Abstract
Decreased availability or efficacy of neurotrophic factors may underlie an increased susceptibility of mesencephalic dopaminergic cells to age-related degeneration. Neuregulins (NRGs) are pleotrophic growth factors for many cell types including mesencephalic dopamine cells in culture and in vivo. The functional NRG receptor ErbB4 is expressed by virtually all midbrain dopamine neurons. To determine if levels of the NRG receptor are maintained during aging in the dopaminergic ventral mesencephalon, expression of ErbB4 mRNA and protein was examined in young (3 months), middle-aged (18 months), and old (24–25 months) Brown Norway/Fischer 344 F1 rats. ErbB4 mRNA levels in the substantia nigra pars compacta (SNpc), but not the adjacent ventral tegmental area (VTA) or subtantia nigra pars lateralis (SNl), were significantly reduced in the middle-aged and old animals when compared to young rats. Protein expression of ErbB4 in the ventral midbrain was significantly decreased in the old rats when compared to the young rats. Expression of tyrosine hydroxylase (TH) mRNA levels were significantly reduced in the old rats when compared to young animals in the SNpc, but not in the VTA or SNl. Tyrosine hydroxylase protein levels in the ventral midbrain were also decreased in the old animals when compared to the young animals. These data demonstrate a progressive decline of ErbB4 expression, coinciding with a loss of the dopamine-synthesizing enzyme TH, in the ventral midbrain of aged rats, particularly in the SNpc. These findings may implicate a role for diminished NRG/ErbB4 trophic support in dopamine-related neurodegenerative disorders of aging such as Parkinson’s disease.
Keywords: neuregulins, aging, dopamine, substantia nigra, in situ hybridization, Parkinson’s disease
Neuregulins (NRGs) are a family of structurally related growth and differentiation factors. During development, NRGs play an integral role in many processes including cell survival, migration, and differentiation of both neuronal and non-neuronal cells (Burden and Yarden, 1997; Gassmann and Lemke, 1997; Buonanno and Fischbach, 2001; Yarden and Sliwkowski, 2001; Falls, 2003; Seroogy and Zhang, 2006). Signaling by NRGs occurs through binding to their epidermal growth factor-like receptors, the ErbB receptors (Buonanno and Fischbach, 2001; Yarden and Sliwkowski, 2001; Citri et al., 2003; Falls, 2003). Like NRGs, ErbB receptors are differentially expressed throughout development and play a role in cell growth, migration, differentiation, and myelination in many tissues and organs, including the nervous system (Carraway and Burden, 1995; Burden and Yarden, 1997; Gassmann and Lemke, 1997; Kornblum et al., 2000; Vaskovsky et al., 2000; Fox and Kornblum, 2005; Mechawar et al., 2007; Birchmeier, 2009).
Neuregulins and ErbB receptors are distributed throughout the adult central nervous system (CNS) including cortical areas, hippocampus, thalamus, hypothalamus, amygdala, and ventral midbrain (Chen et al., 1994; Corfas et al., 1995; Steiner et al., 1999; Gerecke et al., 2001; Yurek and Seroogy, 2001; Bruce et al., 2002; Law et al., 2004; Fox and Kornblum, 2005; Bernstein et al., 2006 Thompson et al., 2007). With respect to the ventral midbrain, ErbB4 is expressed in both the substantia nigra and ventral tegmental area (VTA) (Steiner et al., 1999; Gerecke et al., 2001). Moreover, ErbB4 mRNA is localized to dopaminergic neurons in the substantia nigra pars compacta (SNpc) as demonstrated by reduced expression following 6-hydroxydopamine (6-OHDA) lesions and through direct colocalization (Steiner et al., 1999; Yurek and Seroogy, 2001; Thuret et al., 2004; Abe et al., 2009; K.B. Seroogy, unpublished results). Although NRGs and ErbB receptors are present in the adult CNS, their functional roles remain to be fully elucidated, including within the midbrain dopaminergic cells. However, recent findings in fetal mesencephalic dopamine cell cultures demonstrate that the NRG-1 isoform glial growth factor 2 (GGF2) is both neurotrophic and neuroprotective in serum-free and 6-OHDA-challenged cultures (Zhang et al., 2004). Furthermore, when injected directly above the SNpc, GGF2 and another NRG isoform, NRG1-β1, induce striatal dopamine overflow indicating functional effects of NRGs in the nigrostriatal system in vivo (Yurek et al., 2004; Seroogy and Zhang, 2006).
The nigrostriatal system is particularly vulnerable to age-related degeneration as indicated by functional and histological/neurochemical deficits. Some of the changes associated with aging in humans and non-human primates include reduced nigral volume and tyrosine hydroxylase (TH) cell number, and a decrease in striatal dopamine (Irwin et al., 1994; Emborg et al., 1998; Siddiqi et al., 1999; Stark and Pakkenberg, 2004; Collier et al., 2007). In rodent models of aging, some studies have reported decreased function behaviorally and neurochemically (e.g. Emerich et al., 1993; Yurek et al., 1998; Ling et al., 2000; Sanchez et al., 2008), whereas others have been unable to discern any age-related changes (e.g. McNeill and Koek, 1990; Stanford et al., 2002, 2003; Tamás et al., 2005). Nonetheless, taken together most evidence suggests that dysfunction of the nigrostriatal dopaminergic system is not only a consequence of diseases affecting the basal ganglia, but is also a result of the normal aging process. The neural mechanisms responsible for the loss of dopaminergic function during aging remain unknown.
