Abstract
Objectives
To determine the impact of pregnancy on the pharmacokinetics (PK) of nevirapine (NVP) during chronic dosing in HIV-infected women and appropriate NVP dosing in this population.
Methods
Twenty-six pregnant women participating in two open-label Pediatric AIDS Clinical Trials Group studies (P1022 and P1026S) were evaluated. Each patient received 200 mg NVP every 12 h and had PK evaluations during the second or third trimester; these evaluations were repeated postpartum. Paired maternal and cord blood NVP concentrations were collected at delivery in nine patients. Ante- and postpartum comparisons were made using paired t-tests and using a ‘bioequivalence’ approach to determine confidence interval (CI).
Results
The average NVP Area Under the Curve (AUC) was 56 ± 13 mcg*h/mL antepartum and 61 ± 15 mcg*h/mL postpartum. The typical parameters ± standard error were apparent clearance (CL/F)=3.51 ± 0.18 L/h and apparent volume of distribution (Vd/F)=121 ± 19.8 L. There were no significant differences between antepartum and postpartum AUC or pre-dose concentrations. The AUC ratio was 0.90 with a 90% CI of the mean equal to 0.80-1.02. The median ( ± standard deviation) cord blood to maternal NVP concentration ratio was 0.91 ± 0.90.
Conclusions
Pregnancy does not alter NVP PK and the standard dose (200 mg every 12 h) is appropriate during pregnancy.
Keywords: HIV, nevirapine, non-nucleoside reverse transcriptase, pharmacokinetics, pregnancy
Introduction
Mother-to-child transmission of HIV-1 can be greatly reduced if the maternal viral loads are below the limit of detection at delivery [1]. To achieve this target, combination antiretroviral (ARV) therapy, consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI), is recommended. Although efavirenz (EFV) is the preferred NNRTI in the general population, it has been found to cause severe central nervous system defects when given to primates during the first trimester, a finding consistent with retrospectively reported human neural tube defects [2]. These observations have led to increased reliance on nevirapine (NVP) for use in pregnant women and women with child-bearing potential. Despite recent reports of an increased risk of NVP-associated hepatic toxicity among women with CD4 counts >250 cells/μL [3,4], NVP continues to be a mainstay of ARV therapy in pregnancy for women with lower CD4 counts, particularly in resource-limited settings.
Physiological and hormonal changes associated with pregnancy can have profound effects on drug disposition, including increased plasma volume and reduced plasma protein concentration, increased renal blood flow and modified metabolic enzyme activity. Several ARVs have been studied in pregnancy, with some exhibiting significant alterations in pharmacokinetics (PK), requiring drug dose modifications or avoidance [5,6].
The antiviral effects of NVP are rapid, with significant reduction in plasma virus. However, development of viral resistance to NVP can occur with a single mutation in the HIV-1 reverse transcriptase gene [7]. NVP is metabolized primarily in the liver by the cytochrome P450 (CYP) 2B6 and 3A family enzymes [8,9]. Low NVP concentrations have been associated with inadequate viral suppression [10] and may also contribute to NNRTI resistance [11]. The PK of single-dose NVP to mothers at delivery have been described [12,13], but well-designed PK studies of continuous NVP therapy in pregnancy have not been published. In addition, limited cross-sectional analyses of NVP levels in pregnant women have suggested that NVP dose modifications may be needed for pregnant women [14].
The primary objectives of this study were to describe the PK of NVP in HIV-1 infected pregnant women in their second and third trimesters and to determine if the standard dose of NVP produces adequate drug exposure during pregnancy. These data were compared to repeat PK evaluations performed in the same patients postpartum. A secondary objective was to compare NVP concentrations in plasma from cord blood with those in maternal plasma at the time of delivery.
