SUMMARY
Akt is a central regulator of cell growth. Its activity can be negatively regulated by the phosphatase PHLPP that specifically dephosphorylates the hydrophobic motif of Akt (Ser473 in Akt1). However, how PHLPP is targeted to Akt is not clear. Here we show that FKBP51 (FK506-binding protein 51) acts as a scaffolding protein for Akt and PHLPP and promotes dephosphorylation of Akt. Furthermore, FKBP51 is downregulated in pancreatic cancer tissue samples and several cancer cell lines. Decreased FKBP51 expression in cancer cells results in hyperphosphorylation of Akt and decreased cell death following genotoxic stress. Overall, our findings identify FKBP51 as a negative regulator of the Akt pathway, with potentially important implications for cancer etiology and response to chemotherapy.
Keywords: FKBP51, Chemotherapy, Akt, PHLPP, Cancer
SIGNIFICANCE
Resistance to chemotherapy is a major hurdle for successful cancer therapy. Therefore, an improved understanding of the mechanisms responsible for chemoresistance will be helpful in the development of strategies to sensitize cancer cells to chemotherapy. We have found that decreased expression of FKBP51 results in resistance to chemotherapy. Mechanistically, we found that FKBP51 negatively regulates Akt through an apparent scaffolding function. Our studies identified FKBP51 as an important determinant for cancer cell response to chemotherapy and revealed one mechanism by which cancer cells are resistant to chemotherapy.
INTRODUCTION
The serine/threonine kinase Akt (also called PKB) is a central module in cell signaling downstream of a variety of stimuli (Manning and Cantley, 2007). Akt is a major kinase downstream of phosphatidylinositol 3-kinase (PI3K). PI3K converts phosphatidylinositol–4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which in turn, recruits Akt and PDK1 to the plasma membrane (Brazil and Hemmings, 2001). PDK1 then phosphorylates the activation loop of Akt at Thr308 (Alessi et al., 1997). Akt is also phosphorylated at Ser473, and the phosphorylation of both Ser473 and Thr308 is required for the full activation of Akt (Alessi et al., 1996) The kinase that phosphorylates Ser473 remained elusive until mTOR complex 2 (mTORC2) was found to directly phosphorylate Akt at Ser473 (Sarbassov et al., 2005). On the other hand, the phosphatase PHLPP (PH domain leucine-rich repeat protein phosphatase) was shown to specifically dephosphorylate the hydrophobic motif of Akt (Ser473 in Akt1) and inhibit Akt activity (Brognard et al., 2007; Gao et al., 2005). However, how Akt is targeted to PHLPP is not clear.
Akt has been shown to inhibit apoptosis and promote cell survival, activities that contribute to its oncogenic potential (Manning and Cantley, 2007). Several mechanisms underlie Akt’s anti-apoptotic effect. Firstly, Akt phosphorylates the proapoptotic protein BAD, preventing binding to its target protein (Datta et al., 1997; del Peso et al., 1997). Secondly, Akt phosphorylates FOXO transcription factors, resulting in their export from the nucleus and the downregulation of FOXO target genes (Biggs et al., 1999; Brunet et al., 1999; Kops et al., 1999; Rena et al., 1999), including the proapoptotic BH3-only protein BIM. Thirdly, Akt phosphorylates MDM2, resulting in its translocation to the nucleus, and facilitating MDM2’s inhibition of p53 (Mayo and Donner, 2001; Zhou et al., 2001). Finally, Akt phosphorylates and inhibits GSK isoforms (Cross et al., 1995) that play a pro-apoptotic role by inhibiting anti-apoptotic protein MCL-1 (Maurer et al., 2006). Therefore, Akt activity needs to be tightly regulated in cells, and hyperactivation of Akt has been linked to cancer predisposition and cancer cell resistance to chemotherapy.
We recently identified FKBP51 (also called FKBP5) as a regulator of cell death in response to gemcitabine (Li et al., 2008), downregulation of FKBP51 results in decreased cell death in response to gemcitabine and Ara-C treatment. FKBP51 is a member of the FK506-binding protein (FKBP) family (Baughman et al., 1995). Like other family members, it contains peptidylprolyl cis/trans isomerase (PPIase) activity and FKBP-C domains (Harding et al., 1989). It is well established that FKBP51 regulates steroid receptor activation (Cheung and Smith, 2000). In addition, FKBP51 has been shown to be required for IKK (IκB kinase) activation (Bouwmeester et al., 2004). However, this action of FKBP51 on NFκB activation cannot explain the increased chemoresistance in cells with decreased FKBP51 expression, as downregulation of FKBP51 should inhibit IKK activation and lead to increased cellular sensitivity to chemotherapy. This suggests the existence of other mechanisms by which FKBP51 regulates cell survival.
