Abstract
Objective
Insulin resistance is increased by inflammation, but the mechanisms are unclear. The present study was undertaken to test the hypothesis that decreased insulin sensitivity is differentially associated with mediators of inflammation by studying 2 chronic inflammatory diseases of different pathogenesis, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Methods
We measured fasting insulin, glucose, and lipid levels, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor α (TNFα), and coronary artery calcification in 103 patients with SLE and in 124 patients with RA. Insulin sensitivity was measured using the homeostasis model assessment (HOMA) index.
Results
The HOMA value was higher in RA patients (median 2.05 [interquartile range (IQR) 1.05–3.54]) than in SLE patients (1.40 [0.78–2.59]) (P = 0.007). CRP and ESR did not differ significantly in RA and SLE patients. Body mass index (BMI) was significantly correlated with the HOMA index in both RA (ρ = 0.20) and SLE (ρ = 0.54), independently of age, sex, race, and current use of corticosteroids. In RA patients, the HOMA index was also significantly positively correlated with IL-6 (ρ = 0.63), TNFα (ρ = 0.50), CRP (ρ = 0.29), ESR (ρ = 0.26), coronary calcification (ρ = 0.26), and Disease Activity Score in 28 joints (ρ = 0.21); associations adjusted for age, sex, race, BMI, and current use of corticosteroids remained significant (P < 0.05). In SLE patients, the HOMA index was also significantly correlated with ESR (ρ = 0.35) and CRP (ρ = 0.25), but not with other variables. The association between the ESR and the HOMA value in patients with SLE remained significant after adjustment for confounding covariates (P = 0.008). In multivariable models, the major contributing factors to the HOMA index were the BMI in SLE patients, and IL-6 and TNFα levels in RA patients.
Conclusion
The pathogenesis of insulin resistance and its contribution to atherogenesis varies in different inflammatory settings.
In recent studies, we showed a marked increase in the prevalence of the metabolic syndrome, a constellation of cardiovascular risk factors that includes central obesity, dyslipidemia, hypertension, and disturbed glucose metabolism, in patients with rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) (1,2). Insulin resistance is a key component of the World Health Organization–defined metabolic syndrome that is increased in patients with RA or SLE (3,4).
Insulin resistance is an important contributor to the increased cardiovascular risk attributed to the metabolic syndrome (5,6), and therefore, it is important to understand its pathogenesis. There are several mechanisms that could contribute to altered insulin sensitivity that may be important in patients with RA or SLE, and they provide insights into the pathogenesis of insulin resistance associated with inflammation. These include obesity (7), the medications used to treat inflammatory diseases (8,9), and inflammatory mediators (3).
Obesity is a highly prevalent and modifiable risk factor associated with insulin resistance and the metabolic syndrome (10). In addition, adipose tissue functions as an endocrine organ and produces several inflammatory mediators, thus contributing to a proinflammatory state and increased cardiovascular risk (10). Glucocorticoids modulate appetite, metabolism and energy partitioning, exacerbate obesity (11), and induce insulin resistance and redistribution of fat, features of the metabolic syndrome (12,13). Inflammation, acting through cytokines such as tumor necrosis factor α (TNFα), facilitates the development of insulin resistance (14).
The concentrations of TNFα and other mediators are elevated in patients with inflammatory diseases, but their role in the mechanisms underlying insulin resistance associated with inflammation is unknown. Thus, we examined the hypothesis that decreased insulin sensitivity is differentially associated with mediators of inflammation by studying 2 chronic inflammatory diseases of different pathogenesis, SLE and RA.
PATIENTS AND METHODS
Patients
We studied 103 patients with SLE and 124 patients with RA. These patients have participated in other studies of cardiovascular risk factors (1,2,15–20), and detailed methods have been previously described. Exclusion criteria for the present study were a history of myocardial infarction, angina, or stroke, and the presence of diabetes. The study was approved by the institutional review committee, and all subjects provided written informed consent.
