Abstract
Anemia of chronic disease, also called anemia of inflammation, is characterized by hypoferremia due to iron sequestration that eventually results in iron-restricted erythropoiesis. During the last decade, the molecular mechanisms of iron sequestration have been found to center on cytokine-stimulated overproduction of the iron-regulatory hormone hepcidin. The inflammatory cytokine IL-6 is a particularly prominent inducer of hepcidin but other cytokines are likely to contribute as well. Hepcidin excess causes the endocytosis and proteolysis of the sole known cellular iron exporter, ferroportin, trapping iron in macrophages and iron-absorbing enterocytes. The supply of iron to hemoglobin synthesis becomes limiting, eventually resulting in anemia. Depending on the details of the underlying disease, other inflammation-related mechanisms may also contribute to anemia.
Hypoferremia in infection and inflammation
As described more than sixty years ago, serum iron concentrations markedly decrease in humans1 and in dogs2 during the first few days of systemic infection or inflammation. More recent experimental studies in human volunteers injected with moderate doses of lipopolysaccharide 3 showed approximately a 50% decrease in serum iron by 24 hours. In another group of human subjects, a 3hr infusion of interleukin 6 (IL-6) was followed by an average 30% drop in serum iron 2 hrs later 4. The rapid development of hypoferremia was also observed in mice with experimental meningococcal infection5 or inflammation induced by turpentine6 or LPS7. As suggested by the deleterious effects of iron supplementation during experimental infections8,9, the hypoferremia probably contributes to host defense against infection, likely by decreasing the iron supply to invading microbes.
Hypoferremia of inflammation is caused by iron sequestration in macrophages
As discussed elsewhere in this issue, normally most of the iron delivered to plasma (about 20–25 mg/day) is provided by macrophages involved in recycling senescent erythrocytes, and only 1–2 mg/day comes from iron absorption in the duodenum, with additional variable amounts delivered from stored iron in hepatocytes. Studies with iron-radiolabeled damaged erythrocytes documented that inflammation or infection led to delayed appearance of radioactive iron in circulation and the accumulation of iron in macrophages (“reticuloendothelial system”) both in humans10 and in experimental animal models11. The inflammation-induced sequestration of iron in macrophages explained the hypoferremia of inflammation but the molecular pathways involved in this response were not known until a few years ago.
Anemia of chronic disease (anemia of inflammation)
Prolonged infection or inflammation often leads to the development of anemia (anemia of chronic disease, more recently called anemia of inflammation, AI). AI is usually a mild to moderate anemia (Hgb 7–12 g/dl) that develops in the setting of many infections and inflammatory disorders, and some malignancies 12. The newer terminology is not only more reflective of the pathophysiology of this anemia but also includes an acute form of this disorder, anemia of critical illness 13, a condition that develops within days of the onset of illness.
AI is characterized by inadequate erythrocyte production in the setting of low serum iron and low iron-binding capacity (i.e. low transferrin) despite preserved or even increased macrophage iron stores in the bone marrow. Direct examination of the bone marrow for iron-containing macrophages has been superseded in medical practice by measurements of serum ferritin. In most patients with iron deficiency serum ferritin is below the normal range but it is normal or high in patients with AI, reflecting the stimulation of ferritin synthesis by both inflammation and macrophage iron loading. The erythrocytes are usually normocytic and normochromic but can be mildly hypochromic and microcytic, especially in AI of long duration or in children, who utilize additional iron for growth. In these settings hypochromia and microcytosis develop, presumably because iron restriction becomes more severe as iron stores are progressively depleted.
Most chronic bacterial, fungal, viral or parasitic infections with systemic manifestations can cause AI. AI is also common in rheumatologic disorders, systemic autoimmune disorders, inflammatory bowel diseases, and chronic kidney diseases. Among malignancies, ovarian cancer14 and multiple myeloma15 are often complicated by AI. Anemia of critical illness 13 may develop acutely (within days) in intensive care settings where the effects of infection or inflammation are exacerbated by disease-related or iatrogenic blood loss or red cell destruction, by themselves not sufficiently severe to cause anemia.