RESULTS
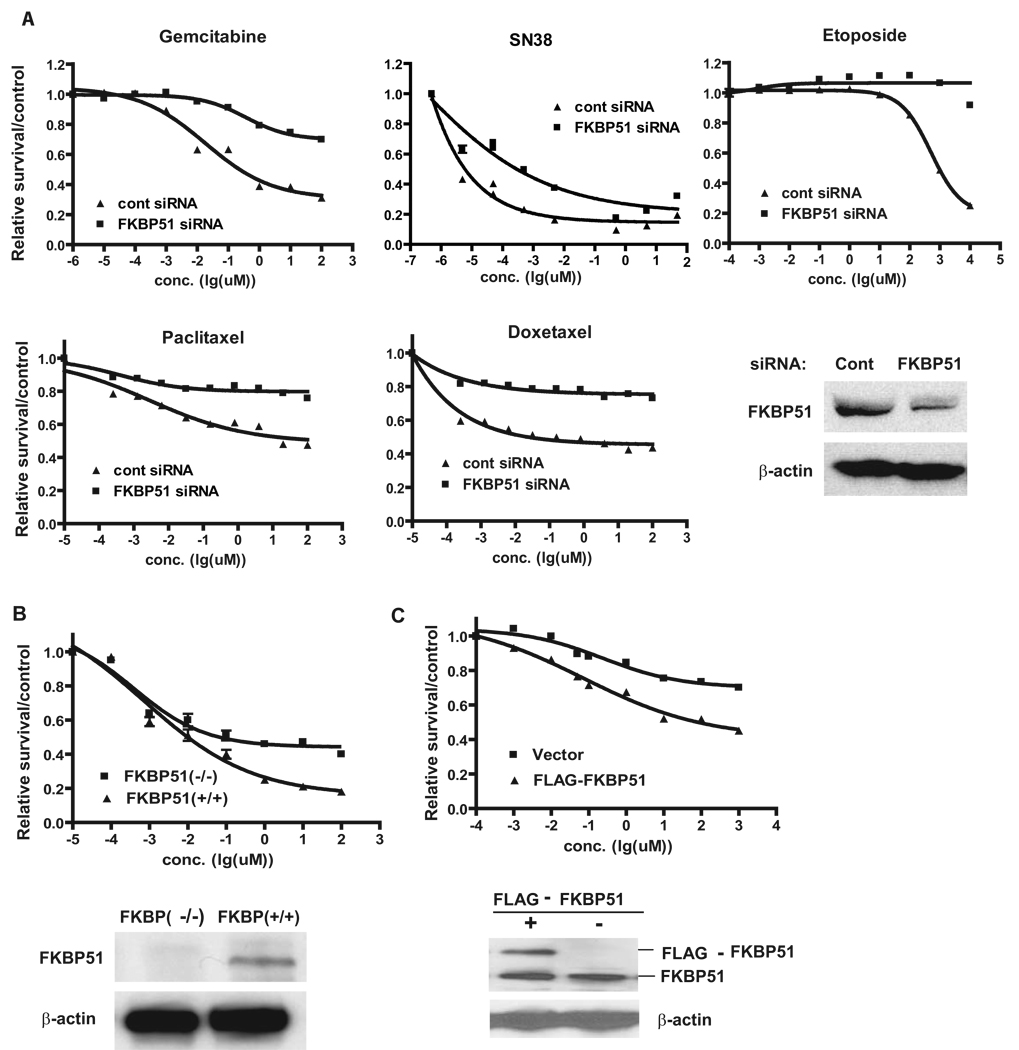
To examine whether FKBP51 regulates cellular response to multiple classes of chemotherapeutic drugs, we used microtubule stabilizers and Topoisomerase I and II inhibitors to treat the pancreatic cancer cell line SU86. Downregulation of FKBP51 with two different siRNAs resulted in increased resistance to these treatments (Figure 1A and Figure S1A). Downregulation of FKBP51 also resulted in resistance to these drugs in the lung cancer cell line A549 and the breast cancer cell line MDA-MB-231 (Figure S2). Furthermore, loss of FKBP51 expression resulted in increased resistance of mouse embryonic fibroblasts (MEFs) to gemcitabine (Figure 1B). In contrast, overexpression of FKBP51 resulted in hypersensitivity to gemcitabine (Figure 1C). Overall, these results have established an important role of FKBP51 in regulating cellular response to a wide range of clinically important anti-neoplastic agents in both transformed and non-transformed cells.
Figure 1. FKBP51 regulates cellular response to genotoxic stress.
(A). SU86 cells were transfected with control or FKBP51 siRNA and then treated with the indicated drugs. Cell survival was determined as described in the Methods. (B). FKBP51+/+ or FKBP51−/− cells were treated with gemcitabine, and cell survival was determined as in (A). (C). SU86 cells were transfected with vector or constructs encoding FLAG-FKBP51. Transfected cells were treated with gemcitabine, and cell survival was determined as in (A). Points, mean values for three independent experiments; Error bars, +/− SEM.