Herein, we examine the effects of aging on the expression of ErbB4 and TH mRNA and protein in the ventral midbrain. The purpose of this study was to determine if there is an age-related decline in the ErbB4 receptor that would suggest a decrease in neurotrophic factor support to mesencephalic dopamine neurons. Diminished NRG/ErbB4 signaling could increase susceptibility of mesencephalic dopamine neurons to age-related degeneration and could have implications into the pathogenesis of Parkinson’s disease.
Experimental procedures
Animals
Male Brown Norway/Fischer 344 F1 (BN/F344 F1) hybrid rats (NIA/Harlan Sprague-Dawley) were used in this study at ages 3 months, 18 months, and 24–25 months (n=10 for each age group). Adult rats weighed 300–650 g and were housed 2 per cage under normal conditions; 12 hr on/off light/dark cycles with free access to food and water. All procedures were conducted in compliance with the University of Cincinnati Institutional Animal Care and Use Committee.
In situ hybridization
Rats (n=4 for each age group) were anesthetized with an overdose of sodium pentobarbital and rapidly decapitated. Fresh-frozen, cryostat-cut, slide-mounted semi-adjacent sections through the rostrocaudal level of the ventral mesencephalon were hybridized with 35S-labeled cRNA probes for detection and localization of ErbB4 and TH mRNAs using in situ hybridization, as previously described in detail (Seroogy and Herman, 1997; Numan and Seroogy, 1999; Numan et al., 2005). The TH cDNA plasmid (provided by J. Herman, University of Cincinnati) was contained in a pCR-TOPO vector and consisted of 366 bp. The ErbB4 plasmid (provided by H. Kornblum, UCLA) was contained in a pCR 2.1 vector and consisted of 1.8 kb (Kornblum et al., 2000). Sections were hybridized overnight at 60°C in hybridization solution with the 35S-labeled probe at a concentration of 1×106 cpm/50 μl. After post-hybridization washes and ribonuclease A treatment, the slides were exposed to BioMax MR film (Kodak, Rochester NY) for 2 days (TH cRNA) or 13 days (ErbB4 cRNA) for generation of film autoradiographs. The films were developed with Kodak GPX developer and fixer (Kodak, Rochester NY).
Western Blots
Rats (n=6 for each age) were euthanized by CO2 asphyxiation for the protein analysis. The ventral midbrain was carefully dissected out of each brain in chilled artificial CSF (Harvard Apparatus, Holliston, MA), frozen on dry ice, and stored at −80°C. The Western blotting procedure was performed as described previously (Wolf et al., 1999), with slight modifications. To solubilize the proteins within the samples, the frozen ventral mesencephalic tissue was probe-sonicated in a buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 10 u g/ml aprotinin, and 10 ug/ml leupeptin. The protein concentration of each sample was determined in triplicate using Bio-Rad’s DC protein assay kit (Hercules, CA). The final volume of each sample was 25 ul and protein concentration was 3.86 ug/ul. For ErbB4 analysis 20 ul of each sample was loaded onto a 7.5% acrylamide gel. The remaining 5 ul of each sample was loaded onto a separate 7.5% acrylamide gel for TH. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) analysis was included as a loading control. Following SDS-polyacrylamide gel electrophoresis the proteins were transferred to nitrocellulose. The nitrocellulose membranes were blocked in 5% milk PBS/tween for 1 hour and then incubated at 4°C overnight in primary antibody [rabbit anti-ErbB4; 1:500 (Santa Cruz Biotechnology, Inc; Santa Cruz, CA); mouse anti-TH; 1:4000 (Chemicon; Temecula, CA); mouse anti-GAPDH; 2 ug/ml (Ab-cam; Cambridge, MA)]. Following primary antibody removal, the membranes were washed in 2% milk PBS/tween (1 hour) and then incubated in the appropriate secondary antibody (anti-mouse IgG for TH and GAPDH; anti-rabbit IgG for ErbB4) diluted in 2% milk PBS/tween for an hour. The membranes were washed in PBS/tween for one hour. Antibody binding was visualized by enhanced chemiluminescence (ECL; GE Healthcare, Piscataway, NJ) and exposure to Hyperfilm ECL (GE Healthcare).
Analysis
For the semi-quantitative in situ hybridization analysis, the mean corrected gray levels were obtained for each age group to compare densities of hybridization. The level of mRNA expression of ErbB4 or TH in the SNpc, SNl, VTA, or retrosplenial cortex was determined using optical density (OD) measurements with Scion Image software (National Institutes of Health). Figure 1 depicts the SNpc, SNl, and VTA as they were delineated for densitometric analysis in this study. Control measurements of the background OD were taken within each section (from the corpus callosum), and then subtracted from the OD value for each analyzed area to give a corrected value for each section. Values for the hybridization data are expressed as mean corrected grey levels ± the standard error of the mean (S.E.M.). Densitometric measurements were taken of each area from 6 sections per animal for each probe. The data were analyzed using one-way ANOVA, followed by the Newman-Keuls post-hoc test.
Figure 1.
