Abstract
In this review we discuss the clinical manifestations, pathogenesis, and treatment of hepatitis C virus (HCV)-related cryoglobulinemia. HCV is a major cause of liver-related morbidity and is increasingly recognized as an instigator of B cell lymphoproliferative disorders such as mixed cryoglobulinemia and non-Hodgkin lymphoma. Cryoglobulinemia is characterized by the clonal expansion of rheumatoid factor-expressing B cells in the liver, lymph nodes and peripheral blood, resulting in the presence of cryoglobulins in the circulation. Cryoglobulins are cold-insoluble immune complexes containing rheumatoid factor, polyclonal IgG, and HCV RNA that precipitate and deposit on vascular endothelium, effecting a vasculitis in organs such as the skin, kidneys, and peripheral nerves. A subset of patients develops a low-grade lymphoma comprised of B cells that are immunophenotypically similar to the expanded B cells seen in cryoglobulinemia. HCV-related B cell lymphoproliferative disorders likely comprise a spectrum of disease, ranging from asymptomatic clonal B cell expansions to pathogenic cryoglobulinemia and lymphoma. It is unclear how B cells become dysregulated during the course of chronic HCV infection, and continued patient-centered research is necessary to elucidate the pathogenesis of HCV-related B cell dysregulation.
Introduction
Hepatitis C virus (HCV) chronically infects over 170 million people worldwide and is the leading indication for liver transplantation in the U.S. and Europe. HCV is a positive strand RNA virus with a 9.6 kb genome that replicates predominantly in the liver. HCV frequently causes extrahepatic manifestations, the most common and severe of which is mixed cryoglobulinemia (MC), a systemic vasculitis affecting small- and medium- sized arteries and veins. MC is characterized by the deposition of immune complexes containing rheumatoid factor (RF), IgG, HCV RNA, and complement on endothelial surfaces, eliciting vascular inflammation through poorly understood mechanisms. It is clear, however, that HCV MC is a B cell lymphoproliferative disorder (LPD). It is unknown how B cells activate, clonally expand and differentiate to produce pathologic quantities of self-reactive RF during chronic HCV infection.
Classification and Serologic Detection of Cryoglobulinemia
In 1966, Meltzer described MC as the clinical triad of palpable purpura, arthralgia, and asthenia accompanied by organ involvement (e.g. nephropathy and neuropathy) and elevated serum RF, defined as Ig capable of binding IgG(1). It is now known that this triad is rare; many MC patients are asymptomatic, and the most common clinical manifestation is palpable purpura, which is often transient. Cryoglobulins are classified as type I (monoclonal Ig only), type II (mixture of monoclonal Ig which is usually IgM RF, and polyclonal IgG) and type III (mixture of polyclonal Ig that is usually IgM, and polyclonal IgG)(2). “Essential” MC refers to the subset of MC patients for whom no cause has been identified; since the identification of HCV in 1989, it has been recognized as the cause of >90% of MC, and <5% of cases are now considered “essential”(3-5). HCV is primarily associated with type II MC (which typically has an IgMκ RF with anti-idiotypic activity(6)), and to a lesser extent, with type III MC. Type I MC is rarely seen in HCV. Serum RF, which is elevated in 16-70% of HCV+ patients(7, 8), is usually increased in the setting of HCV MC, and levels of complement, particularly C4, may be profoundly decreased. Non-HCV causes of MC include infectious agents (e.g., HIV, HBV) and autoimmune disorders (e.g., systemic lupus erythematosus (SLE), Sjögren syndrome and systemic sclerosis)(9). A shared feature of these disorders is chronic inflammation in the setting of high antigenic load, suggesting that antigen-driven B cell dysregulation is a prerequisite for the development of MC.
Serologic testing for cryoglobulins is straightforward, but serum preparation must be performed at 37° C to prevent premature immune complex precipitation. Serum is stored at 4° C for seven days, inspected daily for a precipitate, and spun in a Wintrobe tube for calculation of cryocrit, the percentage of cryoglobulin in the serum. A cryocrit ≥ 2% is positive. The cryocrit is then typed using immunofixation(9). Some chronic HCV patients with MC may be HCV Ab+ yet have undetectable plasma HCV RNA(10). In such cases, examination of the cryocrit for HCV RNA is warranted.