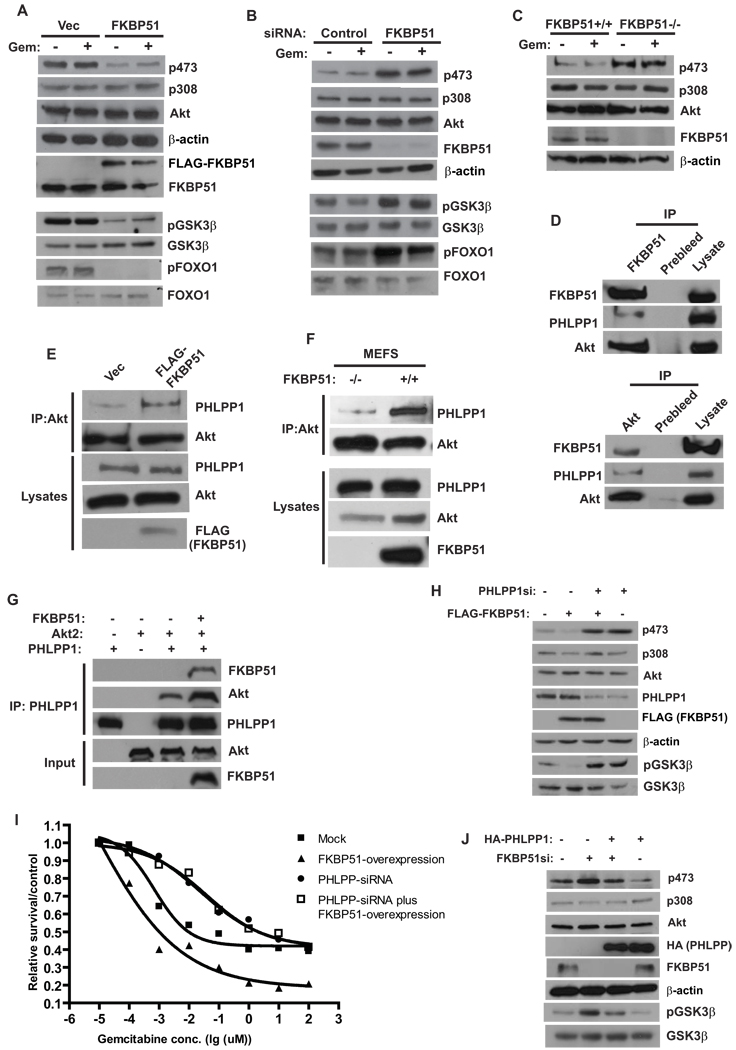
This raised the question, how does FKBP51 regulate cellular response to these therapeutics? We found that overexpression of FKBP51 resulted in a reduced phosphorylation of Akt at Ser473, but had no effect on the phosphorylation of Thr308 (Figure 2A). There was no visible difference in Akt phosphorylation with or without gemcitabine treatment. On the other hand, downregulation of FKBP51 resulted in increased Ser473 phosphorylation, with no effect on Thr308 phosphorylation (Figure 2B and Figure S1A). Furthermore, hyperphosphorylation of Ser473 was observed in FKBP51−/− MEFs (Figure 2C). These results suggest that FKBP51 negatively regulates Akt phosphorylation, which might account for its effects on cell survival.
Figure 2. FKBP51 regulates Akt phosphorylation at Ser473 by promoting Akt-PHLPP interaction.
(A). SU86 cells were transfected for 48 hr with indicated constructs. Cells were treated with DMSO or gemcitabine (Gem, 20 nM, 12 hrs), and the phosphorylation of Akt, FOXO and GSK-3β in cell lysates was detected by Western Blot. (B). SU86 cells were transfected with indicated siRNA. Cells were then treated and harvested as in (A). (C). The phosphorylation of Akt in cell lysates from FKBP51+/+ or FKBP51−/− cells was examined. (D). The coimmunoprecipitation of Akt, PHLPP and FKBP51 was examined. (E). 293T cells were transfected with indicated constructs, and the interaction between Akt and PHLPP was examined. (F). The PHLPP-Akt interaction was examined in FKBP51+/+ and FKBP51−/− cells. (G). Purified recombinant Akt, PHLPP1 and FKBP51 were incubated in vitro as indicated. The Akt-PHLPP interaction was then examined by coimmunoprecipitation. (H–I). SU86 cells were transfected with FLAG-FKBP51 and/or PHLPP siRNA as indicated. The phosphorylation of Akt, GSK-3β, as well as sensitivity to gemcitabine were then examined. Points, mean values for three independent experiments; Error bars, +/− SEM. (J). SU86 cells were transfected with HA-PHLPP and/or FKBP51 siRNA as indicated. The phosphorylation of Akt and GSK-3β was then examined.
To further confirm that FKBP51 regulates Akt activity, we examined the phosphorylation of downstream substrates of Akt, such as GSK-3β and FOXO1. We found that overexpression of FKBP51 decreased the phosphorylation of GSK-3β (pSer9GSK-3β) and FOXO1 (pThr24 FoxO1) (Figure 2A, lower panels), which was consistent with decreased Akt phosphorylation. In contrast, downregulation of FKBP51 significantly increased the phosphorylation of GSK-3β and FOXO1 (Figure 2B, lower panels). These results confirmed that FKBP51 inhibits Ser473 phosphorylation and Akt activity.
