Abstract
Cholecystokinin (CCK) is a peptide neurotransmitter whose production requires proteolytic processing of the proCCK precursor to generate active CCK8 neuropeptide in brain. This study demonstrates the significant role of the cysteine protease cathepsin L for CCK8 production. In cathepsin L knockout (KO) mice, CCK8 levels were substantially reduced in brain cortex by an average of 75%. To evaluate the role of cathepsin L in producing CCK in the regulated secretory pathway of neuroendocrine cells, pituitary AtT-20 cells that stably produce CCK were treated with the specific cathepsin L inhibitor, CLIK-148. CLIK-148 inhibitor treatment resulted in decreased amounts of CCK secreted from the regulated secretory pathway of AtT-20 cells. CLIK-148 also reduced cellular levels of CCK9 (Arg-CCK8), consistent with CCK9 as an intermediate product of cathepsin L, shown by the decreased ratio of CCK9/CCK8. The decreased CCK0/CCK8 ratio also suggests a shift in the production to CCK8 over CCK9 during inhibition of cathepsin L. During reduction of the PC1/3 processing enzyme by siRNA, the ratio of CCK9/CCK8 was increased, suggesting a shift to the cathepsin L pathway for production of CCK9. The changes in ratios of CCK9 compared to CCK8 are consistent with dual roles of the cathepsin L protease pathway that includes aminopeptidase B to remove NH2-terminal Arg or Lys, and the PC1/3 protease pathway. These results suggest that cathepsin L functions as a major protease responsible for CCK8 production in mouse brain cortex, and participates with PC1/3 for CCK8 production in pituitary cells.
Keywords: cholecystokinin, protease, cathepsin L, prohormone convertase, brain, pituitary
1. Introduction
Cholecystokinin (CCK) is among the most abundant and widely distributed neuropeptide in mammalian brain [1, 2, 5, 11, 18, 27, 30, 31, 33, 34, 36]. The active CCK8 sequence within proCCK is flanked by a short carboxyl-terminal segment and a long amino-terminal segment (figure 1). During the passage of proCCK through the secretory pathway, it undergoes proteolytic processing at multiple single and double basic residues to generate CCK8. The carboxyl-terminal glycine is converted to an amide by the amidating enzyme [2, 12, 29] to generate active CCK8.
Figure 1. Procholecystokinin (proCCK) and CCK peptides.
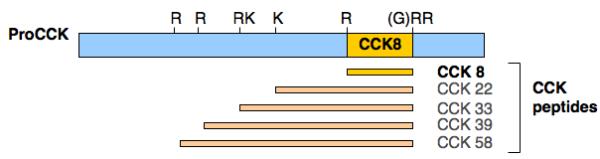
The proCCK precursor contains CCK8 and extended CCK peptide forms (CCK22, CCK33, CCK39, and CCK58) that are generated by proteolytic processing of proCCK at dibasic and monobasic residues. CCK8 (DYMGWMDF) represents the primary brain CCK peptide form, involved in learning, memory, anxiety, and pain perception among several CNS-mediated behaviors [1, 30, 33, 36]. Predicted cleavage sites of proCCK to generate CCK peptide forms are indicated by arrows. CCK peptides are numbered from the COOH-terminus of CCK8.
Until recently, the best candidates for the endoproteases that process proCCK was the prohormone convertase (PC) family of protease enzymes [19, 38, 40, 42]. Members of this expanded enzyme family are responsible for processing a number of proproteins for generating peptide hormones, growth factors, neuropeptides, as well as bacterial and viral virulence factors. Among them, the most likely candidates for CCK8 production are PC1/3, PC2, and PC5 [28, 40, 42, 43]. Analysis of CCK8 levels in brain regions of PC1/3 and PC2 knockout mice support the hypothesis that these enzymes are partially responsible for CCK8 production [4, 6, 9, 32]. PC1/3 KO mice show reduced CCK8 in hippocampus, amygdala, and pons/medulla, and PC2 KO mice show reduced CCK8 in hippocampus, septum, thalamus, and pons. PC1/3 plays a major role for CCK production in the intestine, while PC2 seems to be more important for CCK8 production in the brain [32]. These findings suggest utilization of processing proteases for CCK8 production in tissue-specific manner. Notably, none of the PC1/3 and PC2 KO mice showed a complete loss of CCK8 in the brain. The presence of CCK in the absence of PC1/3 or PC2 suggests the possibility for another protease(s) for the production of CCK8.
Cathepsin L in secretory vesicles has recently been demonstrated to participate in the proteolytic processing of proenkephalin to generate the enkephalin peptide neurotransmitter [22, 19, 46]. Notably, cathepsin L knockout (KO) mice showed substantial decreases in enkephalin and NPY in brain [16, 46], and pronounced decreases in pituitary ACTH, alpha-MSH, and beta-endorphin peptide hormones derived from the common POMC precursor [17]. Cathepsin L is colocalized with enkephalin and NPY in secretory vesicles, and undergoes regulated secretion from these cells induced by nicotinic receptor activation or KCl depolarization [20, 22, 46]. Cathepsin L is localized in secretory vesicles with the POMC-derived peptide hormones ACTH and beta-endorphin in AtT-20 cells [17]. These studies demonstrate a role for cathepsin L in the production of several peptide neurotransmitters and hormones in secretory vesicles.
While both the PC (PC1/3 and PC2) and cathepsin L proteases cleave at dibasic prohormone processing sites, they differ somewhat in their specific cleavage sites. PC1/3 and PC2 are known to cleave at the COOH-terminal side of dibasic residue processing sites (and occasionally at monobasic residues), while cathepsin L prefers to cleave at the NH2-terminal side of dibasic or monobasic sites as well as between the dibasic residues [19, 40, 42] (figure 2). It is predicted that cathepsin L cleavage of proCCK between Asp-Arg adjacent to the NH2-terminal side of CCK8 could produce CCK9 (Arg-CCK8). An aminopeptidase would then be required to remove NH2-terminal Arg to produce CCK8. Aminopeptidase B (AP-B) has been identified as an exopeptidase step following cathepsin L for neuropeptide production in secretory vesicles [7, 8, 13, 23]. Cathepsin L cleavage at the COOH-terminal side of CCK8 within proCCK may be predicted to generate CCK8Gly and/or CCK8GlyArg. CCK8Gly can be directly converted to CCK8 amide by the amidating enzyme [12]; CCK8GlyArg could be converted to CCK8Gly by carboxypeptidase E [14, 45] and then amidated.
Figure 2. Cathepsin L and prohormone convertases: predicted proteolytic pathways for CCK and neuropeptide production.