Epidemiology of HCV-Related MC
Estimates of MC prevalence in HCV infection vary widely, ranging from 10-70%; many of these differences may be due to population selection and lead time biases. Additionally, the clinical assessment of MC is not standardized, and the laboratory test for MC is prone to false negative results. MC is associated with increased duration of HCV infection; HCV+MC+ patients have an apparent duration of HCV infection that is almost twice as long as in HCV+MC- patients(11), and an Italian HCV+ population was found to have a yearly incidence rate of 3%(12). It is controversial whether MC is an independent risk factor for the development of cirrhosis. It was reported early on that MC was a risk factor for cirrhosis (reviewed in(6)), and a meta-analysis of studies predominantly carried out in areas where > 40% of HCV+ individuals have MC has shown MC to confer an OR 4.87 for cirrhosis, independent of age, gender, and estimated duration of HCV infection(13). All HCV genotypes have been found in HCV MC, and there is no clear association with a particular genotype. Female gender may predispose towards MC, although this association is not strong(8, 14).
MC is more prevalent in Southern Europe than in Northern Europe and North America; up to 60% of HCV-infected individuals in Southern Europe have MC(13), whereas the prevalence among HCV+ patients in the US (where no ethnic bias for MC has been reported) is considerably lower (10-50%)(15), and the prevalence of symptomatic MC is much lower. It is unclear whether this difference is due to unidentified genetic factors. Although genomewide association scans have not been performed, several groups have focused on HLA alleles. These studies are limited by their small sample sizes, and comparisons among studies are complicated by varying HLA typing methods and different geographical populations with dissimilar HLA frequencies. Nevertheless, several studies conducted primarily in Italy, France and China have associated DRB1*11 alleles and DR3, DR5 and DR6 serological clusters with MC(16-21). However, a group in Japan failed to detect any significant associations between HLA and HCV MC(22).
Clinical Manifestations
Cryoglobulinemic Vasculitis
HCV MC vasculitis primarily affects the small and medium-sized vessels of the skin, kidneys, and peripheral nerves. Histology typically reveals a leukocytoclastic vasculitis, with deposition of IgM RF, IgG, C3, and neutrophils in the vessel wall. A necrotizing vasculitis, with fibrinoid necrosis of the intima and the inflammation of the entire vessel wall and perivascular space, may also occur. Palpable purpura (Fig. 1A), primarily of the lower legs, occurs in more than 90% of patients with symptomatic HCV MC, is frequently intermittent, and is often the initial manifestation of HCV MC(5). These purpuric lesions may occasionally progress to chronic ulcers and frank gangrene. HCV-MC renal involvement is usually Type I membranoproliferative glomerulonephritis (MPGN)(23), and it frequently heralds a poor clinical course. Manifestations range from isolated proteinuria to nephritic syndrome with variable progression towards chronic renal insufficiency. The MPGN is characterized by endocapillary mesangial cell proliferation, monocytic infiltration, double contour membranes, glomerular IgM, IgG and C3 deposition, eosinophilic PAS-positive intraluminal deposits, and vasculitis of the small and medium sized renal arteries (24, 25). The incidence of neurological involvement is variable. Sensorimotor neuropathy arises from cryoglobulin deposition in the vasa vasorum. Painful paresthesias and concomitant weakness, particularly in the lower limbs may occur(26), as may isolated mononeuritis, manifested by foot or wrist drop.
Figure 1.
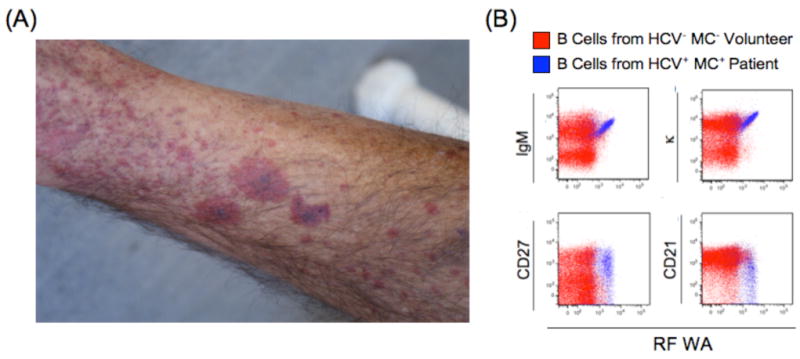
Palpable purpura in a patient with HCV vasculitis (A). This patient has a marked clonal expansion of RF WA-bearing IgM+κ+CD27+CD21lo peripheral B cells (B).
