Abstract
Tau function is regulated by phosphorylation, and abnormal tau phosphorylation in neurons is one of the key processes associated with development of Alzheimer’s disease and other tauopathies. In this study we provide evidence that phospholipid transfer protein (PLTP), one of the main lipid transfer proteins in the brain, significantly reduces levels of phosphorylated tau, and increases levels of the inactive form of glycogen synthase kinase-3β (GSK3β) in HCN2 cells. Furthermore, inhibition of the phosphatidylinositol-3 kinase (PI3K) reversed the PLTP-induced increase in levels of GSK3β phosphorylated at serine 9 (pGSK3βSer9) and partially reversed the PLTP-induced reduction in tau phosphorylation. We provide evidence that the PLTP-induced changes are not due to activation of Disabled-1 (Dab1), since PLTP reduced levels of total and phosphorylated Dab1 in HCN2 cells. We have also shown that inhibition of tyrosine kinase activity of insulin receptor (IR) and/or insulin-like growth factor 1 (IGF1) receptor (IGFR) reverses PLTP-induced increase in levels of phosphorylated Akt (pAktThr308 and pAktSer473), suggesting that PLTP-mediated activation of the PI3K/Akt pathway is dependent on IR/IGFR receptor tyrosine kinase activity. Our study suggests that PLTP may be an important modulator of signal transduction pathways in human neurons.
Keywords: Alzheimer’s disease, signal transduction pathways, phosphorylation, neuronal cells, in vitro
Introduction
Phospholipid transfer protein (PLTP) is a versatile, nearly ubiquitously expressed protein, involved in transfer of cholesterol and phospholipids, lipoprotein metabolism, inflammatory processes, apoptosis and transfer of vitamin E among lipoproteins, and between lipoproteins and cells (Barlage et al 2001; Desrumaux et al 2005; Hailman et al 1996; Wehinger et al 2007; Wolfbauer et al 1999). Expression of PLTP in the brain likely plays a significant role in the brain lipid and lipoprotein metabolism (Vuletic et al 2003). However, most studies of PLTP thus far have focused on PLTP functions in extracellular lipoprotein metabolism.
Previously published studies have shown that PLTP binds and stabilizes ATP-binding cassette A1, ABCA1 (Oram et al, 2003), indicating that PLTP is capable of binding cell surface receptors. We have recently shown that PLTP is localized in the nucleus, that its nuclear export is chromosome region maintenance-1 (CRM1) dependent, that secreted PLTP can re-enter cells and be translocated to the nucleus, and that intranuclear PLTP is active in phospholipid transfer (Vuletic et al, 2009). Thus PLTP could be involved in regulation of intracellular pathways and processes both by binding of extracellular PLTP to the receptors on the cell surface, as well as by internalization at the plasma membrane either by insertion of the putative transmembrane domain or by whole protein uptake. Alternatively, PLTP synthesized by cells could have distinct intracellular functions. These putative PLTP functions have yet to be studied.
PLTP levels in the central nervous system are altered in neurodegenerative and inflammatory brain disorders, including Alzheimer’s disease (AD). Patients with AD have significantly higher levels of PLTP in brain tissue, and significantly lower PLTP-mediated phospholipid transfer activity in cerebrospinal fluid than neurologically healthy controls, suggestive of sequestration of PLTP in brain tissue of patients with AD (Vuletic et al 2003; Vuletic et al 2005). Pathophysiological consequences of these observed alterations are currently unknown.
The hyperphosphorylation of tau and consequent development of neurofibrillary tangles (NFTs) in the affected neurons represent one of the hallmarks of histologically verified changes in the brain tissue of patients with AD, originally discovered by Alois Alzheimer in 1907 (reviewed by Iqbal and Iqbal 2006). Physiological regulation of tau phosphorylation is an important mechanism that ensures undisturbed intracellular transfer through the microtubule system, which is essential for regulation of synaptic functions in neurons (Mandelkow et al 2003). Understanding the pathways that are involved in regulation of tightly controlled tau phosphorylation is relevant for our understanding of pathophysiological processes that lead to tau hyperphosphorylation, disintegration of the tubular system, and formation of neurofibrillary tangles in neurons of patients who suffer from AD and other tau-related neurodegenerative diseases. For example, cerebrospinal fluid levels of tau phosphorylated at threonine 231 highly positively correlate with cognitive decline both in people with mild cognitive impairment, as well as in those with AD (Buerger et al 2002; Gauthier et al 2006). These findings strongly suggest that regulation of tau phosphorylation is a critical process in maintenance of normal cognitive functions in humans. In this in vitro study we tested the hypothesis that PLTP is directly involved in modulation of tau phosphorylation in neuronal cells.
Materials and Methods
Materials
Cell culture media were obtained from Lonza (Walkersville, MD). Fetal calf serum with low endotoxin levels was obtained from HyClone (Logan, UT). Affinity-purified antibodies against human total Dab1 (Cat. No. 3328), Dab1 phosphorylated at tyrosine 232 (Cat. No. 3325), Dab1 phosphorylated at tyrosine 220 (Cat. No. 3327), GSK3β phosphorylated at serine 9 (Cat. No. 9336), total Akt (Cat. No. 4691), Akt phosphorylated at threonine 308 (Cat. No. 2965) and serine 473 (Cat. No. 4060), as well as the phosphatidylinositol-3 kinase (PI3K) inhibitor, LY294002, were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against Dab1 phosphorylated at serine 491 (catalog No. D0900-15) and tau phosphorylated at serine 356 (catalog No. T1032-64) were obtained from US Biological (Swampscott, MA). Total tau (Cat. No. 577801), tau phosphorylated at threonine 231 (Cat. No. 577813), and tau phosphorylated at serine 199/202 (Cat. No. 577802) were detected using affinity-purified antibodies from Calbiochem-EMD BioSciences (La Jolla, CA). Trypan Blue dye to test viability and antibody against β-actin (Cat. No. A2066) were obtained from Sigma-Aldrich (St. Louis, MO). Secondary antibodies that react only with native IgG (TrueBlot), thus eliminating false-positive staining, were obtained from eBiosciences (San Diego, CA). Proteins were isolated in the presence of protease inhibitors (Halt protease inhibitor cocktail, Invitrogen, Carlsbad, CA) using PhosphoSafe protein isolation solution (EMD Chemicals, Gibbstown, NJ), which preserves phosphorylated sites. Criterion XT gels, sample buffer and MOPS running buffer were purchased from Bio-Rad (Hercules, CA). Western blot blocking reagent, PhosphoBlocker, was obtained from Cell Biolabs Inc. (San Diego, CA). SuperSignal West Femto Maximum Sensitivity substrate was purchased from Thermo Scientific/Pierce (Rockford, IL). Inhibitor of insulin receptor (IR) tyrosine kinase activity, HNMPA(AM)3(hydroxy-2-naphthalenylmethylphosphonic acid tris acetoxymethyl ester), was obtained from Enzo Life Sciences International (Plymouth Meeting, PA). Human recombinant PLTP was expressed in baby hamster kidney cells (BHK), isolated, prepared and evaluated for activity and purity, as previously reported (Wolfbauer et al 1999).