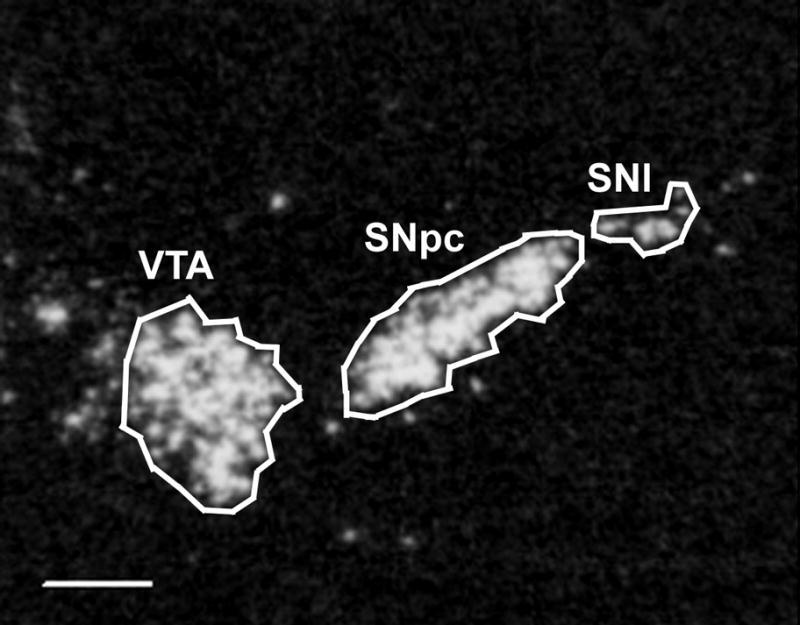
Reverse image film autoradiogram showing a section through the mid-rostrocaudal level of the ventral midbrain hybridized with TH cRNA. The areas of the ventral tegmental area (VTA), substantia nigra pars compacta (SNpc), and substantia nigra pars lateralis (SNl) are outlined as delineated for densitometric analysis in this study. Scale bar = 500 μm.
Western blot films were analyzed by densitometry using Scion Image software. The mean corrected grey levels were determined by measuring the OD of each band and subtracting background (an area in the same lane just below the band). The mean from the bands of young animals was set at 100% (control). Data are expressed as mean corrected grey level (percent control) ± S.E.M. The Western blot data were analyzed using one-way ANOVA followed by the Dunnett’s multiple comparison post-hoc test.
Results
Expression of ErbB4 and TH mRNAs during aging
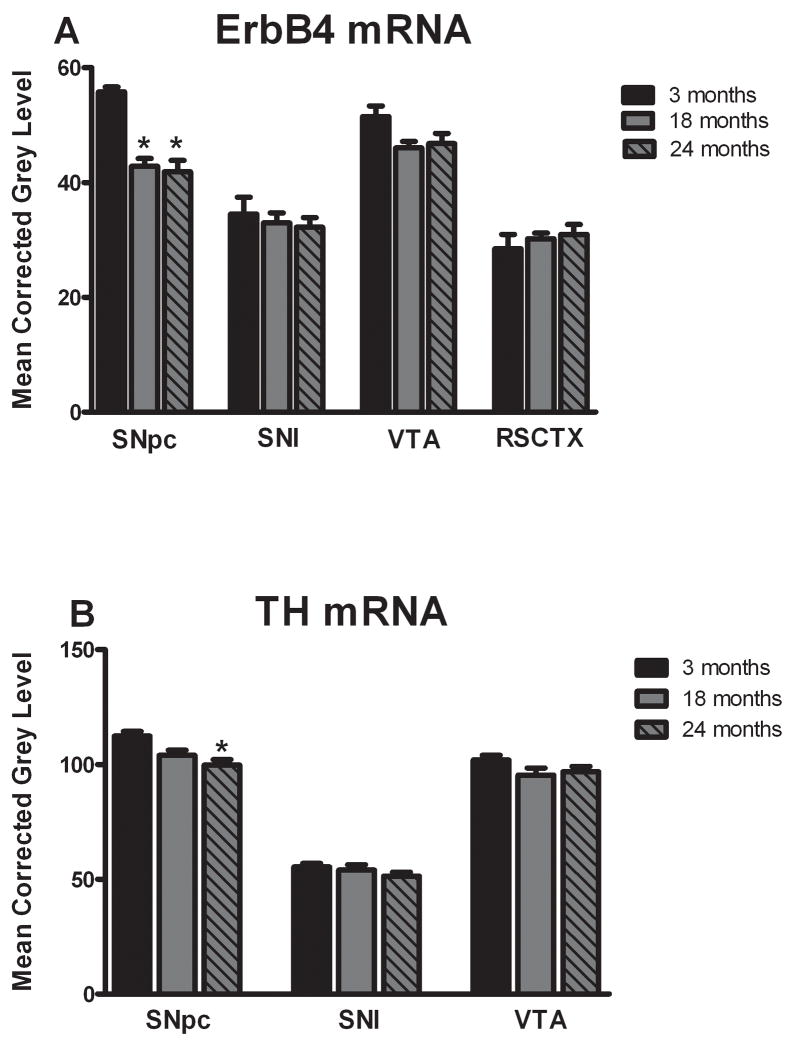
Representative autoradiographs of ErbB4 mRNA hybridization in rostral sections of the ventral midbrain are shown in Figure 2. Numerous areas throughout the brain contained cRNA-hybridizing cells including the SNpc, SNl, VTA, hippocampus and most neocortical regions. Decreased expression of ErbB4 mRNA was found in the SNpc of middle-aged and old BN/F344 animals when compared to the young rats (F(2,9) = 27.24, p<0.01; Figure 3A). Although only rostral sections of the ventral midbrain are shown in Figure 2, the decrease in ErbB4 mRNA was evident throughout the entire rostrocaudal extent of the SNpc. There was no difference in expression levels between the middle-aged and old animals. Expression of ErbB4 mRNA did not differ significantly with aging in the adjacent VTA (F(2,9) = 3.38, p>0.05) or SNl (F(2,9) = 0.28, p>0.05) (Figure 3A). Thus, age-related reduction in ErbB4 mRNA is not evident in all dopaminergic cell populations and appears to be specific for the SNpc.