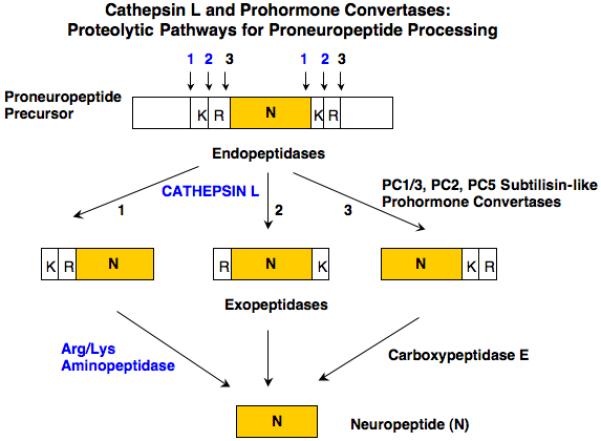
Dual protease pathways are proposed for proneuropeptide processing consisting of the cysteine protease cathepsin L with aminopeptidase B (AP-B), and the subtilisin-like prohormone convertases (PC1/3, PC2, and related PCs) combined with carboxypeptidase E (CPE). Studies have shown differences in cleavage specificities among these proteases. Cathepsin L prefers to cleave at the NH2-terminal side, and between, dibasic residue processing sites. The PC enzymes prefer to cleave at the COOH-terminal side of dibasic residues. These cleavage specificities for the cathepsin L and PC pathways are predicted to each yield mature neuropeptides such as CCK8.
To explore the potential role of cathepsin L in CCK8 production, this study evaluated CCK8 levels in brains of cathepsin L knockout (KO) mice and assessed the role of cathepsin L in the regulated secretory pathway of pituitary AtT-20 cells for CCK8 production. Significantly, CCK8 levels in brain cortex were substantially reduced in cathepsin L KO mice. In CCK-producing AtT-20 cells, treatment with the specific cathepsin L inhibitor CLIK-148 [25] resulted in decreased amounts of CCK8 produced in the regulated secretory pathway. Moreover, analyses of CCK8 and CCK9 peptides in AtT-20 cells during inhibition of cathepsin L, or during reduced expression of PC1/3 by antisense, demonstrated changes in ratios of CCK9 to CCK8 that are consistent with the joint roles of cathepsin L combined with PC1/3 for CCK8 production.
2. Experimental Materials and Methods
2.1. Cathepsin L knockout mice and CCK8 in brain cortex
Cathepsin L deficient mice were generated by gene targeting in mouse embryonic stem cells as described previously [35, 37]. Genotyping established wild-type (+/+) and cathepsin L gene knockout (−/−) mice. Cathepsin L and age-matched wild-type control mice were generated in the C57BL/6J mouse strain. The absence of cathepsin L in the knockout mice has been confirmed by anti-cathepsin L western blots [37]. Brain cortexes were collected from adult mice of approximately 3 months of age consisting of 4 female and 4 male cortexes for cathepsin L KO mice, and 5 female and 5 male cortexes for wild type mice. The neural tissue was homogenized in 1 N acetic acid, heated at 95% for 10 min., centrifuged (15,000 × g for 15 min.), and the supernatant was analyzed for CCK (amidated CCK) by radioimmunoassay (RIA) as described previously [4, 5]. CCK was assayed with a CCK RIA which detects all the major amidated CCK peptide forms (consisting of CCK8, CCK9, CCK12, CCK22, CCK23 and CCK33) [1]; the RIA does not cross-reacts with proCCK. Previous studies have shown that CCK8 is the major form expressed in brains of rodents, human, and canine [2, 5, 11, 18, 27] and, therefore, brain CCK8 is being measured by the CCK RIA in these studies of the cathepsin L knockout and wild-type mouse brains.
The CCK standards were synthesized [26], HPLC purified, and verified by mass spectrometry. Cell culture media samples for HPLC were prepurified by passage and elution over C18 SepPak columns. The columns were washed first with 3 ml of methanol, 20 ml 0.1% TFA, the media samples were passed three times through the columns, were washed with 20 ml 0.1% TFA and eluted with 3 ml 90% acetonitrile/water containing 0.1% TFA. They were evaporated to about 100 microliters in a Savant Speed Vac and were then analyzed by HPLC.
Control experiments found that PC1/3 and PC2 were not altered in brains of cathepsin L knockout mice. Protein concentration in tissue extracts was measured (DC protein assay kit, Biorad, CA). Tissue content of CCK per unit amount of protein was calculated.
2.2. CCK production in pituitary AtT-20 cells: CLIK-148 inhibitor of cathepsin L, CCK peptides analyzed by RIA and HPLC, and PC1/3 reduction by siRNA
AtT-20 cells were cultured as previously described [3, 21]. These AtT-20 cells were stably transfected with the rat proCCK cDNA containing the G418 resistance gene and stable cell lines were selected with 0.7 mg/ml G418. CCK peptide is produced in these AtT-20 cells that stably express proCCK [44]. Cells were treated with the specific inhibitor of cathepsin L known as CLIK-148 (50 μM) [25] for 24 hours, in the presence and absence of forskolin (5 μM) and IBMX (0.5 mM), and the media was collected and assayed for CCK by RIA as previously described [3, 4].
For HPLC and RIA analyses of CCK peptides, media from the treated AtT-20 cells was concentrated and processed by adsorption and elution from Millipore C18 SepPak cartridges (Waters, Bedford, MA). HPLC of CCK peptides was carried out with a Waters Alliance HPLC system using a 4.6 × 250 Symmetry Shield RP 18 column. The column was eluted with a 60 minutes gradient of 22.5% to 31.5% acetonitrile in 0.1% trifluoroacetic acid at 1 ml/min. One minute fractions were collected, aliquots of each fraction were dried down in a Speed Vac for CCK RIA assays. The elution position of fractions showing RIA positive CCK was compared to standard CCK peptides consisting of CCK8, CCK9, as well as CCK22 and CCK33 (synthesized by Dr. Kouki Kitagawa); CCK8 sulfate was obtained from Neosystem (Strasbourg, France).
CCK peptides were also analyzed by HPLC and RIA from AtT-20 cells with reduced levels of PC1/3, achieved by siRNA procedures as described previously [6]. The siRNA procedure resulted in substantial reduction of PC1/3 evaluated by western blots [6].
2.3. Cathepsin L and aminopeptidase B (AP-B) in AtT-20 cells: western blots and immunofluorescence confocal microscopy
The presence of cathepsin L and AP-B in AtT-20 cells was evaluated by western blots with anti-cathepsin L (Athens Research and Technology, GA) and anti-AP-B [8, 22]. AtT-20 cell homogenates were prepared and subjected to SDS-PAGE and western blotting as we have described previously [22].
