Abstract
We report an age-dependent increase in nonimmunohematopoietic cells (CD45neg) in regenerating muscle characterized by high stem-cell antigen (Sca-1) expression. In aged regenerating muscle, only 14% of these CD45negSca-1pos cells express MyoD, whereas 82% of CD45negSca-1pos cells are MyoDpos in young adult muscle. In vitro, CD45negMyoDnegSca-1pos cells overexpress fibrosis-promoting genes, potentially controlled by Wnt2. The cells are proliferative, nonmyogenic, and nonadipogenic, and arise in clonally derived myoblast cultures from aged mice. MyoDneg Sca-1pos nonmyogenic cells also emerge in C2C12 myoblast cultures at late passage. Both in vitro and in vivo studies suggest that MyoDnegSca-1pos cells from aged muscle are more susceptible to apoptosis than myoblasts, which may contribute to depletion of the satellite cell pool. Thus, with age, a subset of myoblasts takes on an altered phenotype, which is marked by high Sca-1 expression. These cells do not participate in muscle regeneration, and instead may contribute to muscle fibrosis in aged muscle.
Keywords: Sca-1, Skeletal muscle regeneration, Muscle stem cells, Fibrosis, Aging
Muscle satellite cells are resident progenitors, located beneath the basal lamina of myofibers, that are primarily responsible for postnatal growth, repair, and maintenance of skeletal muscle (1,2). Quiescent satellite cells express Pax-7 and can be activated by muscle injury to produce progeny myoblasts that proliferate and migrate to the site of damage, followed by withdrawal from the cell cycle in order to fuse and differentiate into new myofibers or fuse with existing myofibers. Clearly, deficits in the ability of satellite cells and myoblasts to regenerate and/or repair myofibers following injury would contribute to decreased myofiber number and size with age, and considerable data obtained in rodents support this idea. Alterations in rodent myoblast metabolic activity (3) and proliferative capacity (3-6) occur with age. Further, changes in differentiation potential (7) may contribute to intramyocellular lipid accumulation (8,9) and muscle fibrosis (10,11), both processes regulated by Wnt signaling. The Wnt family of genes, encoding at least 19 lipid-modified signaling proteins (12), have been implicated in regulating proliferation and differentiation through the canonical β-catenin and noncanonical pathways [for review, see (13)]. Although some age-dependent phenotypes appear stable in vitro, suggesting inherent changes in cell potential, myoblast function is strongly influenced by the aging muscle environment (14,15).
On the other hand, two recent studies show that satellite cell number is reduced with age, but the cells that remain retain proliferation and differentiation capacity (16,17). An age-dependent loss in cells destined for self-renewal from the satellite cell/myoblast population would result in reduced regenerative capacity. A population of myofiber-associated cells, termed reserve cells, that are Pax7posMyoDneg emerge in culture and have been proposed to reflect the quiescent, self-renewing population (18-21). CD34, like Pax7, is expressed heterogeneously in quiescent satellite cells, and has been proposed to be a key regulator of the choice among proliferation, quiescence, and differentiation (22,23). Further, loss of Myf5 expression may also distinguish self-renewing from committed satellite cells (22,24). Sca-1 also shows heterogeneity in expression in myoblasts and has been proposed to distinguish myoblasts capable of contributing to myofiber repair (Sca-1neg) and those that will self-renew (Sca-1pos) during muscle regeneration in vivo (25). Sca-1 may also regulate the balance between proliferation and differentiation in myoblasts (26,27). However, changes in Sca-1 activity in muscle as a function of age have not been characterized. Based on the recent work that the satellite cell pool is not adequately replenished with age (16,17), we hypothesized that Sca-1-expressing myoblasts may be absent or depleted in aged muscle.
Sca-1, the Ly-6A/E member of the Ly-6 multigene family of glycosylphosphatidylinositol (GPI)-anchored membrane proteins, was originally identified on activated lymphocytes, but more recently has been used extensively to enrich for hematopoietic and other stem-cell populations [reviewed in (28)]. Sca-1 null mice appear to be impaired in self-renewal of both hematopoietic and osteogenic stem cells and display immune defects and osteoporosis (29,30). In muscle, Sca-1 is expressed on side population cells (31,32), early progenitors (23,33), myoblasts (25,26,34), and endothelial cells (35,36). Sca-1-expressing myoblasts proliferate more slowly (25), and do not form myotubes compared with nonexpressing myoblasts (25,34), although Sca-1-expressing cells have been shown to undergo myogenic differentiation upon injection into mdx mice (37). Myoblasts in Sca-1 null mice appear hyperproliferative, resulting in delayed muscle repair following injury (27). As a ligand for Sca-1 has not been identified, it has been proposed that Sca-1 co-regulates lipid raft signaling to control stem-cell fate decisions (28).
In this article, we demonstrate that, following injury in aged muscle, the abundance of Sca-1-expressing, non-immunohematopoietic cells increases and that these cells are nonmyogenic (CD45negMyoDnegSca-1pos), whereas Sca-1 is expressed on activated satellite cells following injury in adult muscle (CD45negMyoDposSca-1pos). The nonmyogenic Sca-1pos cells arise from clonally derived aged myoblasts and overexpress genes associated with fibrosis, potentially regulated by Wnt2. Further, the cells are more susceptible to apoptosis than myoblasts in vitro, and Sca-1posTUNELpos (terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling) cells are readily detectable in aged muscle following injury. Primary myoblasts isolated from aged mice, as well as long-term passaged C2C12 myoblasts, heterogeneously lose MyoD expression, and concomitantly upregulate the expression of Sca-1. This expression pattern is characterized by lack of both myogenic and adipogenic differentiation potential and appears to mimic the age-associated myogenic-to-fibrogenic conversion of activated satellite cells (11), independent of the aged muscle environment.
Materials and Methods
Animals
All studies were approved by the University Animal Care and Use Committee and overseen by the Division of Laboratory Animal Medicine (University of Kentucky and University of Arkansas for Medical Sciences). All studies were performed on 6-month-old and 23–24-month-old female DBA/2JNIA mice obtained from the NIA Aging Rodent Colony at Harlan Sprague Dawley and mice were maintained on normal chow.
