SUMMARY
Impaired degradation of amyloid beta (Aβ) peptides could lead to Aβ accumulation, an early trigger of Alzheimer’s disease (AD). Regulation of Aβ-degrading enzymes remains largely unknown. Cystatin C (CysC, CST3) is an endogenous inhibitor of cysteine proteases, including cathepsin B (CatB), a recently discovered Aβ-degrading enzyme. The CST3 polymorphism is also associated with an increased risk of late-onset sporadic AD. Here we identified CysC as the key inhibitory mechanism of CatB-induced Aβ degradation in vivo. Genetic ablation of CST3 in hAPP-J20 mice significantly lowered soluble Aβ levels, the relative abundance of Aβ1–42, and plaque load. CysC removal also attenuated Aβ-associated cognitive deficits, behavioral abnormalities, and restored synaptic plasticity in the hippocampus. Importantly, the beneficial effects of CysC reduction were abolished on a CatB null background, providing direct evidence that CysC regulates soluble Aβ and Aβ-associated neuronal deficits through inhibiting CatB-induced Aβ degradation.
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that causes loss of cognitive function (Hardy and Selkoe, 2002; Mattson, 2004; Roberson and Mucke, 2006). Amyloid beta (Aβ) peptides play a central role in AD pathogenesis (Hardy and Selkoe, 2002), and soluble Aβ assemblies may be early triggers of amyloid toxicity (Kayed et al., 2003; Klein, 2002; Klyubin et al., 2004; Lesne et al., 2006; Shankar et al., 2008). Aβ accumulation in AD brains could reflect overproduction or inefficient clearance (Tanzi et al., 2004). In most sporadic and late-onset AD cases lacking familial mutations in APP or presenilin (PS) 1, high cerebral Aβ levels could result from age-related defects in Aβ clearance (Selkoe, 2001; Tanzi et al., 2004). Thus, promoting the endogenous Aβ degradation and clearance machinery may be an effective strategy to reduce Aβ levels. Several Aβ-degrading enzymes, including insulin-degrading enzyme, neprilysin, endothelin-converting enzyme, plasmin, metalloprotease 9, and angiotensin-converting enzyme have been implicated in cerebral Aβ clearance (Eckman et al., 2001; Iwata et al., 2001; Qiu et al., 1998; Tucker et al., 2000; Yin et al., 2006; Zou et al., 2007). However, little is known about how their Aβ-degradation activities are regulated in the brain (Saito et al., 2005), and strategies to enhance their activities in vivo are lacking.
We identified cathepsin B (CatB) as a protease that effectively degrades Aβ assemblies (Mueller-Steiner et al., 2006). Ablation of CatB in mice overexpressing human amyloid precursor protein (hAPP) with familial AD-linked mutations increased plaque load, the relative abundance of Aβ1–42, and neuronal deficits (Mueller-Steiner et al., 2006). Importantly, CatB reduces Aβ levels by inducing C-terminal truncation of Aβ1–42 (Mueller-Steiner et al., 2006). These findings suggest that enhancing endogenous CatB activity could reduce levels of Aβ, especially Aβ1–42, and protect against AD-related deficits.
We hypothesize that CatB enzymatic activity can be enhanced by reducing levels of cysatin C (CysC, CST3) (Cimerman et al., 1999; Turk et al., 1995), a cysteine protease inhibitor. Genetic linkage studies showed an association between a CST3 polymorphism and increased risk of late-onset sporadic AD, which was later supported by systematic meta-analyses (Bertram et al., 2007; Beyer et al., 2001; Crawford et al., 2000; Finckh et al., 2000). Interestingly, overexpression of human CysC in hAPP-J20 mice reduces plaque load by inhibiting Aβ fibril formation (Kaeser et al., 2007; Mi et al., 2007). The effects of endogenous CysC on levels of soluble Aβ and Aβ1–42 and associated functional deficits, however, remained unknown. In the current study, we tested the hypothesis that CysC regulates levels of soluble Aβ and associated neuronal and behavioral deficits through its inhibition of CatB.
RESULTS
CysC Reduction Elevates CatB Activity and Lowers Soluble Aβ Levels
To directly assess the effect of CysC on CatB activity in the brain, we compared CatB’s enzymatic activities in the hippocampus of CST3+/+, CST3+/−, and CST3−/− mice (Huh et al., 1999). Removal of CST3 alleles yielded significantly higher hippocampal CatB activities in a gene dose–dependent manner (Figure 1A).
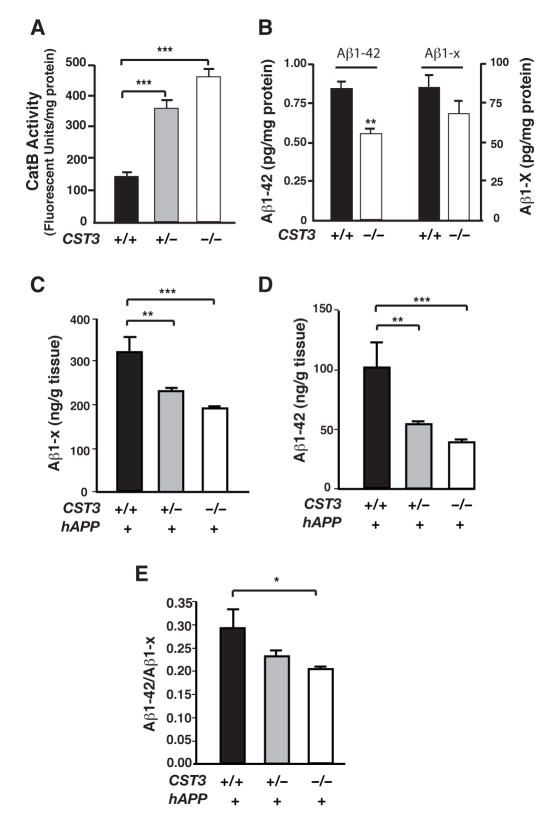
Figure 1. CysC Reduction Increases CatB Activity and Lowers Soluble Aβ Levels.
(A) CatB activities in hippocampal lysates from CST3+/+, CST3+/− or CST3−/− mice (n= 6, ***, P < 0.001, one-way ANOVA with Tukey-Kramer post hoc test).
(B) ELISA measurements of Aβ1–42 or Aβ1-x in the supernatants of primary cortical cultures (CST3+/+ or CST3−/−) infected with hAPPV642I adenovirus. All measurements were normalized to protein concentrations in the cell lysates (n= 4, **, P < 0.01, Mann-Whitney U test. Average Aβ levels in CST3+/+ cultures were arbitrarily set as 1).
(C–E) ELISA measurements of hippocampal levels of soluble Aβ1-x and Aβ1–42 in 2–4-month-old hAPP-J20 mice. Deleting one or both alleles of CST3 significantly reduced levels of soluble Aβ1-x (C) and Aβ1–42 (D). Ablating CysC also reduced Aβ1–42/Aβ1-x ratios significantly (E). (n = 9–21 mice/genotype, ***, P < 0.001; **, P < 0.01, *, P < 0.05, one-way ANOVA with Tukey-Kramer post hoc test). Bars = means ± SEM (A–E).
We next examined the effects of CysC removal on Aβ levels in primary cortical cultures. Since CysC is mainly expressed by glia, we used mixed primary cortical cultures with neurons and glia (Chen et al., 2005). CST3−/− or CST3+/+ cultures were infected with an adenoviral vector encoding hAPP cDNA with the familial London mutation (V642I) but lacking the Swedish mutations. Levels of Aβ1–42, but not Aβ1–x, were significantly lower in supernatants from CST3−/− cultures than those from CST3+/+ cultures (Figure 1B), consistent with higher CatB activity in CST3−/− cultures.
To determine the effects of endogenous CysC on soluble Aβ levels in vivo, we genetically reduced CysC levels by crossing CST3−/− mice with hAPP-J20 mice (Mucke et al., 2000). Soluble Aβ levels were quantified in hAPP/CST3+/+, hAPP/CST3+/−, and hAPP/CST3−/− mice at 2–4 months of age, before Aβ deposition. Reducing CysC significantly lowered levels of both soluble Aβ (Aβ1-x) (Figure 1C) and Aβ1–42 (Figure 1D) in a gene dose–dependent manner. Removing one CST3 allele reduced levels of total Aβ and Aβ1–42 significantly, although to lesser extents than removing both alleles. Notably, CysC ablation significantly reduced the relative abundance of Aβ1–42 (Figure 1E), suggesting that CysC ablation exerted a more robust effect on Aβ1–42 than total Aβ, as in the case of primary cortical cultures.