Figure 2.

Reverse image film autoradiograms showing sections at rostral levels of the SNpc hybridized with either ErbB4 cRNA (A–C) or TH cRNA in semi-adjacent sections (D–F). Note the progressive decrease in expression of ErbB4 mRNA in the SNpc (arrows) among the young (A), middle-aged (B), and old (C) animals. In the same sections, no apparent loss of ErbB4 mRNA labeling is observed in the adjacent VTA, SNl, or in cortical regions (retrosplenial cortex, RSCTX). TH mRNA expression is also reduced in the old animals (F) when compared to the young animals (D) in the SNpc but not in the SNl or VTA. Scale bar = 1000 μm.
Figure 3.
Densitometric analysis of ErbB4 and TH cRNA hybridization during aging. A: Quantification of hybridization signal revealed a decrease in expression of ErbB4 mRNA in the SNpc of the middle-aged and old animals when compared to the young animals. Other areas within the same sections including the VTA, SNl and RSCTX exhibited no significant changes due to aging. B: Expression of TH mRNA was significantly decreased in the SNpc of old animals versus young animals, but no changes were found in the VTA or SNl. Data are expressed as mean corrected grey level ± S.E.M. *p<0.01 compared to the 3-month (young) age group.
To determine if this age-related decrease in ErbB4 mRNA expression was present in non-dopaminergic areas of the brain, the retrosplenial cortex, present at the same level (and in the same section) as the ventral midbrain, was analyzed across the aging spectrum. Representative autoradiographs are shown in Figure 2. There were no significant differences among any of the age groups in the retrosplenial cortex (F(2,9) = 0.48, p>0.05; Figure 3A). Taken together with the VTA and SNl data, these results suggest that the reduction in ErbB4 mRNA in the aged brain is not global, but rather is restricted to the SNpc.
Semi-adjacent sections through the ventral mesencephalon were hybridized for TH mRNA detection. Representative autoradiographs of TH cRNA hybridization in the rostral ventral midbrain are shown in Figure 2. Labeling for TH mRNA was analyzed for changes in expression during aging and is also useful in delimiting the area of the SNpc, SNl, and VTA (Figure 1). Expression levels for TH mRNA in the SNpc were significantly reduced between the young and the old animals (F(2,9) = 8.45, p<0.01; Figure 3B). Although no significant changes in TH mRNA during aging were found in the VTA, a trend toward a decrease in expression was noted in this structure (F(2,9) = 1.84, p=0.12; Figure 3B). There were no significant changes in TH mRNA levels among any of the age groups in the SNl (F(2,9) = 1.08, p>0.05; Figure 3B).
Expression of ErbB4 and TH protein during aging
Changes in mRNA do not always translate into equivalent changes in protein, thus we assessed expression of ErbB4 and TH protein in each age group using Western blot analysis. Figure 4A shows representative autoradiographs of Western blots from young, middle-aged, and old animals for ErbB4, TH, and GAPDH (housekeeping protein used as a loading control). The expression of ErbB4 protein (migrating at 185 kDA) was significantly decreased between the young and the old rats, and a strong trend was found toward a decrease in the middle-aged animals when compared to the young animals (F(2,15) = 5.80, p<0.05 for old versus young rats, p=0.054 for middle-age versus young rats; Figure 4B). Expression of TH protein (migrating at 60 kDa) in the ventral midbrain during aging was also examined. There was a significant decrease in TH protein between the young and old rats (F(2,15) = 4.75, p<0.05; Figure 4B). No differences in the protein levels of GAPDH were observed in any of the animals regardless of age (F(2,15) = 0.53, p>0.05; Figure 4B).
Figure 4.
