Abstract
Here we report a number of novel JS-K structural analogues with sub-micromolar anti-proliferative activities against human leukemia cell lines HL-60 and U937; JS-K is the anti-cancer lead compound O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate. The ability of these compounds to generate intracellular nitric oxide correlated well with their observed anti-proliferative effects: analogues that had potent inhibitory activity against leukemia cells formed elevated levels of intracellular nitric oxide.
Keywords: Nitric oxide, JS-K, Cancer, Prodrug, Glutathione, Glutathione S-transferase, Homopiperazine, Diazeniumdiolate
O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) is a member of the diazeniumdiolate class of nitric oxide prodrugs that has shown promise as an anti-cancer drug.1-11 For example, the in vitro anti-proliferative activity of JS-K was found to be comparable to that of Ara-C and better than that of etoposide against the HL-60 human leukemia cell line.1 Furthermore, in mouse xenograft leukemia and multiple myeloma models, a significant inhibition of tumor growth in animals treated with JS-K was observed.1,5
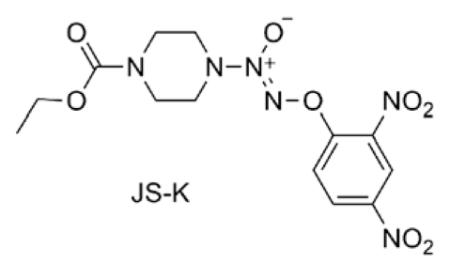
In previous reports, a series of analogues were prepared and their in vitro anti-proliferative activities in HL-60 leukemia cells were established and compared with those of JS-K.4,7,8 The ability to inhibit cancer cell proliferation by this class of compounds was sensitive to structure. Bulkier substituents on the piperazine ring,8 or removal of the carbamate functionality altogether in moving to a piperidine ring,4 or an acyclic secondary amine bearing the arylated diazeniumdiolate resulted in diminution of inhibitory activity.7,8 Furthermore, the size of the carbamate group was also an important determinant of the cytotoxicity; an alkyl group longer than ethyl decreased the inhibitory activity.4,8 Finally, two nitro groups on the aryl ring were essential for nitric oxide release or any observable anti-proliferative activity in the in vitro cell viability assay.4,8 In a recent study, it was found that JS-K and its homopiperazine analogue were identical in activity against a number of cancer cell lines; these compounds did not display any cytotoxicity against a normal renal epithelial cell line at concentrations where they inhibited the proliferation of a panel of renal cancer cell lines.8 In order to explore the stereoelectronic accommodation at N-4 of the (homo)piperazine ring of such compounds, a number of variably N-substituted heterocyclic O2-(2,4-dinitrophenyl) diazeniumdiolates were prepared and studied.
Using a reported method, compound 1a was prepared and deprotection of 1a afforded the amine hydrochloride, 2a.4 A similar procedure was used to prepare the homopiperazines, 1b and 2b (Scheme 1).4
Scheme 1.
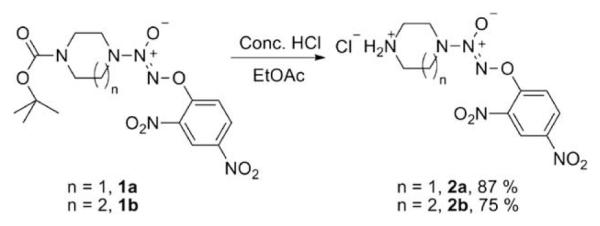
Synthesis of ammonium salts 2a and 2b.
Compounds 2a and 2b were then independently treated with a range of corresponding electrophiles to form the desired compounds 3–15 (Table 1).4,7,8 Compound 16 was prepared using a reported method from N-carboethoxy(homopiperazine) in two steps (Fig. 1).4 The quaternary ammonium salt 17 was obtained by methylation of 1c12 with excess methyl iodide in dichloromethane (Scheme 2).
Table 1.