Muscle Regeneration
Mice (4 adult [6-month-old] and 4 aged [24-month-old]) were anesthetized by isoflurane inhalation and muscle necrosis induced using cardiotoxin injection. A 5 mm incision was made over the tibialis anterior muscle, which was then exposed. Twenty-five microliters of 10 μM cardiotoxin (snake venom from Naja Mossambica, Sigma, St. Louis, MO) was injected into the muscle under direct vision (9). The incision was closed and the mice euthanized 1 week following injury. All in vivo analyses were performed in the areas immediately adjacent to and surrounding the major area of massive infiltration of CD45posSca-1pos immunohematopoietic cells.
Isolation, Maintenance, and Differentiation of Primary Myoblasts
Myoblasts from the tibialis anterior pooled from four adult (6-month-old) and four aged (23-month-old) DBA/2JNIA mice characterized previously (8) were used. Additional myoblast isolates were obtained from all hindlimb muscles of two 6-month-old and two 24-month-old DBA/2JNIA mice following mechanical disruption and enzymatic digestion with 1.5 U/ml collagenase D (Sigma) and 2.4 U/ml dispase (Sigma). All experiments were performed in duplicate on each of the three myoblast isolates. Myoblasts were plated on collagen-coated plates (Sigma) and maintained in growth medium containing Ham's F-10 (BioWhittaker, Walkersville, MD) supplemented with 20% fetal bovine sera (FBS) (BioWhittaker), 0.5% pen-strep (Invitrogen, Carlsbad, CA) and 5 ng/mL basic fibroblast growth factor (bFGF, Promega, Madison, WI) at 37°C in a humidified 5% CO2–95% air atmosphere. Sca-1-expressing cells were depleted from aged myoblast cultures using the Sca-1 antibody and the Dynal CELLection PAN Mouse IgG (immunoglobulin G) kit with the magnetic particle concentrator (Invitrogen) and expanded. For differentiation, cells were cultured on E-C-L (Upstate Biotechnology, Lake Placid, NY), grown to confluence, and then switched to differentiation medium consisting of Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) with 2% horse serum (Hyclone, Logan, UT) and 0.5% penstrep. Myogenic differentiation was assayed by myotube formation and myosin heavy chain (MyHC) expression after 72 hours (see Immunocytochemistry below).
To assess adipogenic potential, cells were grown to >95% confluence and then switched to medium composed of DMEM and 20% FBS supplemented with a cocktail of IBMX (115 mg/mL iso-butylmethylxanthine; 5 × 10−4 M dexamethasone (Sigma); and 25 U/mL insulin (Novolin, Clayton, NC) (8) for 3 days with daily feeding. On day 4, this medium was replaced with growth medium supplemented with insulin only. On day 5, lipid accumulation was assessed by Oil Red O staining (8).
For clonal analysis, limiting dilutions were performed resulting in approximately 20 clones from each of the 3 aged myoblast isolates. When composed of approximately 50–100 cells, clones were replica-plated and one replica immunostained for MyoD and Sca-1. For those that were MyoDposSca-1neg, the other replica was expanded. When the clones contained a few thousand cells (5–10 additional population doublings), clones were immunostained for Sca-1 and/or MyoD.
C2C12 Cell Culture
C2C12 myoblasts were grown in DMEM supplemented with 20% FBS at 37°C in a humidified 10% CO2–90% air atmosphere. Cells were maintained at <60% confluence requiring passaging every 3 days. Intermediate passage C2C12s had undergone approximately 50 population doublings, whereas late passage C2C12s had undergone more than 300 population doublings and had been maintained in culture continuously for more than 1 year. Differentiation was induced as described above.
Fluorescence-Activated Cell Sorting (FACS)
Cells for FACS analysis were isolated by enzymatic digestion as for direct culture and pooled from 6 adult (6-month-old) and 6 aged (23–24-month-old) mice. Following digestion, cells were pelleted by centrifugation, resuspended in DMEM with 10% FBS, and filtered sequentially through 70 μm and 40 μm nylon mesh filters (BD Biosciences, San Jose, CA). Sca-1-expressing cells are rare in both young adult and aged muscle and following muscle injury, CD45posSca-1pos cells increase in abundance, which overwhelms the increase in muscle cell-derived Sca-1-expressing cells. To promote satellite cell activation and Sca-1 expression, but avoid immune cell infiltration, the filtrate was incubated at 37°C in 5% CO2 for 6 hours after which cells were spun down and dissolved in PBS with 0.5% FBS (PF buffer). All subsequent steps until collection of cells after FACS were performed at 4°C. Cells were counted, repelleted, and resuspended in viral supernatant 2.4G2 (HB197, ATCC, Manassas, VA) at a concentration of 5×106 cells/ml for 15 minutes to block nonspecific antibody binding. Cells were incubated for 30 minutes with two primary antibodies: CD45.2, clone 104 antibody conjugated with APC-Alexa Fluor® 750 (eBiosciences, San Diego, CA), and a Sca-1, Ly-6A/E antibody conjugated with PE (BD Biosciences). Labeled cells were washed in PF buffer, filtered through a 30 μm filter (BD Biosciences), and subjected to FACS analysis on a BD Biosciences FACS Vantage cell sorter. Sca-1-positive and -negative cells from the CD45 negative pool were collected and cultured for RNA isolation. The entire FACS analysis was performed twice.