Cell culture
Human neuronal cells, HCN2 (ATCC CRL-10742) were plated in 6-well plates, and grown in DMEM supplemented with 12% fetal calf serum at 37°C, 5% CO2 until 80% confluent. Human neuroblastoma cells, SK-N-SH, were used for gene expression analyses, and grown under identical conditions in DMEM supplemented with 4% fetal calf serum. For most experimental purposes, cells were incubated under serum-free conditions, while some experiments were conducted with serum supplementation to exclude possibility that our findings are due to serum starvation. Viability of cells was evaluated using Trypan Blue (Sigma-Aldrich). Briefly, HCN2 cells were grown in serum-free media for 48 hours without or with rPLTP (2, 5 and 10 μg/ml). Following incubation, cells were washed, trypsinized and stained with Trypan Blue for 3 minutes; number of total and positively stained cells was established by counting in five separate fields for each experimental condition. Under all experimental conditions, cell viability was above 90%. Following incubation, cells were extensively washed to remove dead cells and cell debris, and scraped.
Electrophoresis and Western blot analyses
Proteins were isolated using PhosphoSafe protein isolation solution (EMD Chemicals), which preserves phosphorylated sites in the presence of protease inhibitors (Halt protease inhibitor cocktail, Invitrogen), according to the manufacturer’s instructions. Cell proteins were subjected to denaturing sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. Samples were loaded on 4–12% gel (BioRad Bis-Tris Criterion XT) for SDS-PAGE, and following electrophoresis, transferred to nitrocellulose membrane using BioRad semi-dry transfer. Blots were incubated overnight with primary antibodies at 4°C with agitation in PhosphoBlocker, a specialized blocker that preserves availability of phosphorylated sites for antibody binding (the same blocker was used in all incubation steps). Loading control was evaluated using β-actin. The blots were incubated with the appropriate TrueBlot secondary antibody for two hours at room temperature. The membranes were developed using West Femto SuperSignal chemiluminescent kit, scanned using Kodak CF400 imaging system, and evaluated by densitometry scanning.
PI3K Inhibition
Cells were grown in serum-free media for 48 hours, in the presence or absence of the PI3K inhibitor, LY294002 (50 μM). Following incubation, cells were washed, and cell protein isolated using PhosphoSafe with protease inhibitor cocktail. Cell proteins were separated by SDS-PAGE, and levels of phosphorylated GSK3β and tau were established by Western blotting.
Inhibition of Insulin Receptor Tyrosine Kinase Activity
HCN2 cells were incubated with HNMPA(AM)3, an inhibitor of the insulin receptor (IR) and insulin-like growth factor 1 (IGF1) receptor (IGFR) tyrosine kinase activity (Saperstein et al, 1989). The inhibitor or vehicle (DMSO) was added to serum-free media 2 hr before addition of rPLTP at concentrations previously shown to inhibit IR (50 μM) and IGFR (100 μM; Diaz et al., 2007) and incubated with rPLTP (5 μg/ml) for 10 and 30 min. Cells were then washed and scraped. Isolated proteins were evaluated for total and phosphorylated Akt/PKB by SDS-PAGE/Western blot.
RNA Isolation
RNA was isolated using Qiagen RNEasy kit, according to the manufacturer’s instructions. To remove trace genomic DNA, samples were treated with DNase and DNA-Shredder kit (Qiagen). Following assessment of 260/280 nm ratio by spectrometry, RNA samples were stored at −70°C until use. Total RNA was quantified on the Mx4000® Multiplex QPCR System (Stratagene, La Jolla, CA) using the RiboGreen® RNA Quantitation Kit (Molecular Probes, Eugene, OR).
Quantitative PCR
Quantitative PCR was performed on an Mx4000® Multiplex QPCR System with samples loaded in triplicate using 40 ng of total RNA. Quantitative PCR was run in a 10 μl reaction using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) (5μl 2× Master Mix, 400 nM each primer, 0.5 units of StrataScript RT, 0.5 units of RNase Block) with PCR cycling conditions of 48°C for 30 minutes, 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds, 60°C for 1 minute. After each assay, a dissociation curve was run to confirm specificity of all PCR amplicons. Resulting Ct values were converted to nanograms, normalized to total RNA and 18S, and expressed as a ratio of gene expression and total RNA/18S. Pooled sample total RNA was used for standard curves in 1:2 serial dilutions.
Primers were from Operon (Huntsville, AL) and designed using Applied Biosystems Primer Express 2.0 software. Primers used in the study are listed in Table 1.
Table 1.