Iron restriction is a major contributor to anemia of inflammation
The limitation of iron supply to erythropoiesis is a major factor in the development of AI. Other factors that variably contribute to AI include increased destruction of erythrocytes, diagnostic phlebotomy or other blood loss, suppression of the maturation of erythrocyte precursors by cytokines and cytokine-mediated interference with erythropoietin production or signaling. Several biochemical and histochemical observations support the importance of iron restriction as a key mechanism in AI. During the synthesis of heme, iron is incorporated into protoporphyrin IX with zinc as a minor alternative protoporphyrin ligand. As would be expected, the amount of zinc incorporated into protoporphyrin IX is increased in iron deficiency. In AI, zinc protoporphyrin is also increased 16, indicating that insufficient iron is reaching the sites of heme synthesis in the developing erythrocytes leading to the substitution of zinc. The number of sideroblasts, nucleated erythrocyte precursors that stain for iron with Prussian blue, is decreased in AI 12, again suggesting iron restriction. As a further indication of the limiting role of iron in patients with AI but no evidence of iron deficiency, the resistance of AI to erythropoietin therapy can sometimes be overcome by the co-administration of parenteral iron 17,18. The mechanism by which high doses of intravenous iron preparations ameliorate resistance to pharmacologic doses of erythropoietin19, even in situations where ferritin is elevated20,21, is not yet clear. Nearly all iron from existing IV preparations is first processed by macrophages, and its utilization for erythropoiesis is dependent on iron export through ferroportin to transferrin. Macrophage iron overload would be expected to increase membrane ferroportin22 and increase iron availability for erythropoiesis. In parallel, high doses of erythropoietin may partially suppress hepcidin by as yet unknown mechanisms23. Previous attempts to treat AI with more moderate doses of iron alone (without added erythropoietin) have generally been unsuccessful, as iron became rapidly trapped in the macrophage compartment 12,24,25. Although newer iron preparations appear to be well tolerated26, the long term consequences of high dose iron therapy are not known and concerns have been raised about excess iron promoting infections and atherosclerosis27–29 as well as carcinogenesis30,31.
Molecular pathways of iron sequestration
Hepcidin and ferroportin
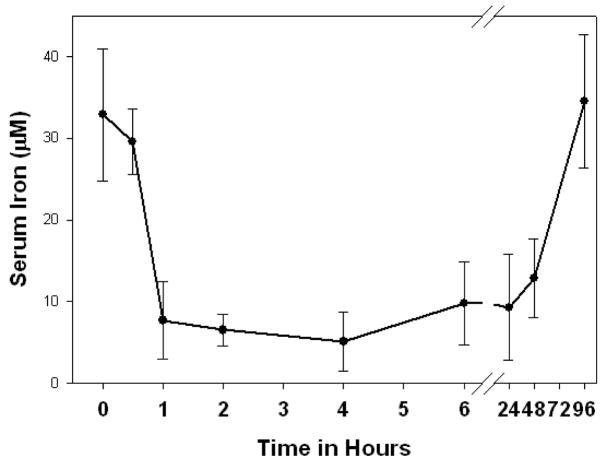
Hepcidin is a small peptide hormone secreted by hepatocytes, circulating in blood plasma and excreted in urine 32. An injection of synthetic hepcidin into mice induces profound hypoferremia lasting 2–3 days (Figure 1)33. The endogenous peptide is essential for iron homeostasis as shown by the consequences of its absence (juvenile hemochromatosis34,35) or its excess (iron restricted anemia36,37). Hepcidin acts by binding to the cellular iron efflux channel ferroportin, and inducing its internalization and degradation38. Ferroportin is a 12-transmembrane segment protein found in all tissues that export iron to blood plasma39–41. It is the only known cellular exporter of elemental iron and it is also essential for iron homeostasis42.
Figure 1.
Mice injected with synthetic hepcidin (50 μg) develop prolonged and severe hypoferremia (from Reference 33).