Assessments
Patients were evaluated using a standardized clinical interview, physical examination, laboratory tests, and chart review. Height and weight were measured and the body mass index (BMI) (weight [kg]/height [m2]) was calculated. Blood pressure was recorded as the mean of 2 measurements obtained 5 minutes apart after subjects had rested for 10 minutes. Blood was collected for the measurement of glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol, triglycerides, Lp(a), homocysteine, and insulin after overnight fasting. Insulin, interleukin-6 (IL-6), and TNFα concentrations were measured using enzyme-linked immunosorbent assay (ELISA) (Millipore, Billerica, MA). C-reactive protein (CRP) and the Westergren erythrocyte sedimentation rate (ESR) were determined at the hospital clinical laboratory. Before 2003, our laboratory did not use a high-sensitivity CRP assay, and low concentrations were reported as <3 mg/dl. In 33 patients with RA and 40 patients with SLE with CRP concentrations <3 mg/dl, concentrations were measured by ELISA (Millipore) with a lower sensitivity limit of 0.125 mg/dl.
In patients with SLE, disease activity and damage were measured using the Systemic Lupus Erythematosus Disease Activity Index and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (21,22). In patients with RA, disease activity was measured using the Disease Activity Score in 28 joints (23), and disease damage was quantified using the Larsen scale (24). Radiographs of the hands and feet were available in posterior-anterior projection in 69 patients, and were graded by 1 researcher (TS) using the Larsen scale.
Insulin sensitivity was determined using the homeostasis model assessment (HOMA) index ([fasting glucose (mmoles/liter) × fasting insulin (μU/ml)]/22.5). Based on the Study of Inherited Risk of Coronary Atherosclerosis, we defined a HOMA index of >2.114 as representing the top quartile of a nondiabetic population and thus indicative of insulin resistance (25).
As a measurement of subclinical atherosclerosis, patients underwent computed tomography (Imatron C-150 scanner; GE/Imatron, South San Francisco, CA) of the chest for coronary artery atherosclerosis, as previously described (15,16). Briefly, imaging was performed with a 100-msec scanning time and a single-slice thickness of 3 mm. Forty slices were obtained during a single breath-holding period, starting at the aortic arch and proceeding to the level of the diaphragm. All scans were read by a single experienced investigator (PR), who was blinded with regard to the subjects’ clinical status. Coronary artery calcium scores were calculated as described by Agatston et al (26).
Statistical analysis
Statistical analyses were performed in 2 phases. In the first phase, clinical characteristics were compared in patients with RA and SLE, using chi-square tests for categorical variables or Wilcoxon’s rank-sum tests for continuous variables. Multiple linear regressions were used to assess the independent relationship between the diseases and the HOMA index with adjustments for age, race, sex, BMI, and current use of corticosteroids.
In the second phase, the effects of clinical characteristics and markers of inflammation on the HOMA index were assessed separately among patients with RA and those with SLE. Spearman’s correlations were used to examine the association between the HOMA index and continuous clinical characteristics. To assess the magnitude of the contribution of BMI and inflammation markers to the relationship with the HOMA index, the R2 value, representing the proportion of variation explained, was calculated for 3 models. The first model included age, sex, race, and current use of corticosteroids (base model); in the second model, BMI was added to the base model; and the final models added individual inflammatory biomarkers (TNFα, IL-6, or CRP) and BMI to the base model.
We also evaluated effect modification using cross-product terms (interaction terms) in the multivariable model to test whether the relationship between BMI or the markers of inflammation and the outcome of insulin resistance differed between SLE and RA. In order to minimize Type I error when assessing multiple interaction terms simultaneously, a global test was performed using a partial F test on the entire model (including all interactions) compared with the reduced model (without interaction terms). When the overall test was statistically significant, each interaction between the diseases and BMI, TNFα, IL-6, or CRP was reported. In addition, stratified analyses by disease (SLE or RA) were conducted. To ensure normality of the residuals in the multiple linear regression, the HOMA values were Box-Cox transformed, the TNFα and IL-6 values were logarithmically transformed, and the CRP value was included as a nonlinear parameter using restricted cubic splines (27). Regression diagnostics were performed to verify assumptions of normality of residuals. All analyses used a 5% 2-sided significance level and were performed using Stata software, version 9.2 (StataCorp, College Station, TX) and R version 2.4.0 (www.r-project.org).