List of primers used in the study.
| Gene | Accession # | Primer Name | Primer | Amplicon (nt) |
|---|---|---|---|---|
| MAPT | NM005910 | HMAPT-4038F | CAGGGATTGGGATGAATTGC | 113 |
| variants 1–4 | HMAPT-4150R | TCTGGTCAAGGCTTTGGGAA | 113 | |
| GSK3β | NM002093 | HGSK3B-1526F | TCCACCTGAACAGTCCCGA | 81 |
| HGSK3B-1606R | CGTGACCAGTGTTGCTGAGTG | 81 | ||
| DAB1 | NM021080 | HDAB1-798F | TACAAAGCCAAATTGATCGGG | 87 |
| HDAB1-884R | GCCCTTGAGTTTCATCATGGA | 87 |
Note: h= human; F= forward; R= reverse.
Abbreviations: DAB1 – Disabled 1; GSK3β – glycogen synthase kinase 3β; MAPT – microtubule-associated protein tau.
Statistical Analyses
Statistical analyses were performed with Statistica for Windows (StatSoft Inc., 2000, Tulsa, OK). Differences were evaluated using Mann-Whitney U-test, Wilcoxon matched-pair test and T-test, and p-values <0.05 were considered statistically significant.
Results
PLTP reduces tau phosphorylation at threonine 231 and serine 199/202
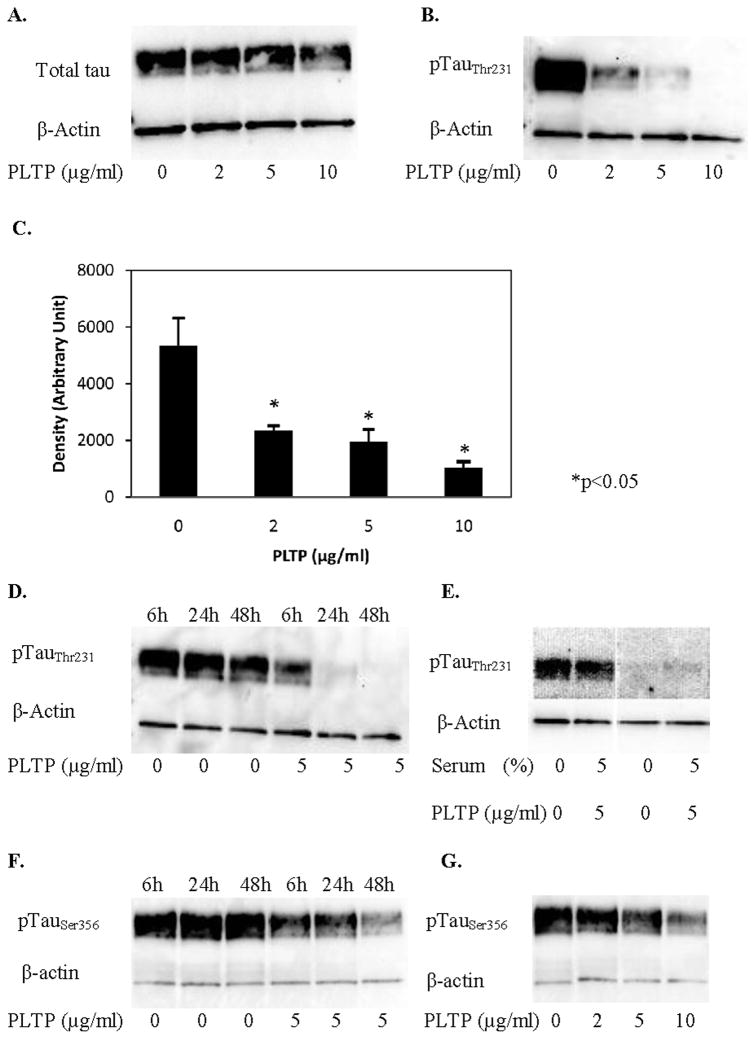
To test whether PLTP affects phosphorylation of tau in neuronal cells, we evaluated the levels of tau phosphorylated at threonine 231, pTauThr231, in HCN2 cells incubated with human rPLTP. pTauThr231 is a known marker for Alzheimer’s disease (Buerger et al 2003; Buerger et al 2006), which correlates with the neurofibrillary tangle burden in the brain of AD patients (Buerger et al 2006). Tau phosphorylated at threonine 231 is dependent on activity of GSK3β following priming by Cdk5 (Platner et al 2006; Sengupta et al 1997; Wang et al 1998). PLTP significantly reduced levels of pTauThr231 (by approximately 55%, 65% and 80% at 2, 5 and 10 μg/ml rPLTP, respectively; Figure 1B and 1C) without reduction in levels of total tau (Figure 1A). This effect was observable after 6h, and further developed after 24 and 48h (Figure 1D). PLTP-induced reduction in pTauThr231 was not affected by the presence or absence of serum (Figure 1E). Similar, but attenuated results were obtained for tau phosphorylated at serine 199 and 202, residues also phosphorylated by GSK3β, with PLTP reducing pTauSer199, 202 by ~20% (data not shown). For comparison, we tested the PLTP effect on levels of pTauSer356, showing that PLTP reduces phosphorylation of tau at serine 356 as well, but not to the same extent (Figure 1F and 1G). In order to establish whether PLTP affects expression of tau, human neuroblastoma cells, SK-N-SH were incubated with PLTP, and isolated mRNA levels were assessed by RT-PCR. Under the employed experimental conditions, PLTP had no effect on tau mRNA levels in SK-N-SH cells (data not shown). These data suggest that PLTP reduces GSK3β-dependent phosphorylation of tau in HCN2 cells in vitro, without affecting levels of total tau.
Figure 1.
