Abstract
Pain results from the complex processing of neural signals at different levels of the central nervous system, with each signal potentially offering multiple opportunities for pharmacological intervention. A logical strategy for developing novel analgesics is to target the beginning of the pain pathway, and aim potential treatments directly at the nociceptors — the high-threshold primary sensory neurons that detect noxious stimuli. The largest group of receptors that function as noxious stimuli detectors in nociceptors is the transient receptor potential (TRP) channel family. This Review highlights evidence supporting particular TRP channels as targets for analgesics, indicates the likely efficacy profiles of TRP-channel-acting drugs, and discusses the development pathways needed to test candidates as analgesics in humans.
Primary sensory neurons are the interface of the nervous system with the external and internal environments that exist outside and inside our bodies. These neurons have a cell body located in the dorsal root ganglion (DRG), a peripheral axon that innervates tissues such as skin and whose terminals react to sensory stimuli, and a central axon that enters the spinal cord to transfer information to the central nervous system (CNS) by synaptic communication. A major function of the sensory apparatus is to detect potentially damaging stimuli and thereby warn of the risk of injury. This key survival tactic has evolved so that the sensations evoked by noxious stimuli are intensely unpleasant and consequently can be avoided the next time they are encountered. However, the threshold for eliciting pain must be high enough so that most activities can be carried out largely pain-free, but sensitive enough so that an alert can be given immediately if an injury is impending. The first neural mediator of this crucial alarm system is the nociceptor1.
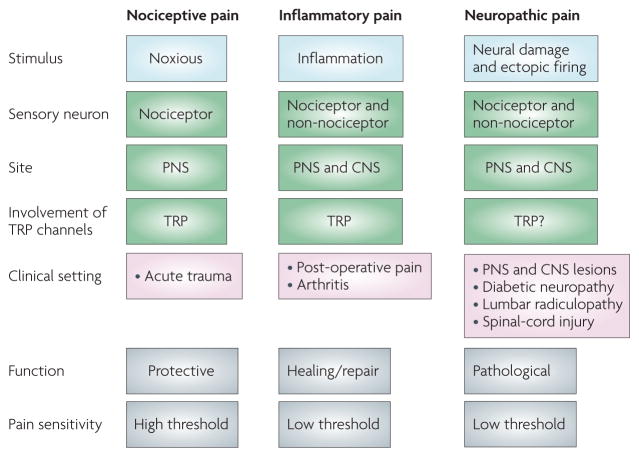
The detection of noxious stimuli by nociceptors elicits nociceptive pain, such as that elicited after touching a hot object or in response to an intense pinch (FIG. 1). However, in various pathological conditions pain can occur in the absence of a noxious stimulus, in response to normally innocuous stimuli (allodynia), and with an exaggerated response to a noxious stimulus (hyperalgesia). After tissue injury the inflammatory response sensitizes nociceptors so that their threshold for activation drops and their responsiveness increases, and this contributes to pain hypersensitivity at an inflamed site, a distinct component of inflammatory pain2 (FIG. 1). Following peripheral nerve damage, nociceptors may begin to fire ectopically, and in this way contribute to the spontaneous component of neuropathic pain3 (FIG. 1).
Figure 1. Classification of major pain syndromes.
Pain can be divided into three broad categories: nociceptive, inflammatory and neuropathic. This division is based on the initiating stimulus (presence of a noxious stimulus, inflammation or neural damage); the neural substrate involved (nociceptors or non-nociceptors and the relative contribution/involvement of the peripheral nervous system (PNS) or central nervous system (CNS)); and the relative involvement of transient receptor potential (TRP) channels; the typical clinical conditions; the biological role of pain; and the pain threshold. Nociceptive pain is generated by noxious stimuli that act on nociceptors in the PNS, and which, for thermal stimuli and chemical irritants, depend on TRP channels. This pain occurs clinically in the setting of acute trauma, is protective and serves to warn of damage. Inflammatory pain occurs in the presence of damaged or inflamed tissue. Inflammatory mediators can sensitize nociceptors, which involves alterations in TRP channel threshold. Central changes are also induced (central sensitization) such that pain can be recruited by activation of non-nociceptors. This clinical pain state is typically reversible and associated with hypersensitivity (noxious stimuli are no longer needed to evoke pain). Neuropathic pain results from damage and lesions to the nervous system. The pathophysiological changes responsible for the spontaneous pain and pain hypersensitivity experienced by patients occur both in the PNS and CNS and represent non-adaptive pathological changes. Some TRP channel antagonists reduce such pain but their involvement is not well understood.
Pharmacological intervention to reduce pain can produce analgesia by either decreasing excitation or increasing inhibition in the nervous system. Opioids, for example, decrease neurotransmitter release presynaptically and hyperpolarize neurons postsynatically in the spinal cord, brainstem and cortex4. Sodium-channel blocking and potassium-channel opening anticonvulsants reduce excitation throughout the nervous system, whereas amine uptake inhibitors potentiate the actions of inhibitory transmitters in the spinal cord and brain5,6. A problem associated with centrally acting drugs — such as opioids, antidepressants, anticonvulsants and sodium-channel blockers that target receptors/channels that are widely expressed — is a higher risk of adverse effects such as sedation, dizziness, somnolence or loss of cognitive function. An alternative strategy for developing novel analgesics is to target the very beginning of the pain pathway and aim treatment directly at receptors and ion channels that transduce noxious stimuli at the peripheral terminals of nociceptors into electrical activity. These nociceptive ion channels, which essentially define the characteristic functional properties of nociceptors, are selectively or mainly expressed in these neurons7–9, thereby potentially reducing the side-effect profiles of drugs that specifically act on them. The largest group of noxious stimulus detectors is the transient receptor potential (TRP) channel family7–9, which this Review will highlight, with a particular emphasis on TRPV1 and TRPA1 as targets for analgesics (FIG. 2).
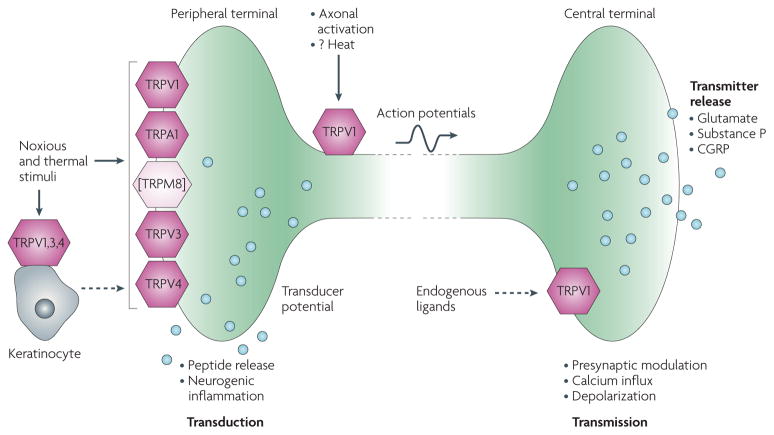
Figure 2. Representation of the roles of TRP channels in the peripheral and central terminals of nociceptor neurons.
Noxious and thermal stimuli act directly on the peripheral terminals of nociceptors to activate these sensory fibres. Many of the transduction channels that convert thermal, mechanical or chemical stimuli into electrical activity are transient receptor potential (TRP) channels. Activation of the peripheral terminal leads to action potential signalling towards the central nervous system (CNS) and a local release of vasoactive peptides that produce neurogenic inflammation. Although TRPV1 and TRPA1 are co-expressed in nociceptors TRPM8 is largely expressed in a unique set of sensory neurons without co-expression of TRV1 and TRPA1 (represented by bracket around TRPM8). Some TRP channels are expressed on keratinocytes and these cells may respond to noxious thermal stimuli by releasing a signal molecule, possibly ATP that then acts on the nociceptor. The nociceptor presynaptic terminal contains excitatory amino acid and peptide transmitters, the release of which is modulated by several TRP channels. The dashed arrows in the figure represent unknown signalling molecules, possibly endovanilloid lipids. CGRP, calcitonin gene-related peptide.
TRP channels as targets for novel analgesics
The first suggestion that TRP channels are key receptors involved in sensory transduction emerged from the identification of the original TRP ion channel from Drosophila melanogaster10. The name of the ion channel is derived from a fly mutant that displays a transient instead of a sustained response to bright light. TRP ion channels from various species are important for vision, olfaction, taste, mechanosensation, osmoregulation and thermosensation7. A role of TRP channels specifically in pain and thermosensation was first suggested by the finding that mammalian TRPV1 is activated by both noxious heat and capsaicin, the active ingredient of chilli peppers11. Importantly, the cloning and characterization of several close and distant relatives of TRPV1, which are activated at distinct thresholds of cool and warm temperatures, established a general role for TRP channels in thermosensation and nociception: TRPV2 (REF. 12), TRPV3 (REFS 13–15), TRPV4 (REFS 16,17), TRPM8 (REFS 18,19) and TRPA1 (REF. 20).