RESULTS
Demographic characteristics, lipid profiles, other cardiovascular risk factors, fasting concentrations of insulin, and the HOMA index for patients with RA (n = 124) or SLE (n = 103) are shown in Table 1. As would be expected, patients with SLE were younger and there was a greater preponderance of women and African Americans. BMI did not differ, but the HOMA index was significantly higher in patients with RA (2.0 [inter-quartile range (IQR) 1.0–3.5]) than in those with SLE (1.4 [0.8–2.6]) (P = 0.007). This difference remained significant after adjustment for age, race, sex, BMI, and current corticosteroid use (P = 0.03). The results were essentially unchanged when hypertension (P = 0.026) or antihypertensive medications (P = 0.030) were added as covariates.
Table 1.
Demographic and clinical characteristics of the RA and SLE study patients*
| Characteristic | RA patients (n = 124) | SLE patients (n = 103) | P |
|---|---|---|---|
| Demographics | |||
| Age, years | 52 (44–61) | 40 (30–46) | <0.001 |
| Sex, % female | 73 | 91 | <0.001 |
| White, % | 89 | 68 | <0.001 |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mm Hg | 130 (116–142) | 117 (106–128) | <0.001 |
| Diastolic blood pressure, mm Hg | 74 (67–82) | 73 (65–81) | 0.28 |
| BMI, kg/m2 | 28 (24–32) | 27 (24–33) | 0.86 |
| Homocysteine, μmoles/liter | 10.2 (8.0–11.9) | 9.2 (7.3–11.1) | 0.02 |
| Cumulative smoking, pack-years | 0 (0–18) | 0 (0–4) | 0.11 |
| Cholesterol, mg/dl | 188 (160–213) | 165 (141–206) | 0.01 |
| Low-density lipoprotein, mg/dl | 114 (90–136) | 96 (81–130) | 0.008 |
| High-density lipoprotein, mg/dl | 44 (38–56) | 48 (36–55) | 0.84 |
| Lipoprotein(a), mg/dl | 6.9 (2.1–24.7) | 12.0 (5.0–37.5) | 0.001 |
| Triglycerides, mg/dl | 108 (76–141) | 99 (71–155) | 0.74 |
| Glucose, mg/dl | 86 (81–92) | 83 (75–90) | 0.003 |
| Insulin resistant, % | 49 | 41 | 0.21 |
| HOMA index | 2.0 (1.0–3.5) | 1.4 (0.8–2.6) | 0.007 |
| Measures of disease activity and damage | |||
| SLEDAI† | NA | 4 (1–6) | NA |
| SLICC/ACR‡ | NA | 0 (0–1) | NA |
| DAS28† | 2.9 (2.1–3.9) | NA | NA |
| M-HAQ§ | 0.4 (0.0–0.8) | 0.1 (0.0–0.6) | 0.03 |
| Current use of corticosteroids, no. (%) | 69 (56) | 61 (59) | 0.59 |
| Cumulative corticosteroid dose, gm | 2.8 (0.5–9.4) | 11.6 (2.7–26.5) | <0.001 |
| Other markers of inflammation | |||
| ESR, mm/hour | 13 (5–30) | 17 (9–37) | 0.06 |
| CRP, mg/liter | 3.6 (1.0–11.0) | 4.0 (0.6–7.0) | 0.10 |
| IL-6, pg/ml | 13.8 (4.0–44.9) | 5.5 (2.1–24.3) | <0.001 |
| TNFα, pg/ml | 4.9 (2.6–11.1) | 4.7 (3.0–7.6) | 0.24 |
Except where indicated otherwise, values are the median (interquartile range). P values were calculated using chi-square tests for categorical values and Wilcoxon’s rank sum tests for continuous variables. RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; BMI = body mass index; HOMA = homeostasis model assessment; SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; NA = not applicable; SLICC/ACR = Systemic Lupus International Collaborating Clinics/American College of Rheumatology; DAS28 = Disease Activity Score in 28 joints; M-HAQ = modified Health Assessment Questionnaire; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; IL-6 = interleukin-6; TNFα = tumor necrosis factor α.
Higher scores indicate greater disease activity.
Higher scores indicate greater disease-related damage.
Lower scores indicate better functional capacity.