Characterization of cathepsin L localization in secretory vesicles that contain peptide hormones was achieved by immunofluorescence confocal microscopy. AtT-20 cells were cultured as previously described [3, 21] on polylysine coated glass chamber slides (Nalge Nunc International, NY) and fixed with 3.7% formaldehyde in PBS for 10 minutes at room temperature. After permeabilization with Triton X-100 (0.1%) cells were incubated with PBS containing 3% BSA for 30 minutes at room temperature. Cells were then double-stained for 2 hours at room temperature in PBS-3% BSA with primary antibodies anti-CCK8 rabbit (1:100, Millipore, MA, USA), anti-cathepsin L-goat (15 μg/ml, R&D Systems, MN, USA), or with anti-AP-B (rabbit, custom antisera to AP-B as previously reported [23]). Immunofluorescence staining was revealed by secondary antibodies in PBS-BSA 3% with donkey anti-rabbit Alexa Fluor 594 (red fluorescence, 1:200, Molecular Probes, OR) and donkey anti-goat Alexa Fluor 488 (green fluorescence, 1:200, Molecular Probes, OR) incubated for 45 minutes at room temperature. For studies of ACTH in secretory vesicles, ACTH was colocalized with CCK8, cathepsin L, and aminopeptidase B using anti-ACTH-mouse (1:100, Abcam, MA) detected with goat anti-mouse Alexa Fluor 594 (red fluorescence, 1:200 dilution), combined with anti-CCK8 rabbit or anti-aminopeptidase B rabbit detected with anti-rabbit Alexa Fluor 488 (green fluorescence, 1:200), and anti-cathepsin L goat detected with anti-goat Alexa Fluor 488 (green fluorescence, 1:200). For controls, cells were incubated with secondary antibodies only, resulting in lack of immunofluorescence staining. Slides were mounted with aqua poly/mount (Polysciences) and images were taken with the microscope Delta Vision Spectris Image Deconvolution System on an Olympus IX70 using the software Softworx Explorer from Applied Precision.
3. Results
3.1. Substantial reduction of CCK8 in cathepsin L knockout mouse brain
Evaluation of CCK8 levels in cortex of mouse brains obtained from cathepsin L knockout mice was conducted to assess the role of cathepsin L in the production of CCK8. CCK8 is the primary CCK form present in rodent brain [2, 5, 11, 18, 27]. Results revealed substantial decreases in CCK8 in brains of cathepsin L knockout mice compared to wild-type mouse brains. CCK8 was decreased by 81% in male cathepsin L KO mice, and was decreased by 69% in female cathepsin L KO mice, compared to age-matched controls of the same gender (figure 3). These data represent an average decrease of CCK8 by 75% in all cathepsin L KO mice (male and female) compared to control wild-type mice. The substantial decrease in CCK8 in brain cortex of cathepsin L KO mice demonstrates an important role of cathepsin L in the production of CCK8.
Figure 3. Cathepsin L knockout mice show substantially reduced CCK8 levels in brain cortex.
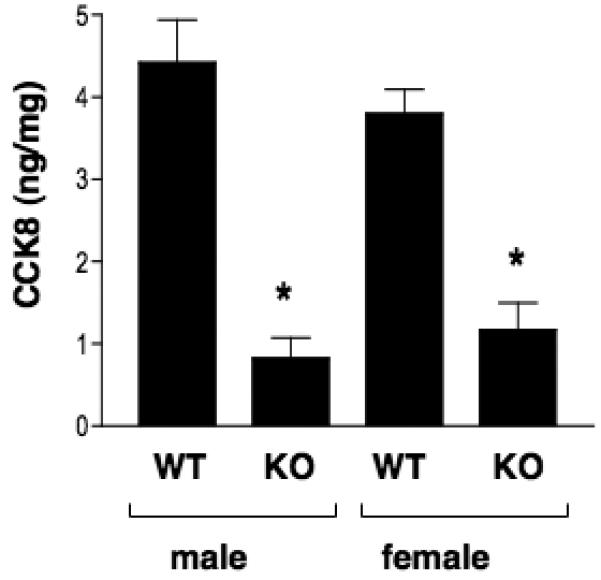
Cathepsin L knockout mice and in wild-type mice were assessed for CCK8 levels in brain cortex. Results show significant reduction of CCK8 in both males and females. Results show CCK8 levels (CCK ng/mg protein) as the mean ± s.e.m. (p < 0.05, by student's t-test).
3.2. CLIK-148, a specific inhibitor of cathepsin L, reduces CCK8 levels in the regulated secretory pathway of AtT-20 cells
The production of CCK and other neuropeptides occurs primarily in the regulated secretory pathway that produces, stores, and releases active CCK. Moreover, cathepsin L in regulated secretory vesicles participates in the production of enkephalin and NPY neuropeptides [16, 17, 46]. To assess the role of cathepsin L to produce CCK in the regulated secretory pathway, AtT-20 cells that produce CCK in the regulated secretory pathway [3] were treated with CLIK-148 that specifically inhibits cathepsin L. During this treatment, cells were treated with or without the secretatogues forskolin and IBMX. Forskolin activates adenyl cyclase to produce cAMP [39], and IBMX inhibits cAMP degradation by phosphodiesterase [41].
Results showed that the amount of CCK secreted from the regulated secretory pathway, induced by forskolin/IBMX treatment, was significantly reduced by CLIK-148 treatment of AtT-20 cells (figure 4). Thus, CCK in the regulated secretory pathway was reduced by approximately 60%. It was also noted that CLIK-148 decreased the amount of CCK secreted in the absence of forskolin/IBMX. These data indicate participation of cathepsin L in the production of CCK in AtT-20 cells.
Figure 4. CLIK-148, a specific inhibitor cathepsin L, reduces CCK production in the regulated secretory pathway of pituitary AtT-20 cells.
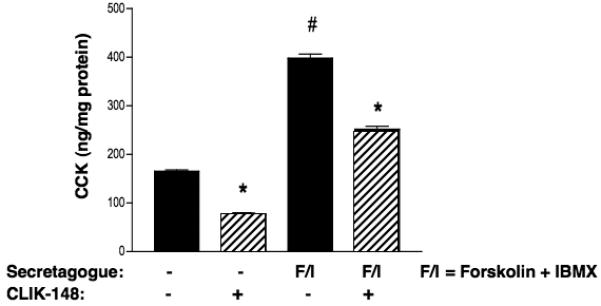
AtT-20 cells that produce CCK by stable expression of proCCK were treated with or without CLIK-148 (50 μM), in the presence or absence of forskolin (5 μM) and IBMX (0.5 mM), for 24 hours. The amount of CCK in the secretion media was measured by RIA, and is shown as the x ± s.e.m. (significance evaluated by student's t-test, *p < 0.05, n = 4-6).
3.3. Alterations in ratios of CCK8/CCK9 peptides during treatment of AtT-20 cells with the cathepsin L inhibitor CLIK-148L, compared to PC1/3 siRNA treatment
The molecular forms of CCK in cell media from proCCK-expressing AtT-20 cells were examined by HPLC and CCK RIA (figure 5). The HPLC elution positions of CCK peptides were monitored by RIA, and were compared to synthetic CCK peptide standards. CCK8 was demonstrated by the HPLC and RIA analyses. However, CCK9 appears to be the major CCK peptide form, which represents Arg-CCK8. Lower levels of CCK22 and CCK23 were also present.