HCV-Related Lymphoproliferation
Since the identification of HCV, many reports have linked HCV infection to the development of B cell non Hodgkin lymphoma (NHL)(27). However, given the low incidence of NHL during HCV infection it has been difficult to ascertain the additive risk HCV confers for NHL. A large retrospective trial conducted among U.S. veterans has recently confirmed that HCV is associated with NHL as well as with other B cell LPD such as Waldenström macroglobulinemia and monoclonal gammopathy of unknown significance(15). It is likely that HCV MC represents an antigen-driven, relatively benign clonal B cell lymphoproliferation that, with continued antigenic stimulation, occasionally progresses towards overt NHL. Pre-malignant B cell clones can be detected in the bone marrow (BM) or in the liver several years before the development of frank lymphoma(28). Typically, the NHL that arises in HCV MC patients is low-grade and is characterized by clonal RF+ B cells that may be present in the liver, spleen, peripheral lymph nodes, peripheral blood and/or BM. It has been proposed that a subset of these low-grade NHL evolve to a high-malignancy phenotype. The most frequent NHL histiotypes are immunocytoma, splenic marginal zone lymphoma, MALT lymphoma and aggressive diffuse large B cell lymphoma (DLBCL)(29-31) suggestive of the expansion of a germinal center (GC) or post-GC B cell during or after antigenic stimulation. It has been reported that higher-grade DLBCL is predominantly associated with MC-, rather than MC+, HCV infection(32). It is possible that a fundamentally different etiologic mechanism underlies the development of aggressive NHL in the absence of MC. Consistent with the hypothesis that continued antigenic presence is required for ongoing clonal B cell proliferation, eradication of HCV can lead to disappearance of the associated low-grade NHL(33), similar to what has been observed with H. pylori-induced MALT lymphomas. An aggressive subset of H. pylori MALT contains t(11;18) translocations and does not regress after H. pylori eradication. In contrast, there is no convincing evidence that chromosomal translocations are frequent in HCV-related NHL. Although several groups have detected t(14:18) in PBMCs from HCV+MC+ patients(34-36), these translocations have been identified in healthy controls(37) as well as in HCV-NHL patients(38).
Pathogenesis of HCV-Induced B Cell Lymphoproliferation
HCV MC is characterized by aberrant RF+ B cell lymphoproliferation (Fig 1B). Multiple groups have demonstrated that clonal B cell expansions are present in the liver and peripheral blood of HCV+MC+ patients(39-42). We have shown that these B cells express memory markers and are markedly biased towards the RF WA-encoding VH1-69, JH4 and Vκ3-20 gene segments(41), which are also preferentially expressed in HCV-related NHL(43, 44). It is unclear why such an expansion occurs more readily in chronic HCV infection, compared to other chronic viral diseases such as HBV or HIV. Increased serum B cell-activating factor (BAFF), a TNF-α family member required for B cell survival, has been reported in SLE, rheumatoid arthritis, and Sjögren syndrome. BAFF inhibits B cell apoptosis, and high levels may allow the survival of autoreactive B cells. Elevated BAFF has been described in HCV MC(45-48), although the underlying mechanism remains unclear. Interestingly, BAFF promoter polymorphisms may predispose to MC(49), but further studies are needed to confirm this association.
It has been proposed that HCV infects B cells, leading to direct malignant transformation. However, this model fails to explain the RF restriction seen in HCV MC, unless it is presumed that HCV preferentially infects RF+ B cells. Nevertheless, a B cell line that productively releases infectious HCV has been reported(50), and several groups have claimed to detect minus strand RNA, the replicative intermediate, associated with lymphocytes from HCV+ patients(51-53); However, it has been demonstrated that artifactual detection may occur, possibly through non-specific priming events(54). One group has failed to detect HCV infection in malignant B cells(55), and we have shown that B cells lack necessary HCV entry receptors and cannot support HCV replication(56), but can associate with low levels of HCV RNA. Furthermore, when HCV MC patients are treated with rituximab, serum HCV RNA levels often increase, arguing against a potential B cell reservoir of HCV infection. Taken together, cumulative data suggest that HCV viral particles may be bound directly or indirectly to B cells, but HCV rarely infects B cells.
It has alternatively been postulated that specific HCV proteins are necessary for clonal B cell expansion. The suggested IgG Fc binding activity of HCV core(57) could possibly lead to enhanced HCV-immune complex generation. Also, NS5A protein may sequester p53 and predispose cells towards proliferation(58); however, as NS5A expression requires active replication, this scenario necessitates B cell infection. High concentrations of HCV E2 protein in vitro polyclonally stimulate B cell expansion via interactions with CD81, a known HCV E2 entry factor(59). It has also been claimed that HCV E2-CD81 interactions trigger activation-induced cytidine deaminase expression resulting in stochastic immunoglobulin hypermutation(60). Neither of these scenarios explains the clonal RF+ B cell expansion seen in HCV MC patients. However, the BCR cloned from an HCV-associated NHL was shown to bind HCV E2, and it was hypothesized that simultaneous engagement of the BCR and CD81 could result in a reduced B cell stimulation threshold(61). Intriguingly, a potent HCV-neutralizing mAb cloned from an asymptomatic HCV+ patient is coded for by VH1-69/JH4 gene segments that also encode RF WA(62, 63). It is unlikely that this mAb has RF activity, as it is extensively hypermutated and utilizes a Vκ4-1 light chain. Another group has reported that a minority of HCV MC patients' IgM RF cross-reacts with IgG Fc and HCV NS3, and they speculate that RF activity is generated during the normal adaptive immune response to HCV(64).