CysC Ablation Reduces Total Plaque Load in hAPP-J20 Mice
The formation of amyloid plaques in aging brains is enhanced by increases in Aβ, the relative abundance of Aβ1–42, and by the fibrillization process. In agreement with the Aβ/Aβ42-lowering effects of CysC ablation (Figure 1), the average amount of 3D6-labeled Aβ deposits in the hippocampus of hAPP/CST3−/− mice was markedly lower than that in hAPP/CST3+/+ mice at both 5–8- and 8–10-months of age (Figure 2A and 2B), Since plaques increased exponentially, we ran multivariate loglinear regressions of plaque load against both age and genotype to adjust for the age effects (Figure 2C). Notably, the linear regression of logarithm-transformed plaque load in hAPP/CST3+/+ mice was markedly different from that in hAPP/CST3−/− mice (Figure 2C). Subsequent statistical analyses performed in SPSS confirmed that age-adjusted plaque load in hAPP/CST3+/+ mice was significantly higher than that in hAPP/CST3−/− mice (genotype coefficient β2 = 1.015, t = 6.217, P < 0.001, See Table-S1 for details). In cortex, CysC ablation also lowered 3D6-positive plaque load in 8–10-month-old hAPP-J20 mice (Figure 2D).
Figure 2. CysC Ablation in hAPP-J20 Mice Lowers Total Plaque Deposition.
(A and E) Photomicrographs of 3D6 immunostaining (A) or thioflavin S staining (E) in the hippocampus of a 5–8-month-old hAPP/CST3+/+ or hAPP/CST3−/− mouse.
(B, D and F) Quantification of plaque load labeled with 3D6 (B and D) or thioflavin S (F) in 5–8-month-old and 8–10-month-old hAPP/CST3+/+ or hAPP/CST3−/− mice (n= 9–15 mice/genotype, **, P < 0.01, *, P < 0.05, unpaired t test). For total plaque load, three hippocampal (B) or cortical (D) sections per mouse were analyzed to determine the percent area covered by 3D6-immunoreactive material. The number of thioflavin S–positive plaques was counted in five hippocampal sections per mouse (F). Bars represent means ± SEM.
(C) The logarithm-transformed plaque load was regressed against both age (5–10-month, n= 23–25 mice/genotype) and genotype (as a dummy variable) and was fit to the following model: Log (Plaque Load) = (−7.912 + (0.892) * Age + (1.015) * Genotype (Dummy). The age-adjusted plaque load in hAPP/CST3+/+ mice (Genotype dummy = 1) was significantly higher than that in hAPP/CST3−/− mice (Genotype dummy = 0), since the β2 coefficient (1.015) is significantly larger than 0 (t = 6.217, P < 0.001).
Unlike 3D6 antibody, which labels both diffused and fibrillar Aβ, thioflavins S predominantly labels Aβ fibrils (Figure 2E). Interestingly, deleting CysC reduced thioflavin S–positive Aβ fibrils in hAPP-J20 mice at 5–8 months of age, but not at 8–10 months of age (Figures 2E and 2F). CysC removal in 8–10-month-old hAPP-J20 mice lowered the levels of Aβ and the relative abundance of Aβ1–42 in the TBS-extractable, but not TBS-insoluble pools (Figure S1). These results indicate that CysC may affect soluble Aβ and the fibrillization processes differently. Indeed, human CysC overexpression inhibits Aβ fibrillization by directly binding to Aβ (Kaeser et al., 2007; Mi et al., 2007), independent of its function as a cysteine protease inhibitor.
CysC Ablation Does Not Affect the Processing of hAPP with or without FAD Mutations
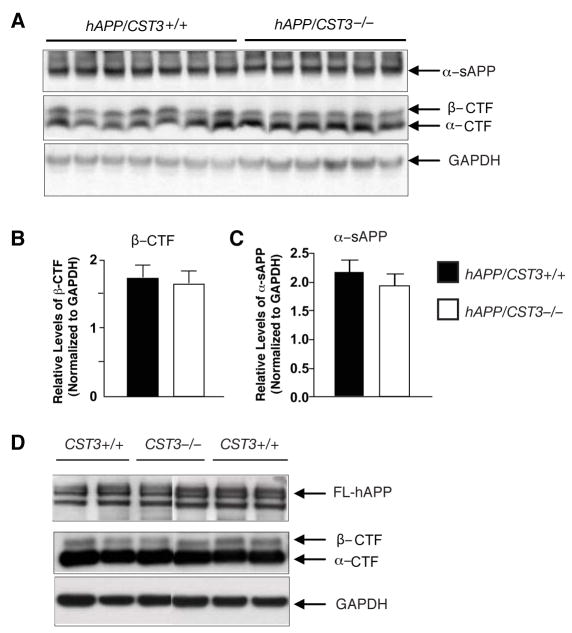
We next examined if CysC ablation affected APP processing by comparing the levels of metabolic fragments of hAPP in hAPP/CST3+/+ and hAPP/CST3−/− mice. Western blot analyses revealed that levels of α-sAPP, detected with 8E5 antibody(Esposito et al., 2004), or those of β-C-terminal fragments (β-CTF), detected with CT-15 antibody, were not affected by CysC ablation in hAPP-J20 mice (Figure 3A–C). The subtle increase in the levels of α-CTF in hAPP/CST3−/− mice did not reach statistical significance (P > 0.05, data not shown). These results indicate that CysC does not affect the processing of hAPP with Swedish and Indiana mutations, consistent with a previous report (Mi et al., 2007). Importantly, CysC ablation had no effects on the processing of wild-type hAPP either. Levels of β-CTF, α-CTF or full-length hAPP (FL-hAPP) were similar in the lysates from CST3−/− and CST3+/+ cultures infected with an adenoviral vector encoding wild-type hAPP cDNA (Figure 3D). Notably, the cleavage of wild-type hAPP at the α-secretase site predominates due to the lack of Swedish mutations at the β-secretase site (Figure 3D).
Figure 3. CysC Ablation Does Not Affect the Processing of hAPP.
(A) Western blot analyses of cortical lysates demonstrate comparable levels of hAPP metabolic fragments in 5–8-month-old hAPP/CST3+/+ and hAPP/CST3−/− mice.
(B–C) Quantification of levels of α-CTFs (B) or α-CTFs (C) relative to those of GAPDH in hAPP/CST3+/+ (n = 9) and hAPP/CST3−/− mice (n = 7). Bars represent means ± SEM.
(D) Western blot analyses demonstrate comparable levels of hAPP metabolic fragments in the lysates of CST3+/+ or CST3−/− primary cortical cultures infected with an adenoviral vector expressing wild-type hAPP. GAPDH was used as a loading standard.
CysC Ablation Prevents Premature Mortality and Aβ-Associated Cognitive Deficits
Mice expressing high levels of hAPP and Aβ die prematurely (Chin et al., 2005; Hsiao et al., 1995; Roberson et al., 2007). Although the etiology is unknown, premature mortality was prevented by lowering Aβ levels or altering downstream pathways (Leissring et al., 2003; Roberson et al., 2007). Indeed, both hAPP/CST3−/− and hAPP/CST3+/− mice, with lower soluble Aβ levels, were protected from the early mortality of hAPP/CST3+/+ mice (Figure 4A).