Patients and methods
The PK data for this analysis were collected prospectively from two Pediatric AIDS Clinical Trials Group (PACTG) studies. The first study was PACTG P1022 (Randomized Trial of Protease Inhibitor-including vs. Protease Inhibitorsparing Regimens for Women Who Initiate Therapy of HIV Infection During Pregnancy). This was a multi-centre prospective evaluation to compare classes of ARV agents during pregnancy. It was halted because of unexpected toxicity seen in women receiving NVP with high CD4 cell counts [4]. Women were eligible for this study if they: (i) were HIV-infected with a plasma viral load >1000 HIV-1 RNA copies/mL within 30 days of study entry; (ii) were pregnant between 10 and 30 weeks’ gestation; (iii) had received <8 weeks of prior ARV therapy [including zidovudine (ZDV) prophylaxis]; and (iv) agreed to continue ARV therapy for 2 years after delivery. Primary exclusion criteria included: (i) active hepatitis; (ii) receiving drugs likely to interact with NVP or PIs; (iii) any clinically significant diseases (other than HIV infection); (iv) laboratory abnormalities; and (v) active substance or alcohol abuse. Patients randomized to NVP therapy received 200 mg initially once daily for 14 days then twice daily in combination with ZDV (300 mg) and lamivudine (150 mg) twice daily.
The second study contributing to this evaluation was PACTG P1026S (Pharmacokinetic Properties of Antiretroviral Drugs During Pregnancy). This is a multi-centre, ongoing, prospective trial to evaluate the PK of currently prescribed ARV drugs and interacting combinations of these drugs in pregnant HIV-infected women. Eligible patients for this analysis were those who were: (i) already enrolled in the parent study (PACTG P1025); (ii) receiving NVP 200 mg orally twice daily as part of routine clinical care for at least 2 weeks prior to PK sampling; and (iii) planning to continue NVP until at least 6 weeks postpartum. Primary exclusion criteria were: (i) multiple gestation pregnancies; and (ii) clinical or laboratory toxicity that, in the opinion of the site investigator, would likely require a change in the ARV regimen during the study. Patients continued to take their prescribed medications during the study, unless changed by their clinical physician because of toxicity or lack of effectiveness or based on the results of the individual woman’s antepartum PK evaluation. Women continued on study until completion of postpartum PK sampling. Institutional review boards approved both P1022 and P1026S at all participating sites and all patients consented prior to participation.
Clinical laboratory monitoring
The clinical and laboratory monitoring for P1022 have been described previously [4]. For P1026S, medical history, clinical and laboratory data were collected in P1025. Data accessed for P1026S included maternal data [age, ethnicity, concomitant ARV medications, plasma HIV RNA concentration (viral load), CD4 cell count] and infant data (infection status, gestational age, birth length and weight). Information collected in P1026S included the time and description of recent ARV consumption, and height and weight on days of PK sampling. On each sampling day, patients underwent an interview to collect the medical history, a physical examination, and laboratory studies including alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, creatinine, blood urea nitrogen (BUN), albumin and haemoglobin. Adverse events were reported according to the Division of AIDS (DAIDS) standardized Toxicity Table for Grading Severity of Adult Adverse Experiences (August 1992) (http://rcc.tech-resintl.com). All toxicities were followed through resolution.
PK sample collection
Plasma samples (5 mL) for PK evaluation were collected at three evaluation times: antepartum, at delivery (P1026S) and postpartum (4-12 weeks after delivery). The antepartum evaluations were conducted during the second trimester (16-24 weeks’ gestation) or third trimester (30-36 weeks’ gestation). Women who did not complete the antepartum evaluation were replaced. Patients had received NVP at a stable dosage for at least 2 weeks prior to PK sampling. In P1026S, five plasma samples were drawn at both the ante- and postpartum PK evaluation visits, starting immediately before the witnessed morning oral NVP dose and at 1, 2, 4 and 6 h after the dose. Women in P1022 had PK samples collected at identical times to women in P1026S plus additional samples collected at 0.5, 1.5 and 8 h after their dose. To assess placental passage, NVP was measured in single maternal plasma and umbilical cord samples obtained at delivery in P1026S patients.
NVP concentrations
NVP concentrations were measured in the PACTG Pharmacology Laboratories. The laboratories are registered with the ACTG Quality Assurance/Quality Control proficiency testing programme, and successfully completed bi-annual proficiency testing for NVP during the study period. For P1022, the samples were analysed at the University of California, San Francisco PACTG Pharmacology Laboratory. The samples were assayed using a validated liquid chromatography/tandem mass spectrometry method. The limit of quantitation was 50 ng/mL, and the inter- and intra-day coefficient of variation for this method was consistently <5%. Overall mean recovery from plasma was 101.6%.