We propose that IgG-bound HCV specifically drives the clonal expansion of RF+ B cells (Fig. 2). Upon chronic HCV infection, immune-complexed HCV stimulates the expansion of VH1-69+ B cells, which upon continued antigenic exposure (usually over several years) become clonally predominant. We postulate that these cells have expanded independently of T cell help in response to dual ligation of BCR and toll like receptor 7 by IgG and HCV RNA, respectively. We have found that the clonal RF+ memory B cells have low levels of somatic hypermutation, likely acquired in response to antigenic exposure, and that they have downregulated CD21, the Complement 3d receptor, which together with CD19 and CD81 forms the B cell co-receptor complex(41). We propose that these cells, although activated and clonally expanded, may have dampened responses to antigenic stimulation. This self-attenuation process, although incomplete in HCV MC, might mitigate otherwise unchecked activation and proliferation pathways and serve to maintain overall clonal B cell numbers at a relatively stable, albeit elevated, steady state level. We are actively investigating requirements for B cell survival, stimulation, and differentiation in HCV MC patients.
Figure 2.
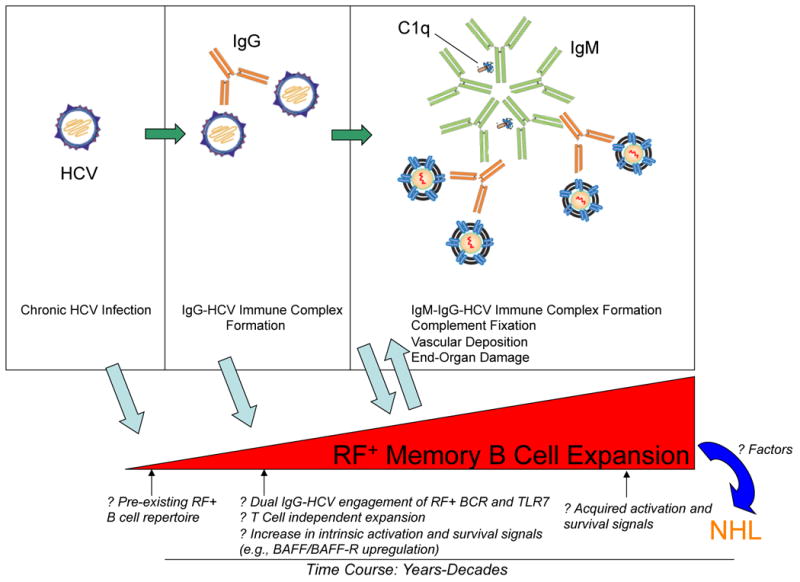
Proposed Mechanism of Clonal B Cell Expansion in HCV-Induced Cryoglobulinemia.
Treatment
Consistent with HCV MC being an antigen-stimulated process, the most effective treatment for HCV MC is eradication of the underlying HCV infection. MC symptoms and evidence of B cell LPD almost always disappear in sustained virologic responders (SVR), although persistence has been rarely described(65, 66). This persistence may be reflective of continued HCV replication below the limit of detection, or long-term persistence of viral antigen in the absence of replicating virus. Purpuric rash resolves more frequently than GN or neuropathy(67-70). HCV RNA relapse is associated with recurrence of MC and clinical symptoms. The current standard of care for HCV infection is pegylated interferon-α−2a/2b (pIFN) in combination with ribavirin (RBV), which results in a SVR in of 45-50% in genotypes 1 and 4, and 70-80% in genotypes 2 and 3 HCV-monoinfected patients. Small pilot studies show that pIFN/RBV is effective in treating HCV in MC+ patients. Importantly, eradication of HCV with IFN/RBV leads to resolution of HCV MC-associated splenic villous lymphomas and immunocytomas(33, 71, 72).
Treatment with antivirals must be individualized, as RBV is contraindicated in individuals with creatinine clearance < 50 ml/min, pIFN/RBV is poorly tolerated in ESRD, even with weekly plasma monitoring(73), and pIFN is contraindicated in renal allograft recipients. For patients unable to tolerate antivirals, or for those with severe renal disease, or for those who have failed to reach SVR after antiviral therapy, symptomatic treatment of vasculitis with plasmapheresis, rituximab, or prednisone should be considered(74).