Table 2 shows the association between the HOMA index and clinical characteristics. BMI was more significantly correlated with the HOMA index in patients with SLE (ρ = 0.54, P < 0.001) than in patients with RA (ρ = 0.20, P = 0.03). Cumulative exposure to hydroxychloroquine and corticosteroids was not associated with the HOMA index in either SLE or RA. In patients with RA, concentrations of HDL cholesterol, measures of disease activity and damage, and markers of inflammation (IL-6, TNFα, ESR, and CRP) were significantly associated with the HOMA index. These associations remained significant after adjustment for age, race, sex, BMI, and current corticosteroid use. In contrast, in patients with SLE, concentrations of IL-6, TNFα, and CRP were not associated with the HOMA index, and only HDL cholesterol and ESR were. Furthermore, the HOMA index was associated with coronary calcification in RA (ρ = 0.26, P = 0.003), as previously described (2), but not SLE (ρ = 0.11, P = 0.27). The relationship between the HOMA index and coronary artery calcification in patients with RA remained significant after adjustment for age, race, sex, BMI, and current corticosteroid use (P = 0.02), and after additional adjustment for CRP (P = 0.018).
Table 2.
Relationship between the HOMA index and clinical characteristics in RA and SLE patients*
| RA patients |
SLE patients |
|||||
|---|---|---|---|---|---|---|
| Correlation coefficient | P | Adjusted P† | Correlation coefficient | P | Adjusted P† | |
| Age, years | 0.06 | 0.53 | NA | 0.06 | 0.56 | NA |
| Systolic blood pressure, mm Hg | −0.01 | 0.89 | 0.26 | 0.17 | 0.08 | 0.56 |
| Diastolic blood pressure, mm Hg | 0.01 | 0.89 | 0.77 | −0.06 | 0.56 | 0.94 |
| BMI, kg/m2 | 0.20 | 0.03 | 0.02‡ | 0.54 | <0.001 | <0.001‡ |
| Homocysteine, μmoles/liter | 0.12 | 0.17 | 0.22 | 0.16 | 0.10 | 0.12 |
| Cumulative smoking, pack-years | 0.14 | 0.12 | 0.64 | 0.04 | 0.72 | 0.54 |
| Cholesterol, mg/dl | −0.03 | 0.74 | 0.99 | −0.02 | 0.87 | 0.30 |
| Low-density lipoprotein, mg/dl | 0.06 | 0.50 | 0.42 | 0.07 | 0.48 | 0.49 |
| High-density lipoprotein, mg/dl | −0.21 | 0.02 | 0.04 | −0.37 | <0.001 | <0.001 |
| Lipoprotein(a), mg/dl | 0.10 | 0.25 | 0.40 | 0.23 | 0.02 | 0.04 |
| Triglycerides, mg/dl | 0.06 | 0.54 | 0.44 | 0.21 | 0.03 | 0.28 |
| Disease duration, years | 0.15 | 0.10 | 0.02 | −0.14 | 0.15 | 0.25 |
| Disease activity | 0.21 | 0.02 | 0.03 | 0.20 | 0.04 | 0.33 |
| Disease damage | 0.26 | 0.03 | 0.02 | 0.01 | 0.94 | 0.76 |
| Cumulative corticosteroid dose | 0.15 | 0.09 | 0.15 | 0.01 | 0.94 | 0.80 |
| Cumulative dose of HCQ | 0.16 | 0.08 | 0.24 | −0.04 | 0.66 | 0.69 |
| ESR, mm/hour | 0.26 | 0.003 | 0.002 | 0.35 | <0.001 | 0.008 |
| CRP, mg/liter | 0.29 | 0.002 | 0.001 | 0.25 | 0.01 | 0.30 |
| IL-6§ | 0.63 | <0.001 | <0.001 | 0.16 | 0.10 | 0.18 |
| TNF§ | 0.50 | <0.001 | <0.001 | 0.11 | 0.28 | 0.24 |
| Agatston score | 0.26 | 0.003 | 0.02 | 0.11 | 0.27 | 0.89 |
Disease activity is expressed as the DAS28 in patients with RA and as the SLEDAI score in patients with SLE. Disease damage is expressed as the Larsen score in RA (available in 69 patients) and as the SLICC/ACR score in SLE. HCQ = hydroxychloroquine (see Table 1 for other definitions).
Adjusted for age, race, sex, BMI, and current corticosteroid use.
Adjusted for age, race, sex, and current corticosteroid use.
IL-6 and TNFα were log-transformed in the multivariable analysis.
