Abstract
Anemia of inflammation (AI) is a complex multi-organ response to inflammatory disorders. Because AI can result from many infectious and non-infectious inflammatory diseases, multiple mechanisms may contribute to its pathogenesis including iron restriction, direct erythropoietic suppression, shortened red cell survival or frank hemolysis. Animal models have been helpful in the study of the mechanisms of AI and its potential treatments but each model reflects distinct aspects of this heterogeneous syndrome. It is therefore important to study a variety of models of AI. This review focuses on the use of infectious and noninfectious mouse models of inflammation that have been shown to manifest anemia. We review many of the models reported in the literature or developed in our laboratory, and discuss their respective merits and drawbacks.
INTRODUCTION
Anemia of inflammation (AI), also known as anemia of chronic disease or anemia of chronic disorders (ACD), is a common syndrome complicating many infectious, non-infectious inflammatory, and neoplastic disorders. The definition, pathogenesis and consequences of AI will be reviewed extensively in other articles in this same issue. What follows is a brief discussion of the definitions of AI and some of the aspects of pathogenesis that are necessary to understand how to model AI in animals for further studies of the condition.
Classically, ACD has been defined as a normocytic or microcytic anemia, without another known cause, developing in the setting of a chronic inflammatory condition. Although the anemia in ACD is often iron restricted, iron stores, as estimated by bone marrow biopsy, are intact. The hypothesis that ACD is an iron restricted anemia caused by inflammatory induction of hepcidin gained popularity with the discovery of the iron regulatory hormone hepcidin, its increase by inflammation and its ability to inhibit iron absorption and the egress of iron from stores. Moreover, the overproduction of hepcidin causes an anemia that fits this classical definition of ACD1–3.
However, iron restriction does not explain all the features of AI. First, AI develops too quickly to be attributed to iron restriction alone. In fact, part of the motivation for abandoning the term ACD is the recognition that anemia often develops quite quickly in severe inflammatory settings. Nguyen and colleagues found that anemia develops at a rate of 5.2 g/L per day in non-bleeding ICU patients4 and that this exceeded what could be accounted for by phlebotomy alone4,5. Iron restriction would account for a much slower fall in hemoglobin since only production would be affected.
In fact, it has long been known that multiple mechanisms contribute to the development of anemia in the setting of inflammation. Destruction is also accelerated due to increased clearance by macrophage or frank hemolysis during severe inflammation. Besides iron restriction, suppression of erythropoiesis and interference with the production of erythropoietin or its signaling could also contribute to decreased production of red blood cells6,7.
Because AI is likely a multifactorial disease, perhaps the broadest definition of AI is that it is anemia that develops in the presence of inflammation without obvious blood loss. Understanding the diverse mechanisms that could be involved requires a variety of animal models that illuminate specific individual mechanisms. Most of this review will focus on mouse models because the mouse is the most extensively studied; there are a large number of important genetically altered mice that aid in the study of AI, and mice have a hematologic and inflammatory system similar to humans. Anemia of cancer will not be discussed since this may represent a separate entity entirely and few well characterized animal models exist.
MOUSE MODELS OF AI
Multiple systems, including the hemopoietic system, macrophages, immune system, liver and kidneys are involved in the development of AI. While some aspects of AI can be modeled in cell culture systems, most studies of AI will require animal models. Furthermore, because so many systems are involved, it is important that the animal chosen is as similar to humans as possible. This must be balanced by practical aspects such as amenability to genetic manipulation, homogeneity of the animals, breeding time and costs. The mouse likely strikes the best balance of similarity to the human and practicality. Therefore, the majority of this review will be devoted to mouse models of AI with a short discussion of non-mouse models at the end.
It is important to recognize that there are important differences between human and murine pathophysiology that could lead to profound differences in the anemic response to inflammation. The lifespan of the mouse erythrocyte is much shorter than that of humans8 so consequently they have a more active erythropoiesis and higher reticulocyte counts at baseline. Mice mount a much more intense reticulocyte response to some inflammatory stimuli and are capable of extensive extramedullary hematopoiesis9,10. A discussion of the differences in the immune systems of mice and humans is beyond the scope of this review but could also affect the anemic response to AI.