Figure 5. HPLC analyses of CCK8 and CCK9 from AtT-20 cells.
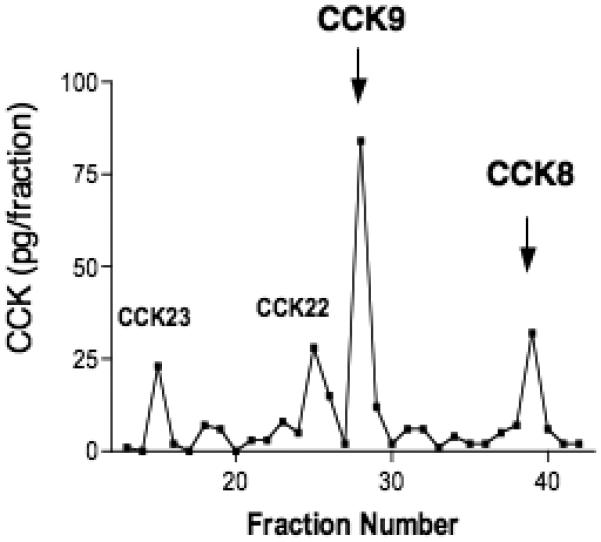
CCK8 and CCK9 peptides from AtT-20 cells (in secretion media) were separated by HPLC, and detected by the CCK RIA, as described in the methods. Elution positions for standard CCK8 and CCK9 peptides are shown by arrows. In addition, elution of CCK22 and CCK33 standards are indicated (gray lettering).
Based on the ability of cathepsin L to cleave at the NH2-terminal side of monobasic and dibasic residue sites, CCK9 (Arg-CCK8) may represent a predicted product of cathepsin L processing. This prediction would suggest that after treatment of cells with CLIK-148 that inhibits cathepsin L, levels of CCK9 would be decreased compared to levels of CCK8 to result in a reduced ratio of CCK9/CCK8. Indeed, HPLC analyses of secretion media showed that CLIK-148 treatment resulted in a reduced ratio of CCK9/CCK8 that was decreased by one-half (Table 1). These data suggest that during inhibition of cathepsin L, its predicted CCK9 product was decreased compared to CCK8.
Table 1.
Comparison of Ratios of CCK9 to CCK8 from AtT-20 Cells Treated with Cathepsin L Inhibitor or PC1/3 Antisense.
| Treatment | Ratio CCK9/CCK8 |
|---|---|
| None (control) | 1.00 ± 0.06 |
| CLIK-148 | 0.49 ± 0.15* |
| PC1/3 antisense | 1.58 ± 0.14* |
AtT-20 cells were treated with the specific cathepsin L inhibitor, CLIK-148 (24 hrs), or with PC1/3 antisense (by stable expression). At the end of the treatment period, secretion media was collected and subjected to HPLC analyses and CCK RIA (as shown in figure 5). The amounts of CCK8 and CCK9 were compared as ratios, with the ratio normalized to 1.00 for untreated control cells. Differences in ratios of CCK9/CCK8 were statistically significant, with p < 0.05 (by student's t-test, n = 4-6).
To examine whether the ratio of CCK9 to CCK8 changes with inhibition of endogenous PC1/3 expression, AtT-20 cells were subjected to PC1/3 siRNA treatment which effectively decreases PC1/3 expression [6]. HPLC analyses showed that inhibition of PC1/3 expression shifted the production of CCK8 to increased levels of CCK9, which resulted in a higher ratio of CCK9/CCK8 that was increased by 50% above untreated control cells (Table 1). These data suggest that with reduced PC1/3 expression, cells shifted to production of CCK9 that may represent a peptide product of cathepsin L.
3.4. Cathepsin L and aminopeptidase B (AP-B) in AtT-20 cells: colocalization with CCK and ACTH
The presence of the cathepsin L and AP-B protease pathway in AtT-20 cells was assessed by western blots. Cathepsin L of ∼25-27 kDa [10, 15, 46] was demonstrated to be present in AtT-20 cells by anti-cathepsin L western blot (figure 6a). Furthermore, AP-B of ∼72 kDa was present in AtT-20 cells, illustrated by anti-AP-B western blot (figure 6b).
Figure 6. Cathepsin L and aminopeptidase B (AP-B) in AtT-20 cells illustrated by western blots.
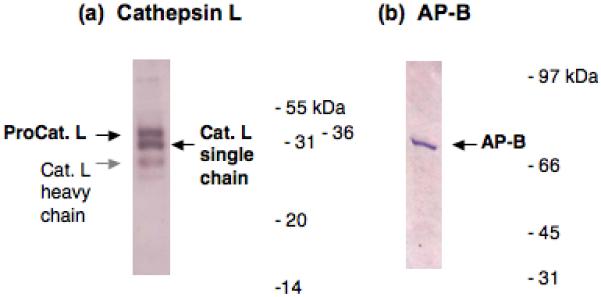
Western blots of cathepsin L (panel a) and AP-B (panel b) were conducted in AtT-20 cells. The presence of cathepsin L and AP-B was demonstrated by western blots with specific antisera to each protease, performed as described in the methods.
(a) Cathepsin L in AtT-20 cells. Cathepsin L bands observed by anti-cathepsin L western blot correspond to procathepsin L (∼38-40 kDa, black arrow), the mature single chain form of cathepsin L (∼28 kDa, black arrow), and a light band corresponding to the heavy chain form of cathepsin L (∼24 kDa, gray arrow) [24].
(b) AP-B in AtT-20 cells. The AP-B visualized by western blot with anti-AP-B corresponds to a band of ∼72 kDa [19].
To participate in CCK production in the regulated secretory pathway of AtT-20 cells, cathepsin L is predicted to be localized with peptide hormone containing secretory vesicles of these cells. Therefore, the colocalization of CCK8 in secretory vesicles with cathepsin L was examined by immunofluorescence confocal microscopy (figure 7). CCK8 showed a clear punctate pattern of immunostaining (red fluorescence, figure 7) that represents its secretory vesicle localization. Similarly, cathepsin L immunostaining (green fluorescence, figure 7) also showed a discrete, punctate pattern of immunostaining that was very well colocalized with CCK (shown by merged image of yellow fluorescence, figure 7). An enlarged view showed colocalization of CCK and cathepsin L in neuritic extensions of these cells (figure 7b). These results demonstrate excellent colocalization of CCK with cathepsin L in AtT-20 cells.
Figure 7. Colocalization of CCK8 and cathepsin L in secretory vesicles of AtT-20 cells illustrated by immunofluorescence confocal microscopy.