The P1026S samples were analysed at the PACTG Pharmacology Laboratory of the University of California, San Diego by a validated, reversed-phase high-performance liquid chromatography method. The lower limit of detection for NVP was 50 ng/mL. In the 24 assay runs for this study, the inter-assay coefficient of variation for the middle and high controls ranged from 0.9 to 7.7% [n=96 and 100 quality control (QC) samples] and from 7.4 to 15.1% for the low controls (n=100). Overall, recovery from plasma ranged from 87 to 98%.
PK analysis
NVP PK was analysed both by the individual patient and using a population approach. Both analyses were performed with the computer program NONMEM version V (Globomax, Hannover, MD, USA) using a one-compartment model with first-order absorption. The first-order conditional estimation subroutine with interaction was used in the estimation process. Because the objective of the study was to assess the impact of pregnancy and the overall patient numbers were limited, an extensive exploratory population analysis for other covariates was not undertaken. Empiric Bayesian PK parameters were estimated for each patient’s antepartum and postpartum evaluation.
Because of the more extensive sampling in P1022 patients, an additional non-compartmental PK analysis was performed in these patients. This included estimation of the individual Area Under the Curve (AUC) over the dosing interval (0-12 h) using the linear trapezoidal rule for ascending concentrations and the logarithmic trapezoidal rule for descending concentrations. Because patients were at steady-state at the time of the PK evaluations, the 0 h concentration measurement was used as an estimate of the concentration at 12 h.
Statistical analysis
Ante- and postpartum NVP exposure measurements from each woman were compared using a repeated-measures design. These comparisons were made at the within-patient level, using geometric mean ratios of ante- to postpartum AUC and Cmin. When the true geometric mean ratio (the antilog of the true mean of the log ratios) of the PK exposure parameters for ante- and postpartum conditions equals 1, this indicates equal PK exposure parameters for the ante- and postpartum conditions. The 90% confidence interval (CI) of the geometric mean ratio was determined and a no-effect window was defined as 0.8-1.25. Thus, if the entire 90% CI for the parameter mean ratio was within the range limits of 0.8 and 1.25, the PK exposure parameters for the pregnant and non-pregnant conditions were deemed to be equivalent [15]. The magnitudes of difference in median values of AUC and Cmin antepartum and postpartum also were assessed with the Wilcoxon signed-rank test. Descriptive statistics, including geometric least-squares means and 90% CIs, were calculated for PK parameters of interest at each study period.
Results
Twenty-six patients were included in this analysis, with seven pregnancy PK evaluations obtained in the second trimester and 19 obtained in the third trimester. Characteristics of the patients are shown in Table 1. Patients completed evaluations between October 2002 and August 2004. The plasma HIV-1 RNA concentration was <400 copies/mL in 20 of the 23 patients with data available during pregnancy, and in 23 of 24 patients postpartum. Of 26 infants, 25 were liveborn; of these, 24 were uninfected and one HIV-infected.
Table 1.
Characteristics of patients in the study population (n = 26)
| Mean ± SD | Range/frequency (%) | |
|---|---|---|
| Age (years) | 29 ± 6.4 | 15-40 |
| Antepartum weight (kg) | 77 ± 22 | 53-145 |
| Postpartum weight (kg) | 71 ± 21 | 45-163 |
| Antepartum CD4 (cells/μL) | 538 ± 300 | 155-1554 |
| Plasma HIV-1 RNA* (<400) | 21/24 | |
| Concomitant ARVs | Lamivudine | 23/26 (88%) |
| Zidovudine | 23/26 (88%) | |
| Abacavir | 2/26 (8%) | |
| Tenofovir | 2/26 (8%) | |
| Stavudine | 1/26 (4%) | |
| Nelfinavir | 1/26 (4%) | |
| Ethnicity | Black, non-Hispanic | 12 (46%) |
| Hispanic/Latino | 11 (42%) | |
| White, non-Hispanic | 2 (8) | |
| Other | 1 (4%) |
Two patients did not have viral loads measured near pharmacokinetics sampling visit.
ARVs, antiretrovirals; SD, standard deviation.