Although there is little amino-acid conservation among distant TRP channels, they share a similar architecture of six-transmembrane domains with cytoplasmic amino and carboxy termini. TRP channels are thought to function as tetramers, mostly as homomers21. Six of the 28 TRP channels from the three distinct TRP family subtypes are activated by temperature (TRPV1–4, TRPM8 and TRPA1), and are expressed in sensory neurons or in skin keratinocyte cells, which are peripheral targets of these nerves8. Three other TRP channels (TRPM2, TRPM4 and TRPM5) are strongly modulated by warm temperatures; however, the lack of expression in nociceptor neurons argues against a role in nociception22,23.
Specific activators of thermoTRP channels
One way to elucidate the function of somatosensory receptors is to examine the behavioural consequences of specifically activating them by chemical ligands or thermal stimuli — a gain-of-function approach.
TRPV1
TRPV1 was identified as a capsaicin- and heat-activated ion channel, and analysis of TRPV1-deficient mice established that capsaicin acts entirely through TRPV1 (REFS 24,25). The sensory qualities of capsaicin are described as burning, itching, piercing, pricking and stinging; sensory qualities that are clearly related to pain26,27. Indeed, most mammals (excluding those humans who find TRP activation enhances culinary experiences) avoid capsaicin and other pungent compounds. In addition to capsaicin, TRPV1 is activated by spider toxins, noxious heat and is modulated by low pH (a common consequence of inflammation)11,28. Therefore, TRPV1 can be defined as a polymodal nocitransducer.
TRPA1
Many pungent chemicals, excluding capsaicin, activate TRPA1. The list includes several compounds found in food, such as isothiocyanates (horseradish, mustard), cinnamaldehyde (cinnamon) and allicin (garlic)29–32. Such pungent compounds are all electrophiles that activate TRPA1 through covalent modification of reactive amino acids such as cysteines33,34. Therefore, this form of chemical TRPA1 modulation is not dictated by the structure of the activating molecule per se, but rather by its chemical reactivity. Indeed, all potent cysteine-reactive chemicals tested seem to activate TRPA1, irrespective of overall shape33,34. Other TRPA1 activators include the alkylating agent iodoacetamide; the environmental irritant acrolein, found in cigarette smoke and an industrial pollutant; formaldehyde, the most commonly used agent to assay chemical nociception in rodents; acetaldehyde, an intermediate substrate of ethanol metabolism; and the endogenous 4-hydroxynonenal (4-HNE)35–38. Reactive oxygen and nitrogen species have also been described to activate TRPA1 (REFS 39–41). 4-HNE is of particular interest as an activator of TRPA1, as it is produced by lipid peroxidation in cells and may be responsible for the pathological effects of oxidative stress. Similar to other TRPA1 activators, 4-HNE can bind to cysteine, histidine and lysine residues in proteins via a Michael addition reaction42 with an EC50 value of 13 μm. This is physiologically relevant as oxidative stress causes accumulation of 4-HNE at concentrations of 10 μm to 5 mm in membranes42. Although many of these chemicals irreversibly react with cysteines, it has been argued that the covalent modification of cysteines in TRPA1 by mustard oil is rapidly reversible34,43. However, the half-life of isothiocyanate–cysteine adducts is in the order of 1 hour at physiological pH and temperature44. Furthermore, the successful labelling of mustard oil alkyne adducts on TRPA1 by click reaction suggests that these adducts remain stable, as the click reaction itself takes 1 hour to complete33. TRPV1 can also be activated by cysteine modification by allicin, although it is less promiscuous than TRPA1, and many cysteine modifiers do not activate this channel45.
There is strong evidence that the covalent binding mechanism of TRPA1 activation is physiologically relevant. Although these reactive chemicals modify proteins non-discriminately, the nociception they cause is dramatically reduced or eliminated in Trpa1−/− mice36–38. Therefore, TRPA1 is a key sensor of chemical damage in vivo and could be an exciting target for treating various pain states as well as bronchial hyperresponsiveness to inhaled irritants in asthma. In addition to reactive chemicals, TRPA1 is activated in some experimental settings by noxious cold temperatures20,31,46,47 (although this remains controversial) and by calcium46,48, and is, therefore, also a polymodal nocisensor. Calcium is not a noxious stimulus per se, nevertheless, the calcium activation of TRPA1 in nociceptive neurons raises the possibility that TRPA1 acts as an amplifier of other signals that increase intracellular calcium, including TRPV1 (FIG. 3). However, the role of calcium on TRPA1 modulation is complicated by the fact that it also causes a desensitization of this channel49.
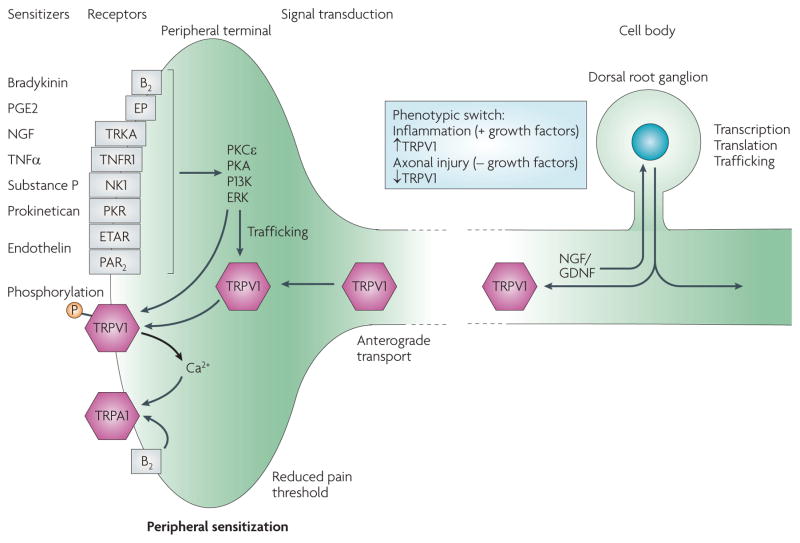
Figure 3. Changes in TRP channels produced by inflammation.
Peripheral inflammation produces multiple inflammatory mediators (sensitizers) that act on their cognate receptors expressed by nociceptors to activate intracellular signal transduction pathways. These pathways can phosphorylate transient receptor potential (TRP) channels and thereby alter their trafficking to the membrane and their thresholds and kinetics. Several growth factors produced during inflammation, most notably nerve growth factor (NGF), are retrogradely transported to the cell body of the nociceptors in the dorsal root ganglion. At the dorsal root ganglion, these growth factors — through intracellular signalling pathways, such as p38 MAP kinase — increase the expression of TRP channels, which are then transported to the peripheral terminal. Changes in transcription and translation of TRP channels and other proteins can switch the chemical phenotype of the neurons from their state in naive conditions to an altered state during inflammation. The net effect of these changes is peripheral sensitization; that is, a reduction in the pain threshold at the site of inflamed tissue. B2, bradykinin receptor B2; ERK, extracellular signal-regulated kinase; ETAR, endothelin receptor type A; GDNF, glial-cell-derived neurotrophic factor; NK1, neurokinin receptor 1; PAR2, protease-activated receptor 2; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; PK, protein kinase; PKR, prokineticin receptor; TNFα, tumour necrosis factor α; TNFR1, TNF receptor 1; TRKA, tyrosine kinase receptor A.
Other temperature-activated TRP channels
TRPM8 is activated by innocuous cold temperatures and by cooling compounds such as menthol18,19. These TRPM8-activating stimuli are not, however, noxious, although they could indirectly influence nociceptive signalling and may contribute to cold allodynia in inflammatory and neuropathic pain (see knockout studies below). Specific activators of the other thermoTRP channels (TRPV2, TRPV3 and TRPV4) with a clear nociceptive quality have not yet been identified (but see below for some tentative evidence)8.