PLTP reduces levels of tau phosphorylated at threonine 231 in HCN2 cells. Cells were incubated without or with (2, 5 or 10 μg/ml) human rPLTP, and cell proteins (30 μg) were resolved using SDS-PAGE. A) Total tau in cells incubated for 48 hours in serum-free media. B) Levels of pTauThr231 in cells incubated for 48 hours in serum-free media. C) PLTP effect on levels of pTauThr231; results of three separate experiments, each performed in triplicate (n=9; *p<0.05 compared to control cells). D) Time-dependent changes in pTauThr231 levels in cells incubated without or with (5 μg/ml) human rPLTP for 6, 24 and 48h. E) pTauThr231 levels in cells incubated without or with (5 μg/ml) rPLTP for 48h under serum-free conditions, and in presence of serum. F) Time-dependent changes of pTauSer356 in cells incubated without or with rPLTP (5 μg/ml) for 6, 24 and 48h. G) Dose-dependent changes of pTauSer356 in cells incubated for 48h with 2, 5 or 10 μg/ml rPLTP. Antibodies against tau (total and phosphorylated) were used at 1:800, while anti-actin antibody was used at 1:12,000 dilution.
PLTP increases levels of inactive form of GSK3β (pGSK3βSer9) in human neuronal cells
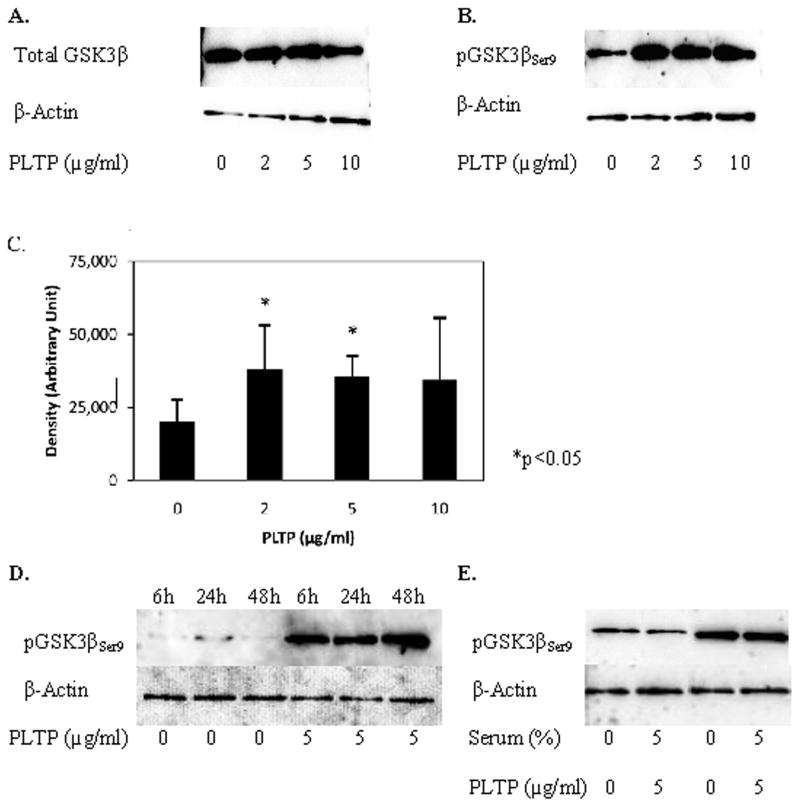
Previously published studies have shown that phosphorylation of tau at threonine 231 is at least partially regulated by GSK3β (Platner et al 2006; Sengupta et al 1997; Wang et al 2007). We, therefore, tested the effects of human PLTP on levels of GSK3β phosphorylated at serine 9, pGSK3βSer9, the inactive form of GSK3β, in HCN2 cells. PLTP induced a significant increase in pGSK3βSer9 (by approximately 70–85%; Figure 2B, 2C), without an increase in either GSK3β mRNA levels (data not shown) or levels of total GSK3β in neuronal cells (Figure 2A). The PLTP-mediated change in pGSK3βSer9 levels was observed after 6h incubation (Figure 2D), and was not affected by addition or removal of serum (Figure 2E). These data suggest that PLTP increases the amount of the inactive form of GSK3β in HCN2 cells.
Figure 2.
PLTP increases levels of glycogen synthase kinase (GSK3β) phosphorylated at serine 9 in HCN2 cells. Cells were incubated without or with (2, 5 or 10 μg/ml) human rPLTP, and cell proteins (30 μg) were resolved using SDS-PAGE. A) Total GSK3β in cells incubated for 48 hours in serum-free media. B) Levels of pGSK3βSer9 in cells incubated for 48 hours in serum-free media. C) PLTP effect on levels of pGSK3βSer9; results of three separate experiments, each performed in triplicate samples (n=9; *p<0.05 compared to control cells). D) Time-dependent changes in pGSK3βSer9 levels in cells incubated without or with (5 μg/ml) human rPLTP for 6, 24 and 48h. E) pGSK3βSer9 levels in cells incubated without or with (5 μg/ml) rPLTP for 48h under serum-free conditions, and in presence of serum. Antibodies against GSK3β (total and phosphorylated) were used at 1:800; anti-actin antibody was used at 1:12,000 dilution.
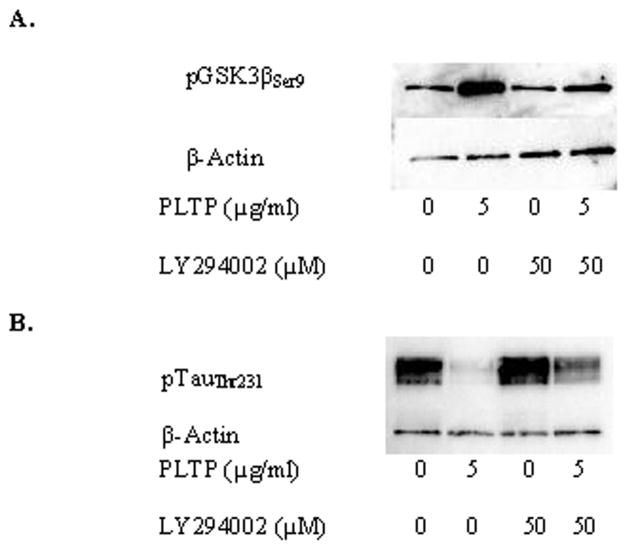
Phosphorylation of GSK3β at serine 9 is regulated by the PI3K pathway (Krasilnikov 2000). Inhibition of PI3K by LY294002 reversed the PLTP-induced increase of pGSK3βSer9 and thereby partially reversed the PLTP-induced reduction in tau phosphorylation (Figure 3).