Synthesis of structural analogues of JS-K
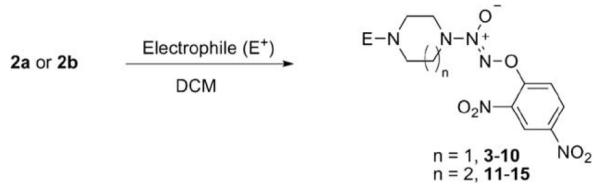 | |||
|---|---|---|---|
| n | E | Compound | Yield (%) |
| 1 | 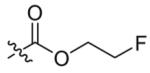 |
3 | 87 |
| 1 | 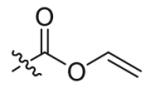 |
4 | 72 |
| 1 | 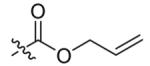 |
5 | 89 |
| 1 | 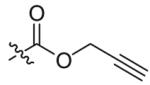 |
6 | 86 |
| 1 | 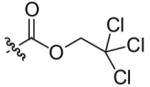 |
7 | 78 |
| 1 | 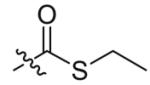 |
8 | 93 |
| 1 | 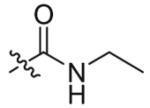 |
9 | 69 |
| 1 | 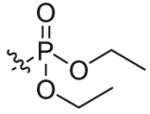 |
10 | 88 |
| 2 | 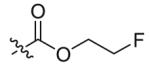 |
11 | 89 |
| 2 | 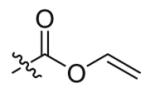 |
12 | 66 |
| 2 | 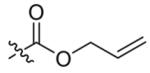 |
13 | 83 |
| 2 | 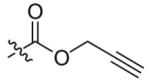 |
14 | 89 |
| 2 | 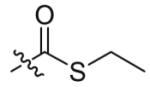 |
15 | 84 |
Figure 1.
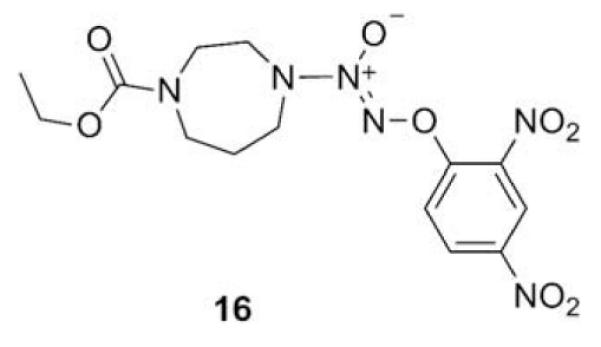
The JS-K analogue 16.
Scheme 2.

Synthesis of the quaternary ammonium salt 17.
JS-K was designed to be activated by glutathione (GSH) to release potentially cytotoxic nitric oxide intracellularly (Scheme 3).1 This reaction was found to be catalyzed by glutathione S-transferase (GST), a class of enzymes frequently over-expressed in certain cancers.1,8
Scheme 3.
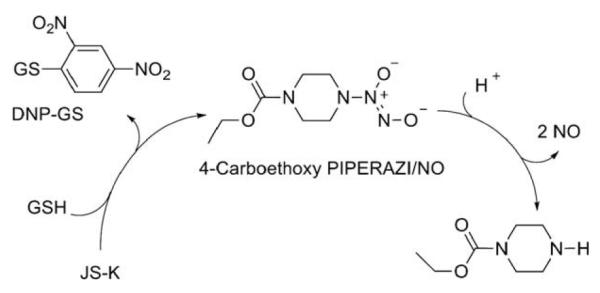
Proposed mechanism of glutathione-activated nitric oxide release from JS-K.
First, glutathione-activated nitric oxide yields from these compounds were determined using a chemiluminescence assay. All compounds were found to release nearly quantitative amounts of NO on reaction with GSH (Table 2).
Table 2.
Nitric oxide release and in vitro anti-proliferative activities of JS-K and its structural analogues
| Compound | Nitric oxide yielda(%) | IC50b (μM) | IC50c (μM) |
|---|---|---|---|
| JS-K | 85 | 0.2–0.5 | 0.3 |
| 1b | 90 | 4.1 | 9.8 |
| 2b | 79 | 1.9 | 1.9 |
| 3 | 88 | 0.4 | 0.7 |
| 4 | 90 | 0.3 | 0.4 |
| 5 | 83 | 1.1 | 1.0 |
| 6 | 86 | 1.2 | 1.1 |
| 7 | 84 | 1.6 | 1.4 |
| 8 | 98 | 1.4 | 1.1 |
| 9 | 74 | 1.9 | 1.9 |
| 10 | 85 | 5.7 | 5.1 |
| 11 | 87 | 0.4 | 0.5 |
| 12 | 93 | 0.2 | 0.3 |
| 13 | 98 | 0.4 | 0.6 |
| 14 | 99 | 0.6 | 0.6 |
| 15 | 90 | 0.5 | 0.4 |
| 16 | 100 | 0.2 | 0.3 |
| 17 | 68 | 10.8 | 9.6 |
Determined by measuring NO release from the compound (70–220 μM) in the presence of glutathione (1–3.5 mM) in 0.1 M pH 7.4 phosphate buffer at 37 °C by chemiluminescence analysis. Yield reported assuming 2 mol of NO per mole of compound.
Cell viability studies carried out on HL-60 human leukemia cells.
50% inhibitory concentration against proliferation of U937 human leukemia cells; same procedure as that for HL-60.