Function follows expression
TRPV1 and TRPA1 are co-localized in a subgroup of DRG and trigeminal neurons that constitute polymodal nociceptors, which are specialized sensors of a variety of noxious stimuli20,31. However, unambiguous expression of the other thermoTRP channels in nociceptors has not been established. TRPM8, for example, is mainly expressed in a separate group of sensory neurons that respond to innocuous cool temperatures (although a minor overlap between TRPV1-expressing neurons is observed)50,51. TRPV2 marks a very poorly characterized subpopulation12. Low levels of TRPV3 and TRPV4 expression have been reported in DRG neurons (including in humans), but seem to be more prominently expressed in keratinocytes13,14,16,52–54. Among the thermoTRP channels, TRPM8 and TRPA1 seem to be exquisitely specific to sensory neurons, whereas the other TRP channels are expressed in several neuronal and non-neuronal tissues8.
TRP channels in the CNS
Although TRP channels are the archetypal transducer receptor — converting thermal and chemical stimuli into electrical activity on the peripheral terminals of sensory neurons — they are also located on axons, which respond to noxious heat stimuli55. TRP channels are also found on the central terminals of nociceptors that make synaptic contact with neurons in the dorsal horn of the spinal cord56. Activation of central terminal TRPV1 by capsaicin, and of TRPA1 by mustard oil applied to isolated spinal cord preparations, results in an increase in synaptic release of both glutamate and neuropeptides57–59. These results indicate a possible role for the TRP channels as synaptic modulators, both directly (by controlling calcium influx and thereby synaptic vesicle release) and indirectly (by producing a depolarization of the terminal). However, what activates central TRP channels — which could include temperature, a chemical ligand or intracellular calcium — is unknown. One set of candidates for TRPV1 are the endocannabinoids, including anandamide, which has a modulatory action on the channel60. For TRPA1 it could conceivably be an endogenous oxidation product, such as 4-HNE, or the coupling of bradykinin through its B2 G-protein-coupled receptor (GPCR) with TRPA1 (REF. 61). Bradykinin is produced in the spinal cord in response to noxious inputs and causes an increase in glutamate release62. A recent surprising finding is that in the hippocampus, TRPV1 receptor activation selectively modifies synapses into inhibitory interneurons to produce long-term depression. This TRPV1-mediated long-term depression may have a role in long-term changes in physiological and pathological circuit behaviour during learning and epileptic activity, and might reveal unexpected side effects or uses for TPV1 antagonists63. TRPV1 also seems to be tonically activated by endovanilloids in brainstem neurons where it may modulate descending antinociceptive pathways64. The specificity of putative TRPV1 actions beyond primary sensory neurons, where its expression levels are by far the highest7–9, need to be confirmed.
Amplifying pain sensitivity
Increases in TRPV1 expression
Inflammatory signals and nerve injury alter TRPV1 expression and function by multiple mechanisms — which include transcriptional and translational regulation, post-translational changes and altered trafficking — and in this way contribute to pathological pain states by increasing sensitivity to noxious stimuli2 (peripheral sensitization) (FIG. 3). Retrograde transport of nerve growth factor (NGF) acts via p38 to increase translation of TRPV1 in the cell body during inflammation, resulting in an increased transport of TRPV1 to peripheral terminals65. NGF also has more immediate actions that increase TRPV1-mediated currents in nociceptor peripheral terminals. NGF acts on tryosine receptor kinase A (TRKA; also known as NTRK1) to activate a phosphoinositide 3-kinase (PI3K)–SRC kinase signalling pathway to phosphorylate intracellular stores of TRPV1, which leads to the insertion of TRPV1 into the cell membrane66. NGF also alters the threshold of TRPV1 via PI3K and p42/p44 mitogen-activated protein kinases67. Many inflammatory mediators that sensitize TRPV1 do so via protein kinase Cε (PKCε)68, which enables TRPV1 to be a major integrator of diverse inflammatory signals on nociceptors (FIG. 3). Indeed, it is this body of evidence that points to the possible efficacy of TRPV1 antagonists in diverse inflammatory pain conditions, including post-surgical pain, cystitis, ulcerative colitis, oesophagitis, asthma and rheumatoid arthritis. Phospholipase C (PLC) and the membrane phospholipid phosphatidylinositol 4,5-bisphosphate it produces, seem to have both inhibitory and activating effects on TRPV1 (REF. 69).
Decreases in TRPV1 expression
In contrast to inflammation and bone cancer, in which TRPV1 levels increase substantially70, expression of TRPV1 in neurons decreases in response to peripheral axonal damage71,72. TRPV1 levels are reduced in the skin of patients with diabetic neuropathy but are increased in intraepidermal fibres in some patients with pain hypersensitivity and proximal to some forms of traumatic nerve injury, where they may accumulate because transport to the periphery is disrupted71. Unmyelinated axons respond to noxious heat with a threshold similar to their peripheral terminals and to heterologous TRPV1 at ~41 °C55. It is not known whether TRPV1 located in axonal membranes and in neuroma are activated by endovanilloids or become sensitized so that their threshold falls to body temperature in neuropathic pain. The contribution of TRPV1 to neuropathic pain may include sensitization of intact peripheral terminals, ectopic activity in injured axons or a role in modulating transmitter release. Interestingly, TRPV1 antagonists reduce pain sensitivity in some models of neuropathic pain73, although a knockout of TRPV1 generally has no effect24,25. However, global knockout of a gene may not always be a useful predictor by itself of a role zin disease states (BOX 1).
Box 1. How do we improve TRP transgenic models?
How useful are transgenic models in predicting therapeutic benefit? Approximately 50% of the molecular targets of the top 100 selling drugs have been knocked out in mouse transgenic models, as have many targets of drugs in full development. In both cases approximately 85% of knockout phenotypes showed a sound biological rationale for the disease indication, with a direct correlation between knockout phenotype and the effect of drug. This therefore demonstrates that knockout models are likely, in most cases, to provide a productive source of validated targets for future drug development142.
Nevertheless, with respect to pain phenotyping, mouse gene knockouts are subject to many potential pitfalls, including functional compensation by related channels and receptors, and significant differences between mouse strains143. Significant advances have been made to improve knockout mouse technology that may help improve their utility for target validation. Transgenic mouse models need to be generated on a specific genetic background for phenotypic analysis, primarily strain C57BL/6. Until quite recently, however, routine model generation has only been possible through gene targeting in the robust 129 embryonic stem cells, followed by successive backcrossing onto the C57BL/6 background. This adds up to 12 months to model generation timelines and is resource intensive.
It is now possible to generate transient receptor potential (TRP) knockout mice by gene targeting in C57BL/6 embryonic stem cells, which obviates the need for a year of backcrossing and allows behavioural phenotyping on a pure genetic background144. Derivation of transgenic mice using C57BL/6 cells is becoming routine practice in many laboratories145. This advance coupled with advances in conditional and tissue specific models will improve the timeliness, utility and application of future TRP transgenic models.
Modulation of other heat-activated TRP expression
The extent to which inflammatory signals also upregulate and sensitize other thermoTRP channels has been less extensively investigated. TRPA1 is activated by high concentrations and sensitized by low concentrations of bradykinin downstream of G-protein-coupled signalling32,61. The bradykinin sensitization is modulated by both PLC and PKA61. TRPA1 is also activated by protease-activated receptor 2 (PAR2; also known as F2RL1) through PLC74. There is also evidence that TRPA1 is transcriptionally regulated. Nerve injury, complete Freund’s adjuvant and NGF increase TRPA1 expression and activity in sensory neurons75,76 (see also loss-of-function studies below). Studies have similarly highlighted the sensitizing effects of inflammatory signals such as prostaglandin E2 (PGE2) and PAR2 on TRPV4 activity77,78. TRPV3 is sensitized by repeated stimulation, and calcium plays an important role in this process13,79. Furthermore, TRPV3 activity is potentiated by PLC-dependent GPCR activation80. However, a role for TRPV3 in the sensitization of nociceptors has not yet been shown in vivo. In contrast to the sensitizing effects of inflammatory signals on other thermoTRP channels, these signals mainly suppress TRPM8 function. For example, PKC activation and phosphatidylinositol 4,5-bisphosphate depletion (which occur in response to inflammatory signals such as bradykinin) abolish or reduce TRPM8 activity81–83. TRPM8 responses are abolished by inhibitors of the calcium-independent form of phospholipase A2, whereas lysophospholipids — one of the enzyme’s products — activate the ion channel84,85. Whether this pathway is involved in nociceptor sensitization in vivo is not clear.