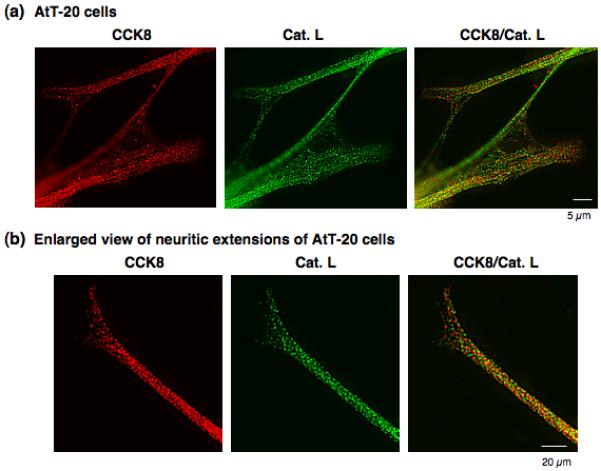
The colocalization of CCK8 and cathepsin L in secretory vesicles was visualized in AtT-20 cells by immunofluorescence confocal microscopy (panel a). CCK (red fluorescence) and cathepsin L (green fluorescence) were colocalized as demonstrated by the merged image showing yellow fluorescence. The punctate pattern of immunofluorescence staining of CCK8 and cathepsin L are consistent with the secretory vesicle localization of CCK8. An enlarged view (panel b) of neuritic-like extensions demonstrated the colocalization of CCK and cathepsin L in punctate secretory vesicle pattern in the extended regions of AtT-20 cells.
The ongoing hypothesis of this study that aminopeptidase B (AP-B) follows cathepsin L for CCK8 production predicts colocalization of AP-B and cathepsin L in AtT-20 cells. Indeed, AP-B and cathepsin L showed excellent, punctate colocalization demonstrated by immunofluorescence confocal microscopy (figure 8). The discrete punctate pattern was similar to that of CCK and cathepsin L colocalization (figure 7). Furthermore, an enlarged view of cells showed colocalization of AP-B and cathepsin L in neuritic extensions (figure 8b). These findings demonstrate the localization of both cathepsin L and aminopeptidase B to secretory vesicles that produce CCK8 and other neuropeptides.
Figure 8. Colocalization of aminopeptidase B (AP-B) and cathepsin L in AtT-20 cells illustrated by immunofluorescence confocal microscopy.
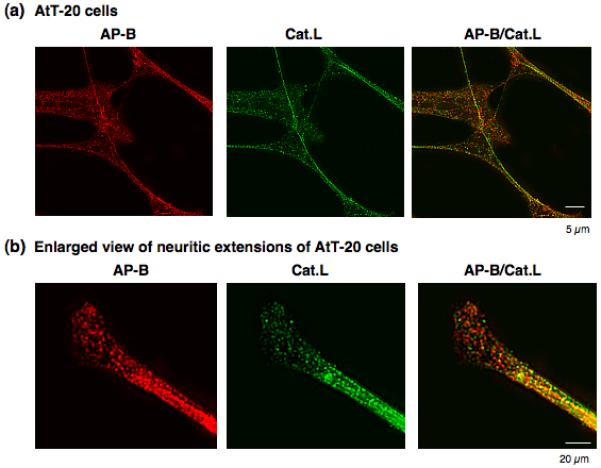
The colocalization of AP-B and cathepsin L was demonstrated by immunofluorescence microscopy (panel a). AP-B (red fluorescence) and cathepsin L (green fluorescence) were colocalized as shown by the merged image showing yellow fluorescence. The punctate pattern of immunofluorescence of AP-B and cathepsin L are consistent with a secretory vesicle pattern of localization, with illustration that cathepsin L is colocalized with CCK in secretory vesicles, shown in figure 7. An enlarged view (panel b) of neuritic-like extensions of AtT-20 cells shows the colocalization of AP-B and cathepsin L in a punctate pattern illustrating their discrete compartmentalization.
CCK8 in AtT-20 cells is colocalized with the peptide hormone ACTH (figure 9) that is known to be present in secretory vesicles of AtT-20 cells. CCK8 and ACTH were colocalized in a punctate pattern consistent with localization in secretory vesicles. Furthermore, ACTH was colocalized with both cathepsin L and aminopeptidase B in neuritic-like extensions of cells in a discrete fashion (figure 9). These data demonstrate that CCK8- and ACTH-containing secretory vesicles show the presence of both cathepsin L and aminopeptidase B. These results are supportive of experiments in this study for a role of cathepsin L and aminopeptidase B in the production of CCK8.
Figure 9. Colocalization of ACTH in secretory vesicles with CCK8, cathepsin L, and aminopeptidase B in neuritic extensions of AtT-20 cells.
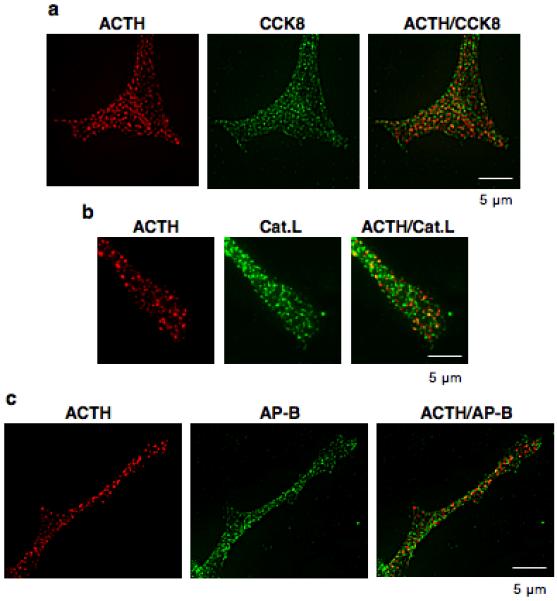
The colocalization of ACTH with CCK8, cathepsin L, and aminopeptidase (AP-B) was examined in neuritic-like extensions of AtT-20 cells which contain secretory vesicles. Panel ‘a’ shows the colocalization of ACTH (red fluorescence) with CCK8 (green fluorescence) shown by the merged yellow fluorescence. Panels ‘b’ and ‘c’ show the colocalization of ACTH (red fluorescence) with cathepsin L and aminopeptidase B (green fluorescence) indicated by the yellow fluorescence in the merged images.
As control, cathepsin L localization in lysosomes of AtT-20 cells was compared its localization with CCK8 and ACTH in secretory vesicles. The reason for this study is that cathepsin L is known to be present in lysosomes and secretory vesicles [17]. Immunofluorescence microscopy with anti-lamp-1, a marker for lysosomes, and anti-cathepsin L was conducted to examine their subcellular distribution (figure 10). Results show partial colocalization of cathepsin L with lamp-1, and much subcellular localization of cathepsin L resides outside of the lysosomes. These findings are consistent with data from this study showing a large extent of cathepsin L colocalized with CCK8 and ACTH in secretory vesicles.