Rituximab is a chimeric monoclonal antibody that binds to the pan B cell marker, CD20, and effects B cell destruction via complement-dependent and antibody-mediated cellular cytotoxicity(75). Following a standard course of 375 mg/m2/week × 4 weeks, B cells are typically undetectable in the periphery, and take 6-18 months to recover. Several small studies have shown that 80-93% of patients with HCV MC vasculitis respond to rituximab(76), and the reduction of peripheral VH1-69+ memory B cells persists 12 months after treatment(77). Symptoms usually reappear with reconstitution of peripheral B cells. Although rituximab is generally safe and well tolerated(74), its use has been associated with modestly elevated (up to two-fold) levels of HCV viremia(78), presumably due to reduction of partially protective humoral control(79). The safety and efficacy of multiple courses of rituximab in the setting of HCV MC are unknown. Studies are needed to determine the optimum duration, dosage, and readministration of rituximab in HCV MC. Prednisone 1-1.5 mg/kg/d has successfully been used to treat vasculitic symptoms. However, systemic corticoseroids are not curative, have significant side effects, and may lead to increases in HCV viral load. There are extremely limited data on the palliative use of plasmapheresis to treat HCV MC vasculitic symptoms. However, case reports suggest that it is safe, and may result in improvement in glomerulonephritis(80), neuropathy(81) and purpura(82, 83). Importantly, randomized controlled trials are needed to determine the appropriate use of these alternative therapies for HCV MC.
Conclusion
HCV cryoglobulins contain RF, polyclonal IgG, and HCV RNA that precipitate and deposit on vascular endothelium, causing an end organ vasculitis. Patients often have striking clonal expansions of RF-bearing IgM+κ+CD21low memory B cells with restricted usage of RF-encoding Ig gene segments. Most of these activated B cells have low to moderate levels of somatic hypermutations, suggestive of a response to antigenic stimulation. A smaller subset of patients with MC develop a low-grade NHL comprised of B cells that are immunophenotypically similar to the expanded B cells seen in MC. The antigenic dependence of these B cells is supported by evidence that HCV-related MC and NHL disappear after successful treatment of HCV infection. However, the mechanisms leading to B cell activation and expansion are unknown. It has been hypothesized that immune complexes of HCV bound to IgG stimulate RF-expressing B cells through the B cell receptor and act in concert with undefined accessory molecules. Continued patient-centered studies are necessary to elucidate the pathogenesis of HCV MC and to devise improved therapeutic strategies for affected patients.
Acknowledgments
This study was supported in part by the National Institutes of Health/National Institute of Allergy and Infectious Disease grants K08AI075031 (E.D.C.) and RO1AI60561 (L.B.D), and the Irma T. Hirschl/Monique Weill-Caulier Trust (L.B.D).
Footnotes
Disclosure The authors declare no competing interests.
References
- 1.Meltzer M, Franklin EC, Elias K, et al. Cryoglobulinemia--a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40:837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- 2.Brouet JC, Clauvel JP, Danon F, et al. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 3.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 4.Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Dammacco F, Sansonno D, Piccoli C, et al. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628–638. doi: 10.1046/j.1365-2362.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Agnello V. The etiology and pathophysiology of mixed cryoglobulinemia secondary to hepatitis C virus infection. Springer Semin Immunopathol. 1997;19:111–129. doi: 10.1007/BF00945029. [DOI] [PubMed] [Google Scholar]
- 7.Pawlotsky JM, Ben Yahia M, Andre C, et al. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994;19:841–848. [PubMed] [Google Scholar]
- 8.Cicardi M, Cesana B, Del Ninno E, et al. Prevalence and risk factors for the presence of serum cryoglobulins in patients with chronic hepatitis C. J Viral Hepat. 2000;7:138–143. doi: 10.1046/j.1365-2893.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Gorevic PD. Cryopathies: Cryoglobulins and Cryofibrinogenimia. In: Frank MM, Austen KF, Claman HN, editors. Samter's Immunologic Diseases. 5. Vol. 2. Little, Brown and Company; Boston: 1995. p. 951. [Google Scholar]
- 10.Bichard P, Ounanian A, Girard M, et al. High prevalence of hepatitis C virus RNA in the supernatant and the cryoprecipitate of patients with essential and secondary type II mixed cryoglobulinemia. J Hepatol. 1994;21:58–63. doi: 10.1016/s0168-8278(94)80137-1. [DOI] [PubMed] [Google Scholar]