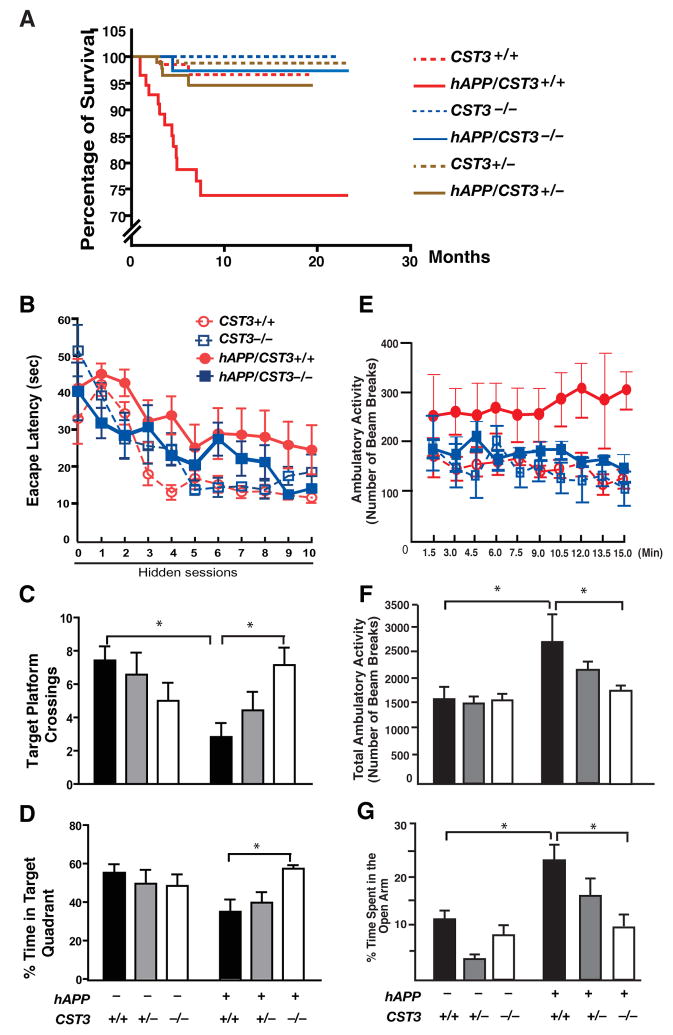
Figure 4. CysC Reduction Prevents Functional Deficits in hAPP-J20 Mice.
(A) Kaplan-Meier survival curves showing the effect of CysC reduction on survival of hAPP-J20 mice. All genotyped mice in the colony (n = 466) were included in the analysis. By log-rank comparison, only hAPP/CST3+/+ mice differed from all other groups (P < 0.0001).
(B–D) Effects of CysC ablation on learning and memory in water maze testing (n= 6–7 male mice/genotype, 7–10 months of age).(B) Hidden platform learning curves differed by genotype (Repeated measure ANOVA: P < 0.0001). In Tukey-Kramer post-hoc comparisons, hAPP/CST3+/+ differed from CST3+/+ and CST3−/− mice (P < 0.001); hAPP/CST3−/− differed from hAPP/CST3+/+ (P < 0.01) but not from CST3+/+ or CST3−/− mice. (C) Number of target platform crossings differed by genotype in the probe trials (Two-way ANOVA, hAPP and CST3 interaction, P < 0.05). In bonferroni post-hoc comparisons, hAPP/CST3+/+ differed from CST3+/+ mice (*, P < 0.05); hAPP/CST3−/− differed from hAPP/CST3+/+ (*, P < 0.05), but not from CST3+/+ or CST3−/− mice. (D) Percentage of time spent in the target quadrant differed by genotype (Two-way ANOVA, hAPP and CST3 interaction, P < 0.05). In bonferroni post-hoc comparisons, hAPP/CST3−/− differed from hAPP/CST3+/+ mice (*, P < 0.05), but not from CST3+/+ or CST3−/− mice. (E–G) Effects of CysC reduction in open field tests and elevated plus maze (n= 5–10 male mice/genotype; 3–4 months of age). (E) The ambulatory activity per min differed by genotype (Repeated-measures ANOVA, P < 0.001). In Tukey-Kramer post-hoc comparisons, hAPP/CST3+/+ differed from CST3+/+ or CST3−/− (P < 0.001), hAPP/CST3+/+ differed from hAPP/CST−/− (P < 0.001). (F) Total ambulatory activity over 15 min differed by genotype (Two-way ANOVA; bonferroni post-hoc comparisons, hAPP/CST3+/+ vs. CST3+/+ *, P < 0.05; hAPP/CST3−/− vs. hAPP/CST3+/+, *, P < 0.05). (G) The percentage of time spent in the open arms of an elevated plus maze differ by genotype (Two-way ANOVA; bonferroni post-hoc comparisons, hAPP/CST3+/+ vs. CST3+/+, *, P < 0.05; hAPP/CST3−/− vs. hAPP/CST3+/+, *, P < 0.05). Error bars represent SEM (B–G).
We next asked if CysC reduction improved cognitive function in hAPP-J20 mice, which develop Aβ-associated deficits in spatial learning and memory (Cheng et al., 2007; Chin et al., 2005; Roberson et al., 2007). In the Morris water maze, spatial learning was measured in the hidden-platform version of the water maze in 10 sessions over 5 days. Control mice that do not express hAPP learned this task by day 3 (session 6), regardless of the CST3 genotype (Figure 4B). The escape latency of hAPP/CST3+/+ mice was considerably longer than that of control mice over the course of 5-day training (Figure 4B), confirming that hAPP/CST3+/+ mice exhibited Aβ-associated spatial learning deficits. Deleting CysC in hAPP-J20 mice significantly reduced the escape latency and improved the learning curve (Figure 4B). As CST3+/+, CST3−/−, hAPP/CST3+/+ and hAPP/CST3−/− mice swam at similar speed (Figure S2), the differences in their learning curves were not confounded by their swim speed.
To measure spatial memory retention, we performed probe trials by removing the hidden platform one day after the training. In agreement with previous observations, hAPP/CST3+/+mice crossed the target platform much fewer times than wild-type mice (CST3+/+), exhibiting impaired memory retention (Figure 4C). CysC ablation in hAPP-J20 mice markedly increased the number of target platform crossings, suggesting that spatial memory was restored in hAPP/CST3−/− mice (Figure 4C). Indeed, hAPP/CST3−/− mice spent significantly more time in the target quadrant than hAPP/CST3+/+ mice, similar to mice without hAPP (Figure 4D). Thus, Aβ-associated deficits in spatial learning and memory was prevented by CysC removal. In the cued (non-spatial) session after the probe trials, mice learned to find the target platform that was clearly visible with a marker placed directly above it. In agreement with previous studies (Cheng et al., 2007; Roberson et al., 2007), hAPP/CST3+/+ mice took longer time than CST3+/+ mice to locate the visible platform (Figure S2). Removing CysC significantly improved the ability of hAPP-J20 mice to learn this task (Figure S2).
Other behavioral abnormalities in hAPP-J20 mice were also attenuated by CysC reduction. Increased exploratory locomotor activity was observed in several independent AD animal models (Kobayashi and Chen, 2005), including Tg2576 (Ognibene et al., 2005) and hAPP-J20 mice (Cheng et al., 2007; Chin et al., 2005; Roberson et al., 2007). In an open field test, hAPP-J20 mice exhibited sustained locomotor hyperactivity over the course of 15 min (Figure 4E). The lack of habituation to the novel environment suggests impaired non-associative learning. Removing CysC in APP-J20 mice restored habituation, suggesting improved non-associative learning in this paradigm (Figure 4E). The total locomotor hyperactivity of hAPP-J20 mice was also significantly attenuated by CysC reduction (Figure 4F). In the elevated plus maze, hAPP-J20 mice exhibited a disinhibition phenotype, spending more time in the open arms than the control mice (Figure 4G), as expected from previous studies (Cheng et al., 2007; Chin et al., 2005; Roberson et al., 2007). The relative amount of time spent in the open arms was markedly decreased in hAPP/CST3−/− mice and, to lesser extent, in hAPP/CST3+/− mice (Figure 4G). Thus, the CysC reduction ameliorated anxiolytic responses of hAPP-J20 mice in a gene dose-dependent manner.
CysC Ablation Ameliorates Aβ-Associated Neuronal and Synaptic Deficits in Hippocampal Circuits
Cognitive deficits in hAPP-J20 mice were associated with depletion of a calcium-binding protein, calbindin-D28k, in the DG (Palop et al., 2007; Palop et al., 2003; Roberson et al., 2007). Reducing CysC in hAPP-J20 mice significantly increased the levels of calbindin in the DG (Figure 5A and 5B), confirming improved hippocampal function. Removal of one or both alleles of CysC also significantly increased the number of Fos-positive neurons in the DG of hAPP-J20 mice (Figure S3).