Figure 10. Subcellular localization of cathepsin L within lysosomes and outside of lysosomes in AtT-20 cells.
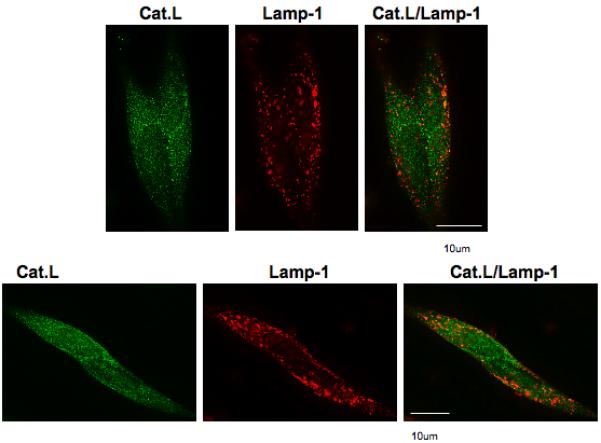
Evaluation of cathepsin L subcellular distribution in lysosomes was achieved by immunofluorescence confocal microscopy of cathepsin L (Cat.L, green fluorescence) and the lysosomal marker lamp-1 (Lamp-1, red fluorescence). The merged images show that cellular cathepsin L is partially colocalized with lamp-1 (yellow fluorescence). But, importantly, a large portion of cellular cathepsin L is located outside of lamp-1 labeled lysosomes, consistent with data in this study showing cathepsin L localization within secretory vesicles.
4. Discussion
Our results demonstrate a primary role for cathepsin L in the production of the neuropeptide cholecystokinin (CCK). In brain cortex of cathepsin L knockout mice, CCK8 levels were substantially reduced by an average of 75%, compared to wild-type controls. Reductions in brain CCK8 were found in both male and female cathepsin L knockout mice. These amounts of decreased CCK8 in cathepsin L KO mice are greater than the modest decreases of CCK8 found in brain regions of PC1/3 or PC2 KO mice [4, 9, 32]. Furthermore, brain cortex of PC1/3 knockout mice shows no change in CCK8, and brain cortex of PC2 knockout mice show an increase in CCK8. Therefore, data from the cathepsin L knockout results provide strong evidence for cathepsin L as the major endoprotease responsible for CCK8 production in brain cortex.
Furthermore, CCK8 production was substantially reduced in the regulated secretory pathway of AtT-20 cells treated with the specific cathepsin L inhibitor, CLIK-148 [25]. These AtT-20 cells stably express proCCK, and possess the appropriate proteolytic activities for production of CCK8. Treatment of these cells with CLIK-148 to reduce the amounts of CCK secreted from regulated secretory vesicles, induced with forskolin and IBMX, is consistent with participation of cathepsin L for the production of CCK that is then secreted.
Analyses of CCK peptide forms from AtT-20 cells by HPLC showed that CLIK-148 decreased the ratio of CCK9/CCK8, suggesting a decrease in CCK9 (Arg-CCK8) as a peptide product generated by cathepsin L. Moreover, reduction of PC1/3 expression by siRNA led to a shift to greater levels of CCK9 compared to CCK8, as shown by the increase in the ratio of CCK9/CCK8; these results suggest that during reduced expression of PC1/3, production of CCK8 is shifted to production of CCK9 that may occur by cathepsin L. This scheme for joint roles of cathepsin L and PC1/3 as endoproteases for production pf CCK8 from proCCK may occur based on knowledge of the differences in cleavage specificities of these proteases for NH2- or COOH-terminal sides of dibasic or monobasic processing sites.
These CLIK-148 inhibitor results are consistent with cathepsin L production of CCK9 as an intermediate peptide that can be converted to CCK8 by a Lys/Arg aminopeptidase such as aminopeptidase B (figure 11). Data are also consistent with production of CCK8 by PC1/3, which utilizes carboxypeptidase E (CPE) to generate CCK8. Notably, the amidating enzyme is present in AtT-20 cells [29] for COOH-terminal amidation to form CCK8. The exopeptidases AP-B and CPE have been demonstrated to be localized within neuropeptide-containing secretory vesicles of pituitary, adrenal medullary chromaffin granules, and secretory vesicles in related neuroendocrine tissues [7, 14, 23].
Figure 11. Hypothesized production of CCK8 from proCCK by cathepsin L and PC1/3 protease pathways.
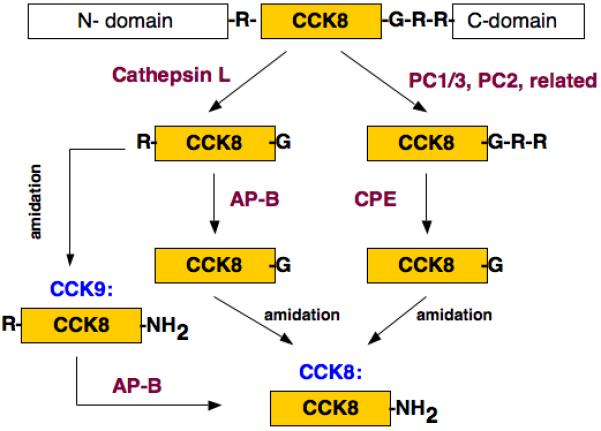
It is proposed that production of CCK8 utilizes both the cathepsin L and PC1/3 proteases for proCCK processing. Results from this study support the model whereby cathepsin L cleaves proCCK to generate Arg-CCK-Gly that then utilizes aminopeptidase B to generate CCK8-Gly; CCK8-Gly is a substrate for C-terminal amidation to form CCK-amide. In parallel, proCCK is proposed to be cleaved by the subtilisin-like prohormone convertase (PC1/3) that generates CCK8-Gly-Arg-Arg; C-terminal basic residues of CCK8-Gly-Arg-Arg are removed by carboxypeptidase E to generate CCK8-Gly, a substrate for C-terminal amidation for the production of CCK8-amide.
The data in this study demonstrates that cathepsin L participates in the production of CCK8, illustrated by studies of cathepsin L knockout mouse brain cortex, and by selective inhibition of cathepsin L in pituitary AtT-20 cells. Consideration of possible involvement of cathepsin L in CCK8 metabolism has been ruled out for two reasons. Firstly, if cathepsin L may be involved in CCK8 degradation, then CCK8 levels would be increased in the cathepsin L knockout condition but this was not the result. Rather, the data showed substantial decrease in brain CCK8 that illustrates participation of cathepsin L in CCK8 production (not its degradation). Secondly, the possibility of degradation of proCCK to CCK8 peptides by cathepsin L in lysosomes has been ruled out because CCK8 is present with ACTH in secretory vesicles, and CCK8 is not located outside of secretory vesicles in subcellular regions such as lysosomes. Since CCK8 is not present in lysosomes, catehspin L degradation to proCCK in lysosomes is unlikely. Overall, data of this study are consistent with participation of cathepsin L in CCK8 production.