- 11.Lunel F, Musset L, Cacoub P, et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994;106:1291–1300. doi: 10.1016/0016-5085(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 12.Adinolfi LE, Utili R, Attanasio V, et al. Epidemiology, clinical spectrum and prognostic value of mixed cryoglobulinaemia in hepatitis C virus patients: a prospective study. Ital J Gastroenterol. 1996;28:1–9. [PubMed] [Google Scholar]
- 13.Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36:978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- 14.Cacoub P, Poynard T, Ghillani P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 16.Congia M, Clemente MG, Dessi C, et al. HLA class II genes in chronic hepatitis C virus-infection and associated immunological disorders. Hepatology. 1996;24:1338–1341. doi: 10.1002/hep.510240603. [DOI] [PubMed] [Google Scholar]
- 17.Amoroso A, Berrino M, Canale L, et al. Are HLA class II and immunoglobulin constant region genes involved in the pathogenesis of mixed cryoglobulinemia type II after hepatitis C virus infection? J Hepatol. 1998;29:36–44. doi: 10.1016/s0168-8278(98)80176-1. [DOI] [PubMed] [Google Scholar]
- 18.De Re V, Caggiari L, De Vita S, et al. Genetic insights into the disease mechanisms of type II mixed cryoglobulinemia induced by hepatitis C virus. Dig Liver Dis. 2007;39(Suppl 1):S65–71. doi: 10.1016/s1590-8658(07)80014-4. [DOI] [PubMed] [Google Scholar]
- 19.Lenzi M, Frisoni M, Mantovani V, et al. Haplotype HLA-B8-DR3 confers susceptibility to hepatitis C virus-related mixed cryoglobulinemia. Blood. 1998;91:2062–2066. [PubMed] [Google Scholar]
- 20.Sebastiani GD, Bellisai F, Caudai C, et al. Association of extrahepatic manifestations with HLA class II alleles and with virus genotype in HCV infected patients. J Biol Regul Homeost Agents. 2005;19:17–22. [PubMed] [Google Scholar]
- 21.Hwang SJ, Chu CW, Huang DF, et al. Genetic predispositions for the presence of cryoglobulinemia and serum autoantibodies in Chinese patients with chronic hepatitis C. Tissue Antigens. 2002;59:31–37. doi: 10.1034/j.1399-0039.2002.590106.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagasaka A, Takahashi T, Sasaki T, et al. Cryoglobulinemia in Japanese patients with chronic hepatitis C virus infection: host genetic and virological study. J Med Virol. 2001;65:52–57. [PubMed] [Google Scholar]
- 23.Saadoun D, Landau DA, Calabrese LH, Cacoub PP. Hepatitis C-associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology (Oxford) 2007;46:1234–1242. doi: 10.1093/rheumatology/kem132. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 25.D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 26.Authier FJ, Pawlotsky JM, Viard JP, et al. High incidence of hepatitis C virus infection in patients with cryoglobulinemic neuropathy. Ann Neurol. 1993;34:749–750. doi: 10.1002/ana.410340524. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo K, Kusano A, Sugumar A, et al. Effect of hepatitis C virus infection on the risk of non-Hodgkin's lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Vita S, De Re V, Gasparotto D, et al. Oligoclonal non-neoplastic B cell expansion is the key feature of type II mixed cryoglobulinemia: clinical and molecular findings do not support a bone marrow pathologic diagnosis of indolent B cell lymphoma. Arthritis Rheum. 2000;43:94–102. doi: 10.1002/1529-0131(200001)43:1<94::AID-ANR12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Ferri C, Caracciolo F, Zignego AL, et al. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994;88:392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman E, Zuckerman T, Levine AM, et al. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127:423–428. doi: 10.7326/0003-4819-127-6-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Talamini R, Montella M, Crovatto M, et al. Non-Hodgkin's lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380–385. doi: 10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 32.De Vita S, Sacco C, Sansonno D, et al. Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood. 1997;90:776–782. [PubMed] [Google Scholar]
- 33.Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 34.Zuckerman E, Zuckerman T, Sahar D, et al. bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol. 2001;112:364–369. doi: 10.1046/j.1365-2141.2001.02573.x. [DOI] [PubMed] [Google Scholar]
- 35.Zignego AL, Ferri C, Giannelli F, et al. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571–580. doi: 10.7326/0003-4819-137-7-200210010-00008. [DOI] [PubMed] [Google Scholar]
- 36.Libra M, Gloghini A, Malaponte G, et al. Association of t(14;18) translocation with HCV infection in gastrointestinal MALT lymphomas. J Hepatol. 2008;49:170–174. doi: 10.1016/j.jhep.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Limpens J, Stad R, Vos C, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85:2528–2536. [PubMed] [Google Scholar]