Function through loss-of-function
TRPV1
Trpv1−/− mice demonstrate a profound requirement for TRPV1 for thermal hyperalgesia in response to inflammatory signals, although a reduction but not elimination of acute thermal pain is also observed in these mice24,25 (BOX 2). Since these initial findings, analysis of Trpv1−/− mice has also established a role of TRPV1 in bladder function and inflammation, serum osmolarity, diabetes, pancreatitis, cough, arthritis, anxiety, and fever86–91. Acute pharmacological block of protein activity in adult animals sometimes deviates from the effects of germline ablation of the encoding gene. For example, pharmacological inhibition of TRPV1 induces hyperthermia in wild-type mice in a TRPV1-dependent manner, whereas Trpv1−/− mice do not display this phenotype92,93. Similarly, many TRPV1 antagonists seem to reduce mechanical nociception, whereas Trpv1−/− mice have normal mechanical responses (reviewed in REF. 94). Clearly, compensatory mechanisms are at play in mice that lack TRPV1 throughout development. Overall, the genetic and pharmacological studies on TRPV1 establish potential clinical uses for targeting TRPV1 for pain indications, but also raise concerns regarding potential side effects, such as a diminished response to damaging heat stimuli, altered body temperature and a reduction in the perception of taste.
Box 2. Phenotypes of Trpv1−/− knockout mice.
Below is a list of the published phenotypes of Trpv1−/− knockout mice:
Reduced response to acute thermal stimuli, but normal response to noxious mechanical stimuli; no vanilloid-evoked pain behaviour (and capsaicin did not cause hypothermia); and reduced inflammatory thermal hyperalgesia24.
Normal acute noxious thermal stimuli; no carrageenan-induced thermal hyperalgesia (inflammation); and lacked capsaicin and acid-gated responses25.
No acute thermal hyperalgesia induced by activin administration146.
Reduced endothelin-1-induced thermal hyperalgesia70.
No swelling and no hypersensitivity in joint inflammation86.
Higher frequency of low-amplitude, non-voiding bladder contractions87.
No changes in response to ambient temperature variations, and attenuated fever in response to lipopolysaccharide (impairment in thermoregulation)88.
Attenuated water intake in response to systemic hypertonicity, and no osmosensory signal transduction cascade (in organum vasculosum lamina terminalis neurons)89.
Less anxiety-related behaviour in the light–dark test and in the elevated plus maze, and less of a ‘freezing’ response to a tone after auditory fear conditioning and stress sensitization90.
Enhanced insulin sensitivity91.
No AMG-0347(antagonist)-induced hyperthermia; normal response to needle prick; and no response (hypothermia) to resiniferatoxin (agonist)92.
No electrophysiological responses to oleoylethanolamide (OEA) in neurons; no rise in intracellular calcium to OEA (neurons); and no visceral pain-related behaviours to OEA administration (mice)147.
No heat hyperalgesia after incision (normal mechanical hyperalgesia)148.
Less swelling of the knee and hyperpermeability in inflammation, and no thermal hyperalgesia in response to complete Freund’s adjuvant-induced inflammation149.
No acute nocifensive behaviour after injection of a protein kinase C activator; earlier and greater chronic mechanical hyperalgesia evoked by toxic polyneuropathy; normal formalin-induced acute nocifensive behaviour, carrageenan-evoked inflammatory mechanical hyperalgesia and partial sciatic nerve lesion-induced neuropathic mechanical hyperalgesia; and reduced thermal and mechanical hyperalgesia induced by mild heat injury150.
Normal mechanical hypersensitivity by tumour necrosis factor α (TNFα), and no thermal hypersensitivity by TNFα151.
Streptozocin-induced diabetes does not alter thermal pain sensitivity152.
No cystitis-induced bladder mechanical hyperreactivity and no increased mechanical sensitivity of hind paws153.
What is the anatomical site of action of TRPV1 antagonists — the peripheral or central end of the nociceptor? Intrathecal administration of capsazepine or iodoresiniferatoxin reduces intraplantar formalin-evoked pain-related behaviour95. By contrast, when a TRPV1 antagonist, SB-366791, is applied to an isolated spinalcord preparation there is a reduction in glutamatergic transmission after peripheral inflammation, implying that in these situations the central TRPV1 receptor is tonically active96. Furthermore, a TRPV1 antagonist with poor CNS penetrance (A-795614) has equal potency to one with good CNS penetration (A-784168) for peripheral capsaicin-evoked pain-like behaviour and thermal hyperalgesia after peripheral inflammation, implying action at the peripheral terminal97. However, the compound with greater CNS penetration is more potent when given systemically for mechanical allodynia and osteoarthritic pain than the compound that does not cross the blood–brain barrier. These findings suggest, perhaps somewhat surprisingly, that targeting TRP channels on the central terminals of nociceptors is required to achieve maximal analgesic benefit. The challenge for the pharmaceutical industry, therefore, is to identify the next generation of compounds that have the ability to cross the blood–brain barrier, while retaining the necessary potency, selectivity and developability properties and which minimize adverse effects. Although these would be centrally acting compounds they would still act primarily on nocicptors, albeit at their central and not peripheral terminal, and in consequence may retain a high therapeutic index.
TRPA1
Two groups have independently shown that Trpa1−/− mice have strong deficits in somatosensory chemosensation98,99, thereby confirming the role of this receptor in sensing noxious stimuli. One group observed deficits in acute nociceptive mechanosensation and cold thermosensation in Trpa1−/− mice98. The mechanosensory deficit was not examined by the other group; however, an independent study was able to reproduce the specific mechanosensory deficit100. For cold thermosensation this result is disputed and more investigations including analysis of Trpm8/Trpa1 double mutant mice are required, as the presence of TRPM8 might be masking any subtle phenotype present in TRPA1-deficient mice. However, in vivo antisense experiments have shown a requirement for TRPA1 for cold hypersensitivity in response to inflammation and injury in rats76. A selective small-molecule inhibitor of TRPA1 that is ineffective in Trpa1−/− mice reverses complete Freund’s adjuvant-induced mechanical hyperalgesia in wild-type mice37,100. These experiments collectively suggest that TRPA1 plays a part in establishing or maintaining mechanical hyperalgesia, and that compensation in Trpa1−/− mice may mask this requirement. In addition, Trpa1−/− mice have deficiencies in oxidant-induced respiratory depression, nasal obstruction, sneezing, cough and pain, emphasizing that TRPA1 activation has consequences beyond pain perception101 (BOX 3). More studies are needed to elucidate the potential effectiveness of targeting TRPA1 in various pain indications as well as in asthma and other respiratory problems.
Box 3. Phenotypes of other TRP channel knockout mice.
Below is a list of the published phenotypes of transient receptor potential (TRP) channel knockout mice that have been generated. Further information on the effect of TRP channel knockouts on pain phenotype can be obtained from the Pain Genes database (see Further information).
TRPV3
Strong deficits in responses to innocuous and noxious heat118.
TRPV4
Prevented protease-activated receptor 2 agonist-induced mechanical hyperalgesia and sensitization77.
No inflammation-induced mechanical or osmotic hyperalgesia108.
Strong preference for 34 °C over 30 °C110.
Impaired response to both hyperosmolar and hypo-osmolar stimuli111.
Reduced sensitivity to tail pressure and acidic nociception114.
Significantly longer latency to escape from 35–45 °C surface temperature when hyperalgesia was induced by carrageenan115.
Strongly reduced mechanical hyperalgesia induced by paclitaxel, vincristine or diabetes154.
Reduced nociceptive behaviour caused by mild hypertonic stimuli, 2% saline in the presence of inflammatory mediator155.
TRPM8
Lack of behavioural response to cold-inducing icilin application; attenuated response to acetone evaporative cold stimuli; severe behavioural deficits in response to cold stimuli; and cold-induced analgesia is reduced50.
Innocuous cold sensation and icilin response severely disrupted, and reduced firing of cold fibres103.
Decreased number of neurons responding to cold (18 °C) and menthol (100 μM) (sensory neurons); deficiencies in certain cold-related behaviours, including icilin-induced jumping and cold sensation; and reduction in injury-induced responsiveness to acetone cooling102.
TRPA1
Dramatically reduced formaldehyde-induced pain responses, and abolished painful responses to iodoacetamide, a nonspecific cysteine-alkylating compound38.
Lack of formalin sensitivity37.
Behavioural deficits in response to mustard oil, cold temperature (~0 °C) and to punctate mechanical stimuli, and normal startle reflex to loud noise, a normal sense of balance, a normal auditory brainstem response, and normal transduction currents in vestibular hair cells98.
Pronounced deficits in bradykinin-evoked nociceptor excitation and pain hypersensitivity, and normal cold sensitivity and unimpaired auditory function99.