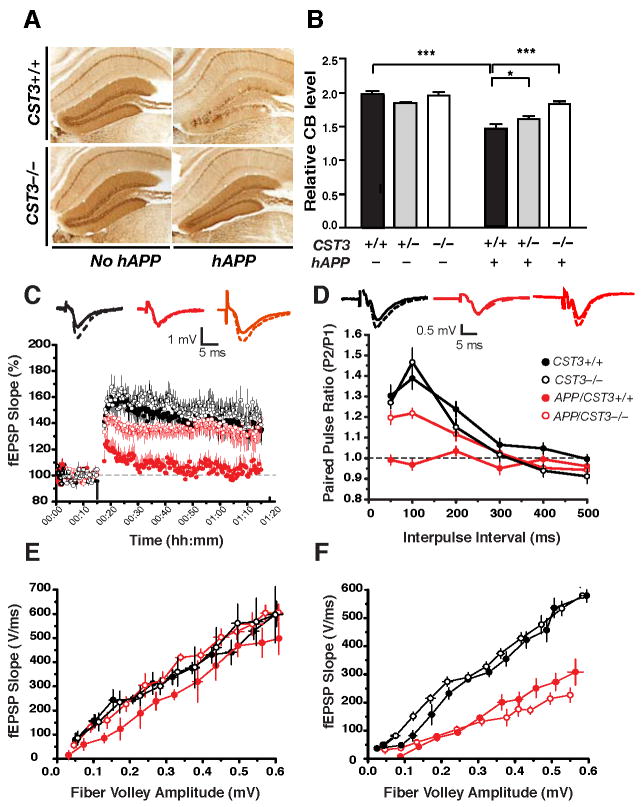
Figure 5. CysC Ablation Ameliorates Aβ-Associated Neuronal and Synaptic Deficits in Hippocampal Circuits.
(A) Photomicrographs of calbindin immunostaining in the hippocampus of a 5–8-month-old hAPP/CST3+/+, hAPP/CST3−/−, CST3+/+, or CST3−/− mouse.
(B) Effects of CysC reduction on the relative calbindin levels in the DG (n= 27–46 mice/genotype, age 2–8 months; two-way ANOVA: P < 0.0001, hAPP and CST3 interaction). In post-hoc comparisons, hAPP/CST3+/+ differed from groups without hAPP (***, P < 0.001); hAPP/CST3−/− differed from hAPP/CST3+/+ (***, P < 0.001), but not from all groups without hAPP; hAPP/CST3+/− differed from hAPP/CST3+/+ (*, P < 0.05). CB, cabindin.
(C–F) Effects of CysC ablation on the synaptic functions at the perforant path to DG synapses (C–E) or at the Schaffer collateral to CA1 synapse (F) (n=8–12 slices from 2–3 mice/genotype). (C) LTP at the medial perforant path was significantly depressed in hAPP/CST3+/+ mice; deleting CysC in hAPP-J20 mice led to a significant recovery (P < 0.05, CST3+/+ vs. hAPP/CST3+/+; P < 0.05, hAPP/CST3+/+ vs. hAPP/CST3−/−; Tukey’s post-hoc analysis on repeated-measures ANOVA for data collected from minutes 61–70). (D) Paired pulse facilitation at the lateral perforant pathway was markedly reduced in hAPP/CST3+/+ mice; deleting CysC in hAPP-J20 mice led to a significant recovery (P < 0.01, CST3+/+ vs. hAPP/CST3+/+; P < 0.01, hAPP/CST3+/+ vs. hAPP/CST3−/−; One-way ANOVA at 50 and 100 ms interpulse intervals). (E) The synaptic strength at the medial perforant path was not affected by expression of hAPP or deletion of CST3. (F) CysC deletion did not restore the synaptic transmission along the Schaffer collateral-CA1 synapse, which was significantly impaired in hAPP/CST3+/+ mice (P < 0.01, CST3+/+ vs. hAPP/CST3+/+, ANOVA). Error bars represent SEM (B–F).
To directly assess the effects of CysC reduction on functional deficits in hippocampal circuits, we performed extracellular recordings of field excitatory postsynaptic potentials (EPSPs) of two hippocampal circuits, perforant path to DG granule cell synapses and Schaffer collateral to CA1 pyramidal cell synapses. In the medial perforant path (MPP) to granule cell synapses, the long-term potentiation (LTP) induced by theta burst stimulation was significantly depressed in hAPP-J20 mice compared with non-transgenic controls (Palop et al., 2007) (Figure 5C). CysC removal in hAPP-J20 mice led to a robust recovery of LTP (Figure 5C), suggesting improved long-term synaptic plasticity in the DG. In the lateral perforant path (LPP) to DG synapses of hAPP-J20 mice, alterations of paired pulse facilitation (PPF), which reflect deficits in short-term plasticity, were also prevented by CysC removal (Figure 5D).
Interestingly, the strength of basic synaptic transmission in the DG, as measured by input/output (I/O) relationships at the MPP pathway was not affected by either hAPP overexpression or CysC ablation (Figure 5E). In contrast, the I/O ratios of the Schaffer collateral to CA1 synapses were markedly reduced in hAPP-J20 mice, but ablating CysC failed to improve the synaptic strength of this synaptic pathway (Figure 5F). These results demonstrated that CysC deletion in hAPP-J20 mice specifically restored both long-term and short-term synaptic plasticity in the hippocampal circuits involving DG, providing plausible molecular mechanisms underlying the improvements in spatial learning and memory and other hippocampal-associated behaviors.
CysC Regulates Soluble Aβ in a CatB-Dependent Manner
CysC has multiple biological activities, and its regulation of Aβ levels could still be independent of CatB. To directly prove that beneficial effects of CysC reduction are mediated by enhancing CatB activity, we examined the effects of CysC reduction in the absence of CatB. CatB was genetically deleted by backcrossing hAPP/CST3+/+, hAPP/CST3+/−, hAPP/CST3−/− mice and their littermate controls (CST3+/+, CST3+/−, CST3−/−) onto a CatB-null background.
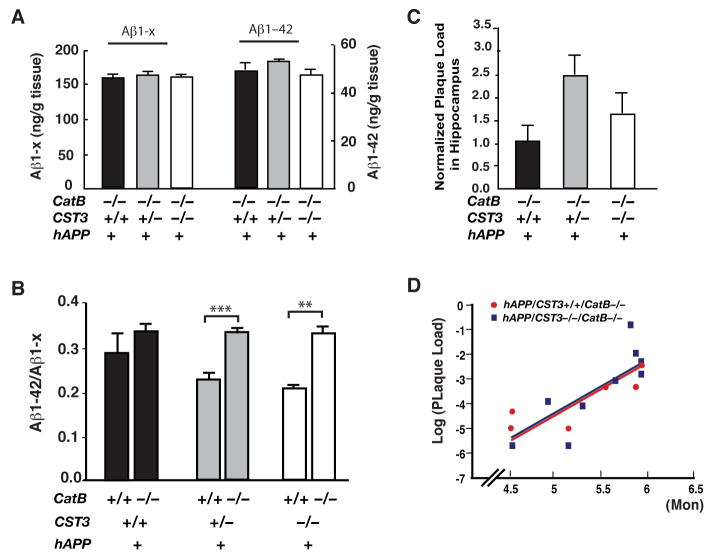
In 2–3-month-old hAPP-J20 mice lacking CatB, CysC reduction had no effect on the levels of soluble Aβ1-x, Aβ1–42, or the relative abundance of Aβ1–42 (Figure 6A and 6B). These results demonstrated that CysC reduction lowered Aβ levels in a CatB-dependent manner. Notably, CatB ablation elevated the relative abundance of Aβ1–42 only when CysC levels were reduced (Figure 6B), further supporting that CatB-induced proteolytic truncations of Aβ1–42 are normally suppressed by endogenous CysC. In addition, CysC reduction failed to lower 3D6-positive plaque load in 4–6-month-old hAPP mice without CatB (Figure 6C). In contrast to the distinctive regressions of the plaque load in hAPP/CST3+/+ and hAPP/CST3−/− mice with CatB (Figure 2C), the logarithm-transformed plaque load in hAPP/CST3+/+CatB−/− mice regressed indistinctively from that in hAPP/CST3−/−CatB−/− mice (Figure 6D). Multivariate loglinear regression analyses demonstrated no difference between age-adjusted plaque load of these two genotypes (genotype coefficient β2 = −0.111, t = −0.258, P = 0.8, See Table-S2 for statistical details). These results identified enhanced Aβ-degrading activity of CatB as the direct mechanism underlying the Aβ-lowering effects of CysC reduction.