- 38.Libra M, De Re V, De Vita S, et al. Low frequency of bcl-2 rearrangement in HCV-associated non-Hodgkin's lymphoma tissue. Leukemia. 2003;17:1433–1436. doi: 10.1038/sj.leu.2402968. [DOI] [PubMed] [Google Scholar]
- 39.Franzin F, Efremov DG, Pozzato G, et al. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–552. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 40.Sansonno D, De Vita S, Iacobelli AR, et al. Clonal analysis of intrahepatic B cells from HCV-infected patients with and without mixed cryoglobulinemia. J Immunol. 1998;160:3594–3601. [PubMed] [Google Scholar]
- 41.Charles ED, Green RM, Marukian S, et al. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–1356. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbonari M, Caprini E, Tedesco T, et al. Hepatitis C virus drives the unconstrained monoclonal expansion of VH1-69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol. 2005;174:6532–6539. doi: 10.4049/jimmunol.174.10.6532. [DOI] [PubMed] [Google Scholar]
- 43.Ivanovski M, Silvestri F, Pozzato G, et al. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–2442. [PubMed] [Google Scholar]
- 44.Chan CH, Hadlock KG, Foung SK, Levy S. V(H)1-69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood. 2001;97:1023–1026. doi: 10.1182/blood.v97.4.1023. [DOI] [PubMed] [Google Scholar]
- 45.Toubi E, Gordon S, Kessel A, et al. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134–139. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Fabris M, Quartuccio L, Sacco S, et al. B-Lymphocyte stimulator (BLyS) up-regulation in mixed cryoglobulinaemia syndrome and hepatitis-C virus infection. Rheumatology (Oxford) 2007;46:37–43. doi: 10.1093/rheumatology/kel174. [DOI] [PubMed] [Google Scholar]
- 47.Sene D, Limal N, Ghillani-Dalbin P, et al. Hepatitis C virus-associated B-cell proliferation--the role of serum B lymphocyte stimulator (BLyS/BAFF) Rheumatology (Oxford) 2007;46:65–69. doi: 10.1093/rheumatology/kel177. [DOI] [PubMed] [Google Scholar]
- 48.Landau DA, Rosenzwajg M, Saadoun D, et al. The B lymphocyte stimulator receptor-ligand system in hepatitis C virus-induced B cell clonal disorders. Ann Rheum Dis. 2009;68:337–344. doi: 10.1136/ard.2007.085910. [DOI] [PubMed] [Google Scholar]
- 49.Giannini C, Gragnani L, Piluso A, et al. Can BAFF promoter polymorphism be a predisposing condition for HCV-related mixed cryoglobulinemia? Blood. 2008;112:4353–4354. doi: 10.1182/blood-2008-07-170613. [DOI] [PubMed] [Google Scholar]
- 50.Sung VM, Shimodaira S, Doughty AL, et al. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J Virol. 2003;77:2134–2146. doi: 10.1128/JVI.77.3.2134-2146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zignego AL, Ferri C, Giannini C, et al. Hepatitis C virus infection in mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma: evidence for a pathogenetic role. Arch Virol. 1997;142:545–555. doi: 10.1007/s007050050100. [DOI] [PubMed] [Google Scholar]
- 52.Takyar ST, Li D, Wang Y, et al. Specific detection of minus-strand hepatitis C virus RNA by reverse-transcription polymerase chain reaction on PolyA(+)-purified RNA. Hepatology. 2000;32:382–387. doi: 10.1053/jhep.2000.9094. [DOI] [PubMed] [Google Scholar]
- 53.Sansonno D, Lauletta G, Montrone M, et al. Virological analysis and phenotypic characterization of peripheral blood lymphocytes of hepatitis C virus-infected patients with and without mixed cryoglobulinaemia. Clin Exp Immunol. 2006;143:288–296. doi: 10.1111/j.1365-2249.2005.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerat H, Berby F, Trabaud MA, et al. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J Clin Invest. 1996;97:845–851. doi: 10.1172/JCI118485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Vita S, De Re V, Sansonno D, et al. Lack of HCV infection in malignant cells refutes the hypothesis of a direct transforming action of the virus in the pathogenesis of HCV-associated B-cell NHLs. Tumori. 2002;88:400–406. doi: 10.1177/030089160208800510. [DOI] [PubMed] [Google Scholar]
- 56.Marukian S, Jones CT, Andrus L, et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology. 2008;48:1843–1850. doi: 10.1002/hep.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maillard P, Lavergne JP, Siberil S, et al. Fcgamma receptor-like activity of hepatitis C virus core protein. J Biol Chem. 2004;279:2430–2437. doi: 10.1074/jbc.M311470200. [DOI] [PubMed] [Google Scholar]
- 58.Majumder M, Ghosh AK, Steele R, et al. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J Virol. 2001;75:1401–1407. doi: 10.1128/JVI.75.3.1401-1407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosa D, Saletti G, De Gregorio E, et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci U S A. 2005;102:18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machida K, Cheng KT, Pavio N, et al. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinn ER, Chan CH, Hadlock KG, et al. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001;98:3745–3749. doi: 10.1182/blood.v98.13.3745. [DOI] [PubMed] [Google Scholar]