Profound deficiencies in hypochlorite-induced and hydrogen-peroxide-induced respiratory depression as well as decreased oxidant-induced pain behaviour101.
Abolished pain-related responses induced by general anaesthetics156.
Greatly reduced nocifensive behaviour in response to isovelleral157.
Strongly reduced calcium response induced by 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and allyl isothiocyanate (dorsal root ganglion (DRG) neurons), and no acute nociceptive responses by 15d-PGJ2 (REF. 158).
Strongly reduced calcium response induced by hydrogen peroxide or 15d-PGJ2 (DRG neurons), and no nocifensive/pain response to injection of hydrogen peroxide or 15d-PGJ2 (REF. 159).
Strongly reduced calcium response induced by cyclopentenone prostaglandins (DRG neurons); absent early and transient nociceptive response to injection of cyclopentenone prostaglandins; normal nociceptive response to classical pro-algesic prostaglandin160.
Overall, these early studies on TRPV1 and TRPA1 underscore the importance of considering both acute pharmacological blockade and genetic ablations in assigning gene function and potential clinical utility.
TRPM8
Analysis of Trm8−/− mice clearly establishes that this ion channel is the major sensor of peripheral innocuous cool temperatures50,102,103 (BOX 3). However, sensing noxious cold nociception is only partially affected in these mice, which suggests the existence of at least one other receptor. A role of TRPM8 in cold allodynia after nerve injury is suggested in rats and in mice102,104. Paradoxically, TRPM8 activation has also been shown to have analgesic properties50,105. It is proposed that TRPM8 expression in functionally distinct DRG neurons can account for multiple (even opposing) roles in somatosensation106. TRPM8 is mainly expressed in DRG neurons that respond to cool temperatures, but most do not express any nociceptive markers. About 10–20% of TRPM8-positive neurons also express TRPV1 and are most likely to be nociceptors51,107. Therefore, TRPM8-expressing neurons could send nociceptive as well as cool signals to the spinal cord, depending on the context. Whether TRPM8 is a useful target for treating cold allodynia in patients with neuropathic pain or for other indications is difficult to assess at this time. Pharmacological disruption of TRPM8 in adult animals should shed light on this.
Other TRP channels
Trpv4−/− mice display various defects including impaired thermal, mechanical and osmotic responses, which are mostly consistent with the different known modes of TRPV4 activation77,108–115 (BOX 3), but are also incontinent and deaf. These studies also validate that TRPV4 is involved in nociceptor sensitization. One outstanding question is whether keratinocytes or sensory neurons are the site of TRPV4 action53,54,78,112. Interestingly, TRPV4 may have a particular role in colonic afferents where it is involved in mechanotransduction, and is expressed in nerve fibres of patients with inflammatory bowel disease116. Given the wide-ranging phenotypes described for TRPV4-deficient mice, it is unclear whether systemic TRPV4 blockade for analgesia would be accompanied by severe side effects110, and whether a non-absorbable TRPV4 blocker could be used for colonic pain. Once again, pharmacological blockade in adult animals should directly address these issues. TRPV4 is also involved in the generation of pulmonary oedema in response to increased microvascular pressure and this can be blocked by cyclic GMP117. Therefore, inhaled TRPV4 antagonists, like those for TRPV1 and TRPA1, may have utility in respiratory diseases.
Acute noxious heat thermosensation is diminished in Trpv3−/− mice, but there is no evidence to date for a role of TRPV3 in inflammatory and neuropathic pain118. Trpv2−/− mice have not yet been described. Testing knockout mice for these TRP channels in clinically relevant pain models, and in particular identifying and testing specific antagonists against all thermoTRP channels for efficacy in pain states, is necessary to validate which of these ion channels are potential targets for novel analgesics (BOXES 2,3).
Acute sensors of damage as pain targets
Will blocking TRPV1 and TRPA1 or other thermoTRP channels be useful for pain indications? One could argue that such alarm signals for tissue damage have evolved for protective effects, and blocking them would therefore not be desirable. However, in pathological states, changes in the chemical milieu of nerve endings could overactivate and sensitize the pain pathway through nociceptive TRP channels (FIG. 3), thereby leading to pain hypersensitivity in many disease states119. For example, patients suffering from diabetic neuropathy have elevated levels of reactive chemicals that are known TRPA1 activators120,121. Could this explain the ectopic pain present in this condition and in other painful neuropathies? Does the threshold of TRPV1 expressed in unmyelinated axons ever drop to a point that it is activated at body temperature55? These questions will hopefully be addressed within the next 2–5 years, both in animal models and in Phase IIA proof-of- concept trials with suitable specific antagonists. These specific antagonists will also help address whether TRP antagonists need to be targeted only to the periphery to produce maximum analgesia (FIG. 4).
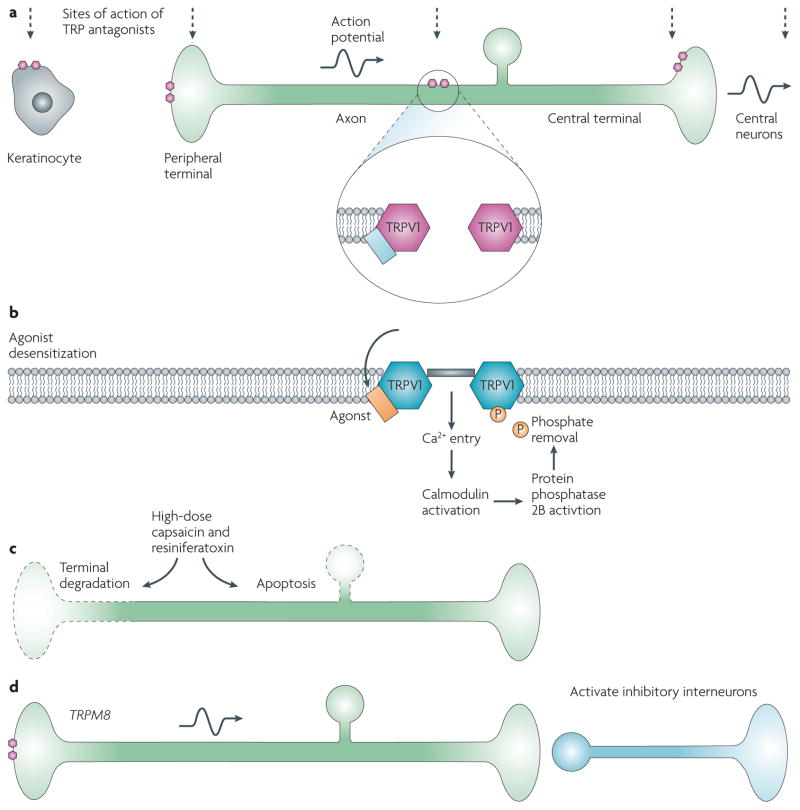
Figure 4. TRP channel antagonists and agonists as analgesics.
Transient receptor potential (TRP) channel antagonists as analgesics (a). Inhibition of TRP channels may produce analgesia by preventing transduction in the periphery, acting on channels expressed in keratinocytes or nociceptors, reducing ectopic activity generated by TRP channels along the axon and by reducing transmitter release as well as possibly acting on central neurons. The small hexagons represent TRPV1 channels at different anatomical locations, the vertical line share the different sites of actions of TRPV1 antagonists and the horizontal line shows the flow of nociceptive information from the periphery to the central nervous system. TRP channel agonists as analgesics (b–d). Activation of TRP channels may reduce pain by desensitizing the TRP channel to reduce pain transduction (this effect is transient and fully reversible; b); by producing sufficient calcium influx to ablate the axon at the site of application (represented by dotted lines on the left; c), producing long-lasting but potentially reversible morphological change, or ablating the whole neuron as a result of death of the cell body (represented by dotted circle), which is irreversible (c); and by driving inhibitory circuits, as for activation of cool-sensing TRPM8 expressing neurons, which represent the channels on the peripheral terminal (d). For approaches b and c the agonist will cause pain until desensitization is achieved or neuronal ablation occurs and may require a general anaesthetic. Evidence for efficacy for topical capsaicin in rheumatic conditions is lacking161, and that for resiniferatoxin for lower urinary tract symptoms is inconclusive162. However, there seems to be efficacy for topical low-dose capsaicin for post-herpetic neuralgia163 and high-dose capsaicin for pain experienced in HIV127.