One patient had no measurable NVP concentration postpartum, suggesting non-adherence; this visit was excluded from the analysis. The remaining NVP concentrations data were described well by the model shown with no bias. The PK parameters from the population analysis are shown in Table 2. The inter-patient variability for apparent clearance (CL/F) was 35%. Adding pregnancy as a covariate to clearance did not improve the model significantly (antepartum/postpartum CL/F ratio = 1.11; P = 0.09). There were no significant differences seen between individual patients’ antepartum and postpartum AUC or pre-dose concentrations (Figs 1-3). The mean NVP AUC from the population analysis was 56 ± 13 mcg*h/mL antepartum and 61 ± 15 mcg*h/mL postpartum. Non-compartmental NVP AUC determinations in P1022 patients resulted in nearly identical values to those resulting from the compartmental modelling approach. The individual antepartum/postpartum AUC ratio was 0.90, with a 90% CL/F of 0.80-1.02.
Table 2.
Pharmacokinetics (PK) parameters [ ± standard error (SE)] from the population analysis
| Parameter estimate | SE of estimate | |
|---|---|---|
| CL/F (L/h) | 3.4 | 0.17 |
| Vd/F (L) | 79.2 | 6.6 |
| Ka (/h) | 0.42 | 0.11 |
| Inter-patient variability | ||
| CL/F (%) | 35 | |
| Ka (%) | 139 | |
| Residual variability | ||
| Proportional (%) | 8 | |
| Additive (mcg/mL) | 0.46 |
CL/F, apparent clearance; Ka, absorption rate constant; Vd/F, apparent volume of distribution.
Fig. 1.
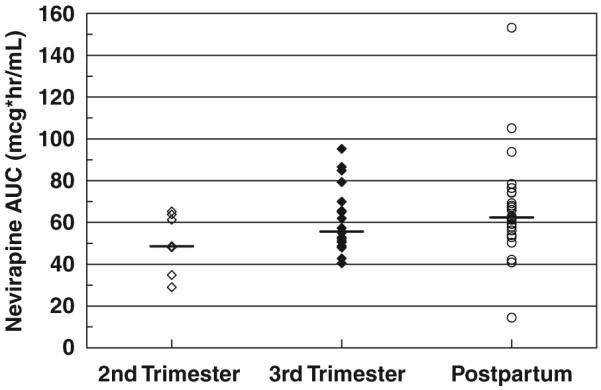
Nevirapine AUC by stage of pregnancy. No significant differences were present.
Fig. 3.
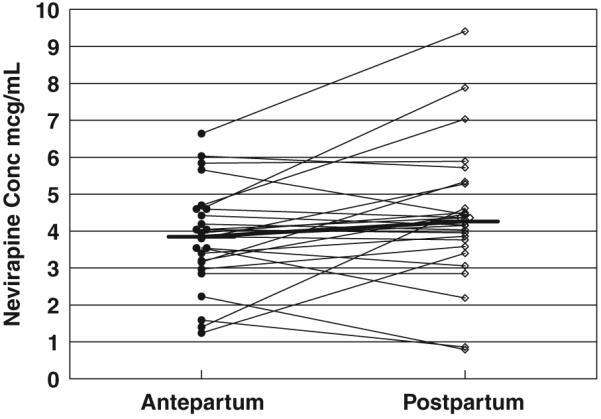
Paired, observed, minimum nevirapine (NVP) (Cmin) concentrations. There was no significant difference between antepartum and postpartum levels. Horizontal bars represent the median NVP trough concentrations.
In the nine P1026S patients where PK samples could be obtained at delivery, cord and maternal plasma concentrations (mean ± standard deviation) were 2.9 ± 0.9 and 3.1 ± 1.3 mcg/mL, respectively. The cord/maternal ratio was 0.91 ± 0.9.
Discussion
PK disposition for some compounds differs between men and women, with women displaying distinct therapeutic and toxic responses. For example, PK and virological responses to saquinavir, a CYP3A4 substrate, were found to be linked to gender [16]. These sex-based differences may be amplified during pregnancy. Previously, the impact of gender and pregnancy on NVP PK has undergone limited investigation. A population PK analysis of NVP indicated reduced clearance in non-pregnant females, but the impact of this effect was small (<15%) and may have been confounded by ethnic differences in the study populations [17].
In contrast, pregnancy may have profound effects on drug disposition, including induction of cytochrome P450 isoenzymes such as CYP2D6 and CYP3A4 [18]. This is supported by additional findings from P1026 including the reduced lopinavir exposure observed in 17 patients during pregnancy [6]. Thus, the development of appropriatedosing regimens of ARVs specifically for pregnant women is critical to the health of both the mother and the foetus.