Since FKBP51 specifically regulates Ser473 phosphorylation, but not Thr308 phosphorylation, it is likely that FKBP51 regulates signaling events that directly control Akt phosphorylation at Ser473. The Ser473 of Akt is specifically phosphorylated by mTORC2 and dephosphorylated by PHLPP phosphatases (PHLPP1 and PHLPP2) (Brognard et al., 2007; Gao et al., 2005; Sarbassov et al., 2005). Another FKBP family member, FKBP38, has previously been shown to bind and inhibit mTORC1 activity (Bai et al., 2007). However, we could not detect any interaction between FKBP51 and mTOR. Instead, we found that PHLPP1 and Akt were coimmunoprecipitated with FKBP51 (Figure 2D, upper panels). Similarly, FKBP51 and PHLPP1 were coimmunoprecipitated with Akt (Figure 2D, lower panels). These results suggest that FKBP51, Akt and PHLPP could exist as a complex in cells.
The findings that FKBP51 interacts with PHLPP and Akt led us to hypothesize that FKBP51 acts as a scaffolding protein that promotes the interaction between Akt and PHLPP, thereby enhancing the dephosphorylation of Akt. To test this hypothesis, we overexpressed FKBP51 and found that the interaction between PHLPP and Akt increased in cells with FKBP51 overexpression (Figure 2E). Furthermore, there was less interaction between Akt and PHLPP1 in FKBP51−/− cells, than in FKBP51+/+ cells (Figure 2F). To test whether FKBP51 directly 7promotes the Akt-PHLPP interaction, we expressed and affinity purified FKBP51, PHLPP , and Akt. As shown in Figure 2G, FKBP51 can increase the interaction between Akt and PHLPP in vitro.
In the absence of FKBP51, Akt becomes hyperphosphorylated at Ser473 due to inefficient binding of PHLPP to Akt, which may contribute to the chemoresistance observed in cells depleted of FKBP51. Indeed, in cells expressing AktS473D, which mimics Ser473 phosphorylation, cells became resistant to gemcitabine (Figure S1B). Similarly, depletion of PHLPP rendered cells resistant to gemcitabine (Figure S1C). These results support the hypothesis that FKBP51 regulates chemoresistance through the Akt pathway.
To further confirm that FKBP51 regulates Akt Ser473 phosphorylation through PHLPP, we overexpressed FKBP51 while downregulating PHLPP. As we demonstrated earlier, overexpression of FKBP51 alone decreased Akt Ser473 phosphorylation and downstream GSK-3β phosphorylation. However, these effects were reversed by reducing PHLPP (Figure 2H and Figure S1D). Overexpression of FKBP51 did not have further effect on Akt Ser473 phosphorylation in cells depleted of PHLPP. Consistent with these observations, although FKBP51 overexpression alone sensitized cells to gemcitabine treatment, downregulation of PHLPP reversed this sensitizing effect (Figure 2I). Furthermore, PHLPP overexpression blocked the effects of FKBP51 knockdown on Akt Ser473 and GSK-3β phosphorylation (Figure 2J). These results establish that FKBP51 regulates Akt Ser473 phosphorylation through PHLPP.
It is possible that FKBP51 not only increases the Akt-PHLPP interaction but also enhances the dephosphorylation of Akt by stimulating PHLPP activity. FKBP51 has peptidylprolyl isomerase activity thus could affect PHLPP activity. However, overexpression of the FKBP51 mutant FD67/68DV, which lacks peptidylprolyl isomerase activity (Barent et al., 1998), had similar effect on Akt Ser473 phosphorylation as WT FKBP51 (Figure S3A), suggesting that FKBP51 regulates Akt phosphorylation in a peptidylprolyl isomerase-independent manner. Although it is possible that the binding of FKBP51 to PHLPP directly stimulates PHLPP activity, we found that overexpression or depletion of FKBP51 did not affect PHLPP phosphatase activity in vitro (Figure S3B–C). PHLPP has been shown to regulate the levels of PKC βII by dephosphorylating the hydrophobic motif of PKC βII, resulting in rapid degradation of PKC βII (Gao et al., 2008). However, FKBP51 did not interact with and did not significantly affect the phosphorylation or levels of PKC βII, (Figure S3D–E). These results further confirm that FKBP51 regulates Akt Ser473 phosphorylation mostly through its scaffolding function.