ThermoTRP channels are more than just acute damage sensors that mediate acute nociceptive pain; they are also important players in inflammatory pain and possibly also neuropathic pain. Indeed, TRPV1 and TRPA1 activation does not only send an afferent (sensory) signal to the CNS but also an efferent (motor) signal via secretion of inflammatory agents such as substance P and calcitonin gene-related peptide (CGRP)9 (FIG. 2). This efferent signal at the peripheral terminal causes local neurogenic inflammation with vasodilation and oedema owing to increased capillary permeability9. TRPV1-expressing afferents may trigger the auto immune inflammatory response in type 1 diabetes91. As nociceptive TRP channels are themselves targets of inflammatory signals (FIG. 3), they are also mediators of peripheral sensitization. The challenge clinically will be to minimize disruption of the acute damage sensor role while maximizing reduction of the TRP channel role in pathological conditions. Given their participation in pain transduction and inflammatory signalling, their sensitization properties and their expression pattern, targeting these ion channels could possibly ameliorate many inflammatory conditions including arthritis, some forms of neuropathic pain such as diabetic neuropathy, itch, asthma, interstitial cystitis, gastrointestinal disorders such as reflux oesophagitis and colitis, and even cancer. For example, TRPM8 is expressed in prostate cancer cells and therefore may have utility as a diagnostic marker122 and may even offer opportunities for therapy. The next few years should witness the direct testing in the clinic of the role of the different TRP channels in many of these indications. These results will help further define both the biology of TRP channels in sensory systems and highlight the most promising clinical indications for TRP channel blockers.
Using TRP agonists as analgesics
Potent TRPV1 agonists produce pain but also desensitize the receptor123. This inactivation reduces sensitivity to heat and other ligands, which can be used, with limited efficacy, to reduce pain124 — albeit with the problem of the pain that is produced before the desensitization (FIG. 4). This approach is being tested to treat several bladder conditions such as interstitial cystitis. Furthermore, agonists such as capsaicin and resiniferatoxin can lead to such a large calcium influx that they can produce degeneration of nociceptor axons at the site of application (topically) into joints or onto nerves. They may even cause a loss of the sensory neuron itself by calcium-mediated mitochondrial damage and cytochrome c release, leading to apoptosis125,126 when exposed close to the cell body (FIG. 4). High-dose topical capsaicin-induced peripheral terminal axonal loss does produce long-lasting analgesia in patients with HIV, but at the cost of intense initial pain in some patients127 and with the potential problems inherent in any neuroablative strategy128. Resiniferatoxin is more potent than capsaicin and can selectively ablate nociceptors when delivered intrathecally, which may have special utility for uncontrolled pain in a palliative setting129. Activation of TRPM8 by icilin is reported to produce analgesia by activating central inhibitory pathways50,105, and cold-induced analgesia is attenuated in TRPM8-deficient mice50,105 (FIG. 4).
TRP channels for drug delivery
TRP channels are non-selective cation channels with pores that seem to be large enough to allow cationic molecules with molecular masses of ~500 daltons to enter the cell130. TRPV1 shows a time-dependent and agonist-dependent increase in permeability to large cations owing to changes in the TRPV1 selectivity filter that are PKC-dependent and reflect increases in pore diameter131. This feature of the channels can be exploited to target small membrane-impermeable cationic drugs into cells that only express a particular TRP channel. Indeed, this approach can be used to produce analgesia. A quaternary derivative of the local anaesthetic lidocaine — QX-314 — that is ineffective in blocking sodium channels when administered extracellularly, because it cannot gain access to the inner face of the channels, inhibits sodium currents only in TRPV1-expressing nociceptors. This produces a dense (complete block of response to noxious stimuli), long-lasting local analgesia when co-administered with capsaicin, without impairing motor function or innocuous tactile sensitivity in the way that local anaesthetics do132. This approach produces local analgesia when injected subcutaneously, close to a peripheral nerve, and intrathecally. Because QX-314 is trapped in the nerve fibres the duration of effect is about ten-times longer than lidocaine and offers, therefore, a new approach to manage surgical and post-operative pain as well as labour pain. Because this strategy produces regional or local analgesia instead of a non-specific local anaesthesia with blockade of all sensory, motor and automatic fibres, patients will be able to mobilize earlier with less risk of hypotension due to autonomic blockade. To successfully use the technology in patients a non-pungent activator of TRPV1 would be ideal to minimize patient discomfort until sodium-channel blockade is achieved on entry of the charged membrane-impermeant sodium-channel blocker. Although this strategy uses a TRPV1 agonist, it is only as a means of targeting delivery of a drug to particular cells and not as an intrinsic analgesic as when used by itself. In principle, the technology should work for any TRP channels for which pore size is sufficient to allow entry of small cationic drugs, and for which expression is limited to nociceptors, such as TRPA1.
Moving into the clinic
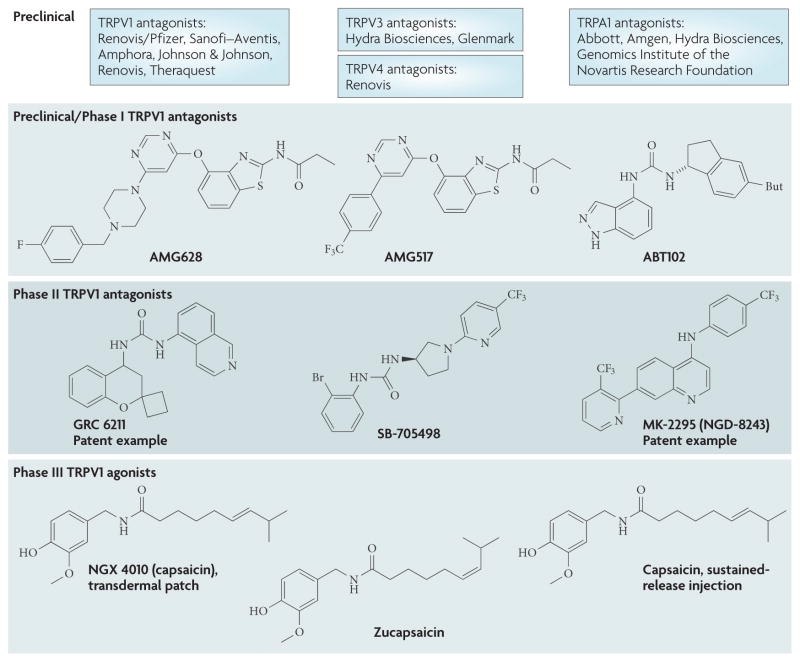
It is often difficult to obtain exact information regarding the status of molecules in early stages of clinical development. However, through a review of patent literature and company press releases we have noted significant activity, in particular with TRPV1 modulators (FIG. 5). Several companies, including GlaxoSmithKline, Amgen, Merck/Neurogen Astra Zeneca, Evotec/Pfizer and Glenmark, are conducting or have now completed Phase I studies with oral TRPV1 antagonists and some have progressed into Phase II for pain and/or migraine indications133. Other companies are exploring local application of TRPV1 agonists, including NeurogesX for neuropathic pain and Anesiva for post-surgical pain (see Further information for data from individual companies). There is considerable preclinical activity, not only with TRPV1 antagonists but also with TRPA1, TRPV3 and TRPV4 antagonists. Of note are two TRPV3 antagonists from Glenmark, GRC 15133 and GRC 17173, which demonstrated a significant reduction in thermal and mechanical hyperalgesia in models of neuropathic and inflammatory pain (see Further information).
Figure 5. TRP drugs currently under development as analgesics.
Current state of transient receptor potential (TRP) channel drug discovery, illustrated by phase of development. Chemical structures of lead molecules have been inserted where known (see REFS 133,134). Patent examples are drawn where the structure is not published (from REFS 135,136).
Target occupancy studies
The discovery of novel analgesics relies heavily on behavioural observations in animal models of pain before their testing in Phase II proof-of-concept clinical trials. However, there are concerns about the predictive value of animal models in pain. It is well known that most drug candidates fail in early clinical development phases, and poor translation from the pre-clinical to clinical setting is a likely reason for this low success rate. How can we improve the success ratio in the clinic? Perhaps of most importance is to ensure that when novel mechanisms such as TRP channel modulators are tested, the target mechanism is fully engaged by the drug molecule. We have postulated, for example, that a central site of action may be required in order to achieve maximum therapeutic benefit for TRPV1 modulators in certain inflammatory and neuropathic pain states. Target occupancy studies such as positron emission tomography (PET) scans can be used to demonstrate interactions between a drug and the relevant sites of action in the nervous system. PET studies could be performed as part of the Phase I studies, and will also provide valuable information on receptor distribution, receptor density and endogenous neurotransmitter release. The level of target occupancy can then be compared with pharmacological effects.