- 62.Keck ZY, Xia J, Cai Z, et al. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol. 2007;81:1043–1047. doi: 10.1128/JVI.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machida K, Kondo Y, Huang J, et al. HCV-induced Immunoglobulin Hypermutation Reduces the Affinity and Neutralizing Activities of Antibodies against HCV Envelope Protein. J Virol. 2008 doi: 10.1128/JVI.02582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Re V, Sansonno D, Simula MP, et al. HCV-NS3 and IgG-Fc crossreactive IgM in patients with type II mixed cryoglobulinemia and B-cell clonal proliferations. Leukemia. 2006 doi: 10.1038/sj.leu.2404201. [DOI] [PubMed] [Google Scholar]
- 65.Levine JW, Gota C, Fessler BJ, et al. Persistent cryoglobulinemic vasculitis following successful treatment of hepatitis C virus. J Rheumatol. 2005;32:1164–1167. [PubMed] [Google Scholar]
- 66.Landau DA, Saadoun D, Halfon P, et al. Relapse of hepatitis C virus-associated mixed cryoglobulinemia vasculitis in patients with sustained viral response. Arthritis Rheum. 2008;58:604–611. doi: 10.1002/art.23305. [DOI] [PubMed] [Google Scholar]
- 67.Ferri C, Marzo E, Longombardo G, et al. Interferon alfa-2b in mixed cryoglobulinaemia: a controlled crossover trial. Gut. 1993;34:S144–145. doi: 10.1136/gut.34.2_suppl.s144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Misiani R, Bellavita P, Fenili D, et al. Interferon alfa-2a therapy in cryoglobulinemia associated with hepatitis C virus. N Engl J Med. 1994;330:751–756. doi: 10.1056/NEJM199403173301104. [DOI] [PubMed] [Google Scholar]
- 69.Casato M, Agnello V, Pucillo LP, et al. Predictors of long-term response to high-dose interferon therapy in type II cryoglobulinemia associated with hepatitis C virus infection. Blood. 1997;90:3865–3873. [PubMed] [Google Scholar]
- 70.Cresta P, Musset L, Cacoub P, et al. Response to interferon alpha treatment and disappearance of cryoglobulinaemia in patients infected by hepatitis C virus. Gut. 1999;45:122–128. doi: 10.1136/gut.45.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazzaro C, Franzin F, Tulissi P, et al. Regression of monoclonal B-cell expansion in patients affected by mixed cryoglobulinemia responsive to alpha-interferon therapy. Cancer. 1996;77:2604–2613. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2604::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 72.Vallisa D, Bernuzzi P, Arcaini L, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin's lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Carriero D, Fabrizi F, Uriel AJ, et al. Treatment of dialysis patients with chronic hepatitis C using pegylated interferon and low-dose ribavirin. Int J Artif Organs. 2008;31:295–302. doi: 10.1177/039139880803100404. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed MS, Wong CF. Should rituximab be the rescue therapy for refractory mixed cryoglobulinemia associated with hepatitis C? J Nephrol. 2007;20:350–356. [PubMed] [Google Scholar]
- 75.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 76.Cacoub P, Delluc A, Saadoun D, et al. Anti-CD20 monoclonal antibody (rituximab) treatment for cryoglobulinemic vasculitis: where do we stand? Ann Rheum Dis. 2008;67:283–287. doi: 10.1136/ard.2006.065565. [DOI] [PubMed] [Google Scholar]
- 77.Saadoun D, Rosenzwajg M, Landau D, et al. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- 78.Sansonno D, De Re V, Lauletta G, et al. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818–3826. doi: 10.1182/blood-2002-10-3162. [DOI] [PubMed] [Google Scholar]
- 79.Lake-Bakaar G, Dustin L, McKeating J, et al. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood. 2007;109:845–846. doi: 10.1182/blood-2006-08-041525. [DOI] [PubMed] [Google Scholar]
- 80.Koziolek MJ, Scheel A, Bramlage C, et al. Effective treatment of hepatitis C-associated immune-complex nephritis with cryoprecipitate apheresis and antiviral therapy. Clin Nephrol. 2007;67:245–249. doi: 10.5414/cnp67245. [DOI] [PubMed] [Google Scholar]
- 81.Murai H, Inaba S, Kira J, et al. Hepatitis C virus associated cryoglobulinemic neuropathy successfully treated with plasma exchange. Artif Organs. 1995;19:334–338. doi: 10.1111/j.1525-1594.1995.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 82.Stefanutti C, Di Giacomo S, Mareri M, et al. Immunoadsorption apheresis (Selesorb) in the treatment of chronic hepatitis C virus-related type 2 mixed cryoglobulinemia. Transfus Apher Sci. 2003;28:207–214. doi: 10.1016/s1473-0502(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 83.Vila AT, Barnadas MA, Ballarin J, et al. Cutaneous ulcers with type I cryoglobulinemia treated with plasmapheresis. Eur J Dermatol. 2004;14:186–189. [PubMed] [Google Scholar]