Hepcidin-induced ferroportin degradation inhibits iron release from macrophages
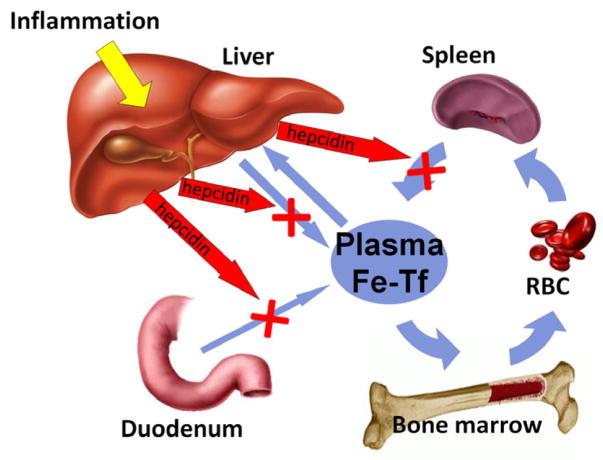
Macrophages recycle iron from senescent erythrocytes by a mechanism reviewed in detail in another chapter in this issue. The recycled iron is released to plasma through macrophage cell-membrane-associated ferroportin. During inflammation, hepcidin concentrations are high, which triggers increased endocytosis and proteolysis of ferroportin38. The efflux of ferrous iron from major iron transporting tissues (duodenal enterocytes, iron-recycling macrophages and iron-storing hepatocytes) into plasma is reduced (Figure 2) and the iron accumulates in their cytoplasmic ferritin. Continued consumption of iron by erythropoiesis then depletes the extracellular iron compartment leading to hypoferremia and iron-restricted erythropoiesis. The prominent effects of the loss of ferroportin on iron supply for erythropoiesis are illustrated by the consequences of moderate ferroportin deficiency or dysfunction due to autosomal dominant mutations in ferroportin (“ferroportin disease”). Despite iron-overloaded macrophages, the affected patients are susceptible to mild anemia, especially when phlebotomized43.
Figure 2.
Inflammation-induced hepcidin causes hypoferremia by inhibiting the major iron flows into plasma (mainly recycled iron from splenic and hepatic macrophages, but also dietary iron from the duodenum and stored iron from hepatocytes). Prolonged hypoferremia limits the availability of iron for hemoglobin synthesis and erythropoiesis, causing AI.
Iron sequestration and anemia in noninflammatory overproduction of hepcidin
Genetic disorders and noninflammatory diseases that give rise to excessive hepcidin production include iron-refractory iron deficiency anemia (IRIDA, discussed elsewhere in this issue) and hepatic adenomas that overproduce hepcidin37,44. Both disorders cause hypoferremia and anemia which is refractory to oral iron supplementation and is only partially corrected by parenteral iron. Moderate overproduction of hepcidin in transgenic mice or in mice bearing hepcidin-producing tumors also causes an iron-restricted anemia45,46. These diseases and models demonstrate that the essential features of AI are reproduced by overproduction of hepcidin. Unlike in most AI, some affected patients have severe microcytosis, probably a consequence of chronicity and severity of hepcidin overproduction.
Resistance to erythropoietin
Hyporesponsiveness to therapeutic erythropoietin has emerged as an important consequence of inflammation, especially in chronic kidney diseases47. This feature of inflammation is reproduced in the hepcidin-overexpressing mouse46 suggesting that increased hepcidin and iron limitation contribute to erythropoietin resistance. Neutralization of hepcidin by monoclonal antibody has been shown to restore responsiveness to erythropoietin in a mouse model of AI48.