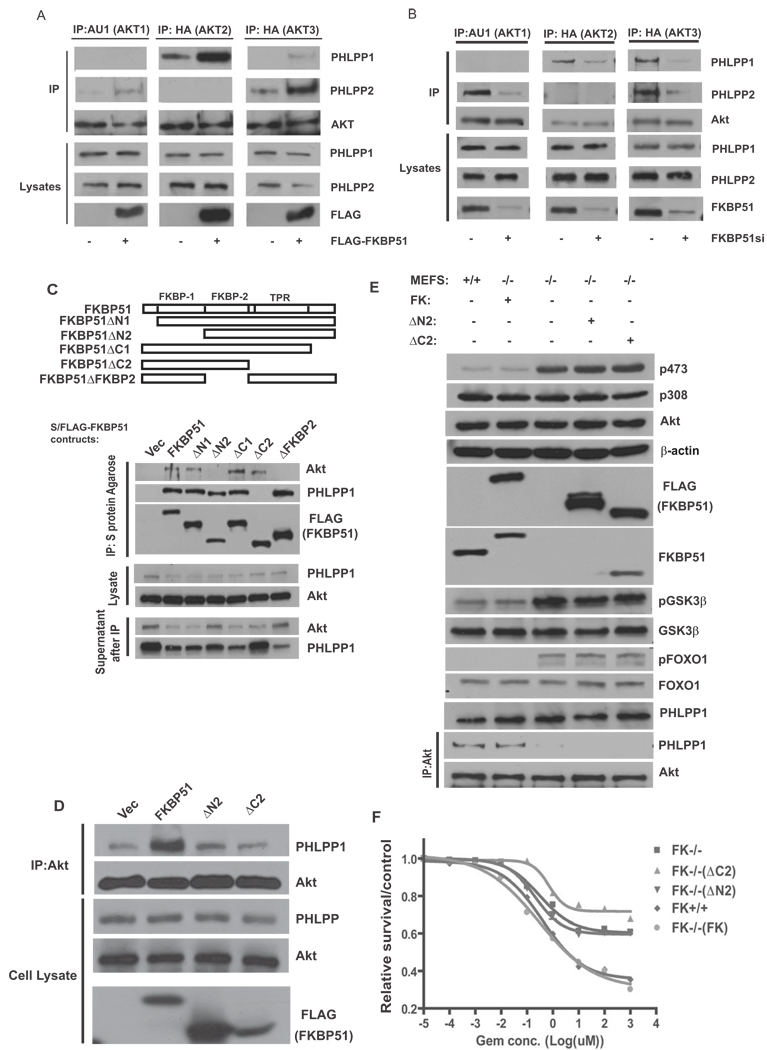
Akt has three isoforms (Akt1, Akt2 and Akt3) (Manning and Cantley, 2007). The antibodies that we used in previous experiments recognize all three isoforms. In addition, PHLPP has 2 isoforms (PHLPP1 and PHLPP2) (Brognard et al., 2007; Gao et al., 2005). Previous studies have established that both PHLPP1 and PHLPP2 dephosphorylate the same hydrophobic phosphorylation motif on Akts (Ser473 on Akt1), but they inhibit Akt signaling differently by interacting with distinct Akt isoforms (Brognard et al., 2007). PHLPP1 specially regulates Akt2 and Akt3, and PHLPP2 regulates Akt1 and Akt3. We next examined whether the isoform-specific effects of PHLPP on Akt are regulated by FKBP51. Consistent with previous studies (Brognard et al., 2007), Akt1 and Akt3 were coimmunoprecipitated with PHLPP2; while Akt2 and Akt3 were coimmunoprecipitated with PHLPP1 (Figure 3A–B). Overexpression of FKBP51 increased the interaction between both PHLPP isoforms with their corresponding Akt isoforms (Figure 3A); while downregulation of FKBP51 decreased these interactions (Figure 3B). These results suggest that FKBP51 facilitates isoform-specific interaction between Akt and PHLPP.
Figure 3. FKBP51 scaffolding function regulates Akt phosphorylation and cell survival.
(A). 293T cells or 293T cells stably transfected with FKBP51 were transfected with AKT isoforms (lane 1,2, AU1-AKT1; lane 3,4, HA-AKT2, lane5,6 HA-AKT3). Cells were lyzed, and lysates were subjected to immunoprecipitation with indicated antibodies. PHLPP1, PHLPP2 and AKT in the immunoprecipitates or cell lysates were detected by immunoblotting. (B). SU86 cells were transfected with control or FKBP51 siRNA together with AKT isoforms. The interaction between Akt isoforms and PHLPP isoforms was then examined as in A. (C). 293T cells were transfected with different S/FLAG-tagged FKBP51 truncated mutants. Lysates from transfected cells were subjected to immunoprecipitation with S protein agarose, PHLPP1 and Akt in the immunoprecipitates were then detected by immunoblotting. (D). 293T cells were transfected with WT FKBP51 or FKBP51 truncation mutations. Transfected cells were then lyzed, and lysates were subjected to immunoprecipitation with anti-Akt antibodies. PHLPP1 and Akt in the immunoprecipitates or cell lysates were detected by immunoblotting. (E–F). FKBP51+/+, FKBP51−/− or FKBP51−/− MEFs stably expressing WT or mutant FKBP51 were used to examine Akt, GSK-3β and FOXO1 phosphorylation as well as the Akt-PHLPP interaction (E). cells were examined for gemcitabine sensitivity using the MTS assay (F). Points, mean values for three independent experiments; Error bars, +/− SEM.