Human experimental pain research
Over the past few years there has been significant progress in human experimental pain research, and new techniques have been developed for the induction and measurement of pain134. Thermal, mechanical, electrical and chemical stimuli can be applied to different tissues and then the responses recorded by a combination of imaging technology such as functional magnetic resonance imaging and PET, electrophysiological recordings such as electroencephalography and evoked potentials, and behavioural measurements135,136. Human experimental medicine studies were used to assess the pharmacodynamic and antihyperalgesic properties of the TRPV1 antagonist SB-705498 (REF. 133). To date, this is the only published TRPV1 clinical study with efficacy data; however, there are several other TRPV1 antagonists in early development (FIG. 5) and data from these studies are eagerly anticipated. The experimental protocol measured heat-evoked pain and skin sensitization, which were induced by either capsaicin or ultraviolet B irradiation. In comparison with placebo, SB-705498 was shown to reduce the area of capsaicin-evoked flare and to increase pain tolerance at the site of ultraviolet B irradiation. Both of these pharmacodynamic effects correlated with plasma exposure of SB-705498. This profile, which compared well with preclinical animal model studies, allowed this molecule to enter Phase II trials with increased confidence135. A further study has shown that PGE2 potentiates nociceptor activation by protons in human skin and that this is caused by TRPV1 sensitization137. This and other translational human experimental pain models will help the transition from preclinical to clinical pain studies for TRP channel modulator drugs.
Potential side effects of TRPV1 antagonists
TRPV1 seems like a highly exciting clinical opportunity based on early efficacy readouts, but potential side effects should be considered. TRPV1-expressing primary afferent neurons project to cardiovascular and renal tissues where they surround blood vessels in vascular beds138. A subset of these neurons release the neuropeptides CGRP and substance P following TRPV1 activation. These neuropeptides are potent vasodilators and natriuretic/diuretic factors, and activation of cardiac afferents that contain CGRP and substance P may be associated with cardio-protective effects138. TRPV1 antagonists may potentially produce unwanted cardiovascular side effects, although there is no data as yet from the early clinical trials that this will be an issue. Recent studies with the TRPV1 antagonist AMG-517 have demonstrated a long-lasting hyperthermia, with core body temperature rising to as much as 40 °C, in patients undergoing a dental pain clinical trial139.This effect seemed to be dose-dependent with some individuals more susceptible than others, and unfortunately the efficacy group of this trial could not be completed owing to this significant adverse effect. The magnitude of this effect seemed to be larger in the patient population than in healthy volunteers, suggesting a correlation with tonically active TRPV1 receptors. Trpv1−/− mice show an impaired response to hyperthermia, further implicating these ion channels in thermosensitivity and core body temperature regulation140. Although TRPV1 is expressed in thermosenstive neurons in the hypothalamus140 TRPV1 antagonists that are peripherally restricted still produce hyperthermia93, which implies that the site of action is outside the blood– brain barrier, and may be due to a blockade of tonically active TRPV1 receptors in abdominal viscera92.
At least two lines of evidence suggest this is not a potential ‘showstopper’ for TRPV1 antagonists. The first involves a study with AMG-8562, a TRPV1 antagonist that is selective for capsaicin and pH activation, but does not block heat-evoked activation of the receptor. AMG-8562 does not elicit a hyperthermic response in preclinical species at doses that are effective in rodent models of inflammatory pain141. Encouragingly, advances in ion-channel screening and the emergence of robust high-throughput electrophysiology systems open up the potential for identifying high quality, modality-specific TRP-channel blockers of this type. The second line of evidence comes from the study on SB-705498 in which hyperthermia was not observed in humans given 400 mg of this TRPV1 antagonist133. Given that this molecule antagonizes all modalities of TRPV1 activation, there may be further compensatory mechanisms that under-pin this apparent lack of hyperthermia. However, it is clear that there are significant challenges for drug companies to develop safe and effective medicines that target TRPV1. For example, clinical development of GRC 6211 — a TRPV1 antagonist that was in Phase II trials for osteoarthritis — has been suspended, with no indication, so far, as to why (Glenmark press release; see Further information).
Conclusions
The discovery that the capacity to detect many forms of noxious stimuli is mediated by high-threshold TRP channels expressed selectively in nociceptors has provided insights into the molecular basis of sensory labelled lines — neural input channels devoted to specific types of stimuli and in the peripheral nervous system. It has also provided an opportunity for the development of analgesics that are different from the non-steroidal anti-inflammatory drugs, opiates, anticonvulsants and antidepressants currently used.
The field has moved relatively quickly, from the cloning of TRPV1 to clinical trials within 10 years. Studies on TRPV1 raise the intriguing possibility that these ion channels have functional roles greater than initially anticipated, which may provide problems and opportunities. For example, while TRPV1 is an acute noxious heat receptor, it also seems to be a major player in various pain states such as bone cancer pain and neuropathic pain, as well as in thermoregulation and hippocampal plasticity. TRPA1 also seems to be a major player is sensing damage, and has great clinical potential as an analgesic and perhaps even more so for several respiratory diseases.
However, more studies are required to identify which TRP channels will be appropriate targets for which specific pain and other indications. Armed with genetic deletions for each of these ion channels, and specific small-molecule agonists and antagonists against many of them, the important questions should be addressed in the next few years.
We are poised to discover whether drugs that act on sensory TRP channels provide greater efficacy and fewer adverse effects in the treatment of pain than the currently available analgesics. In addition to targeting TRP channels with agonists to activate, desensitize and ablate nociceptors, and with antagonists to reduce TRP activity in the periphery and CNS, we can also use TRP channels as a drug delivery system to target, with TRP agonists, small cationic compounds selectively into those specific cells that express these ion channels. It seems likely that somehow, from these multiple and diverse therapeutic opportunities, TRP channels will provide a new means of reducing pain.
Acknowledgments
- Nociceptor
A high-threshold primary sensory neuron that detects or responds to noxious stimuli
- Inflammatory pain
Pain associated with tissue injury and inflammation characterized by reduced threshold and increased responsiveness
- Neuropathic pain
Pain associated with a lesion to the nervous system
- Michael addition reaction
Nucleophilic addition to an alpha or beta unsaturated carbonyl group
- Click reaction
Copper(I)-catalysed azide-alkyne cycloaddition reaction that can be used for in vivo labelling of molecules
- Endocannabinoids
endogenous agonists of cannabinoid receptors in animals
- Endovanilloid
An endogenous ligand of the TRPV1 receptor
- Peripheral sensitization
Reduction in the threshold for activation of the peripheral terminal of nociceptors produced by inflammatory mediators
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details.