Because AI is a multifactorial disease, the mouse model used depends on what aspects of the disease are most important to the investigator. The models will be categorized based on their underlying mechanism of inflammation.
Infectious Models
Anemia is a common feature of a broad spectrum of infectious diseases. It has been suggested that the hypoferremia of AI evolved as a host defense mechanism against infection, inhibiting microbial growth by depriving microbes of iron6,11. The inflammatory response to different infections is diverse, as is the involvement of additional mechanisms that contribute to anemia. Therefore, it will be difficult to extrapolate the findings in a bacterial peritonitis model, for example, to a model based on a murine red cell parasite.
Intestinal Perforation
The large intestine is colonized with a large array of organisms that are pathogenic when allowed to spill into the peritoneum. The most common method of introducing fecal contents into the peritoneum is by cecal ligation and puncture (CLP). CLP is performed by making a midline incision in the lower abdomen, expressing the cecum, ligating it at its junction with the large intestine or below, and puncturing it with a needle. Traditionally, CLP has been used to model severe sepsis or septic shock 12. However, the severity and potential for chronicity of the model depend on the size of the perforations, the number of punctures, the use of catheters to maintain patency of the perforation(s) and the volume and composition of stool contents expressed into the peritoneum 13.
The nature of the inflammatory response in response to CLP has been extensively studied 12 but very few studies reported using CLP to study the pathogenesis or treatment of AI. Schubert and colleagues studied the role of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in a CLP model of AI and found that neither of these cytokines played a significant role in the pathogenesis of AI in this model14,15.
CLP has several significant technical drawbacks that make it less appealing for studying AI. It is technically difficult, time consuming to perform, has variable outcomes and is associated with significant morbidity. However, in experienced hands CLP-induced anemia is an informative model of the anemia in the setting of severe infections.
Pyogenic Bacterial Infections
AI is a common feature of most chronic infections with pyogenic bacteria (gram positives and enteric gram negatives) such as osteomyelitis, indwelling catheter infections and endocarditis. However, there is a relative paucity of literature on animal models of anemia caused by chronic infections of these types. Gallimore and colleagues reported mild chronic anemia in response to a Staphylococcus epidermidis coated catheter implanted in the peritoneum for a period as long as six months16. We modified this technique somewhat to create a very easy model of chronic peritoneal catheter infection that is well tolerated by the mice. Standard intravenous catheter-over-needle assemblies were soaked in a broth culture of S. epidermidis overnight. The catheters were then cut circumferentially 1 cm from the tip. The catheter-over-needle assemblies were passed into the peritoneum using standard IP injection technique under isoflurane anesthesia. The catheter was then slid over the needle to the hub and then the needle and hub were removed. Because of the cut in the catheter, the distal 1 cm would remain implanted in the peritoneal cavity.
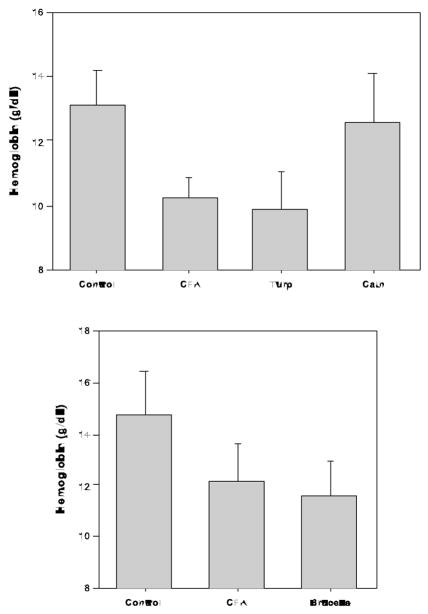
The mice tolerated this procedure very well and showed almost no signs of being chronically ill up to 6 weeks after the procedure. Similar to Gallimore, we found that approximately 90% of the mice developed a large, walled abscess around the catheter and that persisting bacteria could be cultured from the pus and catheter. Although the anemia was mild (see Figure), the mice had a significant fall in hemoglobin when compared to controls (data not shown). We found this anemia to be mildly microcytic but not associated with terminally elevated hepcidin levels (unpublished data).