Next, JS-K and its analogues were tested for their in vitro anti-proliferative activities against human leukemia cell lines HL-60 and U937 (Table 2). Compounds 3–9 were found to display nearly identical anti-proliferative activities (Table 2) but changing the ethyl carbamate to a diethyl phosphamate group resulted in a lowering of inhibitory activity (Table 2, compound 10). All the homopiperazine analogues prepared in this study were found to have activity comparable with that of JS-K in both HL-60 and U937 leukemia cell lines (Table 2, compounds 11–16). The quaternary ammonium salt 17 that had improved aqueous solubility was lower in potency than JS-K (Table 2).
Finally, in order to test the role of cell permeability and NO in cytotoxicity of these compounds, we measured the levels of intracellular nitric oxide formed upon treating cells with selected compounds and compared these with JS-K.13,14 The intracellular NO was estimated using the nitric oxide-sensitive fluorophore, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate); briefly, human leukemia HL-60 cells were pre-loaded with DAF-FM diacetate, followed by treatment with DMSO solutions of various JS-K analogues; fluorescence measurements after 40 min provided estimates of levels of intracellular NO (Fig. 2).
Figure 2.
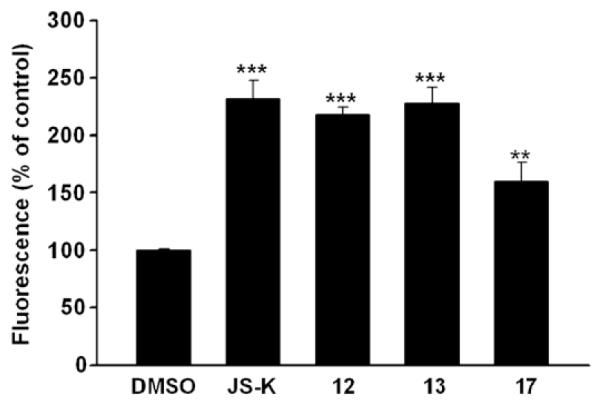
Intracellular NO release after treating HL-60 cells pre-loaded with DAF-FM diacetate with various compounds (5 μM) for 40 min; p values <0.001 (***) and p value = 0.03 (**).
A plot of the relative fluorescence suggests that compounds forming NO at levels comparable with JS-K showed similar anti-proliferative activity profiles (Fig. 2). The quaternary ammonium salt 17 with an IC50 value of 10.8 μM against HL-60 cells showed diminished levels of intracellular nitric oxide relative to JS-K in this assay.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as by National Cancer Institute contract NO1-CO-2008-00001 to SAIC-Frederick.
Footnotes
Supplementary data Supplementary data (preparation procedures and analytical data for all new compounds, and NMR spectra of 2b and 10) associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2009.03.115.
References and notes
- 1.Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK. Mol. Cancer Ther. 2003;2:409. [PubMed] [Google Scholar]
- 2.Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI. J. Cell Physiol. 2003;197:426. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS, Saavedra JE, Keefer LK, Waalkes MP. Mol. Cancer Ther. 2004;3:709. [PubMed] [Google Scholar]
- 4.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. J. Med. Chem. 2006;49:4356. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 5.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li C-Q, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. Blood. 2007;110:709. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udupi V, Yu M, Malaviya S, Saavedra JE, Shami PJ. Leukemia Res. 2006;30:1279. doi: 10.1016/j.leukres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Chakrapani H, Goodblatt MM, Udupi V, Malaviya S, Shami PJ, Keefer LK, Saavedra JE. Bioorg. Med. Chem. Lett. 2008;18:950. doi: 10.1016/j.bmcl.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrapani H, Kalathur RC, Maciag AE, Citro ML, Ji X, Keefer LK, Saavedra JE. Bioorg. Med. Chem. 2008;16:9764. doi: 10.1016/j.bmc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simeone A-M, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM, Tari AM. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitagaki J, Yang Y, Saavedra JE, Colburn NH, Keefer LK, Perantoni AO. Oncogene. doi: 10.1038/onc.2008.401. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shami PJ, Maciag AE, Eddington JK, Udupi V, Kosak KM, Saavedra JE, Keefer LK. Leukemia Res. doi: 10.1016/j.leukres.2009.01.002. in press, doi:10.1016/j.leukres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saavedra JE, Booth MN, Hrabie JA, Davies KM, Keefer LK. J. Org. Chem. 1999;64:5124. doi: 10.1021/jo9901539. [DOI] [PubMed] [Google Scholar]
- 13.Chakrapani H, Maciag AE, Citro ML, Keefer LK, Saavedra JE. Org. Lett. 2008;10:5155. doi: 10.1021/ol8020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrei DA, Maciag AE, Chakrapani H, Citro ML, Keefer LK, Saavedra JE. J. Med. Chem. 2008;51:7944. doi: 10.1021/jm800831y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