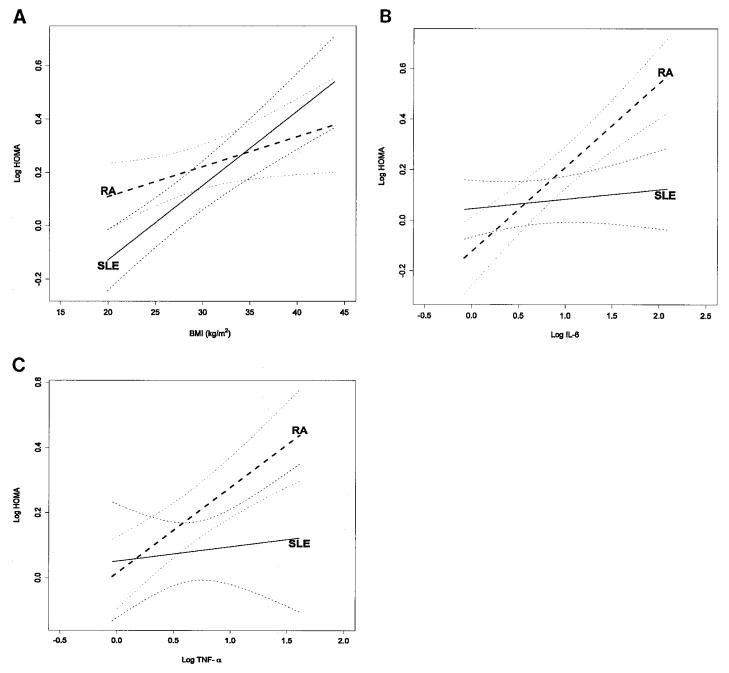
Table 3 shows the proportion of the variation of the HOMA index (expressed as R2) explained by the addition of different sets of variables to the model. In patients with SLE, BMI was the main contributor to the prediction of the HOMA index; the addition of TNFα and IL-6 to the model had no material effect. In contrast, in patients with RA, BMI contributed little, and TNFα and IL-6 were the important contributors. Figures 1A, B, and C show the interactions in the association between BMI, IL-6, and TNFα, respectively, and the HOMA index. After adjustment for age, sex, race, and current use of corticosteroids, there was evidence of an overall modification of the association between BMI, IL-6, TNFα, CRP, and insulin resistance that differed between patients with RA and SLE (P <0.001). The effect of modification of diseases on the relationship between BMI and each marker of inflammation was further assessed with a test for interaction for BMI (P = 0.04), TNFα (P = 0.16), IL-6 (P < 0.001), and CRP (P = 0.71).
Table 3.
Magnitude of the contribution of BMI and markers of inflammation in the HOMA index response models*
| R2 |
||
|---|---|---|
| RA patients | SLE patients | |
| Age, sex, race, and current use of corticosteroids | 0.03 | 0.02 |
| Age, sex, race, current use of corticosteroids, and BMI | 0.07 | 0.33 |
| Age, sex, race, current use of corticosteroids, BMI, and TNFα | 0.37 | 0.34 |
| Age, sex, race, current use of corticosteroids, BMI, and IL-6 | 0.45 | 0.34 |
| Age, sex, race, current use of corticosteroids, BMI, and CRP | 0.15 | 0.34 |
| Age, sex, race, current use of corticosteroids, BMI, and ESR | 0.15 | 0.37 |
See Table 1 for definitions.
Figure 1.
Relationship between body mass index (BMI) (A), interleukin-6 (IL-6) (B), and tumor necrosis factor α (TNFα) (C) and the homeostasis model assessment (HOMA) index in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). A is adjusted for age, sex, race, and current use of corticosteroids, TNFα, IL-6, and C-reactive protein (CRP). P < 0.001 for SLE slope, P = 0.02 for RA slope, and P = 0.04 for interaction. B is adjusted for age, sex, race, BMI, current use of corticosteroids, TNFα, and CRP. P = 0.17 for SLE slope, P < 0.001 for RA slope, and P < 0.01 for interaction. C is adjusted for age, sex, race, BMI, current use of corticosteroids, IL-6, and CRP. P = 0.21 for SLE slope, P < 0.001 for RA slope, and P = 0.16 for interaction.