Hepcidin and ferroportin regulation in inflammation
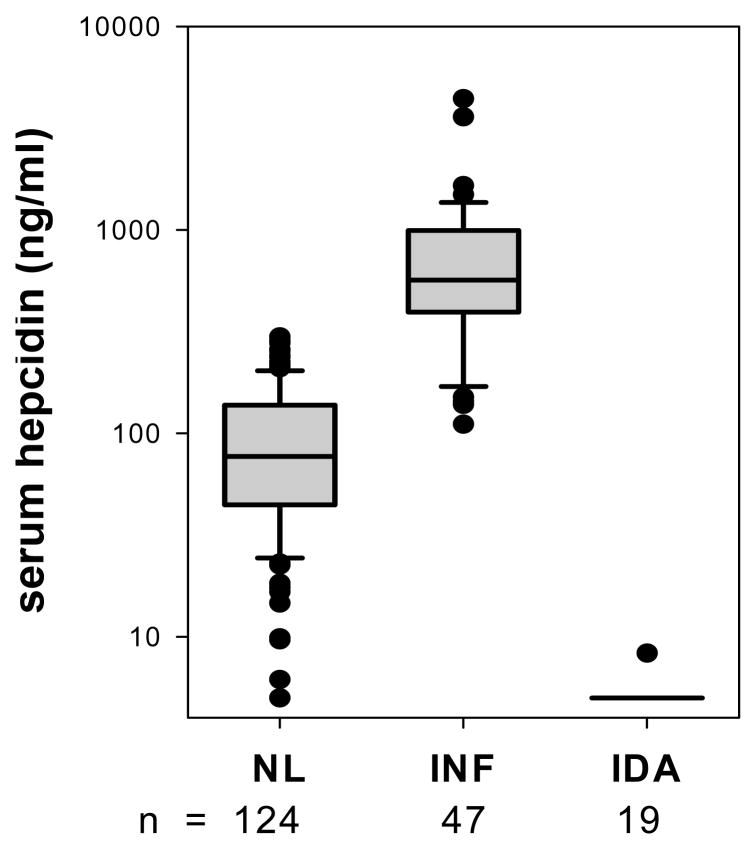
Limited studies in patients with inflammatory diseases document that serum hepcidin or its proxy urinary hepcidin are increased by inflammation (Figure 3). Specific mediators that can cause this increase have been analyzed in human volunteers, mouse models and in cell culture systems but the picture is far from complete.
Figure 3.
Patients with inflammation (defined by CRP greater than 10 mg/dl) have elevated serum hepcidin, in contrast to patients with iron deficiency (defined as ferritin less than 10 ng/ml) in whom hepcidin is low or unmeasurable (from reference 79).
Interleukin-6
Within hours after an inflammatory stimulus, hepcidin levels greatly increase3,4,49, followed by the development of hypoferremia. As indicated by experiments with isolated human hepatocytes treated with macrophage supernatants and studies with IL-6-deficient mice, the acute induction of hepcidin is mediated in large part by IL-63,4,50 but other cytokines may also contribute, especially in the chronic setting. The regulation of hepcidin synthesis is predominantly transcriptional. The STAT3 pathway transduces the effects of IL-6 utilizing a canonical STAT3 binding site in the proximal hepcidin promoter51–53.
In accordance with the role of IL-6 in hepcidin regulation, diseases of IL-6 excess are prominently associated with anemia. Castleman’s syndrome, a lymphoproliferative disorder of uncertain etiology, manifests increased IL-6, increased hepcidin, hypoferremia and iron-restricted anemia54,55, suggestive of a hepcidin-mediated anemia. Treatment with anti-IL6 receptor antibody rapidly decreases hepcidin and reverses the anemia56–59. IL-6 also has an important role in the pathogenesis of multiple myeloma, and IL-6 contributes to the hepcidin-inducing activity of sera from myeloma patients60. Higher hepcidin levels in myeloma correlate with lower hemoglobin60, indicating a likely causal role of hepcidin overproduction in the pathogenesis of anemia in this disease. Another disease where IL-6 excess and iron-restricted anemia are prominent features is systemic onset juvenile chronic arthritis 61. Finally, ovarian carcinomas complicated by pretreatment anemia are also associated with overproduction of IL-6.14 Serial measurements of hepcidin, serum iron and erythrocyte kinetics in these diseases before and during treatment will be informative about the role of hepcidin in these disorders.
Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMP-2, 4, 6 and 9 have been most studied in this regard) are potent inducers of hepcidin synthesis in cultured hepatocyte cell lines62–64, primary hepatocytes65, and in mouse models66. They regulate hepcidin transcription by binding to BMP receptors and activating the SMAD pathway. The essential role of this pathway for hepcidin and iron regulation was first demonstrated by studies of liver-specific SMAD4 knockout mice67 which make very little hepcidin and develop parenchymal iron overload. The principal and specialized endogenous ligand of the iron-regulating BMP receptor in the liver appears to be BMP6 as shown by the development of iron overload in mice lacking BMP668,69 with the absence of significant skeletal or other abnormalities that are associated with the ablation of other BMPs. Whether BMPs contribute to inflammatory regulation of hepcidin and iron is not yet known.