Gene Expression Analysis
Total RNA was prepared from primary cultures using RNAqeous 4PCR (Ambion, Austin, TX), including DNAse treatment, according to the manufacturer's recommendations. RNA quality was assessed with a Bioanalyzer nanochip assay (Agilent Technologies, Santa Clara, CA), and RNA concentration was determined with a spectrophotometer by measuring the absorbance at 260 nm. Expression changes of genes related to the Wnt signaling pathway were screened using a polymerase chain reaction (PCR)-based gene array (SuperArray Bioscience Corporation, Frederick, MD). Abundance of the gene products was calculated using the ΔCT method according to the manufacturer's recommendations using 500 ng of RNA for each array. Changes detected with the PCR array were verified, and additional genes quantified by real-time reverse transcriptase (RT)-PCR utilizing a standard curve from pooled cDNAs on an IQ5 icycler using SYBRGreen (BioRad, Hercules, CA). For these experiments, 1 μg of RNA was converted to cDNA using the iScript reverse transcriptase kit (BioRad). Depending on the gene being analyzed 0.1–10 ng of cDNA/well was used for real-time analysis. In all cases, the primer concentrations were held at 0.3 μM and the annealing temperature was set at 60°C. Reactions were characterized by a single-peak melting curve, 90%–110% amplification efficiency of the standard curve, and no amplification of a nontemplate control. Samples were run in duplicate and experiments repeated three times. Each value was normalized to 18s ribosomal RNA using 18s F primer 5′-AA TGAGCCATTCGCAGTTTC-3′ and R primer 5′-CTCTG TTCCGCCTAGTCCTG-3′. For MyoD F 5′ GGCAGAA TGGCTACGACACC and R 5′ CACTATGCTGGACAG GCAGTC primers were used. All other primer sequences can be found at http://pga.mgh.harvard.edu/primerbank/ using the following Genbank accession numbers: Sca-1 (Ly6/A), NM_010738; Cyclin D1, NM_007631; CyclinD2, NM_009829; Myc, NM_010849; Wnt2, NM_023653; Wnt7b, NM_009528; Wnt10, NM_011718; Twist1, NM_011658; Fibronectin, AF095690; CTGF, NM_010217. Results were analyzed using one-way analysis of variance (ANOVA) (Holm-Sidak method) and are presented as means and standard errors.
Proliferation Assays
To quantify proliferation, cells were seeded at 3 × 104/35 mm on collagen-coated dishes. At 24-hour intervals, cells were trypsinized and 3 separate 35 mm dishes were counted per time point using a hemocytometer under an inverted microscope. Values represent means ± standard errors.
In Vitro Apoptosis Assay
To induce apoptosis, primary myoblasts were seeded in duplicate at 5 × 104 cells/35 mm dish and expanded overnight. Staurosporin was added to growth medium to a final concentration of 5 μM. Cells were placed in a humidified incubator for 2 hours at 37°C, then washed with PBS, fixed with 4% paraformaldehyde, and stained for TUNEL as described below. Cells in three different fields on each plate were counted and the number of TUNELpos cells to the total number counted were expressed as mean percentage ± standard errors. The experiment was repeated three times and results were analyzed using Student's t-test.
TUNEL Staining
Apoptotic cells in vitro and in vivo were detected by the TUNEL reaction according to the manufacturer's recommendations (Roche Diagnostics, Indianapolis, IN). Six micron tissue sections or cells were fixed with 4% paraformaldehyde, blocked in 3% hydrogen peroxide in methanol, and permeabilized with 1% Triton X-100 in 0.1 sodium citrate. TUNEL reaction mix was added at a dilution of 1:5 and incubated for 1 hour at 37°C. Cells were reacted with the antifluorescein antibody, Fab fragment, conjugated with horse-radish peroxidase for 30 minutes at 37°C. The Tyramide Signal Amplification Kit (TSA Fluorescein, NEL701, Perkin Elmer, Boston, MA) was used to visualize TUNEL positive nuclei through HRP catalyzed deposition of FITC-labeled tyramide according to the manufacturer's protocol.
Immunohistochemistry
Tibialis anterior muscles were cut on a cryostat in 6 μm sections, air dried, and stored at −20°C until analysis. Sections were fixed in 4% paraformaldehyde, and endogeneous peroxide activity was blocked by incubating in PBS+0.3% hydrogen peroxide for 30 minutes. Sections were incubated with purified anti-Ly6A/E (Sca-1) antibody (BD Biosciences) overnight at 4°C followed with a horseradish peroxidase (HRP)-conjugated mouse antirat IgG secondary antibody (Zymed, San Francisco, CA) for 1 hour at room temperature. TSA-Fluorescein (NEL701, Perkin Elmer) was used to detect antibody-binding. For all antibodies, cells were counted in the areas immediately adjacent to and surrounding the major area of damage and immune cell inflitration.
For Sca-1/MyoD double-staining, Sca1 immunodetection was performed first as described above. Sections were then permeabilized in PBS + 1% Igepal (Sigma) and incubated with purified anti-MyoD (5.8A, BD Biosciences). Subsequently, the sections were incubated with a rat antimouse, HRP-labeled secondary antibody. TSA-Coumarin (NEL703, Perkin Elmer) was used to detect anti-MyoD binding.
For Sca-1/TUNEL and Sca-1/laminin double-staining, Sca-1 was performed first. TUNEL was performed as described except that the TSA-Cyanine 5 system (NEL705A, Perkin Elmer) was used for detection. For laminin detection, a rabbit polyconal antibody (L9393, Sigma) was used followed by an alkaline phosphate-conjugated secondary antibody (04-1622, Zymed) and the Alkaline Phosphatase substrate kit (SK5100, Vector). For each antibody combination, a minimum of three animals were analyzed. Cells were counted in three separate areas composed of at least 100 myofibers on 3 sections from each animal. Mean percentages and standard errors were compared using Student's t-test.
Immunocytochemistry
Cells in culture were fixed with 4% paraformaldehyde for 15 minutes. For cell surface antigen detection, cells were incubated overnight at 4°C with anti-Sca-1, anti-CD34, or anti-CD45 (BD Biosciences) followed by an HRP-conjugated antirat secondary antibody (Zymed) for 1 hour at room temperature. TSA-Fluorescein was used to detect antibody binding as described above.
For the nuclear antigens MyoD and Pax-7, cells were permeabilized following fixation for 10 minutes with 0.05% Igepal (Sigma), blocked with 2.5% normal horse blocking serum provided in the ImmPress Antimouse Ig staining kit (Vector Laboratories, Burlingame, CA), and incubated overnight at 4°C, with anti-MyoD (BD Biosciences) or anti-Pax-7 (Developmental Studies Hybridoma Study Bank [DSHB], University of Iowa). Subsequently, cells were incubated for 30 minutes with the HRP-coupled ImmPress reagent, followed by TSA-Fluorescein. For Sca-1 and MyoD double-staining, Sca-1 immunodetection was performed first followed by MyoD staining as described above except that TSA-Coumarin (NEL703, Perkin Elmer) was used to detect anti-MyoD staining.