NVP CL/F found in this study is significantly greater than the level reported previously following single-dose administration to pregnant women in labour [12]. This is expected because chronic NVP administration leads to auto-induction of its metabolism. The PK parameter estimates in this study compare favourably to those found in a large population PK study of chronic NVP use in adults [19], with the estimate for apparent volume of distribution (Vd/F) in this study a bit smaller, while the estimates for CL/F were almost identical (3.3 L/h vs. 3.4 L/h).
One previous report compared NVP PK levels in 16 pregnant women to those in men (n = 13) and non-pregnant women (n = 10) using a cross-sectional analysis [14]. While NVP AUC and Cmax were 29 and 32% lower, respectively, in pregnant women than in non-pregnant women, there was no difference in NVP Cmin (3.0 vs. 3.1) nor was there a difference between pregnant women and male patients. Patient selection and slower NVP absorption during pregnancy may have resulted in these observations.
The current study did not find a significant impact of pregnancy during the second or third trimester on NVP PK compared to values found postpartum in the same patients. NVP exposure was not affected by pregnancy using any comparative method including mixed-effects modelling, paired t-test or the ‘bioequivalence’ approach. Assessing the impact of pregnancy as a fixed effect on CL/F in the population model change did not improve the model fit. The median maximum a posteriori (MAP) Bayesian CL/F values were also similar during pregnancy (3.23 L/h) and postpartum (3.56 L/h). In addition, if one only compares the third trimester and postpartum AUCs, the values become nearly identical. If a minor pregnancy effect exists, one would have expected the maximal difference to occur during the third trimester.
Female gender and high CD4 cell count have been shown to increase the risk for hepatotoxicity with NVP [20]. Hepatitis, including fatal hepatic failure, has been seen with NVP use during pregnancy [4]. The lack of altered NVP PK during pregnancy suggests that elevated NVP exposure does not contribute to adverse effects that may be encountered in pregnant women.
While none of the patients with PK evaluations had Grade 2 AST elevations, 5/17 patients in P1022 experienced NVP-associated toxicity, including fulminant fatal hepatic failure. While toxicity has tempered enthusiasm for overall NVP use during pregnancy, the teratogenic effects of EFV and sometimes erratic absorption of PIs during pregnancy are concerns for the use of these agents. Given these considerations, NVP remains a recommended treatment option for women with low CD4 cell counts during pregnancy [21]. The wide availability NVP formulations, including generics (some in combination with NRTIs), also make NVP a cost-effective option in resource-limited settings.
Placental transfer of ARVs is an important consideration for drug selection during pregnancy. High placental expression of p-glycoprotein and possibly other drug transporters limit foetal exposure to PIs [22,23]. This characteristic reduces the potential for foetal ARV toxicity but may leave infants ‘under-protected’ against HIV infection in utero and during birth. Our study indicates that NVP - unlike PIs - readily crosses the placenta, achieving therapeutic concentrations in foetal circulations to help protect against early transmission. The near unity between cord and maternal blood NVP concentrations is consistent with a prior evaluation of NVP cord blood concentrations in women undergoing Caesarean section [24].
We used population PK and empiric Bayesian compartmental approaches to allow combination of PK data from two studies with different sampling designs to determine appropriate NVP dosing in pregnancy. CIs were used to assess the power of this PK study and the potential for pregnancy-induced changes in NVP disposition. This approach demonstrates that the 90% CI for NVP AUC during pregnancy falls entirely within 80-125% of reference AUC (postpartum). Therefore, this study had sufficient power to exclude any change in NVP exposure during pregnancy that would be considered clinically relevant and require a dose modification.
Although this analysis was well powered to address the impact of pregnancy on NVP AUC, only one of the two studies included in this analysis had frequent sampling to precisely determine Cmax. This translates into more limited precision in the Vd/F estimates. Also, we also did not assess patients for CYP 2B6 genotype. Genetic polymorphism for this enzyme has been linked with reduced metabolism of NVP. While patient genotypes were not determined in this study, alterations in activity would have been detected because each patient served as their own control.
In summary, NVP exposure (AUC) at steady state during the second or third trimester of pregnancy is similar to postpartum values. NVP readily crosses the placenta and provides ARV prophylaxis to the newborn at birth. Because pregnancy does not alter NVP clearance, no dose adjustment of NVP is required as a result of pregnancy. The PK properties of NVP make it a useful agent for continuous use in pregnant women with low CD4 cell counts.