Hemojuvelin
A very severe form of hepcidin deficiency leading to iron overload is found in patients70 and mouse models71,72 lacking the GPI-linked membrane protein hemojuvelin. This protein functions as a coreceptor for BMP2, 4 and 673 and the loss of hemojuvelin results in very low basal levels of hepcidin transcription. Unexpectedly, hemojuvelin mRNA levels are potently suppressed by inflammation74, an effect mediated by tumor necrosis factor-alpha but not by IL-675. It is not yet clear how this effect contributes to the regulation of hepcidin and iron during inflammation.
Direct regulation of ferroportin during inflammation
The reduction of ferroportin protein expression during inflammation76 was noted even before the discovery of its posttranslational regulation by hepcidin. Although hepcidin is probably the principal systemic regulator of ferroportin, the production and trafficking of ferroportin may also be regulated by mechanisms independent of hepcidin. Ferroportin mRNA levels in the liver and the spleen were shown to be suppressed after the administration of lipopolysaccharide to mice76. Moreover, ferroportin transcripts contain a 5′ iron regulatory element (IRE)41 that could mediate translational repression of ferroportin synthesis by IRP1 during inflammation and oxidative stress77.
Therapeutic perspectives
Anemia of inflammation can add substantially to the morbidity of the underlying disease and is often a predictor of adverse outcome78. Increased awareness of the key role of iron sequestration in AI has led to the empiric use of high dose IV iron to ameliorate inflammation-induced resistance to erythropoietin19,20,47. Systematic studies of pathways that mediate iron sequestration in AI have pinpointed molecular targets for the treatment of this anemia in those situations when the underlying disease cannot be reversed. Anti-IL-6 treatments, which should alleviate AI along with other IL-6-mediated pathology in a variety of inflammatory disorders, are already undergoing clinical trials59. Because of the specialized involvement of these molecules in the iron pathway, the therapeutic antagonism of hepcidin, membrane hemojuvelin, or BMP6 could have a highly selective beneficial effect in AI.
Conclusion
Anemia of chronic disease develops in a great variety of disease settings but is commonly characterized by inflammation, hypoferremia, iron sequestration in macrophages, and iron-restricted erythropoiesis. At the molecular level, cytokine-stimulated overproduction of hepcidin causes the endocytosis and degradation of the iron efflux channel ferroportin, decreasing the delivery of iron from macrophages and enterocytes to plasma. Hypoferremia ensues, and restricts the supply of iron to hemoglobin synthesis, eventually resulting in anemia. Similar mechanisms may also account for erythropoietin resistance in the setting of chronic kidney diseases. Evolving understanding of its pathogenetic pathways should improve the treatment of this anemia.
Acknowledgments
Supported in part by Roche Foundation for Anemia Research (RoFAR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tomas Ganz, Professor of Medicine and Pathology, Departments of Medicine and Pathology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA 90095-1690
Elizabeta Nemeth, Associate Professor of Medicine, Departments of Medicine, David Geffen School of Medicine, University of California, Los Angeles Los Angeles, CA 90095-1690
References
- 1.Cartwright GE, Lauritsen MA, Jones PJ, Merrill IM, Wintrobe MM. THE ANEMIA OF INFECTION. I. HYPOFERREMIA, HYPERCUPREMIA, AND ALTERATIONS IN PORPHYRIN METABOLISM IN PATIENTS. J Clin Invest. 1946;25:65–80. doi: 10.1172/JCI101690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartwright GE, Lauritsen MA, Humphreys S, Jones PJ, Merrill IM, Wintrobe MM. THE ANEMIA OF INFECTION. II. THE EXPERIMENTAL PRODUCTION OF HYPOFERREMIA AND ANEMIA IN DOGS. J Clin Invest. 1946;25:81–86. doi: 10.1172/JCI101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holbein BE. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980;29:886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaumier DL, Caldwell MA, Holbein BE. Inflammation triggers hypoferremia and de novo synthesis of serum transferrin and ceruloplasmin in mice. Infect Immun. 1984;46:489–494. doi: 10.1128/iai.46.2.489-494.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertini R, Bianchi M, Erroi A, Villa P, Ghezzi P. Dexamethasone modulation of in vivo effects of endotoxin, tumor necrosis factor, and interleukin-1 on liver cytochrome P-450, plasma fibrinogen, and serum iron. J Leukoc Biol. 1989;46:254–262. doi: 10.1002/jlb.46.3.254. [DOI] [PubMed] [Google Scholar]
- 8.Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holbein BE. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981;34:120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NOYES WD, Bothwell TH, Finch CA. The role of the reticulo-endothelial cell in iron metabolism. Br J Haematol. 1960;6:43–55. doi: 10.1111/j.1365-2141.1960.tb06216.x. [DOI] [PubMed] [Google Scholar]