For myosin heavy chain (MyHC)/TUNEL double-staining, cells were fixed in absolute methanol and incubated in A4.1025 supernatant (Helen Blau, Stanford Medical School, Stanford, CA), recognizing an epitope common to all MyHCs, for 1 hour at room temperature after which an HRP-conjugated rat antimouse secondary antibody (Zymed) was added. TSA-coumarin (NEL703, Perkin Elmer) was used for visualization. TUNEL was subsequently performed as described above.
Soft Agar Assay
The soft agar assay for measuring anchorage-independent growth was performed on the MyoDneg Sca-1pos cells. The murine-transformed cell line 4T1 (CRL-2539 ATCC) was used as a positive control. Thirty-five-millimeter dishes were first layered with 0.6% agar in FBS and tryptose phosphate broth. The upper layer containing the cells was prepared by diluting agar to 0.3% with approximately 103 cells in DMEM supplemented with FBS and tryptose phosphate broth. The dishes were kept in a humidified incubator at 37°C with 5% CO2. Colonies greater than 1 mm were counted under an inverted microscope after 2 weeks.
Image Acquisition
All photomicrographs were acquired using a digital Nikon Eclipse E600 microscope, with a Nikon Plan Fluor 20X/0.50 or 40X/0.8 objectives. The specific fluorochromes used with each specific detection system are described in detail above and were captured using a Photometrics CoolSnap black and white camera with MetaVue software (Molecular Devices Corp., Sunnyvale, CA). Images were colorized, optimized globally for contrast and brightness, and assembled into figures using Adobe Photoshop 6.0.
Results
MyoDnegSca-1pos Cells, Which Preferentially Accumulate in Muscle From Aged Mice Following Injury, Undergo Apoptosis
Sca-1-expressing cells were rare in both young adult and aged muscle (<1 Sca-1pos cell/100 myofibers, data not shown), but abundance increased in both tissues following cardiotoxin injection. One week after injury, aged muscle (Figure 1B) contained three times more Sca-1-positive cells than young adult muscle (Figure 1A), quantified in Figure 1C. In an attempt to identify muscle cell-derived Sca-1pos cells, fluorescence-activated cell sorting (FACS) using anti-Sca-1 antibodies, and anti-CD45 antibodies to eliminate immune-hematopoietic-derived cells, was performed. Results suggested that CD45negSca-1pos cells were more abundant in aged (24-month-old) compared to young adult (6-month-old) muscle (Figure 2). FACS analysis on two sets of mice resulted in similar cell profiles with Sca-1pos cells representing approximately 14% of the CD45neg population in aged and 7% in young adult mice. Although overall less abundant, approximately 82% ± 9% of Sca-1pos interstitial cells in young adult muscle (Figure 3A) also expressed MyoD (Figure 3B, overlay in Figure 3C), whereas this percentage dropped to 14% ± 5% in aged muscle (Figure 3D). Double immunohistochemistry with anti-Sca-1 and antilaminin to visualize the basal lamina showed that, of the Sca-1pos cells located between myofibers, less than 1% were found beneath the basal lamina in the satellite cell location (Figure 4). A large fraction of Sca-1pos cells in aged muscle (63% ± 18%, Figure 5A) showed nuclear fragmentation visualized by TUNEL (Figure 5B, overlay in Figure 5C), whereas we were unable to detect Sca-1pos cells that were also TUNEL pos in adult muscle (Figure 5D). The rare TUNEL pos nuclei that were detected in adult muscle were not associated with Sca-1 staining (Figure 5E). Taken together, these results suggest that MyoDnegSca-1pos cells in aged muscle are undergoing apoptosis.
Figure 1.
Sca-1-expressing cells are more abundant in regenerating aged than young adult muscle. Sca-1 immunofluorescent staining in adult (A) and aged (B) muscle was quantified (C). Areas immediately adjacent to the cardiotoxin-induced infiltration of immune cells were analyzed. Sca-1pos cells are three-fold more abundant in aged than young adult muscle. Cells were counted in 3 separate areas composed of at least 100 myofibers on 3 sections from each animal. Means and standard errors were compared using Student's t-test. Asterisk (*) indicates significant difference, p ≤ .05. Scale bar = 50 μm.
Figure 2.
Fluorescence-activated cell-sorting suggests increased CD45negSca-1pos cells in muscle from aged mice. For each experiment, cells were harvested from hind-limb muscle of six 24-month-old and six 6-month-old mice, pooled and labeled with anti-CD45 and anti-Sca-1 antibodies. Sorting gates were set by using cells labeled with one antibody at a time. The experiment was performed twice.
Figure 3.

Sca-1pos cells expressing MyoD are more abundant in regenerating adult than aged muscle. Double immunofluorescent staining was quantified in adult (A–C) and aged (D) muscle immediately adjacent to the area of cardiotoxin-induced degeneration to avoid the massive infiltration of immune cells that occurs in that area. Although overall less abundant in young adult muscle, approximately 82% ± 9% of Sca-1pos cells were also MyoDpos, whereas this frequency dropped to 14% ± 5% in aged muscle. (A) Sca-1 is visualized in green; (B) MyoD is visualized in blue; (C) overlay of Sca-1 and MyoD in adult muscle; (D) overlay of Sca-1 and MyoD in aged muscle. Cells were counted in three separate areas composed of at least 100 myofibers on 3 sections from each animal. Mean percentages and standard errors were compared using Student's t-test. Scale bar = 50 μm.
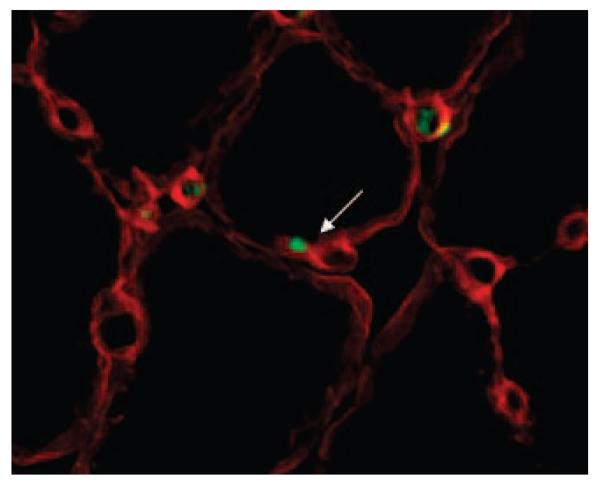
Figure 4.