Figure 3.
Inhibition of phosphatidylinositol-3 kinase (PI3K) reverses PLTP-induced changes on phosphorylation of GSK3β and tau. Cells were incubated without or with (5 μg/ml) human rPLTP for 48h under serum-free conditions, in the presence or absence of the PI3K inhibitor, LY294002 (50 μM). A) pGSK3βSer9. B) pTauThr231. Antibodies against tau and GSK3β were used at 1:800, while anti-actin antibody was used at 1:12,000 dilution.
PLTP-mediated reduction in pGSK3βSer9 is not dependent on the Dab1-dependent pathway
Activation of the PI3K pathway is elicited through a ligand binding to receptors on the cell membrane, and two well-defined pathways involved in the PI3K-mediated regulation of GSK3β have been reported. One involves binding of apoE or Reelin to the apoE receptors APOER2 or VLDLR, and consequent tyrosine phosphorylation of Disabled-1 (Dab1) by an Src-homology kinase, Fyn (Brich et al 2003; Hampel et al 2004; Hiesberger et al 1999; Hoe et al 2006; Howell et al 1999a; Howell et al 1999b; Ohkubo et al 2003; Wang et al 1998). Phosphorylated Dab1 activates the PI3K pathway, leading to reduced activity of GSK3β and reduced tau phosphorylation (idem). The other major pathway involves binding of a ligand to one of the receptor tyrosine kinases (RTK), which leads to activation of the PI3K pathway, and reduced GSK3β activity (Biswas et al 2008; Castri et al 2007). Because PLTP functions as a molecular partner of apoE, we tested the possibility that PLTP reduces tau phosphorylation through the same pathway utilized by apoE, by activation of Dab1.
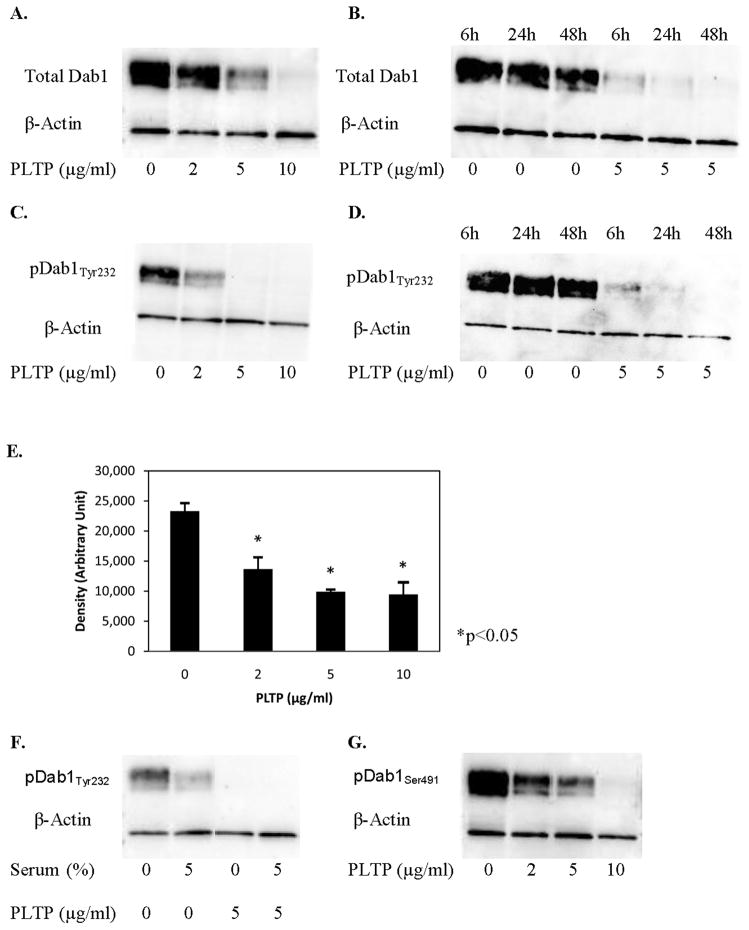
We incubated HCN2 cells with PLTP and following incubation, evaluated levels of total Dab1 and Dab1 phosphorylated at tyrosine 232 and 220, pDab1Tyr232 and pDab1Tyr220. Western blot analyses have shown that PLTP significantly reduced levels of total Dab1 protein levels in neuronal cells (Figure 4A, B). PLTP also reduced DAB1 mRNA expression in human neuroblastoma cells, SK-N-SH, by 26% at 10 μg/ml rPLTP (p<0.02 compared to control). Furthermore, we have shown that PLTP significantly reduced levels of pDab1Tyr232 (by 41%, 58% and 60% at 2, 5 and 10 μg/ml rPLTP, respectively; Figure 4C, 4E), a site known to be phosphorylated by Fyn (Howell et al 1999a). The PLTP-mediated reduction in levels of pDab1Tyr232 was present at 6h, and further developed at 24 and 48h (Figure 4D). Addition or removal of serum did not affect PLTP-mediated reduction of pDab1Tyr232 (Figure 4F). Similar results were obtained for pDab1Tyr220 (data not shown), which is also regulated by Fyn.
Figure 4.
PLTP reduces levels of total Disabled-1 (Dab1), and levels of Dab1 phosphorylated at tyrosine 232 and serine 491 in HCN2 cells. Western blot analyses of cytoplasmic proteins (30 μg) isolated from the HCN2 cells incubated without or with (2, 5 or 10 μg/ml) human rPLTP for up to 48 hours. A) Total Dab1. B) Time-dependent changes in levels of total Dab1. C) Levels of pDab1Tyr232. D) Time-dependent changes in pDab1Tyr232 levels. E) PLTP effect on levels of pDab1Tyr232; results of three separate experiments performed in triple replicates (n=9; *p<0.05 compared to control cells). F) pDab1Tyr232 levels in cells incubated without or with serum. G) Levels of pDab1Ser491 in cells incubated for 48 hours in serum-free media. Antibodies against Dab1 (total and phosphorylated) were used at 1:800, while anti-actin antibody was used at 1:12,000 dilution.