Fig. 2.
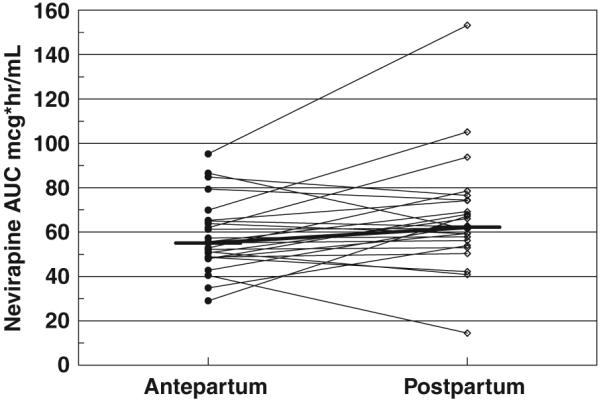
Paired nevirapine (NVP) AUC from the pharmacokinetics model. There was no significant difference between antepartum and postpartum evaluations. Horizontal bars represent the median NVP AUC.
Acknowledgements
The authors thank the participating women and staff of the clinical centres. We appreciate the vital contributions of Elizabeth Sheeran, Carol Elgie, Joanne Schiffhauer and Maureen Shannon to the conduct of this study. This study was supported in part by the Pediatric AIDS Clinical Trials Group of the National Institute for Allergy and Infectious Diseases (grants U01 AI04189 and U01AI41089), the Pediatric/Perinatal HIV-1 Clinical Trials Network of the National Institute of Child Health and Development (contract N01-HD-3-3365) and the General Clinical Research Center Units funded by the National Centre for Research Resources. E. C. and B. B. receive support from the Pediatric Pharmacology Research Unit Network of the National Institute of Child Health and Development (grant U10-HD-031318-11).
Financial support provided by NIAID (grants U01 AI04189 and U01AI41089) and NICHD (contract N01-HD-3-3365 and grant U10-HD-031318-11).
Appendix: contributing sites
University of Miami, Miller School of Medicine, Miami, FL: Amanda Cotter MD, Gwendolyn B Scott, MD.
University of Tennessee Health Science Center, Memphis, TN: Edwin Thorpe Jr MD, Nina K Sublette RN MSN FNP, Pat Flynn MD.
Albert Einstein College of Medicine Bronx-Lebanon Hospital Center, New York, NY: Stefan Hagmann MD, Murli Purswani MD FAAP.
Baylor College of Medicine, Houston, TX: William Shearer MD PhD, Mary E. Paul MD, Hunter Hammill MD, Shelly Buscher CNM.
University of California San Diego Medical Center, San Diego, CA: Andrew D. Hull BMedSci BMBS, Linda Proctor RN MSN CMN, Mary Caffery RN MSN.
The Columbian Presbyterian Medical Center, New York, NY: Marc Foca MD, Alice Higgins RN, Anne Gershon MD.
University of Medicine and Dentistry of New Jersey/ University Hospital, Newark, NJ: Arlene Bardeguez MD, Paul Palumbo MD.
University of Washington, Seattle, WA: Deb Goldman ARNP, Michele Acker ARNP, Jane Hitti MD.
Harbour-UCLA Medical Center, Torrance, CA: Margaret A. Keller MD, Marie Beall MD, Nicole Falgout RN, Spring Wettgen PNP.
Long Beach Memorial Medical Center, Long Beach, CA: Susan Marks RN, Audra Deveikis MD.
State University of New York at Stony Brook, Stony Brook, NY: Jennifer Griffin NP, Denise Ferraro RN, Silvia Muniz, Sharon Nachman MD.
San Juan City Hospital, San Juan, Puerto Rico: Jorge Gandía MD, Rodrigo Díaz MD, Elvia Pérez MEd MA, Lourdes Angel RN.
Los Angeles County Medical Center and University of Southern California Medical Center, Los Angeles, CA: Ana M. Melendrez RN, Yvonne Rodriguez, Françoise Kramer MD, LaShonda Spencer MD.
Brigham and Women’s Hospital, Boston, MA: Ruth Tuomala MD, Arlene Buck RN, Dorothy Pender RN.