The function of cathepsin L in the regulated secretory pathway for CCK8 production represents a novel function for cathepsin L production of a biologically active peptide in secretory vesicles. To directly demonstrate the presence of cathepsin L in peptide hormone-containing secretory vesicles, its colocalization with CCK8 and ACTH was illustrated by immunofluorescence confocal microscopy. Moreover, aminopeptidase B was colocalized with cathepsin L in secretory vesicles that contain CCK8 and ACTH. The secretory vesicle localization of cathepsin L contrasts with its known function in lysosomes for protein degradation [24]. The AtT-20 cells conatin cathepsin L in both secrtory vesicles and lysosomes. The presence of cathepsin L in secretory vesicles represents a newly identified organelle for cathepsin L as a candidate processing enzyme for the production of the CCK8 neuropeptide.
In addition to the production of CCK8, the role of cathepsin L in generating enkephalin peptide neurotransmitters has been demonstrated for conversion of proenkephalin into active enkephalin opioid peptide neurotransmitters [22, 23, 46]. Notably, brains of cathepsin L gene knockout mice show reduced levels of (Met)enkephalin that are 50% lower compared to wild-type controls [46]. In addition to cathepsin L, PC2 also participates in enkephalin production as demonstrated in PC2 knockout mice that show reduction of brain (Met)enkephalin by 50% [19]. These results implicate dual roles for both cathepsin L and PC2 in enkephalin peptide neurotransmitter production. However, little change in enkephalin levels has been observed in brains of PC1/3 knockout mice (Hook et al., unpublished observations). These findings indicate that selected protease components of the dual cathepsin L and PC protease pathways may be utilized for proteolytic processing of proenkephalin or other proneuropeptides.
More recent studies show that cathepsin L also participates in proneuropeptide processing for the production of NPY [16] and the POMC-derived peptide hormones consisting of ACTH, alpha-MSH, and beta-endorphin [17]. These studies showed decreased levels of these neuropeptides by more than 50% in cathepsin L KO mice. Moreover, cathepsin L is colocalized with NPY and POMC-derived peptide hormones in secretory vesicles. These results indicate the importance of cathepsin L as a proneuropeptide processing enzyme for several neuropeptides.
5. Conclusion
In summary, a key role for cathepsin L in the production of CCK8 was demonstrated by the substantial decrease in brain levels of CCK8 in cathepsin L KO mice. Apparently, cathepsin L plays a greater role than PC1/3 and PC2 for CCK8 production in brain cortex, since knockout of PC1/3 or PC2 resulted in only modest changes in brain CCK8 [4, 9, 32] compared to the cathepsin L KO condition. Cathepsin L functions in the regulated secretory pathway of AtT-20 cells for the production of CCK8, shown by reduced amounts of CCK secreted from the forskolin/IBMX stimulated AtT-20 cells in the presence of CLIK-148 that specifically inhibits cathepsin L. Interestingly, cathepsin L inhibition, and reduction of PC1/3 expression, each result in decreased or increased ratios of CCK9/CCK8, respectively. These findings illustrate the dual roles of cathepsin L and PC1/3 in pituitary AtT-20 cells, and the major role of cathepsin L production of CCK8 in brain.
Acknowledgments
The authors appreciate support of this research by grants from the National Institutes of Health (NIH) to V. Hook (R01 DA04271, R01 MH077305, R01 NS24553), and to M. Beinfeld (R01 NS31602).
Abbreviations
- CCK
cholecystokinin
- CLIK-148
N-{L-3-trans-[2-(pyridin-2-yl)ethylcarbamoyl-oxirane-2-calbonyl]-1-phenylalanine dimethylamide
- HPLC
high pressure liquid chromatography
- IBMX
iso-butyl-methyl-xanthine
- PC1/3
prohormone convertase 1/3
- RIA
radioimmunoassay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beinfeld MC. An introduction to neuronal cholecystokinin. Peptides. 2001;22:1197–200. doi: 10.1016/s0196-9781(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 2.Beinfeld MC. Biosynthesis and processing of proCCK: recent progress and future challenges. Life Sci. 2003;72:747–57. doi: 10.1016/s0024-3205(02)02330-5. [DOI] [PubMed] [Google Scholar]
- 3.Beinfeld MC. CCK mRNA expression, pro-CCK processing, and regulated secretion of immunoreactive CCK peptides by rat insulinoma (RIN 5F) and mouse pituitary tumor (AtT-20) cells in culture. Neuropeptides. 1992;22:213–7. doi: 10.1016/0143-4179(92)90048-2. [DOI] [PubMed] [Google Scholar]
- 4.Beinfeld MC, Blum A, Vishnuvardhan D, Fanous S, Marchand JE. Cholecystokinin levels in prohormone convertase 2 knock-out mouse brain regions reveal a complex phenotype of region-specific alterations. J Biol Chem. 2005;280:38410–5. doi: 10.1074/jbc.M500055200. [DOI] [PubMed] [Google Scholar]
- 5.Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212:51–7. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- 6.Beinfeld MC, Vishnuvardhan D, Blum A, Reynolds N, Fannous S, Kitagawa K, et al. Inhibition of prohormone convertase 1 (PC1) expression in cholecystokinin (CCK) expressing At-T20 cells decreased cellular content and secretion of CCK and caused a shift in molecular forms of CCK secreted. Peptides. 2006;27:905–10. doi: 10.1016/j.peptides.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Cadel S, Foulon T, Viron A, Balogh A, Midol-Monnet S, Noel N, et al. Aminopeptidase B from the rat testis is a bifunctional enzyme structurally related to leukotriene-A4 hydrolase. Proc Natl Acad Sci USA. 1997;94:2963–8. doi: 10.1073/pnas.94.7.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadel S, Pierotti AR, Foulon T, Creminon C, Barre N, Segretain D, et al. Aminopeptidase-B in the rat testes: isolation, functional properties and cellular localization in the seminiferous tubules. Mol Cell Endocrinol. 1995;110:149–60. doi: 10.1016/0303-7207(95)03529-g. [DOI] [PubMed] [Google Scholar]
- 9.Cain BM, Connolly K, Blum AC, Vishnuvardhan D, Marchand JE, Zhu X, et al. Genetic inactivation of prohormone convertase (PC1) causes a reduction in cholecystokinin (CCK) levels in the hippocampus, amygdala, pons and medulla in mouse brain that correlates with the degree of colocalization of PC1 and CCK mRNA in these structures in rat brain. J Neurochem. 2004;89:307–13. doi: 10.1046/j.1471-4159.2003.02295.x. [DOI] [PubMed] [Google Scholar]
- 10.Collette J, Bocock JP, Ahn K, Chapman RL, Godbold G, Yeyeodu S, et al. Biosynthesis and alternate targeting of the lysosomal cysteine protease cathepsin L. Int Rev Cytology. 2004;241:1–51. doi: 10.1016/S0074-7696(04)41001-8. [DOI] [PubMed] [Google Scholar]