- 11.Fillet G, Cook JD, Finch CA. Storage iron kinetics. VII. A biologic model for reticuloendothelial iron transport. J Clin Invest. 1974;53:1527–1533. doi: 10.1172/JCI107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright GE. The anemia of chronic disorders. Semin Hematol. 1966;3:351–375. [PubMed] [Google Scholar]
- 13.Corwin HL, Krantz SB. Anemia of the critically ill: “acute” anemia of chronic disease. Crit Care Med. 2000;28:3098–3099. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- 14.Maccio A, Madeddu C, Massa D, Mudu MC, Lusso MR, Gramignano G, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. Blood. 2005;106:362–367. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig H, Pohl G, Osterborg A. Anemia in multiple myeloma. Clin Adv Hematol Oncol. 2004;2:233–241. [PubMed] [Google Scholar]
- 16.Hastka J, Lasserre JJ, Schwarzbeck A, Strauch M, Hehlmann R. Zinc protoporphyrin in anemia of chronic disorders. Blood. 1993;81:1200–1204. [PubMed] [Google Scholar]
- 17.Taylor JE, Peat N, Porter C, Morgan AG. Regular low-dose intravenous iron therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1079–1083. [PubMed] [Google Scholar]
- 18.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823. [PubMed] [Google Scholar]
- 19.Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous Iron Optimizes the Response to Recombinant Human Erythropoietin in Cancer Patients With Chemotherapy-Related Anemia: A Multicenter, Open-Label, Randomized Trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 20.Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, et al. Ferric Gluconate Reduces Epoetin Requirements in Hemodialysis Patients with Elevated Ferritin. J Am Soc Nephrol. 2008;19:372–379. doi: 10.1681/ASN.2007050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, et al. Ferric Gluconate Is Highly Efficacious in Anemic Hemodialysis Patients with High Serum Ferritin and Low Transferrin Saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 22.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411:123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 23.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 24.Hume R, Currie WJ, Tennant M. Anaemia of rheumatoid arthritis and iron therapy. Ann Rheum Dis. 1965;24:451–457. doi: 10.1136/ard.24.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beamish MR, Davies AG, Eakins JD, Jacobs A, Trevett D. The measurement of reticuloendothelial iron release using iron-dextran. Br J Haematol. 1971;21:617–622. doi: 10.1111/j.1365-2141.1971.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. 2007;369:1502–1504. doi: 10.1016/S0140-6736(07)60689-8. [DOI] [PubMed] [Google Scholar]
- 27.Bishu K, Agarwal R. Acute Injury with Intravenous Iron and Concerns Regarding Long-Term Safety. Clin J Am Soc Nephrol. 2006;1:S19–S23. doi: 10.2215/CJN.01420406. [DOI] [PubMed] [Google Scholar]
- 28.Sengoelge G, Sunder-Plassmann G, Horl WH. Potential risk for infection and atherosclerosis due to iron therapy. J Ren Nutr. 2005;15:105–110. doi: 10.1053/j.jrn.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan JL. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp Biol Med (Maywood) 2007;232:1014–1020. doi: 10.3181/0703-MR-54. [DOI] [PubMed] [Google Scholar]
- 30.Corradini E, Ferrara F, Pollicino T, Vegetti A, Abbati GL, Losi L, et al. Disease progression and liver cancer in the ferroportin disease. Gut. 2007;56:1030–1032. doi: 10.1136/gut.2007.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deugnier Y. Iron and liver cancer. Alcohol. 2003;30:145–150. doi: 10.1016/s0741-8329(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 32.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 33.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 36.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proceedings of the National Academy of Sciences. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 38.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 39.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 40.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 41.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 42.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Pietrangelo A. The ferroportin disease. Blood Cells, Molecules, and Diseases. 2004;32:131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Melis MA, Cau M, Congiu R, Sole G, Barella S, Cao A, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008 doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 45.Rivera S, Liu L, Nemeth E, Gabayan V, Sorensen OE, Ganz T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005;105:1797–1802. doi: 10.1182/blood-2004-08-3375. [DOI] [PubMed] [Google Scholar]
- 46.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to Erythropoietin: Causes and Management. Advances in Chronic Kidney Disease. 2009;16:94–100. doi: 10.1053/j.ackd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Sasu B, Hainu M, Boone TC, Bi XJ, Lee KJ, Arvedson T, Winters A, Cooke K, Sheng Z. 12/022,515. HEPCIDIN, HEPCIDIN ANTAGONISTS AND METHODS OF USE. 2009 Amgen Inc. USA. 1-30-2008. Ref Type: Patent.