Figure.
Chronic inflammation was induced in C57BL/6 with intraperitoneal (IP) complete Freund’s adjuvant (CFA), serial injections of subcutaneous turpentine (Turp), peritoneal catheter infection (Cath) or IP brucella antigen (Brucella). Control mice received normal saline injections. Hemoglobin was determined at the end of 3 weeks for all models except Cath at 4 weeks. Mice in the top panel were maintained on a 4 ppm iron diet during the experiment whereas in the lower panel, mice were on 20 ppm iron.
Bunce and colleagues coated dextran microbeads (Cytodex; Sigma Chemical; St. Louis, MO) with Staphylococcus aureus and Streptococcus pyogenes and injected them subcutaneously17. Mice developed large abscesses in proportion to the number of bacteria injected. However, the investigators did not report any hematologic parameters. We modified this technique by using S. epidermidis and injected the bacteria coated beads into the peritoneum. This technique is exceedingly easy and is essentially without morbidity. After 4 weeks, the mice develop 10–30 one to five millimeter abscesses throughout the peritoneum, and these sometimes become confluent. We did not assess whether viable bacteria could be recovered from these abscesses. Mice were statistically more anemic than controls but the anemia was very mild. Regardless, this model may be a good representation of more indolent AI and it is likely that the severity of the anemia could be tuned by altering the number of beads, the inoculum of bacteria or the pathogenicity of the organism.
Mycobacterial Infections
Mycobacterial infections, especially Mycobacterium tuberculosis (MTB), are exceedingly prevalent in the world, responsible for widespread morbidity and mortality and frequently associated with anemia18. Furthermore, the ability to obtain iron is important for the pathogenicity of most mycobacterial infections, especially MTB19. MTB causes infection in several strains of mice including Balb/C20, C57BL/621 and DBA/2J21. One major drawback to working with MTB is that it requires a Biosafety Level-3 (BSL-3) containment. Surprisingly little work has been done to characterize the extent and strain variation of the anemic response to MTB. However, as will be discussed below, we have shown that the inflammatory response to components of MTB causes AI so it is likely that MTB infections would be a good model of AI.
Parasitic Infections
Many parasitic infections are associated with anemia. However, in many cases the cause of the anemia is presumed to be non-inflammatory or have a minor inflammatory component. Enteric parasites, for example, cause anemia by depriving the host of nutrients or causing bleeding. Some parasite associated anemias may be AI or exacerbated by concomitant AI.
Trypanosomes cause a wide variety of diseases in humans and animals, many of which are associated with anemia. T. brucei, the cause of sleeping sickness in humans, causes anemia in mice22,23. Although the mechanism of anemia in T. brucei infection is complicated and involves at least a component of immune-mediated erythrocyte destruction23, iron sequestration and iron restricted anemia, the classical hallmarks of AI, are also present24,25.
Malaria, caused by several Plasmodium species, is a frequent cause of anemia in tropical regions. Although hemolysis is an important contributor to the anemia, iron sequestration and suppression of erythropoiesis, characteristic of AI, are also important contributors. Further evidence of the role of AI in malaria is that urinary excretion of hepcidin is increased in patients with malaria26,27 whereas it would be suppressed by a purely hemolytic process accompanied by increased erythropoiesis28–30. Many Plasmodium species are able to infect mice and cause anemia. P. chabaudi infected red blood cells injected IP causes anemia after about five days that reaches a nadir at 10 days31,32. P. berghei causes severe anemia but is rapidly fatal without treatment33,34. P. yoelii infects mice, has not been shown to be a human pathogen and different strains produce differing severities of anemia and mortality35. Although Plasmodium infection differs significantly from AI because of the importance of hemolysis in the former, it is important to use these models to understand the role of iron sequestration and suppression of erythropoiesis in malaria. Furthermore, these infections may provide important insights into “classical” AI.