Sca-1-expressing cells can be found in the satellite cell position in regenerating aged muscle. Double immunofluorescent staining of aged muscle showed that less than 1% of Sca-1pos cells (green) were located beneath the basal lamina (red), visualized using an antilaminin antibody.
Figure 5.
In regenerating aged muscle, Sca-1-expressing cells are undergoing apoptosis. Double immunofluorescent staining of aged (A–C) and adult (D and E) muscle showed that 63% ± 18% of Sca-1pos cells in aged muscle were also TUNELpos (terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling positive). (A) Sca-1 is visualized in pink; (B) TUNEL is visualized in green; (C) overlay of Sca-1 and TUNEL; (D and E) overlay of Sca-1 and TUNEL in adult muscle. Sca-1pos cells were not TUNELpos (D). The very rare TUNELpos cells detected were Sca-1neg (E). Scale bar = 50 μm.
MyoDneg Sca-1pos Cells Are Derived From Myoblasts In Vitro
To test the hypothesis that myoblasts can transition from MyoDposSca-1neg → MyoDposSca-1pos → MyoDnegSca-1pos in a passage or age-dependent manner, 26 clones of aged primary myoblasts that were initially 100% MyoDpos (Figure 6A) and Sca-1neg (Figure 6B) were followed in culture. By the time each clone contained several thousand cells, all contained Sca-1pos cells at a frequency of 10%–30% (Figure 6C), suggesting that they are derived from myoblasts.
Figure 6.

MyoDnegSca-1pos cells arise from clonally derived MyoDposSca-1neg primary myoblasts. Clonally derived myoblasts were replica-plated and immunostained for MyoD (A) and Sca-1 (B) when clones consisted of 50–100 cells. Twenty-six clones that were 100% MyoDposSca-1neg were expanded and Sca-1-assessed when clones contained several thousand cells (C). All clones contained Sca-1pos cells at this stage and when replicative capacity was declining. Scale bar = 50 μm.
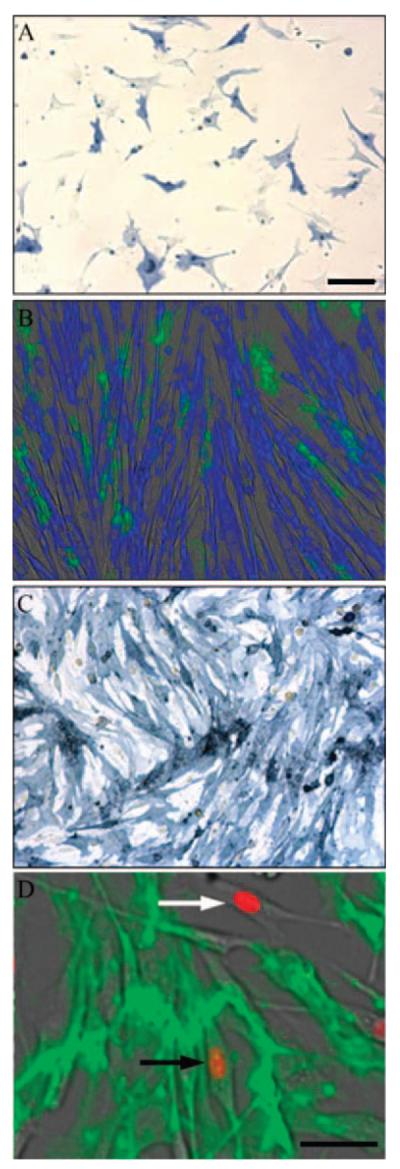
Due to the limited replicative capacity of primary myoblasts following cloning, the C2C12 myoblast cell line, maintained in culture for approximately 50 population doublings (intermediate passage) or greater than 300 population doublings (late passage), was studied. Immunocytochemical analysis of intermediate passage C2C12-derived cells supported the hypothesis that MyoDnegSca-1pos cells can be derived from myoblasts (Figure 7A). C2C12-derived cells with strong nuclear MyoD had little Sca-1 staining (Figure 7A, black arrows). Some cells accumulated both Sca-1 and nuclear MyoD (Figure 7A, white arrow), and in those cells expressing the highest levels of Sca-1, nuclear MyoD was lowest (Figure 7A, open arrows). In fact, in cells expressing a high level of Sca-1 (Figure 8A, black arrows), cytoplasmic MyoD staining was detectable (Figure 8B, black arrows). By contrast, late-passage C2C12s were essentially MyoDneg Sca-1pos (Figure 7B). Under these conditions, the late passage C2C12-derived cells were fusion incompetent and did not express myosin heavy chain (MyHC) upon serum withdrawal (Figure 7E), compared to earlier passage cells that readily fused to form myotubes expressing MyHC (Figure 7C and D). Interestingly, TUNELpos nuclei were readily apparent only in the late passage, MyoDneg Sca-1pos C2C12s (Figure 7E, black arrows), with only rare apoptosing nuclei detected in differentiated earlier passage C2C12s (Figure 7D, white arrow).
Figure 7.
MyoDnegSca-1pos cells are derived from the C2C12 cell line with time in culture. Double immunofluorescence staining of intermediate (A, C, and D) and late (B and E) passage C2C12s. MyoD (blue) and Sca-1 (green) in proliferating cells (A and B) demonstrate reciprocal expression patterns. (A) C2C12-derived cells expressing high levels of MyoD show low Sca-1 expression (black arrows). Cells expressing moderate levels of Sca-1 show reduced MyoD expression (white arrows). Cells with high levels of Sca-1 expression show no nuclear MyoD accumulation (open arrows). (B) Double immunofluorescent staining of late passage C2C12-derived cells shows Sca-1 expression with little MyoD. (C–E) Double immunofluorescence staining for myosin heavy chain (MyHC, blue) and terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) (green) show that intermediate passage C2C12 cells differentiate in low serum (C and D), which contain only very rare TUNELpos nuclei (D, white arrow). (E) Late passage C2C12-derived cells do not differentiate, as evidenced both by lack of fusion and MyHC expression, and contained numerous TUNELpos cells (black arrows). Scale bar = 50 μm.