- 49.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 51.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Verga Falzacappa MV, Vujic SM, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 54.Vinzio S, Ciarloni L, Schlienger JL, Rohr S, Mechine A, Goichot B. Isolated microcytic anemia disclosing a unicentric Castleman disease: The interleukin-6/hepcidin pathway? European Journal of Internal Medicine. 2008;19:367–369. doi: 10.1016/j.ejim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Ohsugi Y. Recent Advances in Immunopathophysiology of Interleukin-6: An Innovative Therapeutic Drug, Tocilizumab (Recombinant Humanized Anti-human Interleukin-6 Receptor Antibody), Unveils The Mysterious Etiology of Immune-Mediated Inflammatory Diseases. Biological & Pharmaceutical Bulletin. 2007;30:2001–2006. doi: 10.1248/bpb.30.2001. [DOI] [PubMed] [Google Scholar]
- 56.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in mice. J Clin Invest. 1990;86:592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–311. doi: 10.1006/cyto.2002.2012. [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 59.Kawabata H, Tomosugi N, Kanda J, Tanaka Y, Yoshizaki K, Uchiyama T. Anti-interleukin 6 receptor antibody tocilizumab reduces the level of serum hepcidin in patients with multicentric Castleman’s disease. Haematologica. 2007;92:857–858. doi: 10.3324/haematol.10794. [DOI] [PubMed] [Google Scholar]
- 60.Sharma S, Nemeth E, Chen YH, Goodnough J, Huston A, Roodman GD, et al. Involvement of Hepcidin in the Anemia of Multiple Myeloma. Clin Cancer Res. 2008;14:3262–3267. doi: 10.1158/1078-0432.CCR-07-4153. [DOI] [PubMed] [Google Scholar]
- 61.Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87:4824–4830. [PubMed] [Google Scholar]
- 62.Huang FW, Babitt JL, Wrighting DM, Samad TA, Xia Y, Sidis Y, et al. Hemojuvelin Acts as a Bone Morphogenetic Protein Co-Receptor To Regulate Hepcidin Expression. ASH Annual Meeting Abstracts. 2005;106:511. [Google Scholar]
- 63.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111:5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proceedings of the National Academy of Sciences. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Andriopoulos JB, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009 doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009 doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 70.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 71.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 74.Krijt J, Vokurka M, Chang KT, Necas E. Expression of Rgmc, the murine ortholog of hemojuvelin gene, is modulated by development and inflammation, but not by iron status or erythropoietin. Blood. 2004;104:4308–4310. doi: 10.1182/blood-2004-06-2422. [DOI] [PubMed] [Google Scholar]
- 75.Constante M, Wang D, Raymond VA, Bilodeau M, Santos MM. Repression of repulsive guidance molecule C during inflammation is independent of Hfe and involves tumor necrosis factor-alpha. Am J Pathol. 2007;170:497–504. doi: 10.2353/ajpath.2007.060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem. 2002;277:39786–39791. doi: 10.1074/jbc.M201485200. [DOI] [PubMed] [Google Scholar]
- 77.Mueller S, Pantopoulos K, Hubner CA, Stremmel W, Hentze MW. IRP1 Activation by Extracellular Oxidative Stress in the Perfused Rat Liver. J Biol Chem. 2001;276:23192–23196. doi: 10.1074/jbc.M100654200. [DOI] [PubMed] [Google Scholar]
- 78.Weiss G, Goodnough LT. Anemia of Chronic Disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 79.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]