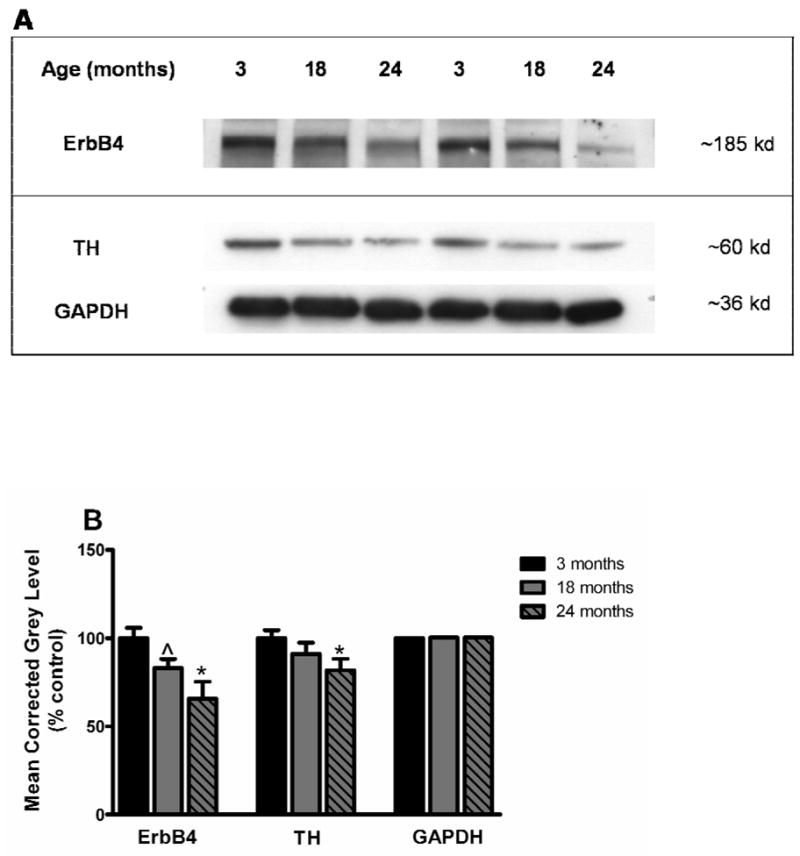
A: Representative autoradiograms from Western blots of the ventral midbrain for two different sets of animals at 3 (young), 18 (middle-aged) and 24 (old) months of age. Bands indicating protein expression of ErbB4 (migrating at 185 kD), TH (migrating at 60 kD), and GAPDH (migrating at 36 kD) are shown. Note the apparent progressive reduction in levels of ErbB4 and TH during aging. B: Quantification of the blots revealed a significant decrease in protein expression between young and old animals for ErbB4 in the ventral midbrain. There was also a strong trend toward a decrease for ErbB4 protein in the middle-aged animals when compared to the young animals. TH protein expression was reduced in the ventral midbrain of old animals when compared to young animals. Quantification of the housekeeping protein GAPDH revealed no differences among the age groups. Data are expressed as mean corrected grey level (represented as percent control) ± S.E.M. *p<0.05, ^p=0.054 compared to the 3-month (young) age group.
Discussion
In the present study we demonstrate an age-related reduction of both message and protein for the ErbB4 receptor and the dopaminergic biosynthetic enzyme TH in the SNpc of BN/F344 rats. The decrease in ErbB4 mRNA was evident in the middle-aged animals and was sustained with advanced age, whereas the significant decline in ErbB4 protein only appeared in the old rats. Tyrosine hydroxylase exhibited decreases in mRNA and protein in the old animals when compared to young animals.
Neuregulins and their ErbB receptors are critical for the development of the CNS and the peripheral nervous system (PNS) (reviewed in Burden and Yarden, 1997; Gassmann and Lemke, 1997; Esper et al., 2006). The importance of NRG signaling through the ErbB4 receptor is emphasized in studies of mutant mice. ErbB4 knockout animals result in embryonic lethality with cardiac and hindbrain defects (Gassmann et al., 1995). Mice rescued from embryonic lethality with cardiac ErbB4 replacement have abnormal neural crest cell migration as well as an increased number of large interneurons in the cerebellum (Tidcombe et al., 2003; Golding et al., 2004). Conditional nervous system ErbB4 knockout mice exhibit behavioral disturbances including lower spontaneous motor activity and reduced grip strength (Golub et al., 2004). Other conditional ErbB4 mutants demonstrate aberrant neuroblast migration from the subventricular zone and deficits in differentiation of olfactory interneurons (Anton et al., 2004). Interestingly, Thuret et al. (2004) were unable to ascertain any behavioral or morphological disturbances in the mesencephalic dopaminergic system in brain-specific ErbB4 null mice. This lack of phenotype is thought due to a strong compensatory response (Thuret et al., 2004). It should also be noted that aged ErbB4 mutant mice were not examined in the Thuret et al. study. After development, the function of NRG/ErbB signaling is less well understood, but has been implicated in the maintenance of neuronal connections and in many disease states including schizophrenia, stroke, multiple sclerosis and peripheral neuropathy (Cannella et al., 1999; Parker et al., 2002; Talmage and Role, 2004; Norton et al., 2006; reviewed in Esper et al., 2006). Furthermore, NRGs in vitro are neurotrophic and neuroprotective in several neuronal culture systems (Vaskovsky et al., 2000; Gerecke et al., 2004; Zhang et al., 2004; Di Segni et al., 2006).
With respect to the nigrostriatal dopaminergic system, ErbB4 is highly expressed in the SN and VTA where it is present within almost all of the dopaminergic cells (Steiner et al., 1999; Yurek and Seroogy, 2001; Abe et al., 2009; K.B. Seroogy unpublished observations). Given the sparse expression of ErbB2 and ErbB3 in the SNpc, it is likely that the known trophic and functional effects of NRGs on mesencephalic dopaminergic cells in vitro and in vivo (Yurek et al., 2004; Zhang et al., 2004) are mediated through ErbB4 receptors. Thus, NRGs signaling specifically via ErbB4 receptor activation could be important not only in the survival and functioning of developing mesencephalic dopamine cells, but also in the maintenance and functioning of midbrain dopamine cells in the adult and aging brain. The current study suggests that a decrease in NRG/ErbB4 support via loss of ErbB4 receptors occurs in aged animals and is specific for the ventral midbrain. This progressive loss of NRG/ErbB4 activation may have important functional implications for the mesostriatal dopaminergic system.
It is unlikely that compensatory changes in expression of ErbB3, the only other ErbB receptor that directly binds NRGs, could replace the diminishing influence of ErbB4 in the aging SNpc. Few ErbB3-expressing cells are located in the adult SNpc and those present are not colocalized to dopaminergic neurons (Steiner et al., 1999; Abe et al., 2009). Moreover, although we did not analyze ErbB3 expression in these BN/F344 rats, we have observed no changes in ErbB3 mRNA levels in the midbrain of aged Sprague Dawley rats (J.W. Dickerson and K.B. Seroogy, unpublished results).