University of California San Francisco, San Francisco, CA: Diane W. Wara MD, Maureen T. Shannon RN MS CNM FNP, Marya G. Zlatnik MD MMS, Shantrice M. Williams BA.
Medical College of Georgia, Augusta, GA: Chitra S. Mani MBBS DCH FAAP, Debra J. Ware MD, Karen Hickman MSN.
Baystate Medical Center, Springfield, MA: Barbara W. Stechenberg MD, Mary Patricia Toye RN, MS.
References
- 1.Mofenson LM, Lambert JS, Stiehm ER, et al. Pediatric AIDS Clinical Trials Group Study 185 Team Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N Engl J Med. 1999;341:385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 2.Saitoh A, Hull AD, Franklin P, Spector SA. Myelomeningocele in an infant with intrauterine exposure to efavirenz. J Perinatol. 2005;25:555–556. doi: 10.1038/sj.jp.7211343. [DOI] [PubMed] [Google Scholar]
- 3.Lyons F, Hopkins S, Kelleher B, et al. Maternal hepatotoxicity with nevirapine as part of combination antiretroviral therapy in pregnancy. HIV Med. 2006;7:255–260. doi: 10.1111/j.1468-1293.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 4.Hitti J, Frenkel LM, Stek AM, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. J Acquir Immune Defic Syndr. 2004;36:772–776. doi: 10.1097/00126334-200407010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Acosta EP, Zorrilla C, Van Dyke R, et al. Pharmacokinetics of saquinavir - SGC in HIV-infected pregnant women. HIV Clin Trials. 2001;2:460–465. doi: 10.1310/PUY3-5JWL-FX2B-98VU. [DOI] [PubMed] [Google Scholar]
- 6.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 7.Vidal C, Arnedo M, Garcia F, et al. Genotypic and phenotypic resistance patterns in early-stage HIV-1-infected patients failing initial therapy with stavudine, didanosine and nevirapine. Antivir Ther. 2002;4:283–287. [PubMed] [Google Scholar]
- 8.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27:1488–1495. [PubMed] [Google Scholar]
- 9.Riska P, Lamson M, MacGregor T, et al. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos. 1999;27:895–901. [PubMed] [Google Scholar]
- 10.Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001;15:1089–1095. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez de Requena D, Bonora S, Garazzino S, et al. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob Agents Chemother. 2005;49:3966–3969. doi: 10.1128/AAC.49.9.3966-3969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirochnick M, Fenton T, Gagnier P, et al. Pediatric AIDS Clinical Trials Group Protocol 250 Team Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. J Infect Dis. 1998;178:368–374. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 13.Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006) AIDS. 1999;13:479–486. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 14.Haberi A, Hentig N, Carlebach A, Kurowski M, Harder S, Staszewski S. Nevirapine plasma exposure is decreased in pregnant women; Proceedings of the XV International AIDS Conference; Bangkok. July 2004; Abstract TuPeB4644. [Google Scholar]
- 15.Food and Drug Administration Draft Guidance for Pharmacokinetic Studies During Pregnancy. 2004 October; www.fda.gov/cder/guidance/5917dft.htm 2004.
- 16.Fletcher CV, Jiang H, Brundage RC, et al. Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis. 2004;189:1176–1184. doi: 10.1086/382754. [DOI] [PubMed] [Google Scholar]
- 17.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther. 2005;10:145–155. [PubMed] [Google Scholar]
- 18.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 19.de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp EC, Beijnen JH. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol. 2002;54:378–385. doi: 10.1046/j.1365-2125.2002.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34(Suppl. 1):21–33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 21.Public Health Service Task Force Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2007 November 2; http://aidsinfo.nih.gov/ contentfiles/PerinatalGL.pdf 2007.
- 22.Camus M, Delomenie C, Didier N, et al. Increased expression of MDR1 mRNAs and P-glycoprotein in placentas from HIV-1- infected women. Placenta. 2006;27:699–706. doi: 10.1016/j.placenta.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Marzolini C, Rudin C, Decosterd LA, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16:889–893. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 24.Gingelmaier A, Kurowski M, Kästner R, et al. Placental transfer and pharmacokinetics of lopinavir and other protease inhibitors in combination with nevirapine at delivery. AIDS. 2006;20:1737–1743. doi: 10.1097/01.aids.0000242820.67001.2c. [DOI] [PubMed] [Google Scholar]