To investigate how FKBP51 enhances the Akt-PHLPP interaction, we generated a series of deletion mutants of FKBP51. As shown in Figure 3C, we found that deletion of either the FKBP1 (Residues 1–138) or FKBP2 (138–251) domain abolished the interaction between FKBP51 and PHLPP, suggesting that both domains are required for this interaction. In addition, we found that the C-terminal TPR domain of FKBP51 was essential for the binding of FKBP51 to Akt. These results suggest that FKBP51 binds PHLPP and Akt using distinct domains, consistent with our hypothesis that FKBP51 acts as a scaffolding protein to promote the Akt-PHLPP interaction. If so, we expected that FKBP51 deletion mutants that could not bind either Akt or PHLPP would not enhance the Akt-PHLPP interaction. Indeed, deletion of the FKBP1 domain or the TPR domain abolished the ability of FKBP51 to enhance the Akt-PHLPP interaction (Figure 3D).
To further confirm that the scaffolding function of FKBP51 is important for the regulation of Akt phosphorylation and cell survival, we reconstituted FKBP51−/− MEFs with wild type FKBP51 (FK), FKBP51 deleted of the FKBP1 domain (ΔN2) or FKBP51 deleted of the TPR domain (ΔC2). As in Figure 2, pSer473, pGSK-3β and pFOXO1 levels were higher in FKBP51−/− cells than in FKBP51+/+ cells (Figure 3E). Reconstitution of the WT FKBP51 in FKBP51−/− cells returned the phosphorylation levels of AktSer473, GSK-3β, and FOXO1 to those observed in FKBP51+/+ cells, whereas reconstitution with either mutant had no effect. This observation correlated with a decreased Akt-PHLPP interaction in the absence of WT FKBP51 (Figure 3E). Importantly, reconstitution of WT FKBP51 in FKBP51−/− MEFS restored cell sensitivity to gemcitabine to a level similar to that of FKBP51+/+ MEFS (Figure 3F), whereas neither mutants had this rescue effect. These results confirmed that the scaffolding function of FKBP51 is important for the regulation of Akt phosphorylation and cell survival.
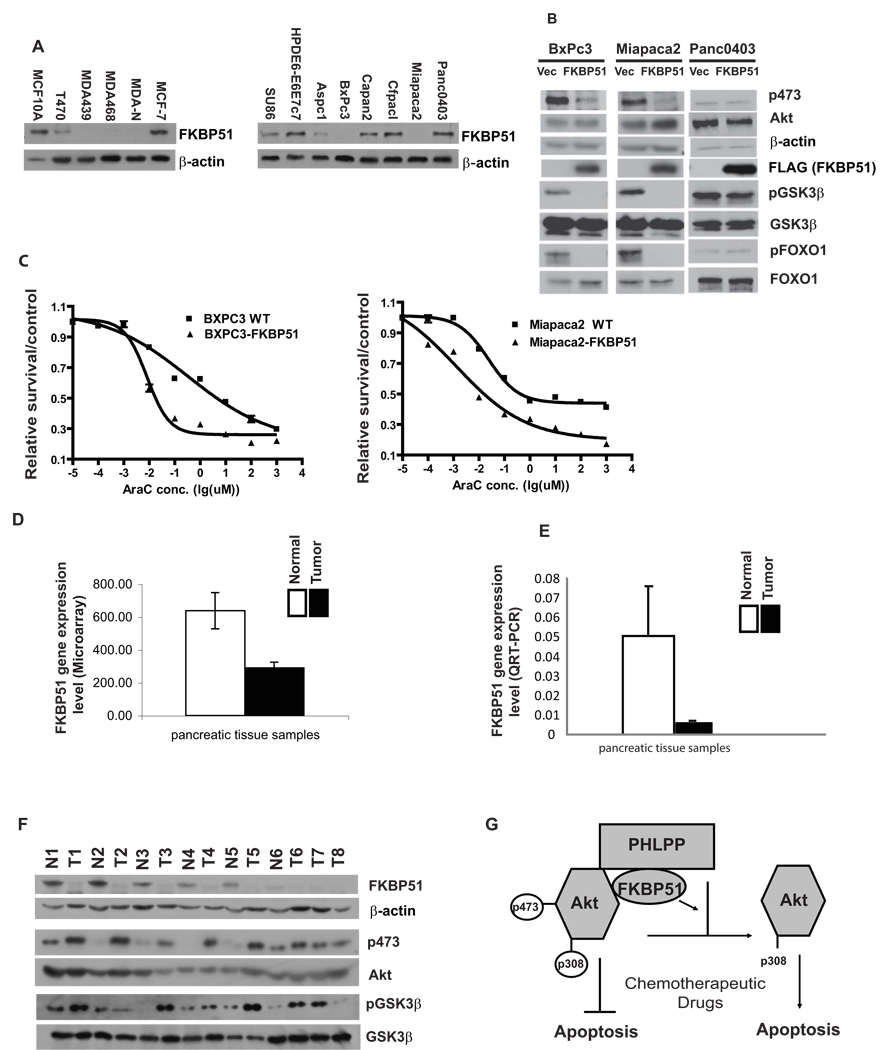
Since decrease or loss of FKBP51 expression results in Akt hyperactivation, which has been observed in many cancers, it is possible that FKBP51 expression is downregulated in cancer cells. Consistent with this notion, we found that FKBP51 expression is lost or significantly decreased in a high percentage of the pancreatic cancer cell lines and breast cancer cell lines that we examined (Figure 4A). Reconstitution of FKBP51 in Miapaca2 or BxPC3 cells decreased Akt phosphorylation at Ser473 (Figure 4B), and sensitized these cells to Ara-C (Figure 4C), supporting the hypothesis that loss of FKBP51 expression renders these cells resistant to chemotherapy. Furthermore, overexpression of FKBP51 in a pancreatic cancer cell line (Panc0403) having high endogenous levels of FKBP51 did not affect Akt phosphorylation (Figure 4B). These results suggest that cancer cell lines having high FKBP51 expression might depend less on the PI3K-Akt pathway to survive. To test this possibility, we treated Miapaca2 (low levels of FKBP51 expression) and Panc0403 (high levels of FKBP51 expression) with the PI3K inhibitor, wortmannin, together with gemcitabine. As shown in Figure S4A, Miapaca2 cells are more sensitive to PI3K inhibition, consistent with the notion that cells expressing low levels of FKBP51 might be more dependent on the PI3K-Akt pathway. However, these two cell lines have different genetic backgrounds, so factors other than FKBP51 expression may contribute to their different responses to the PI3K inhibitor.