It has been theorized that a loss of neurotrophic support underlies the vulnerability of the nigrostriatal dopaminergic system to age-related loss and/or to degeneration in disorders like Parkinson’s disease (Hefti and Weiner, 1986; Hymen et al., 1991; Unsicker 1994; Temlett et al., 1996). Neurotrophic factors (including NRGs) are known to be neuroprotective to cultured dopamine cells against insults with 6-OHDA or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (e.g. Hyman et al., 1991, 1994; Collier and Sortwell, 1999; Yurek and Seroogy, 2001; Zhang et al., 2004). Neurotrophic factors also protect dopaminergic neurons from degeneration and improve functional outcomes in animal models of PD, albeit with differing levels of effectiveness (e.g. Altar et al., 1994a, b; Tomac et al., 1995; Kearns et al., 1995; Gash et al., 1996; Rosenblad et al., 1998; Collier and Sortwell, 1999; Klein et al., 1999; Kordower et al., 2000; Fox et al., 2001; Yurek and Seroogy, 2001; Sun et al., 2005). Thus select neurotrophic factors (including NRGs) are important in the development, maintenance, function, and protection of the nigrostratal dopaminergic system.
There have been only a few studies indicating a decrease in mesostriatal neurotrophic support with age in rats. In Sprague Dawley rats, a decrease in BDNF protein was detected in several areas of the aged brain including a non-significant decrease of 18% in the SN (Croll et al., 1998). A reduction in the BDNF high affinity receptor trkB was also found in the SN of these same rats (Croll et al., 1998). Another study reported a significant 52% reduction of BDNF in the striatum of aged Sprague Dawley rats (Katoh-Semba et al., 1998). Aged F344BN rats exhibited a significant reduction of GDNF protein in the striatum and ventral midbrain (Yurek and Fletcher-Turner, 2001). The current study demonstrates a 25% decrease in ErbB4 message and a 34% decrease in ErbB4 protein, both significant reductions, by 24 months of age in BN/F344 hybrid rats.
Changes in neurotrophic factor support in the nigrostriatal dopaminergic system have also been observed in aged animals following injury or other manipulations. Young animals that receive a neurotoxic lesion of the SNpc have increased expression of BDNF and GDNF protein in the striatum (Zhou et al., 1996; Ling et al., 2000; Yurek and Fletcher-Turner, 2000, 2001; Collier et al., 2005). This compensatory striatal increase of BDNF and GDNF in response to neurotoxin administration in young rats, however, is attenuated in aged rats (Yurek and Fletcher-Turner, 2000, 2001). In addition, GDNF is less effective at preventing 6-OHDA-induced degeneration in old versus young rats, indicating a decreased responsiveness to neurotrophic factors in the aged brain (Fox et al., 2001). Aged animals also display a decreased viability of dopaminergic cell grafts when compared to younger animals, possibly due to a diminished trophic factor environment (Collier et al., 1999; Sortwell et al., 2001). Evidence for this is supported by BDNF and GDNF infusions or Schwann cell cografts, which improve graft survival and outgrowth (Hudson et al., 1995; Sinclair et al., 1996; Yurek et al., 1996; Yurek 1998; Collier et al., 1999). Thus, chronological aging leads to decreased neurotrophic support through the direct loss of neurotrophic factor signaling, the attenuation of compensatory mechanisms, or diminished responsiveness to neurotrophic factor therapy. These deficiencies, alone or in combination, may increase the susceptibility of midbrain dopaminergic cells to neurodegeneration. In comparisons of Parkinson’s disease patients to healthy controls, decreases of GDNF, BDNF, and ciliary neurotrophic factor (CNTF) have been found in the nigrostriatal system, whereas there are little or no changes in neurotrophin-3 (NT-3), NT-4 or nerve growth factor (NGF) (Mogi et al., 1999; Parain et al., 1999; Howells et al., 2000; Seigel and Chauhan, 2000; Chauhan et al., 2001). Although these studies may indicate a role for neurotrophic factors in the diseased-state brain, decreased neurotrophic factor support in the naturally aging brain remains to be fully elucidated.
The animals used in the present study are BN/F344 hybrid rats. These rats are recommended based on National Institute on Aging (NIA) data indicating a normal range of age-related pathologies in these animals including lower incidence of renal pathology and no specific tumor susceptibility (Lipman et al., 1996). Although these hybrid rats exhibit motor deficits, which occur starting at 18 months of age (Yurek et al., 1998), changes in the SNpc have not been reported in the animals. In this study we observe a decrease in ErbB4 mRNA coinciding with the timing of deficits in behavior reported in Yurek et al. (1998), but prior to reduction in TH mRNA and protein indicating the possibility that a loss of NRG/ErbB4 occurs prior to alterations within dopaminergic neurons in the SNpc. Future studies using precise stereological counting methods would be needed to assess any age-related dopaminergic cell loss occurring in the SNpc of BN/F344 animals. Additional studies also need to investigate modulation of other neurotrophic factors, including NRG ligands, in the nigrostriatal dopaminergic system of BN/F344 animals to better understand the changing neurotrophic factor environment of the aging animal.
