | 20.2 ± 0.935 | 31.1 ± 3.12 | 63.1 ± 4.08 | |
| Mouse adipocytes | Control | 68.28 ± 2.05 | 14.81 ± 1.11 | 16.9 ± 3.55 |
| EGTA | 18.64 ± 0.935 | 27.26 ± 2.56 | 65.95 ± 4.67 |
HMW isoform of adiponectin is sensitive to treatment with EGTA in mouse adipocytes
As described by us previously, different species and different cells secrete different proportions of HMW adiponectin.12 To determine whether the requirement for calcium in HMW adiponectin formation is species dependent, we examined adiponectin production in mouse primary adipocyte cultures. Adipocytes were prepared as described in Materials and Methods and incubated for 24 hours, followed by the removal of conditioned medium. Treatment of the medium with EGTA (5 mM) led to a substantial decrease in HMW isomers in the mouse adipocyte conditioned medium (Figure 4A)with a corresponding increase in MMW and LMW isomers compared with controls when analyzed in a sucrose gradient followed by SDS-PAGE and Western blotting.
Densitometric analysis of the different adiponectin isoforms before and after EGTA treatment showed a 73% ± 1.45 decrease (P < 0.05) in the HMW isoforms with a corresponding increase in medium and low molecular weight isoforms. (Figure 4B, Table 1). These results demonstrate that calcium plays a role in stabilizing the HMW isoform of adiponectin in both humans and mice.
Formation of HMW adiponectin in human adipocyte cell cultures is calcium dependent
A useful human adipocyte cell line is the SBGS cell line, which produces primarily LMW isoforms, with little HMW adiponectin detected in cell lysate.12 To determine the effect of calcium on adiponectin isoform formation in this cell line that does not normally form much HMW isoform, we added exogenous CaCl2 (2 mM, 5 mM, and 10 mM) to SGBS cultures for approximately 16 hours. As described in Materials and Methods, cells cultured in medium containing 1.05 mM calcium. The addition of supplemental calcium led to increased formation of HMW adiponectin in a dose-dependent manner while a corresponding decrease in the MMW and LMW bands (Figures 5A and B). These results demonstrate that calcium enhances the formation of HMW adiponectin in a cell type that normally produces very little.
Discussion
Adiponectin is expressed at a high level in adipose tissue and is an important adipokine associated with insulin sensitivity and protection from vascular disease.3 The synthesis and assembly of adiponectin is complex. Following synthesis of the adiponectin monomer, the protein is assembled into trimers, hexamers, and 18-mer HMW isoforms,19–21 In several studies, the HMW isoform of adiponectin was the pre-dominant secreted form in human sera and correlated best with protection from features of metabolic syndrome and correlated strongly with improved insulin sensitivity after treatment of subjects with thiazolidinediones.6,20
The present study was undertaken following the observation of differences in HMW adiponectin complexes on a sucrose gradient when comparing serum and EDTA-plasma. When serum and plasma were fractionated on a sucrose gradient, the HMW isoforms of adiponectin in serum were found in the denser regions of the gradient and migrated slower on the nondenaturing gel than the isoforms from plasma. When the HMW, MMW, and LMW bands from serum and plasma were grouped and quantitated, there were no significant differences between serum and plasma, even though the adiponectin isoforms from plasma were slightly different.
Adiponectin is initially synthesized as a 30 kDa peptide that is rapidly assembled into trimers, hexamers, and HMW octadecamers.7,19 However, other studies have noted that the HMW, MMW, and LMW forms on nondenaturing gels are often composed of multiple closely migrating bands.7 In our studies, the HMW adiponectin from serum was present predominantly as a band at approximately 485 kDa, which would correspond to an 18-mer, given the limitations of molecular weight determination on our gel system. In plasma, however, the HMW complexes were noted to have several faster migrating bands. Although this method is not precise in the determination of molecular weight, the HMW isoforms from plasma appeared to differ from each other by one, two, or more adiponectin subunits, and would suggest that HMW adiponectin in plasma exist as 17-mers, 16-mers, and other forms.