Figure 6. The Aβ-Lowering Effects of CysC Reduction Depend on CatB.
(A) ELISA measurements of levels of soluble Aβ1–42 and Aβ1-x in the hippocampus of 2–3-month-old hAPP/CST3+/+CatB−/−, hAPP/CST3+/−CatB−/−, and hAPP/CST3−/−CatB−/− mice (n= 6–10 mice/genotype). CysC reduction had no effects on the levels of Aβ1-x or Aβ1–42 in hAPP-J20 mice lacking CatB.
(B) CatB ablation increased the ratios of Aβ1–42/Aβ1-x in hAPP-J20 mice with one or both alleles of CST3 deleted (n= 6–21 mice/genotype, age 2–4 months; two-way ANOVA: ***, P < 0.001, **, P < 0.01, bonferroni post-hoc test of the CatB effects).
(C) Normalized plaque load in hippocampus of 4–6-month-old hAPP/CST3+/+CatB−/−, hAPP/CST3+/−CatB−/−, and hAPP/CST3−/−CatB−/− mice (n= 7–14 mice/genotype; one-way ANOVA, P > 0.1).
(D) The logarithm-transformed plaque load was regressed against both age and genotype (as a dummy variable) and was fit to the following model: Log (Plaque Load) = (−14.547) + (2.052) * Age + (−0.111) * Genotype (Dummy). The age-adjusted plaque load in hAPP/CST3+/+CatB−/− mice (Genotype dummy = 1) did not differ from that in hAPP/CST3−/−CatB−/− mice (Genotype dummy = 0), since the β2 coefficient (−0.111) is not significantly different from 0 (t = −0.258, P = 0.8).
Neuroprotective Effects of CysC Ablation Depend on CatB
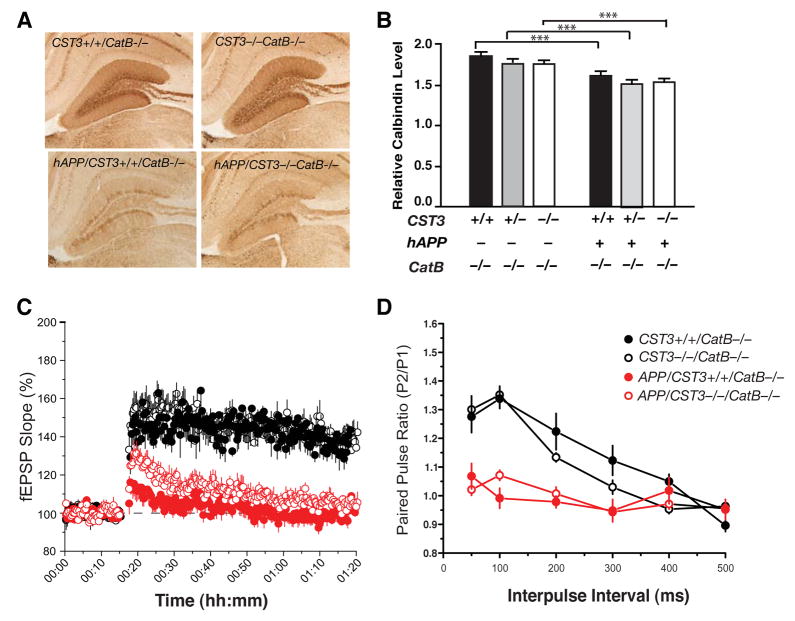
To further establish that the beneficial effects of CysC reduction are due to enhanced CatB activity, we determined if the effects of CysC reduction on the expression of DG neuronal markers in hAPP-J20 mice depended on CatB. Without CatB, CysC reduction failed to increase expression of calbindin (Figure 7A and 7B) or the number of Fos-positive neurons (Figure S4) in the DG. The lack of improvements was expected since levels of Aβ/Aβ1–42 remained similarly high in these animals regardless of the CST3 genotypes (Figure 6A and 6B).
Figure 7. The Neuroprotective Effects of CysC Reduction Depend on CatB.
(A) Photomicrographs of calbindin immunostaining in the hippocampus of 5-month-old hAPP/CST3+/+CatB−/−, hAPP/CST3−/−CatB−/−, CST3+/+CatB−/−, and CST3−/−CatB−/−, mice.
(B) CysC reduction failed to prevent the depletion of DG calbindin levels in hAPP-J20 mice lacking CatB (n= 9–22 mice/genotype, age 2–6 months old; two-way ANOVA: ***, P < 0.001, bonferroni post-hoc test of the hAPP effects; CST3 effects, not significant).
(C–D) Effects of CysC ablation on the synaptic functions in hippocampus of hAPP-J20 mice on CatB null background (n=8–12 slices from 2 mice/genotype). (C) LTP at the medial perforant path was significantly depressed in hAPP/CatB−/− mice regardless of CST3 genotypes (P < 0.01, CST3+/+CatB−/− vs. hAPP/CST3+/+CatB−/− and CST3−/−CatB−/− vs. hAPP/CST3−/−CatB−/−). CysC removal did not alter LTP (P > 0.1, hAPP/CST3+/+CatB−/− vs. hAPP/CST3−/−CatB−/−; Tukey’s post-hoc analysis on repeated-measures ANOVA for data collected from minutes 61–70). (D) The paired pulse facilitations at the lateral perforant pathway were markedly reduced in hAPP/CatB−/− mice regardless of CST3 genotypes (P < 0.01, CST3+/+CatB−/− vs. hAPP/CST3+/+CatB−/− ; CST3−/−CatB−/− vs. hAPP/CST3−/−CatB−/−; One-way ANOVA at 50–100 ms interpulse intervals). Bars represent means ± SEM (B–D).
Electrophysiological recordings further revealed that CatB was also required for the protective effects of CysC ablation against Aβ-associated functional deficits. With CatB, CysC removal restored LTP and PPF in the DG synapses (Figure 5C and 5D). Without CatB, CysC removal failed to restore the LTP at the MPP to granule cell synapses (Figure 7C), or to prevent the alterations of PPF in the LPP to granule cell synapses (Figure 7D). Taken together, our findings established a critical role of CysC-CatB axis in regulating soluble Aβ and associated functional deficits.
DISCUSSION
Our data show that CysC serves as a key inhibitory mechanism of CatB-dependent Aβ degradation in vivo. CysC removal is highly effective in promoting CatB-dependent truncation of Aβ1–42, the most pathogenic form among the different Aβ peptides in the brain (Golde et al., 2000). Levels of Aβ1–42 were significantly lower in cultures from CysC-deficient mice than in cultures from the wild-type mice. CysC removal lowers relative abundance of Aβ1–42 in hAPP-J20 mice with CatB, but not in those lacking CatB. Conversely, inhibiting CatB activity in young hAPP-J20 mice elevated the relative abundance Aβ1–42, but only when CysC levels were reduced. Thus, the relative abundance of Aβ1–42 is modulated by the CysC-CatB interaction, and CatB-induced Aβ1–42 truncation are normally suppressed by endogenous CysC in vivo.
These findings also provide unequivocal confirmation of CatB as an Aβ-degrading enzyme. In apparent contrast to this notion, a recent study reported that non-specific inhibitors of cysteine proteases reduced Aβ levels and β-secretase site cleavage of wild-type hAPP, claiming an involvement of CatB in APP processing (Hook et al., 2008). However, in light of the nonspecific inhibitors used (Bogyo et al., 2000; Montaser et al., 2002) and the results of our studies, it is unlikely that CatB fulfills such a function. Indeed, in CST3−/− cells expressing hAPPV642I without the Swedish mutations, higher CatB activity led to markedly lower Aβ1–42 levels, but had no effects on levels of β-CTF, suggesting that CatB does not induce cleavage at wild-type β-secretase site in primary neurons. Overexpressing CatB in aged hAPP transgenic mice reversed preexisting amyloid pathology, further supporting the efficacy of CatB in degrading Aβ aggregates and the therapeutic potential of enhancing CatB activity (Mueller-Steiner et al., 2006). Furthermore, ablation of BACE1 completely abolished the β-secretase site processing and Aβ generation in PDAPP mice, which overexpress hAPPV717F without the Swedish mutations (McConlogue et al., 2007). These results provide compelling evidence that CatB exhibits no significant β-secretase activity in vivo regardless of the presence or absence of Swedish mutations on hAPP.