In conclusion, we have found decreases in ErbB4 mRNA and protein occurring with advancing age. The aging-induced decrease in ErbB4 mRNA commences prior to indications of nigral dopaminergic changes as measured by TH mRNA and protein analyses. Thus, these findings raise the possibility that a progressive loss of NRG/ErbB4 signaling may contribute to the natural age-related decline of nigrostriatal dopaminergic function and possibly to the pathogenesis of age-related neurodegenerative disorders such as Parkinson’s disease.
Acknowledgments
This work was supported by NIH grant NS39128 to K.B.S. and a Scottish Rite Foundation Fellowship to J.W.D.
Abbreviations
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- BN/F344
Brown Norway X Fischer 344 hybrid rat
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- CSF
cerebral spinal fluid
- DEPC
diethylpyrocarbonate
- ECL
enhanced chemiluminescence
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDNF
glial cell line-derived neurotrophic factor
- GGF2
glial growth factor 2
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NGF
nerve growth factor
- NRG
neuregulin
- NT-3
neurotrophin-3
- NT-4
neurotrophin-4
- OD
optical density
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- PNS
peripheral nervous system
- RSCTX
retrosplenial cortex
- SDS
sodium dodecyl sulfate
- SEM
standard error of the mean
- SNpc
substantia nigra pars compacta
- SNl
substantia nigra pars lateralis
- SSC
standard saline citrate
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- 6-OHDA
6-hydroxydopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Namba H, Zheng Y, Nawa H. In situ hybridization reveals developmental regulation of ErbB1-4 mRNA expression in mouse midbrain: implication of ErbB receptors for dopaminergic neurons. Neuroscience. 2009;161:95–110. doi: 10.1016/j.neuroscience.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Fritsche M, Jackson C, Hyman C, Lindsay RM. The neurotrophins NT-4/5 and BDNF augment serotonin, dopamine, and GABAergic systems during behaviorally effective infusions to the substantia nigra. Exp Neurol. 1994;130:31–40. doi: 10.1006/exnr.1994.1182. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J Neurochem. 1994;63:1021–1032. doi: 10.1046/j.1471-4159.1994.63031021.x. [DOI] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Stauch R, Krell D, Mawrin C, Budinger E, Keilhoff G, Bogerts B. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69:546–559. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315:611–618. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Kornblum HI, Seroogy KB. Comparison of thalamic populations in mammals and birds: expression of ErbB4 mRNA. Brain Res Bull. 2002;57:455–461. doi: 10.1016/s0361-9230(01)00678-5. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Cannella B, Pitt D, Marchionni M, Raine CS. Neuregulin and erbB receptor expression in normal and diseased human white matter. J Neuroimmunol. 1999;100:233–242. doi: 10.1016/s0165-5728(99)00201-5. [DOI] [PubMed] [Google Scholar]
- Carraway KL, 3rd, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Chen MS, Bermingham-McDonogh O, Danehy FT, Jr, Nolan C, Scherer SS, Lucas J, Gwynne D, Marchionni MA. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Dung Ling Z, Carvey PM, Fletcher-Turner A, Yurek DM, Sladek JR, Jr, Kordower JH. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp Neurol. 2005;191(Suppl 1):S60–67. doi: 10.1016/j.expneurol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE. Therapeutic potential of nerve growth factors in Parkinson’s disease. Drugs Aging. 1999;14:261–287. doi: 10.2165/00002512-199914040-00003. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci. 1999;19:5563–5573. doi: 10.1523/JNEUROSCI.19-13-05563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- Di Segni A, Farin K, Pinkas-Kramarski R. ErbB4 activation inhibits MPP+-induced cell death in PC12-ErbB4 cells: involvement of PI3K and Erk signaling. J Mol Neurosci. 2006;29:257–267. doi: 10.1385/JMN:29:3:257. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- Emerich DF, McDermott P, Krueger P, Banks M, Zhao J, Marszalkowski J, Frydel B, Winn SR, Sanberg PR. Locomotion of aged rats: relationship to neurochemical but not morphological changes in nigrostriatal dopaminergic neurons. Brain Res Bull. 1993;32:477–486. doi: 10.1016/0361-9230(93)90294-l. [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51:161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Fox CM, Gash DM, Smoot MK, Cass WA. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 2001;896:56–63. doi: 10.1016/s0006-8993(00)03270-4. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Lemke G. Neuregulins and neuregulin receptors in neural development. Curr Opin Neurobiol. 1997;7:87–92. doi: 10.1016/s0959-4388(97)80125-0. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1beta induces neurite extension and arborization in cultured hippocampal neurons. Mol Cell Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Golding JP, Sobieszczuk D, Dixon M, Coles E, Christiansen J, Wilkinson D, Gassmann M. Roles of erbB4, rhombomere-specific, and rhombomere-independent cues in maintaining neural crest-free zones in the embryonic head. Dev Biol. 2004;266:361–372. doi: 10.1016/j.ydbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hefti F, Weiner WJ. Nerve growth factor and Alzheimer’s disease. Ann Neurol. 1986;20:275–281. doi: 10.1002/ana.410200302. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy GM, Jr, Di Monte DA, Sandy MS, Langston JW. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci Res. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Yanni DS, Easterday MC, Seroogy KB. Expression of the EGF receptor family members ErbB2, ErbB3, and ErbB4 in germinal zones of the developing brain and in neurosphere cultures containing CNS stem cells. Dev Neurosci. 2000;22:16–24. doi: 10.1159/000017423. [DOI] [PubMed] [Google Scholar]
- Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Collier TJ, Sortwell CE, Lipton JW, Vu TQ, Robie HC, Carvey PM. Striatal trophic activity is reduced in the aged rat brain. Brain Res. 2000;856:301–309. doi: 10.1016/s0006-8993(00)01945-4. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill TH, Koek LL. Differential effects of advancing age on neurotransmitter cell loss in the substantia nigra and striatum of C57BL/6N mice. Brain Res. 1990;521:107–117. doi: 10.1016/0006-8993(90)91530-t. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Lacoste B, Yu WF, Srivastava LK, Quirion R. Developmental profile of neuregulin receptor ErbB4 in postnatal rat cerebral cortex and hippocampus. Neuroscience. 2007;148:126–139. doi: 10.1016/j.neuroscience.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O’Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Numan S, Gall CM, Seroogy KB. Developmental expression of neurotrophins and their receptors in postnatal rat ventral midbrain. J Mol Neurosci. 2005;27:245–260. doi: 10.1385/JMN:27:2:245. [DOI] [PubMed] [Google Scholar]
- Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol. 1999;403:295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neuregulin expression after focal stroke in the rat. Neurosci Lett. 2002;334:169–172. doi: 10.1016/s0304-3940(02)01126-6. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Björklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson’s disease. Neuroscience. 1998;82:129–137. doi: 10.1016/s0306-4522(97)00269-8. [DOI] [PubMed] [Google Scholar]
- Sanchez HL, Silva LB, Portiansky EL, Herenu CB, Goya RG, Zuccolilli GO. Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience. 2008;154:1598–1606. doi: 10.1016/j.neuroscience.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Herman JP. In situ hybridization approaches to the study of the nervous system. In: Turner AJ, Bachelard HS, editors. Neurochemistry - A Practical Approach. 2. Oxford: Oxford University Press; 1997. pp. 121–150. [Google Scholar]
- Seroogy KB, Zhang L. Neuregulins. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier Academic Press; 2006. pp. 1401–1407. [Google Scholar]
- Siddiqi Z, Kemper TL, Killiany R. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. J Neuropathol Exp Neurol. 1999;58:959–971. doi: 10.1097/00005072-199909000-00006. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Sinclair SR, Svendsen CN, Torres EM, Martin D, Fawcett JW, Dunnett SB. GDNF enhances dopaminergic cell survival and fibre outgrowth in embryonic nigral grafts. Neuroreport. 1996;7:2547–2552. doi: 10.1097/00001756-199611040-00029. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Camargo MD, Pitzer MR, Gyawali S, Collier TJ. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169:23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: exacerbation by nomifensine. Neurosci Lett. 2002;333:195–198. doi: 10.1016/s0304-3940(02)01105-9. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: relationships with nigrostriatal neurochemical measures. Neurobiol Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92. doi: 10.1007/s00441-004-0972-9. [DOI] [PubMed] [Google Scholar]
- Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159:494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmage DA, Role LW. Multiple personalities of neuregulin gene family members. J Comp Neurol. 2004;472:134–139. doi: 10.1002/cne.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás A, Lubics A, Szalontay L, Lengvári I, Reglodi D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Temlett JA. Parkinson’s disease: biology and aetiology. Curr Opin Neurol. 1996;9:303–307. [PubMed] [Google Scholar]
- Thompson M, Lauderdale S, Webster MJ, Chong VZ, McClintock B, Saunders R, Weickert CS. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Thuret S, Alavian KN, Gassmann M, Lloyd CK, Smits SM, Smidt MP, Klein R, Dyck RH, Simon HH. The neuregulin receptor, ErbB4, is not required for normal development and adult maintenance of the substantia nigra pars compacta. J Neurochem. 2004;91:1302–1311. doi: 10.1111/j.1471-4159.2004.02809.x. [DOI] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Unsicker K. Growth factors in Parkinson’s disease. Prog Growth Factor Res. 1994;5:73–87. doi: 10.1016/0955-2235(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Numan S, Nestler EJ, Russell DS. Regulation of phospholipase Cγ in the mesolimbic dopamine system by chronic morphine administration. J Neurochem. 1999;73:1520–1528. doi: 10.1046/j.1471-4159.1999.0731520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Seroogy KB. Neurotrophic factor protection of dopaminergic neurons. In: Mocchetti I, editor. Neurobiology of the Neurotrophins. New York: F.P. Graham Publishing; 2001. pp. 355–397. [Google Scholar]
- Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153:195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol. 2000;161:392–396. doi: 10.1006/exnr.1999.7274. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Lu W, Hipkens S, Wiegand SJ. BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp Neurol. 1996;137:105–118. doi: 10.1006/exnr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Zhang L, Fletcher-Turner A, Seroogy KB. Supranigral injection of neuregulin1-β induces striatal dopamine overflow. Brain Res. 2004;1028:116–119. doi: 10.1016/j.brainres.2004.08.066. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fletcher-Turner A, Marchionni MA, Apparsundaram S, Lundgren KH, Yurek DM, Seroogy KB. Neurotrophic and neuroprotective effects of the neuregulin glial growth factor-2 on dopaminergic neurons in rat primary midbrain cultures. J Neurochem. 2004;91:1358–1368. doi: 10.1111/j.1471-4159.2004.02817.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pliego-Rivero B, Bradford HF, Stern GM. The BDNF content of postnatal and adult rat brain: the effects of 6-hydroxydopamine lesions in adult brain. Dev Brain Res. 1996;97:297–303. doi: 10.1016/s0165-3806(96)00159-9. [DOI] [PubMed] [Google Scholar]