Because this change in adiponectin isoforms was due to the collection of blood in EDTA, we wondered whether this was due to the chelation of divalent cations. EDTA and EGTA are known to chelate metal ions in 1:1 metal-to- EDTA/EGTA ratio, although EGTA preferentially binds calcium with a significantly greater affinity than other divalent cations. As observed in Figure 2A, the addition of EDTA or EGTA to serum samples was found to cause a reduction in size of the HMW adiponectin by approximately one oligomer, resulting in an adiponectin oligomeric pattern that resembled EDTA plasma. Conversely, the addition of calcium, but not magnesium, to EDTA-plasma samples resulted in a formation of HMW isoforms that were not normally present. Dialysis of plasma samples in the presence of DMEM medium containing calcium at 1.8 mM concentration was also found to reverse the effect of EDTA. Thus, this study clearly demonstrates that the formation of the fully developed, complex HMW structure of adiponectin is influenced by the presence of calcium.
In previous studies, the predominant adiponectin secretory product from human adipose tissue was the HMW form.12 When EGTA was added to medium conditioned from human adipose tissue, again there was a change in the HMW isoforms, as shown in Figure 2C. This was more apparent when the adiponectin isoforms from the conditioned medium of human and mouse adipocytes were separated on a sucrose gradient where more of MMW or LMW isoforms was found to be aggregated at the middle and top of the gradient in the EGTA treated samples (Figures 3A and 4A). Thus we provide here evidence for the requirement of calcium in HMW complex formation of adiponectin.12
The carboxyl domain of adiponectin is homologous to the C1q complement-related proteins which are characterized by a globular head at the C-terminus. C1q plays a major role in immunity and is able to bind a variety of components due to its globular domain (gC1q) 22. The crystal structure of adiponectin revealed a link between tumor necrosis factor (TNF), which is also implicated in the induction of insulin resistance, and gC1q proteins. Adiponectin belongs to this newly designated C1q and TNF superfamily.23,24 Scherer and coworkers have submitted a structure to the protein data bank that indicates the C-terminal region of mouse adiponectin binds calcium and forms the adiponectin 30k Da complex (Protein Data Bank codes 1C28 and 1C3H; gi:40889029). In addition, Ca2+-bound and Ca2+-free forms of mouse adiponectin gC1q domain have been determined showing structural differences.23,24
Calcium-dependent oligomerization of HMW isoforms of adiponectin suggests that a binding site for calcium exists in the adiponectin protein sequence. Different calcium binding motifs are known,25,26 the most common being the helix-loop-helix structural motif27–29 in their calcium binding sites flanked by the two alpha helices termed the EF hand. The calcium-coordinating residues of the loop are commonly aspartates forming a DxDxD motif.30 EF hand motifs direct calcium binding by cellular proteins occurring either upstream of predicted alpha helices and are associated with regulating large conformational changes in the helical domain function in a cooperative manner.30–33 Although there is significant sequence variation in the calcium binding region of different calcium binding proteins, a preference for aspartate at the first, third and fifth position is always evident. Such a sequence (DNDND), beginning amino acid 227 is present in the C-terminal region of adiponectin (Accession # NM 004797; GI: 44890057) that might mediate the role played by calcium in adiponectin oligomerization. Leukert and coworkers14 have shown that mutations in this C terminal EF hand loop of S100A9 decreased calcium binding and tetramerization of dimers in presence of calcium failed to occur. Further Sen and coworkers33 in a recent study in rotavirus NSP5 have shown that the DXDXD motif in the C terminal helix serves as a calcium switch and mutagenesis of any DXDXD motif abolishes the ability to bind calcium and regulate cellular function. As a consequence deletion of the DNDND sequence present on the C-terminal part of the adiponectin gene is required to further elucidate the role of calcium in HMW adiponectin formation.
The effect of Ca2+ and Mg2+ on the interaction of adiponectin with lipopolysaccharide (LPS) was investigated by Peake and coworkers, who demonstrated that the antiinflammatory properties of adiponectin may be mediated through direct binding to LPS 34. Both EGTA and EDTA increased the binding of adiponectin to LPS at pH 5.0 to 6.0, whereas the addition of Ca2+ or Mg2+ inhibited binding. This suggests that quaternary structure is affected by these cations and is an important factor for selective binding and function. However, the authors propose that HMW adiponectin is the predominant LPS-binding isoform, and that reduction and alkylation, resulting in trimer, abolishes such binding. These results appear to be contradictory to our own findings as we report here that Ca2+ increases HMW adiponectin formation and EDTA/EGTA diminishes it. The differences in findings may be explained by variations in experimental procedures, such as pH and the use of recombi- nant adiponectin.