Specific species of soluble Aβ oligomers, including low-n and high-n Aβ assemblies, have emerged as early and potent triggers of AD-related functional deficits (Kayed et al., 2003; Klein, 2002; Klyubin et al., 2004; Lesne et al., 2006; Shankar et al., 2008; Townsend et al., 2006; Walsh and Selkoe, 2007). Removing CysC in hAPP-J20 mice is highly effective to prevent spatial learning and memory deficits and to restore the synaptic plasticity in hippocampal circuits, most likely by enhancing CatB-dependent Aβ degradation and lowering levels of soluble Aβ and Aβ42. Indeed, the protective effects of CysC ablation were abolished in hAPP mice without CatB, which had similarly high levels of soluble Aβ and Aβ42 regardless of CST3 genotype. However, how different species of Aβ oligomers are regulated by the CysC-CatB axis remains to be defined.
Our study showed that not all functional deficits in hAPP-J20 mice were prevented by CysC ablation. For example, CysC deletion improved LTP and ameliorated alterations in the paired-pulse facilitation in the perforant path to granule cells synapse, but it failed to improve the synaptic strength in the Schaffer collateral to CA1 pyramidal cell synapse. One likely explanation is that even at reduced levels, the amount of Aβ in hAPP/CST3−/− mice could already be sufficient to impair the basic synaptic transmission in the CA1. In support of this notion, the I/O ratios of the CA1 synapse were also impaired in hAPP-H6 mice (Hsia et al., 1999), which had only 20–30% of soluble Aβ in APP-J20 mice (Mucke et al., 2000). However, the deficit at CA1 may also be caused by CTFs or certain Aβ oligomeric species that are not affected by CysC ablation. Further studies will be required to differentiate these possibilities.
We found that the marked reduction in plaque load induced by CysC ablation depends on CatB. Surprisingly, overexpressing human CysC also reduced plaque load by inhibiting Aβ fibril formation (Kaeser et al., 2007; Mi et al., 2007). These puzzling effects on plaque load may be explained by different mechanisms underlying CysC’s regulation of soluble and insoluble Aβ—the former by inhibiting CatB and the latter by binding to Aβ (Kaeser et al., 2007; Mi et al., 2007). Depending on the relative levels of CysC and CatB, either mechanism may play a more prominent role. For example, since physiological concentration of endogenous CysC is in large excess over CatB (Turk et al., 2002), CysC reduction would alter CatB activity much more effectively than CysC overexpression. Therefore, in hAPP-J20 mice with reduced CysC, the Aβ-lowering effects prevailed as a result of enhanced CatB activity, leading to a marked decrease in overall Aβ deposits. In contrast, in hAPP-J20 mice overexpressing human CysC, CysC’s inhibitory effects on Aβ fibrillization probably superseded those on CatB activity, also resulting in lower plaque loads without affecting soluble Aβ levels (Kaeser et al., 2007). Furthermore, the balance of CysC’s effects on fibrillization versus those on CatB could be affected by aging. Indeed, CatB activities in the brain of hAPP-J20 mice appeared to decline with aging (Mueller-Steiner et al., 2006), which may help explain why CysC ablation reduced thioflavin S-labeled Aβ fibrils in 5–8-month-old, but not in 8–10-month-old brains.
On the cellular and subcellular levels, where CysC and CatB interacts to regulate Aβ catabolism remains to be defined. CysC is a secreted protein found in high levels in CSF (Abrahamson et al., 1986), suggesting that it might inhibit the Aβ-degrading activity of CatB extracellularly. Notably, CysC and CatB are localized in amyloid plaques (Cataldo and Nixon, 1990; Levy et al., 2001). Moreover, CatB activity in the CSF is much greater in CST3-null than wild-type mice (B. Sun and L. Gan, unpublished observations). However, CatB is mainly localized in lysosomal and endosomal compartments, suggesting that CysC may inhibit CatB-induced Aβ degradation intracellularly. In AD brains, immunoelectron microscopy with anti-CysC antibodies revealed strongly labeled pyramidal neurons that were immunopositive for intracellular Aβx-42 (Levy et al., 2001). In addition, neuronal CysC co-localized with the CatB in endosome/lysosomes (Deng et al., 2001), supporting the notion that CysC could inhibit endosomal CatB-dependent Aβ degradation. The CST3 polymorphisms, which exhibit two common haplotypes (A and B), offer intriguing clues for the involvement of CysC-CatB axis in AD. The B/B homozygosity is associated with an increased risk of developing late-onset sporadic AD (Bertram et al., 2007; Beyer et al., 2001; Crawford et al., 2000; Finckh et al., 2000). Pulse-chase experiments and high-resolution western blots demonstrated that intracellular CysC levels are higher in B/B fibroblasts due to a reduced secretion of CysC (Benussi et al., 2003). The B/B homozygosity-induced increase of intracellular CysC might impair CatB-dependent Aβ degradation in the endosomal/lysosomal pathways and contribute to Aβ accumulation.
The revelation that CysC regulates soluble Aβ via its interaction with CatB may lead to new Aβ-lowering strategies that are highly specific. For example, it might be possible to develop reagents that specifically disrupt the CysC-CatB interactions without affecting CysC’s diverse biological activities, including inhibition of other cysteine proteases or Aβ fibrillization. Interestingly, even partial reduction of CysC levels significantly reduced soluble Aβ levels, neuronal deficits, and premature mortality. These results suggest that CysC plays a rate-limiting role in this newly discovered Aβ clearance pathway, making it an attractive target for therapeutic intervention.
EXPERIMENTAL PROCEDURES
Mice
To remove CST3 genetically, CST3−/− mice (57% C57BL/6 and 43% 129) were crossed with hAPP-J20 mice (C57BL/6). The first cross, resulted in hAPP/CST3+/− mice, which were crossed with CST3+/− (CST3 heterozygotes that do not express hAPP) to generate six genotypes: hAPP/CST3−/−, hAPP/ CST3+/+, hAPP/CST3+/−, CST3−/−, CST3+/+, and CST3+/−. These mice are on a mixed genetic background (77.5% C57BL/6 strain and 22.5% 129 strain). The strain background was determined with a panel of 110 microsatellite markers that distinguishes between C57BL/6 and 129 (Genetic Testing Services, Charles River). PCR-based genotyping for CST3 allele or hAPP transgene was performed as described (Huh et al., 1999; Mucke et al., 2000).
To investigate the genetic interaction of CysC and CatB, we deleted CatB genetically in hAPP/CST3−/−, hAPP/CST3+/+, hAPP/ CST3+/−, CST3−/−, CST3+/+, and CST3+/− mice with the following breeding scheme. 1) CST3+/−CatB+/− mice were generated by crossing CatB−/− mice with CST3−/− mice. 2) CST3+/−CatB+/− mice were then crossed with hAPP/CatB−/−CST3+/+, which were generated from our previous study (Mueller-Steiner et al., 2006), to generate hAPP/CST3+/−CatB−/− or CST3+/−CatB−/−. 3) Non-littermates hAPP/CST3+/−CatB−/− and CST3+/−CatB−/− mice were then crossed with each other to generate hAPP/CST3+/+, hAPP/CST3+/− and hAPP/CST3−/− mice on a CatB−/− background (hAPP/CST3+/+CatB−/−, hAPP/CST3+/−CatB−/− and hAPP/CST3−/−CatB−/−), and their littermate controls that do not express hAPP/CST3+/+CatB−/−, CST3+/−CatB−/− and CST3−/−CatB−/−).
Primary Cortical Culture and Adenoviral Infection
To generate mixed cortical culture, cortices from mouse pups of different genotypes were isolated on postnatal day 0 or 1 and dissociated. Cells were plated at 160,000 cells/ml in plating medium containing Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, 0.5 mM Glutamax, 100 units/ml penicillin, and 100 μg/ml streptomycin. To overexpress hAPP, an adenoviral vector encoding human wild-type APP695 or APPV642I cDNA under the control of the cytomegalovirus promoter was used to infect neurons cultured from CST3+/+ and CST3−/− pups after 6 days in culture, when the medium was replaced with Neurobasal A medium supplemented with N2 (NBA/N2). On day 8, supernatants and cells were harvested for Aβ ELISA, CatB activity assay, and BCA protein assays. Unless otherwise noted, all cell-culture supplies, medium, and antibiotics were from Invitrogen (Carlsbad, CA).