References
- 1.Woolf CJ, Ma Q. Nociceptors-noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Baron R. Mechanisms of disease: neuropathic pain — a clinical perspective. Nature Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 4.Finnegan TF, Chen SR, Pan HL. Effect of the μ opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther. 2005;312:441–448. doi: 10.1124/jpet.104.074633. [DOI] [PubMed] [Google Scholar]
- 5.Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–354. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Wood JN. Ion channels in analgesia research. Handb Exp Pharmacol. 2007;177:329–358. doi: 10.1007/978-3-540-33823-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 8.Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 9.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 10.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. The first cloning of a nociceptive TRP ion channel, TRPV1, and the demonstration that it is both the capsaicin receptor and a noxious heat detector. [DOI] [PubMed] [Google Scholar]
- 12.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 13.Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 14.Smith GD, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 16.Guler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 18.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 19.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. By annotating novel TRP ion channels within the genome and determining their expression and function, the authors cloned TRPM8, the first channel shown to be activated by cool temperatures (15–25°C) and menthol. [DOI] [PubMed] [Google Scholar]
- 20.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 21.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nature Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 22.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 23.Togashi K, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 25.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 26.Philip G, Baroody FM, Proud D, Naclerio RM, Togias AG. The human nasal response to capsaicin. J Allergy Clin Immunol. 1994;94:1035–1045. doi: 10.1016/0091-6749(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 27.Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53:323–334. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- 28.Siemens J, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 29.Macpherson LJ, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 32.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 33.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. This paper shows that TRPA1 is activated through covalent cysteine modification, and that it is the reactivity of these pungent chemicals (not necessarily their shape) that leads to TRPA1 activation. [DOI] [PubMed] [Google Scholar]
- 34.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci. 2007;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- 36.Trevisani M, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara CR, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macpherson LJ, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida T, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nature Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 42.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, et al. Molecular determinants of speciesspecific activation or blockade of TRPA1 channels. J Neurosci. 2008;28:5063–5071. doi: 10.1523/JNEUROSCI.0047-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conaway CC, Krzeminski J, Amin S, Chung FL. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem Res Toxicol. 2001;14:1170–1176. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 45.Salazar H, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nature Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 47.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 48.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 49.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. TRPA1 desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 51.Takashima Y, et al. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, Ookawara S. Localization of mechanosensitive channel TRPV4 in mouse skin. Neurosci Lett. 2003;353:189–192. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann T, Sauer SK, Horch RE, Reeh PW. Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci. 2008;28:6281–6284. doi: 10.1523/JNEUROSCI.1627-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- 57.Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- 58.Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci. 2007;27:4443–4451. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrini F, Salio C, Vergnano AM, Merighi A. Vanilloid receptor-1 (TRPV1)-dependent activation of inhibitory neurotransmission in spinal substantia gelatinosa neurons of mouse. Pain. 2007;129:195–209. doi: 10.1016/j.pain.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Fischbach T, Greffrath W, Nawrath H, Treede RD. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive DRG neurons of rats. J Neurophysiol. 2007;98:929–938. doi: 10.1152/jn.01096.2006. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, et al. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, et al. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J Neurosci. 2005;25:7986–7992. doi: 10.1523/JNEUROSCI.2393-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Starowicz K, et al. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci. 2007;27:13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf C. J p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. Documentation of the changes in TRPV1 that contribute to peripheral sensitization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007;34:689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mandadi S, et al. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCε-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Lukacs V, et al. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawamata T, et al. Contribution of transient receptor potential vanilloid subfamily 1 to endothelin-1-induced thermal hyperalgesia. Neuroscience. 2008;154:1067–1076. doi: 10.1016/j.neuroscience.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Facer P, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lauria G, et al. Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst. 2006;11:262–271. doi: 10.1111/j.1529-8027.2006.0097.x. [DOI] [PubMed] [Google Scholar]
- 73.Culshaw AJ, et al. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J Med Chem. 2006;49:471–474. doi: 10.1021/jm051058x. [DOI] [PubMed] [Google Scholar]
- 74.Dai Y, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 76.Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grant AD, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alessandri-Haber N, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 79.Xiao R, et al. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–6174. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nature Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 81.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nature Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 84.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanden Abeele F, et al. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem. 2006;281:40174–40182. doi: 10.1074/jbc.M605779200. [DOI] [PubMed] [Google Scholar]
- 86.Barton NJ, et al. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Birder LA, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 88.Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 89.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsch R, et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Razavi R, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 92.Steiner AA, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gavva NR, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1, 10 years from channel cloning to antagonist proof-of-concept. Nature Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 95.Kanai Y, Hara T, Imai A. Participation of the spinal TRPV1 receptors in formalin-evoked pain transduction: a study using a selective TRPV1 antagonist, iodo-resiniferatoxin. J Pharm Pharmacol. 2006;58:489–493. doi: 10.1211/jpp.58.4.0008. [DOI] [PubMed] [Google Scholar]
- 96.Lappin SC, Randall AD, Gunthorpe MJ, Morisset V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol. 2006;540:73–81. doi: 10.1016/j.ejphar.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 97.Cui M, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. First evidence that TRP receptor antagonists may act on the central terminals of nociceptors to produce analgesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 99.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. Demonstration that TRPA1 is a receptor-operated channel and that this contributes to the detection of inflammation. [DOI] [PubMed] [Google Scholar]
- 100.Petrus M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. By virtue of its capacity to respond to oxidants and other irritants TRPA1 may have a major role in respiratory diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 103.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 104.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Proudfoot CJ, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 106.Chung MK, Caterina MJ. TRP channel knockout mice lose their cool. Neuron. 2007;54:345–347. doi: 10.1016/j.neuron.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 107.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyper algesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X, Alessandri-Haber N, Levine JD. Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Mol Pain. 2007;3:31. doi: 10.1186/1744-8069-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liedtke W, Friedman JM. Abnormal osmotic regulation in Trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 113.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation with mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 115.Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- 116.Brierley SM, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin J, et al. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res. 2008;102:966–974. doi: 10.1161/CIRCRESAHA.107.168724. [DOI] [PubMed] [Google Scholar]
- 118.Moqrich A, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 119.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 120.Leinninger GM, Edwards JL, Lipshaw MJ, Feldman EL. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nature Clin Pract Neurol. 2006;2:620–628. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- 121.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 123.Novakova-Tousova K, et al. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149:144–154. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 124.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amantini C, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 126.Athanasiou A, et al. Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem Biophys Res Commun. 2007;364:131–137. doi: 10.1016/j.bbrc.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 127.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 128.Nolano M, et al. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 129.Karai L, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meyers JR, et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nature Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 132.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. Demonstrates the use of TRPV1 as a delivery system to target a normally ineffective cationic sodium channel into nociceptors. [DOI] [PubMed] [Google Scholar]
- 133.Chizh BA, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. Preclinical proof-of-concept study in human volunteers of a TRPV1 antagonist. [DOI] [PubMed] [Google Scholar]
- 134.Arendt-Nielsen L, Curatolo M, Drewes A. Human experimental pain models in drug development: translational pain research. Curr Opin Investig Drugs. 2007;8:41–53. [PubMed] [Google Scholar]
- 135.Ravert HT, Bencherif B, Madar I, Frost JJ. PET imaging of opioid receptors in pain: progress and new directions. Curr Pharm Des. 2004;10:759–768. doi: 10.2174/1381612043452992. [DOI] [PubMed] [Google Scholar]
- 136.Schweinhardt P, Bountra C, Tracey I. Pharmacological FMRI in the development of new analgesic compounds. NMR Biomed. 2006;19:702–711. doi: 10.1002/nbm.1076. [DOI] [PubMed] [Google Scholar]
- 137.Rukwied R, et al. Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2. J Pain. 2007;8:443–451. doi: 10.1016/j.jpain.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 138.Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin. 2005;26:286–294. doi: 10.1111/j.1745-7254.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 139.Gavva NR, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. TRPV1-antagonist-induced hyperthermia is a potential important side effect as revealed in this Phase II study. [DOI] [PubMed] [Google Scholar]
- 140.Sharif-Naeini R, Ciura S, Bourque C. W TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron. 2008;58:179–185. doi: 10.1016/j.neuron.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 141.Lehto S, et al. Antihyperalgesic effects of AMG8562, a novel vanilloid receptor TRPV1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008 Apr;17 doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- 142.Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs — will they model the next 100? Nature Rev Drug Discov. 2003;2:38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 143.Hatcher JP, Chessell IP. Transgenic models of pain: a brief review. Curr Opin Investig Drugs. 2006;7:647–652. [PubMed] [Google Scholar]
- 144.Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 145.Keskintepe L, Norris K, Pacholczyk G, Dederscheck SM, Eroglu A. Derivation and comparison of C57BL/6 embryonic stem cells to a widely used 129 embryonic stem cell line. Transgenic Res. 2007;16:751–758. doi: 10.1007/s11248-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 146.Zhu W, Xu P, Cuascut FX, Hall AK, Oxford GS. Activin acutely sensitizes dorsal root ganglion neurons and induces hyperalgesia via PKC-mediated potentiation of transient receptor potential vanilloid 1. J Neurosci. 2007;27:13770–13780. doi: 10.1523/JNEUROSCI.3822-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X, Miyares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol. 2005;564:541–547. doi: 10.1113/jphysiol.2004.081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005;115:296–307. doi: 10.1016/j.pain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 149.Keeble J, et al. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- 150.Bolcskei K, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 151.Jin X, Gereau R. W.t Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pabbidi RM, et al. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 154.Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine J. D Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 156.Matta JA, et al. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Escalera J, von Hehn CA, Bessac BF, Sivula M, Jordt SE. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem. 2008;283:24136–24144. doi: 10.1074/jbc.M710280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cruz-Orengo L, et al. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Materazzi S, et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moore RA, Derry S, McQuay HJ. Topical agents in the treatment of rheumatic pain. Rheum Dis Clin North Am. 2008;34:415–432. doi: 10.1016/j.rdc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 162.Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn. 2007;26:920–927. doi: 10.1002/nau.20479. [DOI] [PubMed] [Google Scholar]
- 163.Watson CP, et al. A randomized vehicle-controlled trial of topical capsaicin in the treatment of post-herpetic neuralgia. Clin Ther. 1993;15:510–526. [PubMed] [Google Scholar]