DISCUSSION
The major novel findings of this study were that insulin resistance in RA was independently associated with markers of inflammation and disease characteristics but not obesity, and in contrast, BMI was the major contributing factor to insulin resistance in patients with SLE. Furthermore, insulin resistance was associated with coronary artery calcification in RA but not SLE. These findings are of interest because they suggest that 2 inflammatory rheumatic diseases have insulin resistance of different pathogenesis and that it is the insulin resistance associated with inflammation that is also associated with coronary atherosclerosis.
In the general population, insulin resistance may be fundamental to the increased cardiovascular risk attributed to the metabolic syndrome (5,6). Indeed, measures of insulin resistance contributed to the association between the metabolic syndrome and coronary atherosclerosis, independently of age, nonmetabolic syndrome cardiovascular risk factors, and CRP (25). Also, individuals in the highest quintile of insulin resistance had more than twice the increased risk for incident cardiovascular events compared with those in the lowest quartile (6). Thus, identification of insulin resistance and the mechanisms underlying it are of interest.
Obesity is an important factor in the pathogenesis of insulin resistance. In the general population, obesity is associated with an inflammatory state and there is a close relationship between obesity and markers of inflammation (28). In the present study, patients with SLE or RA were of similar BMI, yet there was a strong association between the HOMA index and BMI in patients with SLE but not in those with RA, suggesting a differential relationship between BMI and insulin sensitivity in the 2 diseases. This was confirmed by a significant result when statistical tests for interaction were performed (P = 0.04).
Global markers of inflammation, such as CRP and ESR, did not differ significantly in patients with SLE and RA; in fact, ESR tended to be higher in patients with SLE. However, in patients with RA, insulin resistance was associated with several markers of inflammation, including TNFα, IL-6, ESR, and CRP, and measures of disease activity and damage. In contrast, in patients with SLE, reduced insulin sensitivity was associated with an elevated ESR, but not with other markers of inflammation. Thus, these findings suggest not only that inflammatory mediators may contribute to reduced insulin sensitivity in RA, but also that the potential mechanisms underlying increased insulin resistance differ in different inflammatory conditions.
A study evaluating factors associated with glucose metabolism in patients with RA found that obesity correlated best with insulin resistance (29). We found that inflammation, measured by ESR or CRP, and obesity, measured by BMI, both explained a similar proportion of the variance in the HOMA index in RA. However, inflammation, measured by IL-6 and TNFα, explained a substantially larger proportion of the variance, independently of the contribution of age, race, sex, BMI, and current use of corticosteroids. This reinforces the concept that in patients with RA, specific mediators of inflammation play a fundamental role in the pathogenesis of insulin resistance.
The association between TNFα and the HOMA index in patients with RA is consistent with findings in the general population (30), but it is interesting that in SLE, TNFα concentrations were similarly elevated, but were not associated with the HOMA index. TNFα plays a role in the development of insulin resistance (31), and in an animal model of obesity, neutralization of TNFα increased the peripheral uptake of glucose in response to insulin (14). TNFα, although associated with obesity, also has independent actions on insulin sensitivity. In healthy volunteers who were within 10% of their ideal body weight, an infusion of TNF produced an early and sustained increase in plasma glucose concentrations (32). Also, several pathologic conditions unrelated to obesity, such as endotoxemia, cancer, and trauma, are associated with both increased TNFα production and insulin resistance (33). TNFα antagonists improved insulin sensitivity in some studies (34,35), but not all (36), and other inflammatory mediators also affect insulin sensitivity (37).
IL-6 plays a role in regulating glucose metabolism, and concentrations are higher in patients with the metabolic syndrome (38). Potential mechanisms to explain the association between IL-6 and insulin resistance include reductions in adiponectin (an adipocyte-sensitizing adipokine), insulin-dependent glucose transporter 4, and insulin receptor substrate 1 (39). However, the effects of IL-6 are complex, and under particular conditions, it may either increase or decrease insulin resistance (39,40); long-term exposure to IL-6 appears more likely to induce insulin resistance (41). Thus, IL-6 may be of major importance in some diseases characterized by chronic inflammation. We found that IL-6 concentrations were significantly higher in patients with RA than in those with SLE, and this difference may contribute to the differential role of inflammation in the pathogenesis of insulin resistance in the 2 diseases. Thus, we found that IL-6 and TNFα explain a large proportion of the variance in insulin resistance in RA, and consistent with our findings, infliximab reduces insulin resistance in RA (42). This reinforces the concept that in patients with RA, inflammation plays a fundamental role in insulin resistance.