Incubation of HCN2 cells with PLTP also reduced Dab1 phosphorylation at serine 491, pDab1Ser491 (Figure 4G), a residue phosphorylated by cyclin-dependent kinase 5 (Cdk5), both in vivo and in vitro, in a process that previously published studies have shown to be independent of Reelin (Keshvara et al 2002; Ohshima et al 2006). However, because of the similar extent of the PLTP effect on levels of total Dab1, it is not clear whether the reduction in pDab1Ser491 is due to the PLTP-induced reduction in Cdk5-dependent Dab1 phosphorylation, or due to the reduction in total Dab1.
PLTP and Akt/PKB
To evaluate the effect of PLTP on Akt/PKB activation, we incubated HCN2 cells with or without PLTP for ten and thirty minutes. As expected, incubation with PLTP increased levels of pAktThr308 (Figure 5B) and pAktSer473 (Figure 5C) without increasing the levels of total Akt (Figure 5A). PLTP-mediated increase of phospho-Akt was prolonged, and still present at 6h (data not shown).
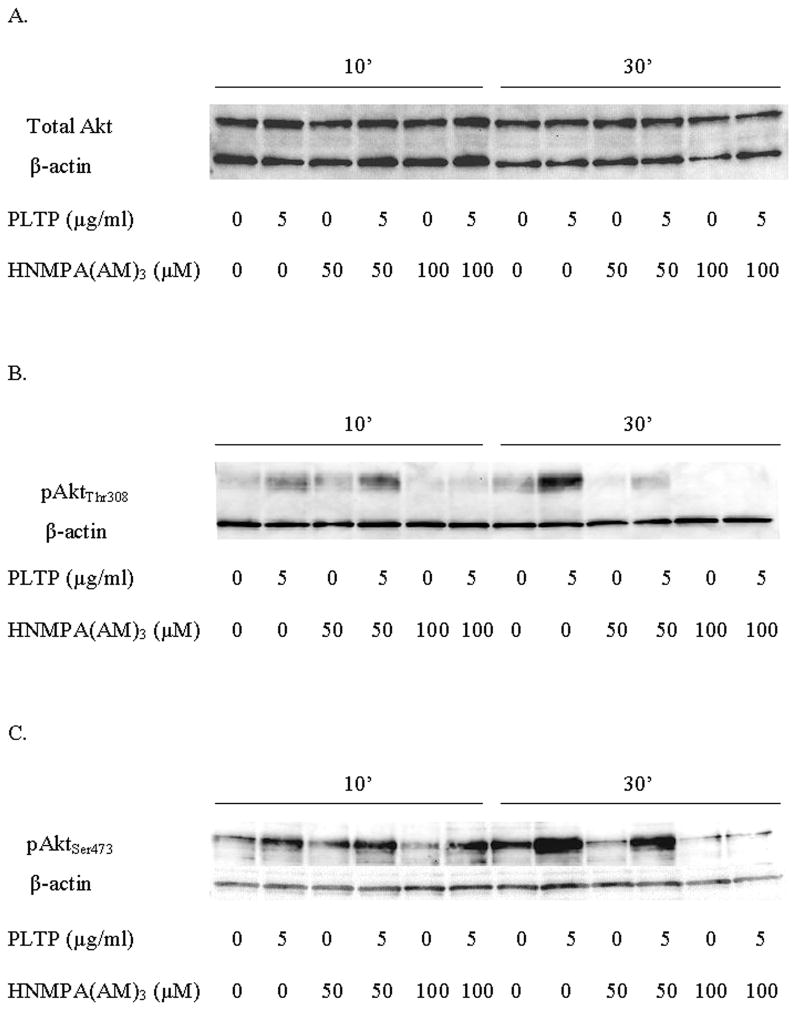
Figure 5.
PLTP increases levels of active Akt/PKB, and this effect is inhibited by HNMPA(AM)3, the insulin receptor and IGF-1 receptor tyrosine kinase activity inhibitor. Western blot analyses of cytoplasmic proteins (30 μg) isolated from the HCN2 cells. The cells were incubated with HNMPA(AM)3 or vehicle (DMSO) for two hours prior to addition of PLTP. The cells were then exposed to PLTP for ten and thirty minutes. A) Total Akt. B) pAktThr308. C) pAktSer473. Antibodies against Akt (total and phosphorylated) were used at 1:1000, while anti- actin antibody was used at 1:12,000.
Additionally, we inhibited tyrosine kinase activity of two receptor tyrosine kinases, insulin receptor (IR) and insulin-like growth factor 1 (IGF-1) receptor (IGFR), using chemical inhibitor HNMPA(AM)3. Inhibition with 50 μM of HNMPA(AM)3, which has been shown to have effect only on IR, but not on IGFR (Diaz et al, 2007), had a limited effect on PLTP-mediated increase in levels of pAktThr308 and pAktSer473. However, inhibition with 100 μM of HNMPA(AM)3, which inhibits tyrosine kinase activity of both IR and IGFR (Diaz et al, 2007), markedly inhibited PLTP-mediated effect on pAktThr308 (Figure 5B) and pAktSer473 (Figure 5C). Our data indicate that PLTP activates Akt at both activation sites, and that this effect is dependent on receptor tyrosine kinase activity of IR/IGFR.