Figure 4. Loss of FKBP51 expression in cancer cells and tissues.
(A). Cell lysates from pancreatic and breast cancer cell lines were blotted with FKBP51 antibodies. Lysates from normal breast (MCF10A) and pancreatic (HPDE6-E6E7c7) epithelial cells were used as controls. (B–C). Miapaca2, BxPC3 or Panc0403 cells were reconstituted with FKBP51, and Akt phosphorylation and sensitivity to genotoxic stress were then determined. Points, mean values for three independent experiments; Error bars, +/− SEM. (D, E). FKBP51 gene expression in tumor and normal pancreatic tissues was determined using microarray analysis (D, 19 normal pancreatic and 36 tumor tissue samples, p= 0.0092) or real-time QRT-PCR (E, 25 pancreatic cancer samples and 12 normal pancreatic tissues, p=0.001). Error bars, +/− SEM. (F). Western blot of lysates from a subset of tumor and normal tissues. T: Tumor; N: Normal pancreatic tissue; 1–8, patient No. (G). A model illustrates how FKBP51 regulates cell survival through the Akt pathway.
Our results also imply that FKBP51 might function as a tumor suppressor. As an initial step to test this hypothesis, we performed microarray analysis using RNA isolated from 36 pancreatic tumor and 19 normal tissue samples. These were fresh frozen samples obtained during surgical procedures. The microarray data revealed that expression levels of FKBP51 were significantly lower in pancreatic tumor tissue than in normal pancreatic tissue (Figure 4D). The comparison of expression profile between normal and tumor tissues identified genes expressed significantly differently (P<10−6) between the two (Figure S4B). Network analysis using Ingenuity Pathway analysis software of the most differentially expressed genes showed that a network surrounding Akt was the top network (Figure S4C). We also performed real time quantitative RT-PCR (QRTPCR) to validate the microarray results and found these results to be similar with the correlation coefficient between the RT-PCR and microarray data approximately 0.8 (P<0.0001) (Figure 4E). Furthermore, lower or loss of FKBP51 protein levels was found in selected pancreatic cancer samples, many of which had increased Akt/GSK-3β phosphorylation (Figure 4F and Figure S4D–E). Decreased expression levels of FKBP51 was also found in ovarian, head and neck, seminoma, leukemia and prostate cancer tissues based on expression data obtained through the Oncomine (www. Oncomine.org). Overall, our results suggest that FKBP51 negatively regulates Akt activation through its scaffolding function and that hyperactivation of Akt caused by the loss of FKBP51 might contribute to tumorigenesis and cancer cell resistance to chemotherapy.
DISCUSSION
Resistance to chemotherapy represents a major challenge for cancer therapy. Therefore, the identification of biomarkers for chemoresistance and understanding mechanisms of chemoresistance will reveal possible strategies to overcome this problem. We have identified FKBP51 as an important determinant of cancer cell response to a wide range of clinically important chemotherapeutic agents. Decreased expression of FKBP51 caused resistance to chemotherapy in cancer cell lines. Mechanistically, FKBP51 acts as a scaffolding protein for Akt and PHLPP, a phosphatase that specifically dephosphorylates Akt Ser473 (Gao et al., 2005), thereby enhancing the phosphatase activity of PHLPP toward Akt.