A similar finding of calcium-induced aggregation has been noticed in the surfactant protein A present in the alveolar fluid and is very similar to adiponectin in that it possesses a collagenous domain and a globular domain.14,35 In plants, GAD (glutamate decarboxylase) has been shown to form higher order oligomerization with transient elevations in cytosolic calcium concentration.36
Calcium is known to play an important role in the differentiation of adipocytes in a biphasic manner. Increasing intracellular calcium inhibits early stage differentiation, while late stage differentiation and lipid accumulation are increased.37 Previously we demonstrated that pioglitazone, a thiazolidinedione (TZD) known to improve insulin sensitivity in humans, increased the secretion of HMW adiponectin from human adipose tissue and adipocytes.12 TZDs are also known to affect calcium channel function resulting in lower blood pressure in hypertensive rats.38,39 Whether pioglitazone modulates intracellular calcium in adipocytes which influences formation of HMW adiponectin remains to be determined. It is known that insulin stimulates adiponectin secretion at both early and late phases in its secretory pathway, possibly corresponding to adiponectin export from the endoplasmic reticulum and to exocytosis of adiponectin secretory vesicles.40 Further studies are required to gain a better understanding of the role of endoplasmic reticulum calcium levels in adiponectin maturation and folding, and its effect on adiponectin secretion in the exocytic pathway.
In summary, this study examined the adiponectin isoforms in serum and plasma, and in the medium from adipocytes, and determined that the formation of the fully developed HMW complex is dependent on the availability of calcium. HMW adiponectin is the isoform most strongly associated with insulin sensitivity, and the stabilization of this complex by calcium may be important in adiponectin biologic activity. This study demonstrates for the first time that analysis of adiponectin in blood can be affected by the specific specimen collection method, and these data suggest that serum, as opposed to EGTA plasma, is a superior specimen type for adiponectin analysis because of the absence of metal chelating agents that can modify the oligomeric structure of adiponectin.
Acknowledgments
This work was supported by a grant from the American Diabetes Association (R.J.O.), a Merit Review Grant (P.A.K.), and a VISN Career Development Award (N.R.) from the Veterans Administration; Grant number M01RR14288 of the General Clinical Research Center; and DK 39176 and DK 71277 (P.A.K.) from the National Institutes of Health.
References
- 1.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 2.Trujillo ME. Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg AH. Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 4.Kern PA. Di Gregorio GB. Lu T. Rassouli N. Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T. Kamon J. Waki H. Terauchi Y. Kubota N. Hara K. Mori Y. Ide T. Murakami K. Tsuboyama-Kasaoka N. Ezaki O. Akanuma Y. Gavrilova O. Vinson C. Reitman ML. Kagechika H. Shudo K. Yoda M. Nakano Y. Tobe K. Nagai R. Kimura S. Tomita M. Froguel P. Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 6.Pajvani UB. Hawkins M. Combs TP. Rajala MW. Doebber T. Berger JP. Wagner JA. Wu M. Knopps A. Xiang AH. Utzschneider KM. Kahn SE. Olefsky JM. Buchanan TA. Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 7.Waki H. Yamauchi T. Kamon J. Ito Y. Uchida S. Kita S. Hara K. Hada Y. Vasseur F. Froguel P. Kimura S. Nagai R. Kadowaki T. Impaired Multimerization of Human Adiponectin Mutants Associated with Diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y. Xu LY. Lam KS. Lu G. Cooper GJ. Xu A. Proteomic characterization of human serum proteins associated with the fat-derived hormone adiponectin. Proteomics. 2006;6:3862–3870. doi: 10.1002/pmic.200500840. [DOI] [PubMed] [Google Scholar]
- 9.Pajvani UB. Du X. Combs TP. Berg AH. Rajala MW. Schulthess T. Engel J. Brownlee M. Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZV. Schraw TD. Kim JY. Khan T. Rajala MW. Follenzi A. Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki Y. Mahankali A. Wajcberg E. Bajaj M. Mandarino LJ. DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–4319. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 12.Bodles A. Banga A. Rasouli N. Ono F. Kern PA. Owens RJ. Pioglitazone increases secretion of high molecular weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291:E1100–E1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ. Bootman MD. Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 14.Leukert N. Vogl T. Strupat K. Reichelt R. Sorg C. Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Vogl T. Roth J. Sorg C. Hillenkamp F. Strupat K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 1999;10:1124–1130. doi: 10.1016/s1044-0305(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 16.Kern PA. Marshall S. Eckel RH. Regulation of lipoprotein lipase in primary cultures of isolated human adipocytes. J Clin Invest. 1985;75:199–208. doi: 10.1172/JCI111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wabitsch M. Brenner RE. Melzner I. Braun M. Moller P. Heinze E. Debatin KM. Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 18.Varma V. Yao-Borengasser A. Rasouli N. Bodles AM. Phanavanh B. Lee MJ. Starks T. Kern LM. Spencer HJ., III McGehee RE., Jr. Fried SK. Kern PA. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipid and inflammation. J Clin Endocrinol Metab. 2007;92:666–672. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S. Wilson-Kubalek EM. Wert D. Tsao TS. Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–814. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lara-Castro C. Luo N. Wallace P. Klein RL. Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 21.Tsao TS. Tomas E. Murrey HE. Hug C. Lee DH. Ruderman NB. Heuser JE. Lodish HF. Role of Disulfide Bonds in Acrp30/Adiponectin Structure and Signaling Specificity: different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 22.Gaboriaud C. Juanhuix J. Gruez A. Lacroix M. Darnault C. Pignol D. Verger D. Fontecilla-Camps JC. Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro L. Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Current Biology. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 24.Tonelli J. Li W. Kishore P. Pajvani UB. Kwon E. Weaver C. Scherer PE. Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 25.McPhalen CA. Strynadka NC. James MN. Calcium-binding sites in proteins: a structural perspective. Adv Protein Chem. 1991;42:77–144. doi: 10.1016/s0065-3233(08)60535-5. [DOI] [PubMed] [Google Scholar]
- 26.Baumann U. Wu S. Flaherty KM. McKay DB. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kretsinger RH. Calcium-binding proteins. Annu Rev Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- 28.Strynadka NC. James MN. Crystal structures of the helix-loop-helix calcium- binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 29.Lewit-Bentley A. Rety S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 30.Rigden DJ. Galperin MY. The DxDxDG motif for calcium binding: multiple structural contexts and implications for evolution. J Mol Biol. 2004;343:971–984. doi: 10.1016/j.jmb.2004.08.077. [DOI] [PubMed] [Google Scholar]
- 31.Huq NL. Cross KJ. Reynolds EC. Nascent helix in the multiphosphorylated peptide alphaS2-casein(2-20) J Pept Sci. 2003;9:386–392. doi: 10.1002/psc.465. [DOI] [PubMed] [Google Scholar]
- 32.Chen B. Mayer MU. Markillie LM. Stenoien DL. Squier TC. Dynamic motion of helix A in the amino-terminal domain of calmodulin is stabilized upon calcium activation. Biochemistry. 2005;44:905–914. doi: 10.1021/bi048332u. [DOI] [PubMed] [Google Scholar]
- 33.Sen A. Sen N. Mackow ER. The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium regulated and directed by a C-terminal helical domain. J Virol. 2007;81:11758–11767. doi: 10.1128/JVI.01124-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peake PW. Shen Y. Campbell LV. Charlesworth JA. Human adiponectin binds to bacterial lipopolysaccharide. Biochem Biophys Res Commun. 2006;341:108–115. doi: 10.1016/j.bbrc.2005.12.162. [DOI] [PubMed] [Google Scholar]
- 35.Ruano ML. Garcia-Verdugo I. Miguel E. Perez-Gil J. Casals C. Self-aggregation of surfactant protein A. Biochemistry. 2000;39:6529–6537. doi: 10.1021/bi000188z. [DOI] [PubMed] [Google Scholar]
- 36.Zik M. Fridmann-Sirkis Y. Fromm H. C-terminal residues of plant glutamate decarboxylase are required for oligomerization of a high-molecular weight complex and for activation by calcium/calmodulin. Biochim Biophys Acta. 2006;1764:872–876. doi: 10.1016/j.bbapap.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Shi H. Halvorsen YD. Ellis PN. Wilkison WO. Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000;3:75–82. doi: 10.1152/physiolgenomics.2000.3.2.75. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F. Sowers JR. Ram JL. Standley PR. Peuler JD. Effects of pioglitazone on calcium channels in vascular smooth muscle. Hypertension. 1994;24:170–175. doi: 10.1161/01.hyp.24.2.170. [DOI] [PubMed] [Google Scholar]
- 39.Heppner TJ. Bonev AD. Eckman DM. Gomez MF. Petkov GV. Nelson MT. Novel PPARgamma agonists GI 262570, GW 7845, GW 1929, and pioglitazone decrease calcium channel function and myogenic tone in rat mesenteric arteries. Pharmacology. 2005;73:15–22. doi: 10.1159/000081070. [DOI] [PubMed] [Google Scholar]
- 40.Bogan JS. Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]