Glucocorticoids induce glucose intolerance (43), and thus are prime candidates to explain the reduced insulin sensitivity in both RA and SLE. However, we (1,2) and others (4,44) found only borderline or no association between corticosteroids and the presence of the metabolic syndrome or insulin resistance in patients with RA or SLE, suggesting that perhaps better control of inflammation (45) may counterbalance the deleterious effect of corticosteroids on glucose metabolism. As reported by others (46), we found that patients with SLE had higher concentrations of Lp(a) than those with RA. A relatively weak relationship between Lp(a) and insulin resistance has been noted in other populations (47). Thus, we believe this difference is consistent with the notion that the mechanisms underlying insulin resistance in RA and SLE differ.
Consistent with findings in the general population (25,48), the HOMA index was associated with coronary artery calcification in patients with RA (2). However, in patients with SLE, this was not the case. This suggests not only that insulin resistance may have a different pathogenesis in RA and SLE, but also that the relationship between insulin resistance and atherosclerosis may differ. Because insulin resistance and its cardiovascular consequences may be differentially associated with inflammatory cytokines, therapeutic agents that target these cytokines may also decrease the risk of cardiovascular outcomes differentially in various inflammatory settings.
In summary, 2 chronic inflammatory diseases, RA and SLE, are both associated with insulin resistance, but the mechanisms and relationship with coronary atherosclerosis differ. The major contributing factors to insulin resistance were BMI in SLE, and IL-6 and TNFα in RA. The pathogenesis of insulin resistance and its contribution to atherogenesis varies in different inflammatory settings.
Acknowledgments
We thank Mrs. Carol Brannon, who assisted with recruitment of subjects and helped enter data.
Supported by the NIH (grants HL-04012, HL-65082, HL-67964, and GM5-M01-RR-00095).
Footnotes
Dr. Sokka has received consulting fees, speaking fees, and/or honoraria from Schering-Plough, Wyeth, UBC, Sanofi-Aventis, and Abbott (less than $10,000 each). Dr. Pincus has received research grants from Amgen and Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
Dr. Stein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Chung, Oeser, Stein.
Acquisition of data. Oeser, Solus, Sokka, Raggi, Pincus, Stein.
Analysis and interpretation of data. Chung, Oeser, Solus, Gebretsadik, Shintani, Raggi, Stein.
Manuscript preparation. Chung, Oeser, Solus, Gebretsadik, Avalos, Raggi, Pincus, Stein.
Statistical analysis. Chung, Gebretsadik, Shintani.
References
- 1.Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, et al. High frequency of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. 2007;66:208–14. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756–63. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Dessein PH, Joffe BI, Stanwix AE. Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J Rheumatol. 2003;30:1403–5. [PubMed] [Google Scholar]
- 4.El Magadmi M, Ahmad Y, Turkie W, Yates AP, Sheikh N, Bernstein RM, et al. Hyperinsulinemia, insulin resistance, and circulating oxidized low density lipoprotein in women with systemic lupus erythematosus. J Rheumatol. 2006;33:50–6. [PubMed] [Google Scholar]
- 5.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 6.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25:1177–84. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 7.Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89:2601–7. doi: 10.1210/jc.2004-0432. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed MH. Chloroquine-induced nitric oxide improves insulin sensitivity in rheumatoid arthritis. Med Hypotheses. 2006;66:208–9. doi: 10.1016/j.mehy.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Dessein P, Joffe B, Stanwix A. Effects of disease modifying agents and dietary intervention on insulin resistance and dyslipidemia in inflammatory arthritis: a pilot study. Arthritis Res. 2002;4:R12. doi: 10.1186/ar597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 11.Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice [published erratum appears in J Clin Invest 2006; 116:842] J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4:17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 13.Fardet L, Cabane J, Kettaneh A, Lebbe C, Flahault A. Corticosteroid-induced lipodystrophy is associated with features of the metabolic syndrome. Rheumatology (Oxford) 2007;46:1102–6. doi: 10.1093/rheumatology/kem062. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 16.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 17.Oeser A, Chung CP, Asanuma Y, Avalos I, Stein CM. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:3651–9. doi: 10.1002/art.21400. [DOI] [PubMed] [Google Scholar]