Figure 8.
MyoD is nonnuclear in cells expressing high levels of Sca-1. (A) and (B) are higher magnification micrographs of cells from Figure 7A. (A) Double immunofluorescence with MyoD (blue) and Sca-1 (green). (B) Single immunofluorescence staining for MyoD of the same cells shown in (A). Whereas nuclei were strongly MyoDpos in cells with low Sca-1 expression, low-level MyoD staining was perinuclear in strongly Sca-1pos cells (arrows).
MyoDneg Sca-1pos Cells From Aged Muscle Display Altered Wnt Gene Expression Associated With Impaired Myogenic Potential and Increased Fibrosis
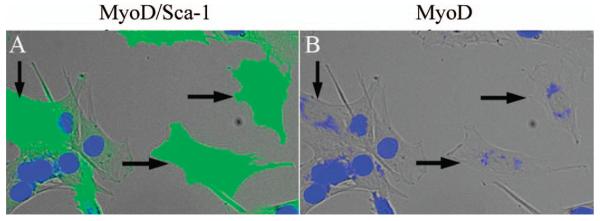
Whereas Sca-1 expression in myoblasts from young animals is dynamic [(25) and data not shown], Sca-1 expression in cells from aged mice was stable, permitting expansion of the cells (Figure 9A). Incubating mixed cultures of Sca-1pos and Sca-1neg cells in myogenic differentiation medium demonstrated that only Sca-1neg cells fused to form myotubes characterized by MyHC expression (Figure 9B), and Sca-1pos cells purified to near homogeneity showed little fusion capability in differentiation medium (Figure 9C). Double-staining with an antibody against MyoD indicated essentially no Sca-1/MyoD coexpression (Figure 9D). Sca-1pos cells also did not express Pax7 (data not shown).
Figure 9.
Sca-1-expressing cells from aged skeletal muscle are deficient in myogenic potential. Sca-1-expressing cells were enriched to approximately 50% in mass cultures of aged myoblasts followed by immunostaining for Sca-1 (blue in A and C). (A) Sca-1pos cells were larger and more flattened than myoblasts. (B) Withdrawal of serum from confluent mixed cultures of Sca-1pos and Sca-1neg cells showed that Sca-1pos cells (green) do not fuse to form myotubes. Myotubes formed by fusion of Sca-1neg cells expressed myosin heavy chain (blue). (C) Sca-1pos cells enriched to >95% purity by Sca-1 coated magnetic beads did not fuse in differentiation medium and demonstrated that Sca-1 expression was stable, but intensity of staining varied between cells. (D) Double immunofluorescent staining for MyoD (orange) and Sca-1 (green) demonstrated that the majority of Sca-1pos cells do not express MyoD. Those cells that do express MyoD (white arrow) were likely contaminating myoblasts with only very rare MyoDposSca-1pos cells present (black arrow). Scale bar = 50 μm.
To characterize the cells in more detail, quantitative real-time RT-PCR was performed on MyoDposSca-1neg myoblasts from young adult and aged mice, and MyoDneg Sca-1pos cells from aged mice (Figure 10). We were unable to isolate stable populations of MyoDnegSca-1pos cells from young animals. Significant differences in the expression of transcripts encoding several Wnt ligands were apparent (Figure 10B). Wnt2 mRNA was expressed at low levels in myoblasts at both ages, and expression was significantly higher in MyoDnegSca-1pos cells. This was associated with overexpression of downstream canonical target genes of Wnt signaling (Figure 10C). Also, target genes shown to be specifically downstream of Wnt2 such as Twist (Figure 10D), and genes associated with fibrosis including connective tissue growth factor (CTGF) and fibronectin were significantly overexpressed in MyoDnegSca-1pos cells compared to myoblasts. Wnt7b mRNA was expressed at lower levels in both cell types from aged mice relative to young adult myoblasts, whereas the Wnt10b gene was down-regulated only in aged myoblasts (Figure 10B), which resulted in activation of an adipocyte-like phenotype in those cells (8,9). Consistent with this observation, MyoDnegSca-1pos cells (Figure 11B) accumulate less lipid than aged myoblasts (MyoDposSca-1neg; Figure 11A), indicated by Oil Red O staining, when cultured under conditions that promote adipogenic differentiation in preadipocytes.
Figure 10.
Gene expression analysis shows altered expression of Wnt pathway genes. Myoblast RNA from young adult (6-month-old) and aged (24-month-old) mice depleted of Sca-1-expressing cells by Sca-1 antibody-coated magnetic beads and from fluorescence-activated cell sorter (FACS) isolated MyoDnegSca-1pos cells pooled from muscles of six 24-month-old mice were analyzed by real-time reverse transcriptase polymerase chain reaction (RT-PCR). Data presented are means and standard errors normalized to 18s, expressed as arbitrary units (AU). (A) MyoD and Sca-1 mRNA levels are consistent with protein expression; (B) specific Wnt genes (Wnt2, 7b, and 10b) showing the greatest difference in gene expression between the cell types; (C) representative downstream genes of canonical Wnt signaling; (D) genes related to fibrosis. Twist1 has been described as a downstream target of Wnt2. Data were analyzed by one-way analysis of variance (ANOVA) (Holm-Sidak method). †, ‡, and *indicate significantly different (p ≤ .05) from adult, aged, and Sca-1+, respectively.
Figure 11.
Phenotypic characterization of MyoDnegSca-1pos cells. Lipid accumulation, as visualized by Oil Red O, was assessed in confluent aged MyoDposSca-1neg myoblasts (A) and MyoDnegSca-1pos cells (B) cultured in medium that promotes lipid accumulation in preadipocytes. Only aged MyoDposSca-1neg myoblasts demonstrated lipid staining. (C) Proliferation rates did not differ between MyoDposSca-1neg and MyoDnegSca-1pos cells. Scale bars=50 μm.