Immunohistochemistry and Quantitation of Immunoreactive Structures
Experimenters were blind to the genotypes of the mice for all immunohistochemical analyses. Expression of calbindin, Fos in the DG and the plaque loads in the hippocampus were quantified as described previously with minor modifications (Palop et al., 2003; Mueller-Steiner et al., 2006). Sections were incubated with rabbit anti-calbindin (1:15,000, Swant, Bellinzona, Switzerland), anti-c-Fos (1:10,000, Ab-5, calbiochem), biotinylated goat anti-rabbit (1:200, Vector Laboratories, Burlingame, CA), or biotinylated mouse monoclonal antibody 3D6 (5 μg/ml, Elan, South San Francisco, CA), after quenching endogenous peroxidase activity. Binding of the antibody was detected with the Elite kit (Vector Laboratories) with diaminobenzidine and H2O2 for development. Images were obtained with a DEI-470 digital camera (Optronics, Goleta, CA). The integrated optical density from three coronal sections was determined with a Bioquant image analysis system and averaged in two areas of the molecular layer of the DG and of the stratum radiatum of the CA1 region, in which calbindin levels remain stable. Calbindin levels were expressed as the ratio of integrated optical density in the molecular layer and in the stratum radiatum of the CA1. The relative numbers of Fos-positive neurons were determined by counting Fos-positive cells in the DG in every 10th serial coronal section throughout the rostrocaudal extent of the DG. At least five coronal sections were analyzed per mouse, and the average of the individual measurements was used to calculate group means. Total plaque load was calculated as the percent area of the hippocampus or cortex covered by 3D6-immunoreactive material. Thiofalvin S–positive mature plaques were quantified by counting the number of plaques in the hippocampus. Three coronal sections were analyzed per mouse, and the average of the individual measurements was used to calculate group means.
CatB Enzymatic Assay
Brain CatB activities were quantified as described (Mueller-Steiner et al., 2006). Briefly, hippocampi from CST3−/−, CST3+/+, and CST3+/− mice were lysed in lysis buffer in the absence of proteinase inhibitors, preactivated with 4 mM cysteine, and incubated with the synthetic substrate Z-Arg-Arg AMC at 37°C for 30 min. Released free AMC was determined fluorometrically. The enzymatic activities inhibited by CA074 were defined as CatB-specific activities. Protein concentrations were measured with the BCA protein assay kit (Pierce, Rockford, IL) and used to normalize CatB activities.
Western Blot Analysis
Mouse cortical samples were individually sonicated at 4°C in buffer containing 10 mM Hepes, pH 7.4, 150 mM NaCl, 50 mM NaF, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1% SDS and centrifuged at 5,000 x g (10 min). Equal amounts of protein (by BCA assay) were resolved by SDS/PAGE and transferred to nitrocellulose membranes. After blocking, membranes were labeled with rabbit anti-CT15 (1:1000, a kind gift of E. H. Koo, UCSD), mouse mAb anti-hAPP (1:1,000, 8E5, Elan), or mouse mAb anti-GAPDH (1:1000, Millipore, Billerica, MA), and incubated with horseradish peroxidase–conjugated goat anti-rabbit IgG (1:5,000, Millipore) or goat anti-mouse IgG (1:10,000, Millipore). Bands were visualized by enhanced chemiluminescence and quantitated by densitometry and QUANTITY ONE 4.0 software (Bio-Rad, Hercules, CA).
Quantification of Aβ
For quantification of Aβ in young animals before plaque formation, snap-frozen hippocampi were homogenized in guanidine buffer. In older animals after plaque formation, soluble and insoluble fractions of Aβ were quantified separately using sequential extraction, as described (Leissring et al., 2003). Briefly, to extract soluble hippocampal Aβ, frozen hippocampi were homogenized with five volumes (w: v) of 50 mM Tris-HCL, pH 7.4, and centrifuges at 100,000 g for 1 h, and the resulting supernatant was denatured with 5 M guanidine (1:3) for ELISA measurement. To extract Tris-insoluble Aβ, the resulting pellet was dissolved in five volumes of guanidine buffer and spun at 100,000 g for 1 h after a 30-min incubation. ELISAs were performed as described (Johnson-Wood et al., 1997). We used antibodies that recognize species referred to as Aβ1–42 and Aβ1-X. The Aβ1–42 ELISA detects only Aβ1–42, and the Aβ1-x ELISA detects Aβ1–40, Aβ1–42, and Aβ1–43, as well as C-terminally truncated forms of Aβ containing amino acids 1–28.
Morris Water Maze
Experimenters were blind to the genotypes of the mice for all behavioral analyses (Morris water maze, elevated plus maze, and open field). Only male mice were used for behavioral assessment due to high levels of variability observed in female mice (Data not shown). The water maze consisted of a pool (122 cm in diameter) containing opaque water (20 ± 1°C) and a platform (14 cm in diameter) submerged 1.5 cm under the water. Mice were first given four pretrainings (90 sec/trial, day 0) in which they had to swim down a channel (15 × 122 cm) and mount a platform hidden 1.5 cm below the water surface at the end of the channel. Hidden platform training (days 1–5) consisted of 10 sessions (two per day, 2 h apart), each with three trials. The platform location remained constant in the hidden platform sessions, and the entry points were changed semirandomly between trials. The maximum trial time was 60 s. Mice that failed to find the platform were led to it and placed on it for 15 s. A day after the last hidden platform training session, a probe trial was conducted by removing the platform and allowing mice to search in the pool for 60 s. For cued training sessions (days 9–11), the platform was marked with a visible beacon, and the mice were trained to locate the platform over five sessions (two per day for the first two days, 4 h apart; one for the last day), each with two trials. The platform location was changed for each session. Time to reach the platform, time in target quadrant, platform crossings, path length, and swim speed were recorded with an EthoVision video tracking system (Noldus, Netherlands).
Elevated Plus Maze
The elevated plus maze consisted of two open (without walls) and two enclosed (with walls) arms elevated 63 cm above the ground (Hamilton-Kinder, Poway, CA). Mice were first allowed to habituate in the testing room under dim light for 30 min before testing. During testing, mice were placed at the junction between the open and closed arms of the plus maze and allowed to explore for 10 min. The distance traveled and time spent in the open arms over the total distance traveled and time spent in both open and closed arms were determined as a measure of disinhibition. The maze was thoroughly cleaned with 70% ethanol between testing sessions.
Open Field Test
An automated Flex-Field/Open Field Photobeam Activity System (San Diego Instruments, San Diego, CA) was used to assess spontaneous motor activity and within-session habituation to a novel environment. Mice were placed in one of four identical clear plastic chambers (41 x41 x 30 cm) for 15 min, with two 16 x 16 photobeam arrays detecting horizontal and vertical movements. Total movements (ambulations) in the outer periphery and center of the open field were reported. The apparatus was thoroughly cleaned with 70% ethanol between trials.
Electrophysiology
Experimenter was blind to the genotypes of the mice for all electrophysiological analyses. Acute hippocampal slices (350 μm, coronal) were prepared from 3–4-month-old mice of indicated genotypes. Briefly, brains were quickly removed and sliced (with a Vibratome 3000; Vibratome, St. Louis, MO) in ice-cold solution oxygenated with 95% O2–5% CO2 containing (in mM) 2.5 KCl, 1.25 NaPO4, 10 MgSO4, 0.5 CaCl2, 26 NaHCO3, 11 glucose, and 234 sucrose (300–305 mOsm). Slices were maintained in continuously oxygenated solution containing (in mM) 2.5 KCl, 126 NaCl, 10 glucose, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, and 26 NaHCO3 (pH ≈ 7.4 when gassed with a mixture of 95% O2–5% CO2) at 30°C for 30 min, and allowed to recover at room temperature for at least 45 min before being transferred to a submerged recording chamber. Field EPSPs were recorded via glass electrodes (~3 MΩ tip resistance) filled with 1 M NaCl and 25 mM HEPES (pH = 7.3), and evoked every 20 s by a bipolar tungsten electrode (FHC, Bowdoin, ME). Recordings were filtered at 2 kHz (−3 dB, eight-pole Bessel), digitally sampled at 20 kHz with a Multiclamp 700A amplifier (Molecular Devices, Foster City, CA), and acquired using a Digidata-1322A digitizer and pClamp 9.2 software. Data were analyzed offline using pClamp9 software and Origin 8.0 (OriginLab, Northampton, MA).