Discussion
PLTP is an important lipid transfer protein present in the brain, expressed and secreted by neurons and glia (Vuletic et al 2003). PLTP levels are significantly higher in brain tissue of early- to mid-stage Alzheimer’s disease patients, with subsequent significant decrease in patients with a severe neuronal loss (idem). Both our immunohistochemical and in vitro studies have shown that PLTP is highly expressed in neurons, and that neuronal PLTP levels are significantly higher in neurons of AD patients compared to controls (idem and unpublished data). However, potential physiological effects of this significant intraneuronal increase of PLTP, as well as the increase in PLTP levels in the brain tissue of AD patients are not known.
In this study, we provide evidence that PLTP significantly reduces levels of tau phosphorylated at residues phosphorylated by GSK3β, and increases levels of the inactive form of GSK3β in vitro. Furthermore, our findings indicate that the increase in pGSK3βSer9 levels does not fully explain the reduction in tau phosphorylation induced by PLTP, suggesting that PLTP activates an additional pathway. Previously published studies have shown that regulation of pTauThr231 is affected by activities of GSK3β, Cdk5 and protein phosphatase 2A (PP2A) (Wang et al 2007). GSK3 plays an important role in pathogenesis of AD (Hooper et al, 2008). Therefore, our finding that PLTP increases levels of pGSK3βSer9 is relevant for Alzheimer’s disease, since GSK3β inhibitors are considered to be among the most promising therapeutic approaches for the treatment of AD (Martinez and Perez, 2008).
Phosphorylation of GSK3β at serine 9, and consequential inactivation of this kinase, involves phosphatidylinositol-3 kinase (PI3K) pathway (Krasilnikov 2000), the downstream effector of the Reelin/pDab1-mediated regulation of GSK3β activity (Beffert et al 2002). In our studies, inhibition of the PI3K pathway reversed the PLTP-induced phosphorylation of GSK3β, and partially reversed PLTP-induced reduction of phosphorylation of tau. However, contrary to our expectations, PLTP reduced protein levels of Dab1, as well as levels of Dab1 phosphorylated at tyrosine residues. These findings suggest that PLTP-mediated reduction in tau phosphorylation is not executed through the Dab1-dependent mechanism, since previously published studies have shown that reduction in Dab1 tyrosine phosphorylation is associated with an increase in GSK3β-dependent tau phosphorylation (Brich et al 2003; Hiesberger et al 1999; Hoe et al 2006). Although PLTP-modulated reduction of tau phosphorylation seems to be independent of Dab1, our findings strongly suggest that PLTP has a significant effect on Dab1 levels and function in human neurons.
Previously published studies have shown that activation of the PI3K pathway is also regulated by ligand-binding to the RTK. It is, therefore, possible that PLTP modulates the PI3K pathway through this mechanism. Indeed, in our experiments we have shown that inhibition of receptor tyrosine kinase activity of the IR/IGFR blocks PLTP-mediated activation of Akt/PKB, suggesting that PLTP effect is dependent on IR/IGFR tyrosine kinase activity.
Additionally, the activation of PI3K is regulated by phosphatidylinositol (PI), and in vivo and in vitro studies have shown that presence of PLTP is required for transfer of PI, with a complete abolition of PI transfer in PLTP-deficient mice (Jiang et al 1999). It is, therefore, reasonable to propose that PLTP might modulate the PI3K pathway also through regulation of substrate delivery to PI3K.
The findings presented in this study also have broader implications. Dab1, PI3K, GSK3β and Cdk5 are important for numerous other processes, such as inflammation, apoptosis and cell cycle, and alterations in their activity have been reported as important factors in development of many diseases, including cancer. Therefore, further investigation of the relationship between PLTP and these molecules is likely to provide new insights into the physiological regulation of these highly relevant intracellular processes associated with numerous human diseases.
In summary, our study suggests that PLTP plays an important role in modulation of tau phosphorylation in neurons. Furthermore, our findings strongly suggest that PLTP-induced inactivation of GSK3β and modulation of Dab1 in neurons are achieved through separate, independent mechanisms and pathways. Based on our findings, we postulate that the increased levels of PLTP observed in the brain tissue of patients with AD would likely have beneficial effects by reducing the burden of tau phosphorylation and the formation of neurofibrillary tangles in neurons.
Acknowledgments
We are thankful to our colleague Dr. Gertrud Wolfbauer for preparation and isolation of recombinant PLTP, and to Hal Kennedy for help in preparation of the manuscript. We appreciate quantitative RT-PCR technical assistance from Brian van Yserloo at the University of Washington Diabetes Endocrinology Research Center (DERC, http://depts.washington.edu/diabetes; supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK-17047).
This work was supported by the National Institutes of Health, National Heart, Lung and Blood Institute grant HL030086.
Grant Information: NIH NHLBI grant HL030086.