- 18.Chung CP, Oeser A, Avalos I, Raggi P, Stein CM. Cardiovascular risk scores and the presence of subclinical coronary artery atherosclerosis in women with systemic lupus erythematosus. Lupus. 2006;15:562–9. doi: 10.1177/0961203306071870. [DOI] [PubMed] [Google Scholar]
- 19.Asanuma Y, Chung CP, Oeser A, Shintani A, Stanley E, Raggi P, et al. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: relationship to cardiovascular risk factors. J Rheumatol. 2006;33:539–45. [PubMed] [Google Scholar]
- 20.Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, Raggi P, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8:R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH the Committee on Prognosis Studies in SLE. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 22.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol. 1995;22:1974–5. [PubMed] [Google Scholar]
- 25.Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803–9. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 26.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Vaimonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 27.Harrel FE. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 28.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 29.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54:2765–75. doi: 10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27 (Suppl 3):S53–5. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 31.Rask-Madsen C, Dominguez H, Ihlemann N, Hermann T, Kober L, Torp-Pedersen C. Tumor necrosis factor-α inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815–21. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 32.Van der Poll T, Romijn JA, Endert E, Borm JJ, Buller HR, Sauerwein HP. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:E457–65. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- 33.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNFα in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–62. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–6. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ. Improved insulin sensitivity by anti-TNFα antibody treatment in patients with rheumatic diseases. Ann Rheum Dis. 2007;66:558–9. doi: 10.1136/ard.2006.062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Rocco P, Manco M, Rosa G, Greco AV, Mingrone G. Lowered tumor necrosis factor receptors, but not increased insulin sensitivity, with infliximab. Obes Res. 2004;12:734–9. doi: 10.1038/oby.2004.86. [DOI] [PubMed] [Google Scholar]
- 37.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 38.Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E. Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care. 2006;29:1363–9. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 39.Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia. 2004;47:1135–42. doi: 10.1007/s00125-004-1447-y. [DOI] [PubMed] [Google Scholar]
- 40.Heliovaara MK, Teppo AM, Karonen SL, Tuominen JA, Ebeling P. Plasma IL-6 concentration is inversely related to insulin sensitivity, and acute-phase proteins associate with glucose and lipid metabolism in healthy subjects. Diabetes Obes Metab. 2005;7:729–36. doi: 10.1111/j.1463-1326.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 41.Mooney RA. Counterpoint: interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102:816–8. doi: 10.1152/japplphysiol.01208a.2006. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, et al. Anti-tumor necrosis factor-α blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–6. [PubMed] [Google Scholar]
- 43.Pagano G, Bruno A, Cavallo-Perin P, Cesco L, Imbimbo B. Glucose intolerance after short-term administration of corticosteroids in healthy subjects: prednisone, deflazacort, and beta-methasone. Arch Intern Med. 1989;149:1098–101. [PubMed] [Google Scholar]
- 44.Dessein PH, Tobias M, Veller MG. Metabolic syndrome and subclinical atherosclerosis in rheumatoid arthritis. J Rheumatol. 2006;33:2425–32. [PubMed] [Google Scholar]
- 45.Svenson KL, Lundqvist G, Wide L, Hallgren R. Impaired glucose handling in active rheumatoid arthritis: effects of corticosteroids and antirheumatic treatment. Metabolism. 1987;36:944–8. doi: 10.1016/0026-0495(87)90129-6. [DOI] [PubMed] [Google Scholar]
- 46.Asanuma Y, Kawai S, Aoshima H, Kaburaki J, Mizushima Y. Serum lipoprotein(a) and apolipoprotein(a) phenotypes in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:443–7. doi: 10.1002/1529-0131(199904)42:3<443::AID-ANR8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 47.Sowers M, Crawford SL, Cauley JA, Stein E. Association of lipoprotein(a), insulin resistance, and reproductive hormones in a multiethnic cohort of pre- and perimenopausal women (the SWAN Study) Am J Cardiol. 2003;92:533–7. doi: 10.1016/s0002-9149(03)00720-3. [DOI] [PubMed] [Google Scholar]
- 48.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–8. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]