The stimulating electrode was placed in stratum radiatum (for CA1 I/O), LPP (for paired pulse) or MPP (for DG I/O and LTP). For recordings in the DG the bathing solution contained 10 μM GABAzine (Tocris) to block inhibitory transmission. After a 15 min stable baseline was established, LTP was induced by theta burst stimulation (TBS; a set of 10 bursts repeated 10 times every 15 s. Each burst, consisted of four pulses at 100 Hz, is repeated at 5 Hz).
Statistical Analysis
Statistical analyses were conducted with Graphpad Prism (San Diego, CA) or SPSS (Chicago, IL). Survival data were analyzed with Kaplan-Meier statistics and post-hoc log rank tests. Differences among multiple means with one variable (CysC genotype) were evaluated by one-way ANOVA and the Tukey-Kramer post-hoc test. Differences among multiple means with two variables (CysC and hAPP genotypes or CysC and CatB genotypes) were evaluated by two-way ANOVA and bonferroni multiple comparison post-hoc test. Differences between two means were assessed with unpaired, two-tailed t test or Mann-Whitney U test. Age-adjusted plaque load was assessed with multivariate loglinear regression analyses performed in SPSS. The logarithm-transformed plaque load was regressed against both age and genotype (as a dummy variable). Only values with P < 0.05 were accepted as significant.
Supplementary Material
Acknowledgments
We thank Drs. Hidde Ploegh and Christoph Peters for cathepsin B null mice, Dr. L. Mucke for insightful discussion, Dr. Katherine Pollard for advice on statistical analyses, Stephen Ordway and Gary Howard for editorial review, and Kelley Nelson for administrative assistance. This work was supported in part by NIH (NIA AG024447 to L.G.), a grant from Hellman Family Fund (to L. G), a grant from California Health Services (to L. G), and NIH/NCRR CO6 RRO18928 (a facility grant to J. David Gladstone Institutes). S.M.S was supported by a fellowship from the Swiss Science Foundation.
Footnotes
Supplemental data for this article can be found at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson M, Barrett AJ, Salvesen G, Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986;261:11282–11289. [PubMed] [Google Scholar]
- Benussi L, Ghidoni R, Steinhoff T, Alberici A, Villa A, Mazzoli F, Nicosia F, Barbiero L, Broglio L, Feudatari E, et al. Alzheimer disease-associated cystatin C variant undergoes impaired secretion. Neurobiol Dis. 2003;13:15–21. doi: 10.1016/s0969-9961(03)00012-3. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Beyer K, Lao JI, Gomez M, Riutort N, Latorre P, Mate JL, Ariza A. Alzheimer’s disease and the cystatin C gene polymorphism: An association study. Neurosci Lett. 2001;315:17–20. doi: 10.1016/s0304-3940(01)02307-2. [DOI] [PubMed] [Google Scholar]
- Bogyo M, Verhelst S, Bellingard-Dubouchaud V, Toba S, Greenbaum D. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogs. Chem Biol. 2000;7:27–38. doi: 10.1016/s1074-5521(00)00061-2. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Nixon RA. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci USA. 1990;87:3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 Protects against Microglia-dependent Amyloid-{beta} Toxicity through Inhibiting NF-{kappa}B Signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, Mucke L. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimerman N, Prebanda MT, Turk B, Popovic T, Dolenc I, Turk V. Interaction of cystatin C variants with papain and human cathepsins B, H and L. J Enzyme Inhib . 1999;14:167–174. doi: 10.3109/14756369909036552. [DOI] [PubMed] [Google Scholar]
- Crawford FC, Freeman MJ, Schinka JA, Abdullah LI, Gold M, Hartman R, Krivian K, Morris MD, Richards D, Duara R, et al. A polymorphism in the cystatin C gene is a novel risk factor for late-onset Alzheimer’s disease. Neurology. 2000;55:763–768. doi: 10.1212/wnl.55.6.763. [DOI] [PubMed] [Google Scholar]
- Deng A, Irizarry MC, Nitsch RM, Growdon JH, Rebeck GW. Elevation of cystatin C in susceptible neurons in Alzheimer’s disease. Am J Pathol . 2001;159:1061–1068. doi: 10.1016/S0002-9440(10)61781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer's amyloid beta peptide by endothelin-converting enzyme. J Biol Chem. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- Esposito L, Gan L, Yu GQ, Essrich C, Mucke L. Intracellularly generated amyloid-beta peptide counteracts the antiapoptotic function of its precursor protein and primes proapoptotic pathways for activation by other insults in neuroblastoma cells. J Neurochem. 2004;91:1260–1274. doi: 10.1111/j.1471-4159.2004.02816.x. [DOI] [PubMed] [Google Scholar]
- Finckh U, von der Kammer H, Velden J, Michel T, Andresen B, Deng A, Zhang J, Muller-Thomsen T, Zuchowski K, Menzer G, et al. Genetic association of a cystatin C gene polymorphism with late-onset Alzheimer disease. Arch Neurol. 2000;57:1579–1583. doi: 10.1001/archneur.57.11.1579. [DOI] [PubMed] [Google Scholar]
- Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce Abeta in transgenic Alzheimer's Disease mice expressing the wild-type, but not the Swedish mutant, beta -secretase APP site. J Biol Chem. 2008 doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- Huh CG, Hakansson K, Nathanson CM, Thorgeirsson UP, Jonsson N, Grubb A, Abrahamson M, Karlsson S. Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol Pathol. 1999;52:332–340. doi: 10.1136/mp.52.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser SA, Herzig MC, Coomaraswamy J, Kilger E, Selenica ML, Winkler DT, Staufenbiel M, Levy E, Grubb A, Jucker M. Cystatin C modulates cerebral beta-amyloidosis. Nat Genet. 2007;39:1437–1439. doi: 10.1038/ng.2007.23. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Klein WL. Aβ toxicity in Alzheimer’s disease: Globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Cullen WK, Fadeeva JV, Anwyl R, Selkoe DJ, Rowan MJ. Soluble Arctic amyloid beta protein inhibits hippocampal long-term potentiation in vivo. Eur J Neurosci. 2004;19:2839–2846. doi: 10.1111/j.1460-9568.2004.03389.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer's disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F. Co-deposition of cystatin C with amyloid-β protein in the brain of Alzheimer disease patients. J Neuropathol Neurol. 2001;60:94–104. doi: 10.1093/jnen/60.1.94. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- Mi W, Pawlik M, Sastre M, Jung SS, Radvinsky DS, Klein AM, Sommer J, Schmidt SD, Nixon RA, Mathews PM, Levy E. Cystatin C inhibits amyloid-beta deposition in Alzheimer's disease mouse models. Nat Genet. 2007;39:1440–1442. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci . 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, Laviola G. Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer's disease. Behav Brain Res. 2005;156:225–232. doi: 10.1016/j.bbr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem . 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer's disease. Science. 2006;314:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med. 2005;11:434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain's amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008 doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S. The plasmin system is induced by and degrades amyloid-β aggregates. J Neurosci. 2000;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B, Bieth JG, Bjork I, Dolenc I, Turk D, Cimerman N, Kos J, Colic A, Stoka V, Turk V. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol Chem Hoppe Seyler. 1995;376:225–230. doi: 10.1515/bchm3.1995.376.4.225. [DOI] [PubMed] [Google Scholar]
- Turk B, Turk D, Salvesen GS. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr Pharm Des. 2002;8:1623–1637. doi: 10.2174/1381612023394124. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Yamaguchi H, Akatsu H, Sakamoto T, Ko M, Mizoguchi K, Gong JS, Yu W, Yamamoto T, Kosaka K, et al. Angiotensin-converting enzyme converts amyloid beta-protein 1–42 (Abeta(1–42)) to Abeta(1–40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27:8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.