MyoDnegSca-1pos Cells Are More Susceptible to Apoptosis In Vitro Than Aged Myoblasts
Results from in vitro and in vivo analyses presented above suggest that cells expressing Sca-1 in aged muscle may be undergoing apoptosis. To test this idea, MyoDpos Sca-1neg (Figure 12A) and MyoDnegSca-1pos (Figure 12B) cells from aged muscle were treated in culture with staurosporine for 2 hours to induce apoptosis, followed by TUNEL-staining to identify apoptotic nuclei. Cell loss did not occur within 2 hours of treatment in either cell type, but there were significantly more TUNEL-positive cells in the MyoDnegSca-1pos population (Figure 12C). Finally, MyoDneg Sca-1pos cells from aged muscle proliferate at a rate comparable to myoblasts (Figure 11E), and are not anchorage independent as they do not grow in soft agar (data not shown).
Figure 12.
Staurosporin treatment demonstrates that MyoDnegSca-1pos cells are more susceptible to apoptosis than myoblasts. MyoDposSca-1neg (A) and MyoDnegSca-1pos (B) cells from aged muscle were treated with 5 μM staurosporin for 2 hours, fixed and stained for terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) (green). (C) Quantification of TUNELpos cells. The experiment was performed three times on duplicate dishes and results analyzed by Student's t-test. Asterisk (*) indicates a significant difference from MyoDposSca-1neg cells, p < .05.
Discussion
Our work suggests that, with advancing age, CD45neg−MyoDnegSca-1pos cells increase in abundance in muscle following injury and on occasion can be found in the satellite cell position. Cells with this phenotype have been described in young adult muscle where they are nonmyogenic in vitro (34); however, cells expressing Sca-1 also have been reported to possess myogenic potential (31,32,37,38). Whereas MyoDnegSca-1pos cells do not readily arise from myoblasts isolated from young mice [(25) and data not shown], they do arise from myoblasts isolated from aged mice, as well as following long-term culture of C2C12 myoblasts. As Sca-1 expression increases, MyoD expression decreases and the MyoD protein that is detectable remains cytoplasmic. Genes associated with fibrosis, including Twist1, CTGF, and fibronectin, are overexpressed in MyoDnegSca-1pos cells compared to both young adult and aged MyoDposSca-1neg myoblasts and this may be regulated by Wnt2, which is also highly overex-pressed in MyoDnegSca-1pos cells. Expression of Wnt2, which signals through a canonical β-catenin-dependent pathway (39), has been shown to induce the expression of the basic helix-loop-helix transcription factor Twist1 in mammary gland cells (40). Twist1 inhibits the expression of MyoD (41) and also induces fibronectin expression both in breast cancer (42) and gastric carcinoma cells (43). Moreover, it has been shown that Wnt2 and Twist1 are highly expressed in a model of kidney fibrosis (44), suggesting that, in aged muscle, Wnt2 and Twist1 may be important regulators of fibrosis.
On the other hand, Wnt7b, which promotes myogenic differentiation (9), is down-regulated in both aged myoblasts (MyoDposSca-1neg) and MyoDnegSca-1pos cells from aged mice, which may contribute to impaired myogenic potential (9). Wnt10b, which inhibits adipogenic differentiation (45,46), declines only in aged myoblasts, potentially accounting for increased adipogenic potential specifically in those cells (8). As overall markers of Wnt/β-catenin signaling appear to be up-regulated in the MyoDnegSca-1pos cells, Wnt7b and 10b likely signal through noncanonical pathways to control differentiation potential.
These results are consistent with a recent report that satellite cells convert to a fibroblastic lineage in aged muscle, contributing to muscle fibrosis, which is associated with increased canonical Wnt signaling (11), and suggest that Sca-1 is a useful marker of this transition. In addition to changes in Wnt signaling, alterations in TGFβ signaling occur with age, which may also contribute to increased expression of fibrosis-promoting genes, particularly CTGF (10). We observe increased expression of canonical Wnt-responsive genes primarily in the MyoDnegSca-1pos cells, suggesting that aged MyoDposSca-1neg myoblasts retain some aspects of normal Wnt signaling apart from changes specifically associated with altered differentiation potential discussed above. However, as these cells are in close proximity in aged muscle and are influenced by both the aged muscle and systemic environment (14,15,47), their behavior in vitro will not accurately reflect behavior in vivo. The fact that age-dependent differences in phenotype are stable in culture suggests that inherent changes in cell potential occur that are clearly exacerbated by the aged muscle environment.
Although all the work presented and discussed have been in rodents, interesting parallels appear to exist with human myoblasts, even though regulation of cell proliferation differs between rodents and humans, and a human homolog of Sca-1 has not been identified. The number of satellite cells declines with age in humans (48), and myoblasts from old donors, as well as myoblasts aged in vitro, display altered myogenic properties (49). Further, fibroblastic markers increase in human myoblasts during replicative senescence in vitro (50).
Myoblasts are heterogeneous based on Sca-1 expression, and Mitchell and colleagues hypothesize that the MyoDposSca-1pos myoblasts represent a self-renewing population, whereas MyoDposSca-1neg myoblasts actively participate in muscle regeneration [(25); see model, Figure 13]. MyoDposSca-1pos myoblasts proliferate more slowly than MyoDposSca-1neg myoblasts and are nonmyogenic, and there is interconversion between these two cell types. Work by Epting and colleagues (26,27) also shows that Sca-1neg myoblasts are more proliferative than those expressing Sca-1, but their work suggests that Sca-1 expression in myoblasts promotes differentiation. It is possible that, in young adult muscle, Sca-1 may be involved not only in self-renewal, but in regulating the balance between proliferation and differentiation in different subpopulations of cells. Work presented here suggests that, with age, transition to a nonmyogenic MyoDnegSca-1pos fibrogenic phenotype occurs (Figure 13). This is unexpected in that, for hematopoietic stem cells, Sca-1 deficiency appears to hasten age-associated changes (28). As enthothelial cells in muscle can also express Sca-1 (35,36), we have not ruled out the possibility that they also undergo an age-associated fibrogenic conversion and contribute to muscle fibrosis. However, as MyoDnegSca-1pos arise from clonally derived myoblasts, results from our in vitro studies suggest that myoblasts are a major source of MyoDnegSca-1pos cells in aged muscle. Some MyoDnegSca-1pos cells may return to the quiescent myofiber-associated pool, but no longer express myogenic markers as proposed by Collins and colleagues (17). The increased susceptibility of MyoDnegSca-1pos cells to apoptosis-inducing agents in vitro, and increased abundance of Sca-1pos apoptotic cells in regenerating aged muscle suggest that these cells are lost from muscle during recovery, potentially contributing to depletion in the number of satellite cells capable of self-renewal in aged muscle (16,17). Taken together, the model (Figure 13) proposes that, with age, myoblasts can contribute directly to two features of aging muscle: increased intramyocellular lipid accumulation and increased fibrosis, and that Sca-1 is a useful marker of the latter.