References
- Barlage S, Frohlich D, Bottcher A, Jauhiainen M, Muller HP, Noetzel F, Rothe G, Schutt C, Linke RP, Lackner KJ, Ehnholm C, Schmitz G. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J Lipid Res. 2001;42:281–290. [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Zhao Y, Nagalingam A, Gardner TW, Sandirasegarane L. PDGF- and insulin/IGF-1-specific distinct modes of class IA IP 3-kinase activation in normal rat retinas and RGC-5 retinal ganglion cells. Invest Ophtalmol Vis Sci. 2008;49:3687–3698. doi: 10.1167/iovs.07-1455. [DOI] [PubMed] [Google Scholar]
- Brich J, Shie FS, Howell BW, Li R, Tus K, Wakeland EK, Jin LW, Mumby M, Churchill G, Gerz J, Cooper JA. Genetic modulation of tau phosphorylation in the mouse. J Neurosci. 2003;23:187–192. doi: 10.1523/JNEUROSCI.23-01-00187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Buerger K, Teipel SJ, Zinkowski R, Blennow K, Arai H, Engel R, Hofmann-Kiefer K, McCullock C, Ptok U, Heun R, Andersen N, DeBernardis J, Kerkman D, Moeller HJ, Davies P, Hampel H. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- Buerger K, Zinkowski R, Teipel SJ, Hirohuki A, DeBernardis J, Kerkman D, McCulloch Cheryl, Padberg F, Faltraco F, Goernitz A, Tapiola T, Rapoport SI, Pirttila T, Moeller HJ, Hampel H. Differentiation of geriatric major depression from Alzheimer’s disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiatry. 2003;160:376–379. doi: 10.1176/appi.ajp.160.2.376. [DOI] [PubMed] [Google Scholar]
- Castri P, Iacovelli L, De Blasi A, Giubilei F, Moretti A, Capone FT, Nicoletti F, Orzi F. Reduced insulin-induced phosphatidylinositol-3-kinase activation in peripheral blood mononuclear leukocytes from patients with Alzhimer’s disease. Eur J Neurosci. 2007;26:2469–2472. doi: 10.1111/j.1460-9568.2007.05869.x. [DOI] [PubMed] [Google Scholar]
- Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, Laplanche H, Guern NL, Blache D, Jiang XC, Tall A, Desor D, Lagrost L. Phospholipid transfer protein PLTP deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005;19:296–7. doi: 10.1096/fj.04-2400fje. [DOI] [PubMed] [Google Scholar]
- Diaz LE, Chuan Y-C, Lewitt M, Fernandez-Perez L, Carrasco-Rodriguez S, Sanchez-Gomez M, Flores-Morales A. IGF-II regulates metastatic properties of choriocarcinoma cells through the activation of the insulin receptor. Mol Hum Reproduct. 2007;13:567–576. doi: 10.1093/molehr/gam039. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leion M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Hailman E, Albers JJ, Wolfbauer G, Tu AY, Wright SD. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J Biol Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moeller HJ, Davies P, Blennow K. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of reelin to VLDL receptor and apoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Freeman J, Rebeck GW. Apolipoprotein E decreases tau kinases and phosphor-tau levels in primary neurons. Mol Neurodegener. 2006;1:18. doi: 10.1186/1750-1326-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999a;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999b;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. J Alz Dis. 2006;9:219–242. doi: 10.3233/jad-2006-9s325. [DOI] [PubMed] [Google Scholar]
- Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest. 1999;103:907–914. doi: 10.1172/JCI5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshvara L, Magdaleno S, Benhayon D, Curran T. Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling. J Neurosci. 2002;22:4869–4877. doi: 10.1523/JNEUROSCI.22-12-04869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasilnikov MA. Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry Mosc. 2000;65:59–67. [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Martinez A, Perez DI. GSK3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J Alz Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]
- Ohkubo N, Lee YD, Morishima A, Terashima T, Kikkawa S, Tohyama M, Sakanaka M, Tanaka J, Maeda N, Vitek MP, Mitsuda N. Apolipoprotein E and Reelin ligands modulate tau phosphorylation through an apolipoprotein E receptor/disabled-1/glycogen synthase kinase-3beta cascade. FASEB J. 2003;17:295–297. doi: 10.1096/fj.02-0434fje. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Suzuki H, Morimura T, Ogawa M, Mikoshiba K. Modulation of Reelin signaling by cyclin-dependent kinase 5. Brain Res. 2006;1140:84–95. doi: 10.1016/j.brainres.2006.01.121. [DOI] [PubMed] [Google Scholar]
- Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transported A1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- Platner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Saperstein R, Vicario PP, Strout HV, Brady E, Slater EE, Greenlee WJ, Ondeyka DL, Patchett AA, Hangauer DG. Design of a selective insulin receptor tyrosine kinase inhibitor and its effect on glucose uptake and metabolism in intact cells. Biochemistry. 1989;28:5694–5701. doi: 10.1021/bi00439a053. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ. Potentiation of GSK-3- catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol Cell Biochem. 1997;167:99–105. doi: 10.1023/a:1006883924775. [DOI] [PubMed] [Google Scholar]
- Vuletic S, Dong W, Wolfbauer G, Day JR, Albers JJ. PLTP is present in the nucleus, and its nuclear export is CRM1-dependent. Biochim Biophys Acta. 2009;1793:584–591. doi: 10.1016/j.bbamcr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuletic S, Jin LW, Marcovina SM, Peskind ER, Moller T, Albers JJ. Widespread distribution of PLTP in human CNS: evidence for PLTP synthesis by glia and neurons, and increased levels in Alzheimer’s disease. J Lipid Res. 2003;44:1113–1123. doi: 10.1194/jlr.M300046-JLR200. [DOI] [PubMed] [Google Scholar]
- Vuletic S, Peskind ER, Marcovina SM, Quinn JF, Cheung MC, Kennedy H, Kaye JA, Jin LW, Albers JJ. Reduced CSF PLTP activity in Alzheimer’s disease and other neurologic diseases; PLTP induces ApoE secretion in primary human astrocytes in vitro. J Neurosci Res. 2005;80:406–413. doi: 10.1002/jnr.20458. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luebbe P, Gruenstein E, Zemlan F. Apolipoprotein E (ApoE) peptide regulates tau phosphorylation via two different signaling pathways. J Neurosci Res. 1998;51:658–65. doi: 10.1002/(SICI)1097-4547(19980301)51:5<658::AID-JNR13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Wehinger A, Tancevski I, Schgoer W, Eller P, Hochegger K, Morak M, Hermetter A, Ritsch A, Patsch JR, Foeger B. Phospholipid transfer protein augments apoptosis in THP-1-derived macrophages induced by lipolyzed hypertriglyceridemic plasma. Arterioscler Thromb Vasc Biol. 2007;27:908–915. doi: 10.1161/01.ATV.0000259361.91267.8c. [DOI] [PubMed] [Google Scholar]
- Wolfbauer G, Albers JJ, Oram JF. Phospholipid transfer protein enhances removal of cellular cholesterol and phospholipids by high-density lipoprotein apolipoproteins. Biochimica et Biophysica Acta. 1999;1439:65–76. doi: 10.1016/s1388-1981(99)00077-3. [DOI] [PubMed] [Google Scholar]