- 11.Dockray GJ, Gregory RA, Hutchison JB, Harris JI, Runswick MJ. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978;274:711–3. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- 12.Eipper BA, Milgram SL, Husten EJ, Yun HY, Mains RE. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993;2:489–97. doi: 10.1002/pro.5560020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontes G, Lajoix AD, Bergeron F, Cadel S, Prat A, Foulon T, et al. Miniglucagon (MG)-generating endopeptidase, which processes glucagon into MG, is composed of N-arginine dibasic convertase and aminopeptidase B. Endocrinology. 2005;146:702–12. doi: 10.1210/en.2004-0853. [DOI] [PubMed] [Google Scholar]
- 14.Fricker LD. Carboxypeptidase E. Ann Rev Physiol. 1988;50:309–21. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, et al. Thyroid functions of mouse cathepsins B, K, and L. The J Clin Invest. 2003;111:1733–45. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funkelstein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Hook V. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem. 2008;106:384–91. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funkelstein L, Toneff T, Mosier C, Hwang SR, Beuschlein F, Lichtenauer UD, et al. Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression. J Biol Chem. 2008;283:35652–9. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goltermann NR, Rehfeld JF, Roigaard-Petersen H. In vivo biosynthesis of cholecystokinin in rat cerebral cortex. J Biol Chem. 1980;255:6181–5. [PubMed] [Google Scholar]
- 19.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Ann Rev Pharmacol Tox. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hook V, Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Troutner K, Toneff T, et al. Cathepsin L and Arg/Lys aminopeptidase: a distinct prohormone processing pathway for the biosynthesis of peptide neurotransmitters and hormones. Biol Chem. 2004;385:473–80. doi: 10.1515/BC.2004.055. [DOI] [PubMed] [Google Scholar]
- 21.Hook VY, Heisler S, Sabol SL, Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Comm. 1982;106:1364–71. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, et al. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–63. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SR, O'Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2007;100:1340–50. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 24.Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. Elsevier Academic Press; New York: 2004. pp. 1097–1102. [Google Scholar]
- 25.Katunuma N, Tsuge H, Nukatsuka M, Asao T, Fukushima M. Structure-based design of specific cathepsin inhibitors and their application to protection of bone metastases of cancer cells. Arch Biochem Biophys. 2002;397:305–11. doi: 10.1006/abbi.2001.2709. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa K, Aida C, Fujiwara H, Yagami T, Futaki S, Kogire M, et al. Facile solid-phase synthesis of sulfated tyrosine-containing peptides: total synthesis of human big gastrin-II and cholecystokinin (CCK)-39. J Org Chem. 2001;66:1–10. doi: 10.1021/jo000895y. [DOI] [PubMed] [Google Scholar]
- 27.Lindefors N, Linden A, Brene S, Sedvall G, Persson H. CCK peptides and mRNA in the human brain. Progr Neurobiol. 1993;40:671–90. doi: 10.1016/0301-0082(93)90010-p. [DOI] [PubMed] [Google Scholar]
- 28.Lusson J, Vieau D, Hamelin J, Day R, Chretien M, Seidah NG. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci USA. 1993;90:6691–5. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mains RE, Glembotski CC, Eipper BA. Peptide alpha-amidation activity in mouse anterior pituitary AtT-20 cell granules: properties and secretion. Endocrinology. 1984;114:1522–30. doi: 10.1210/endo-114-5-1522. [DOI] [PubMed] [Google Scholar]
- 30.Moran TH, Schwartz GJ. Neurobiology of cholecystokinin. Crit Rev Neurobiol. 1994;9:1–28. [PubMed] [Google Scholar]
- 31.Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978;253:4022–30. [PubMed] [Google Scholar]
- 32.Rehfeld JF, Bundgaard JR, Hannibal J, Zhu X, Norrbom C, Steiner DF, et al. The cell-specific pattern of cholecystokinin peptides in endocrine cells versus neurons is governed by the expression of prohormone convertases 1/3, 2, and 5/6. Endocrinology. 2008;149:1600–8. doi: 10.1210/en.2007-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem. 2007;7:1154–65. doi: 10.2174/156802607780960483. [DOI] [PubMed] [Google Scholar]
- 34.Rehfeld JF, Hansen HF. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem. 1986;261:5832–40. [PubMed] [Google Scholar]
- 35.Reinheckel T, Deussing J, Roth W, Peters C. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol Chem. 2001;382:735–41. doi: 10.1515/BC.2001.089. [DOI] [PubMed] [Google Scholar]
- 36.Ritter RC, Covasa M, Matson CA. Cholecystokinin: proofs and prospects for involvement in control of food intake and body weight. Neuropeptides. 1999;33:387–99. doi: 10.1054/npep.1999.0051. [DOI] [PubMed] [Google Scholar]
- 37.Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 2000;14:2075–86. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- 38.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–63. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 39.Seamon KB, Daly JW, Metzger H, de Souza NJ, Reden J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983;26:436–9. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- 40.Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, et al. The activation and physiological functions of the proprotein convertases. Intl J Biochem Cell Biol. 2008;40:1111–25. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Shafer SH, Phelps SH, Williams CL. Reduced DNA synthesis and cell viability in small cell lung carcinoma by treatment with cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1998;56:1229–36. doi: 10.1016/s0006-2952(98)00260-3. [DOI] [PubMed] [Google Scholar]
- 42.Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–9. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 43.Tagen MB, Beinfeld MC. Recombinant prohormone convertase 1 and 2 cleave purified pro cholecystokinin (CCK) and a synthetic peptide containing CCK 8 Gly Arg Arg and the carboxyl-terminal flanking peptide. Peptides. 2005;26:2530–5. doi: 10.1016/j.peptides.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Vishnuvardhan D, Beinfeld MC. Biosynthesis and post-translational processing of site-directed endoproteolytic cleavage mutants of Pro-CCK in AtT-20 cells. Biochemistry. 2002;41:570–8. doi: 10.1021/bi015566u. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Cain BM, Beinfeld MC. Adult carboxypeptidase E-deficient fat/fat mice have a near-total depletion of brain CCK 8 accompanied by a massive accumulation of glycine and arginine extended CCK: identification of CCK 8 Gly as the immediate precursor of CCK 8 in rodent brain. Endocrine. 1998;9:329–32. doi: 10.1385/ENDO:9:3:329. [DOI] [PubMed] [Google Scholar]
- 46.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–5. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon J, Beinfeld MC. Prohormone convertawe 2 is necessary for the formation of cholecystokinin-22, but not cholecystokinin-8, in RIN5F and STC-1 Cells. Endocrinology. 1997;138:3620–23. doi: 10.1210/endo.138.9.5399. [DOI] [PubMed] [Google Scholar]