Figure 13.
Model expanded from the work of Mitchell and colleagues (25) in young animals summarizing the effects of aging on myogenic progenitors (highlighted in yellow). Quiescent satellite cells express neither Sca-1 nor MyoD. Upon activation, most cells express MyoD (blue nuclei), but not Sca-1 (green cell surface), and these myoblasts are highly proliferative and myogenic differentiation competent. A small percentage of cells may coactivate MyoD and Sca-1 and are characterized by low proliferation and an inability to undergo myogenic differentiation. In young animals, these cells have the potential to return to quiescence through down-regulation of both MyoD and Sca-1 expression, thereby renewing the satellite cell population. Alternatively, cells may down-regulate Sca-1, returning to the myoblast pool to contribute to myofiber regeneration. Aging affects both MyoDposSca-1neg and MyoDposSca-1pos myoblast populations. In the latter, down-regulation of MyoD results in cells that are proliferative but differentiation defective. Fibrosis-promoting genes (red lines) are overexpressed and the cells are lost by apoptosis. With age, MyoDposSca-1neg myoblasts express genes characteristic of both myotubes and adipocytes (red droplets).
Acknowledgments
The authors thank Marjorie L. Beggs for technical assistance. This work was supported by grants to C.A.P. from the National Institutes of Health (AG20941 and AG12411, project 4) and the Central Arkansas Veterans Health Care System, and by the UAMS Microarray Facility through Act 1, The Arkansas Tobacco Settlement Proceeds Act of 2000, and by NIH Grant #P20 RR-16460 from the BRIN Program of the National Center for Research Resources.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 3.Barani AE, Durieux AC, Sabido O, Freyssenet D. Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. J Appl Physiol. 2003;95:2089–2098. doi: 10.1152/japplphysiol.00437.2003. [DOI] [PubMed] [Google Scholar]
- 4.Dodson MV, Allen RE. Interaction of multiplication stimulating activity/rat insulin-like growth factor II with skeletal muscle satellite cells during ageing. Mech Ageing Dev. 1987;39:121–128. doi: 10.1016/0047-6374(87)90003-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SE, Allen RE. Proliferating cell nuclear antigen (pcna) is expressed in activated rat skeletal muscle satellite cells. J Cell Physiol. 1993;154:39–43. doi: 10.1002/jcp.1041540106. [DOI] [PubMed] [Google Scholar]
- 6.Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev. 1982;20:377–383. doi: 10.1016/0047-6374(82)90105-1. [DOI] [PubMed] [Google Scholar]
- 7.Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol. 2004;97:1082–1090. doi: 10.1152/japplphysiol.00006.2004. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 9.Vertino AM, Taylor-Jones JM, Longo KA, et al. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFβ signaling pathway in myogenic progenitors with age. Aging Cell. 2004;3:353–361. doi: 10.1111/j.1474-9728.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 11.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 12.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipidmodified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 13.Huelsken J, Behrens J. The wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 14.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 15.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 16.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins CA, Zammit PS, Perez Ruiz A, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 18.Halevy O, Piestun Y, Allouh MZ, et al. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- 19.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zammit PS, Relaix F, Nagata Y, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 22.Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 24.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell PO, Mills T, O'Connor RS, Graubert T, Dzierzak E, Pavlath GK. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol. 2005;283:240–252. doi: 10.1016/j.ydbio.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Epting CL, Lopez JE, Shen X, Liu L, Bristow J, Bernstein HS. Stem cell antigen-1 is necessary for cell-cycle withdrawal and myoblast differentiation in C2C12 cells. J Cell Sci. 2004;117:6185–6195. doi: 10.1242/jcs.01548. [DOI] [PubMed] [Google Scholar]
- 27.Epting CL, Lopez JE, Pedersen A, et al. Stem cell antigen-1 regulates the tempo of muscle repair through effects on proliferation of alpha7 integrin-expressing myoblasts. Exp Cell Res. 2008;314:1125–1135. doi: 10.1016/j.yexcr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 29.Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in sca-1/ly-6a null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Hematopoietic stem cell and progenitor defects in sca-1/ly-6a-null mice. Blood. 2003;101:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 31.Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 32.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol. 2007;311:449–463. doi: 10.1016/j.ydbio.2007.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torrente Y, Tremblay JP, Pisati F, et al. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deasy BM, Lu A, Tebbets JC, et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of b-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 40.Howe LR, Watanabe O, Leonard J, Brown AM. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- 41.Hamamori Y, Wu HY, Sartorelli V, Kedes L. The basic domain of myogenic basic helix-loop-helix (bHLH) proteins is the novel target for direct inhibition by another bHLH protein, Twist. Mol Cell Biol. 1997;17:6563–6573. doi: 10.1128/mcb.17.11.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z, Zhang X, Gang H, et al. Up-regulation of gastric cancer cell invasion by twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Com. 2007;358:925–930. doi: 10.1016/j.bbrc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Kida Y, Asahina K, Teraoka H, Gitelman I, Sato T. Twist relates to tubular epithelial-mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J Histochem Cytochem. 2007;55:661–673. doi: 10.1369/jhc.6A7157.2007. [DOI] [PubMed] [Google Scholar]
- 45.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 46.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 47.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines functional recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 48.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 49.Lorenzon P, Bandi E, de Guarrini F, et al. Ageing affects the differentiation potential of human myoblasts. Exp Gerontol. 2004;39:1545–1554. doi: 10.1016/j.exger.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Bigot A, Jacquemin V, Debacq-Chainiaux F, et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell. 2008;100:189–199. doi: 10.1042/BC20070085. [DOI] [PubMed] [Google Scholar]