Since Akt is a major signaling node within the cell, its activity needs to be tightly regulated. Misregulation of the Akt pathway can disrupt the balance between cell survival and death, affecting cancer development and therapy. Indeed, the PI3K-Akt pathway has been linked to resistance to a variety of chemotherapeutics, such as gemcitabine, irinotecan, etoposide and taxol (West et al., 2002). Hyperphosphorylation of Akt has also been linked to poor prognosis in a variety of cancers (West et al., 2002). Based on our studies, we expect that lower expression of FKBP51 will also associate with poor prognosis in clinical settings. This hypothesis remains to be validated in the future.
Many regulators of the Akt pathway, such as PI3K, PTEN and Akt, are mutated in cancers. It is plausible that downregulation or mutation of FKBP51 contribute to not only chemoresistance but also tumorigenesis. We found that FKBP51 expression is lost in many cancer cell lines and pancreatic cancers, supporting a possible role of FKBP51 in tumor suppression. However, the causal role of FKBP51 in tumor suppression remains to be determined.
Our findings will have a significant impact on the dissection of components in the pathway controlling Akt activity. Furthermore, since dysregulation of the Akt pathway is frequently linked to cancer predisposition and poor prognosis, our findings may also have important implications for cancer etiology and response to therapy.
MATERIALS AND METHODS
Phosphatase Assay
PHLPP1 was immunoprecipitated with PHLPP1 antibody from the indicated cells. HA-Akt2 was expressed and purified from 293T cells with HA tag antibody and subsequent HA peptide elusion. Immunoprecipitated PHLPP1 were incubated in phosphatase buffer (50 mM Tris (pH 7.4), 1 mM DTT, and 5 mM MnCl2 ) with purified phosphorylated Akt at 30 °C for 0–10min as previously described (Gao et al., 2005).
MTS Assay
AraC, paclitaxel and docetaxel were purchased from Sigma-Aldrich (St. Louis, MO) and gemcitabine was provided by Eli Lilly (Indianapolis, IN). Assays with 10-fold diluted concentrations of drugs were performed in triplicate with the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega Corporation, Madison, WI). After incubation with drugs for 72 hrs, the plates were measured in a Safire2 microplate reader (Tecan AG, 14 Switzerland).
Expression Array Data
Total RNA was extracted from pancreatic tumor tissue samples using Qiagen RNeasy Mini kits (QIAGEN Inc. Valencia, CA). These samples were obtained during clinically indicated surgical procedures and were consented for experimental purpose. The present study was reviewed and approved by Mayo Clinic Institutional Review Board. RNA quality was tested using an Agilent 2100 Bioanalyzer, followed by hybridization to Affymetrix U133 Plus 2.0 GeneChips (Affymetrix, Inc., Santa Clara, CA). Expression levels were normalized by GCRMA.
Pancreatic tissue sample preparation for Western blot
Proteins were extracted from portions of the selected fresh frozen pancreatic tumor and normal tissue, samples that were also used to perform microarray and real time RT-PCR. These samples were consented for research use and the present study was reviewed and approved by Mayo Clinic Institutional Review Board. To extract proteins from these tissues, we froze the tissue samples in liquid nitrogen and cut it into small fragments, followed by incubation on ice in NETN buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40) containing 50 mM glycerophosphate, 10 mM NaF, with a protease inhibitor cocktail. The tissues were milled in a blender for 5 min and lyzed on ice for 30mins. The loading buffer for the SDS-PAGE was added and boiled for 20 minutes. Samples were then centrifuged before loading.
Statistical analysis
Gene signatures between tumor and normal tissues were determined with Student’s t test for each probe set. Ingenuity Pathway Analysis was performed with the most differentially expressed genes (P<10−6 ) between normal and tumor tissues by calculating the p-values for the probability of finding a set of genes within a given pathway. Fischer’s exact test was used to calculate the p-values.
The accession number for the microarray data is GSE16515.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Richard Schultz Foundation (Z.L.), CA102701 (The Pancreatic Cancer Specialized Programs of Research Excellence) (Z.L., L.W. G.P. and W.L), R01CA138461 (Z.L., L.W.), K22CA130828 (L.W.), GM61388 (The Pharmacogenetics Research Network) (L.W., B.F. and R.K.) and the PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award (L.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15:4395–4402. doi: 10.1128/mcb.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cheung J, Smith DF. Molecular chaperone interactions with steroid receptors: an update. Mol Endocrinol. 2000;14:939–946. doi: 10.1210/mend.14.7.0489. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Li L, Fridley B, Kalari K, Jenkins G, Batzler A, Safgren S, Hildebrandt M, Ames M, Schaid D, Wang L. Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Res. 2008;68:7050–7058. doi: 10.1158/0008-5472.CAN-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.