Pseudo-infectious Models
Working with true infections has the advantage of mimicking AI that occurs in humans with similar infections. However, there are several technical limitations that make non-infectious models more appealing. Some organisms, such as MTB, are known human pathogens, making them difficult to work with. Even if they are not pathogenic to humans, all the organisms discussed above could be transmitted to other mice requiring an increased level of isolation within the mouse colony. Infections are often less predictable in their timing and manifestation even within littermates. During our studies of peritoneal catheter infection models, we observed surprising variation in the size of the abscess, the intensity of systemic inflammation and the severity of the anemia. We and others have therefore focused on models of AI that employ immunogenic particles from infectious organisms. The chief disadvantage of these models is that the inflammatory response to these particles may not fully reflect the response to the intact organism.
Complete Freund’s Adjuvant (CFA) is an emulsion of dried and inactivated mycobacteria (Mycobacterium butyricum) in oil. As the name suggests, CFA has long been used as an adjuvant to aid in the preparation of antibodies. Intraperitoneal CFA injection causes an acute increase in hepcidin production and hypoferremia in mice36. CFA has long been known to produce anemia in rats37–39 and dogs40. We found that a single IP injection of 0.2 mL CFA emulsified in an equal amount of normal saline produced a fall in hemoglobin in one week that reached its maximum at approximately 3 weeks. Although this fall is mild (see figure), it is reproducible and the anemia is iron restricted as evidenced by a fall in MCV and an increase in blood zinc protoporphyrin. However, we have found significant variability in the potency of CFA between manufacturers and even between different lots by the same manufacturer. We therefore recommend that each batch of CFA be tested for its ability to produce anemia.
Brucella abortus causes brucellosis in cattle and humans. Investigators from Amgen reported that a single injection of the Brucella ring test antigen (U.S. Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories, Ames, Iowa) produces anemia in C57Bl/6 mice and that this anemia was iron restricted and partially prevented by anti-hepcidin antibody41. We have worked with this model as well and found it easy to use. The model is more acute but similar in severity to the CFA (see Figure) model and mice tolerate it well with no mortality and only occasional signs of chronic illness. Like in the CFA model, there is evidence of iron restriction but because of the rapidity with which the anemia develops, we suspect that there may be a significant component of increased red cell destruction.
Lipopolysaccharides (LPS) are components of the outer cell membrane of Gram-negative bacteria and elicit a potent inflammatory response when administered intravenously or IP. LPS induces hepcidin and causes hypoferremia within hours of administration in both humans 42 and mice 43. LPS administration followed by zymosan (a component of yeast cell walls) was reported to cause AI 44.
Non-infectious Models
Several inflammatory models exist that either mimic autoimmune diseases or produce inflammation by mechanisms that are not well understood. As an example of the latter, turpentine has been used to elicit an inflammatory response in animal models for more than 50 years. Turpentine is a distillate of the resins obtained from various trees and is a heterogenous mixture of organic compounds. It is generally injected subcutaneously and causes a brisk inflammatory response that peaks at approximately 16–24 hours after injection but persists for several days. Turpentine acutely induces hepcidin and causes hypoferremia 28,45,46. Repeated turpentine injections, generally on a weekly basis, produce anemia although hepcidin does not remain elevated45,47 (and unpublished data).
Collagen induced arthritis (CIA) is a model of rheumatoid arthritis created by sensitizing mice to their own collagen by injecting them with collagen (autologous or heterologous) in the presence of an adjuvant48. CIA, like active rheumatoid arthritis in humans, causes AI in mice10. Oral feeding of dextran sulfate sodium (DSS) for several days induces a colitis in mice that is a model of inflammatory bowel disease (IBD) in humans. DSS colitis, like IBD, is associated with anemia that resembles AI more than it does simple colonic bleeding10. Both of these models are useful for studying AI due to autoimmune disease but findings may not be generalizable to other forms of AI.
Transgenic Models
Transgenic models of AI use a specific overexpressed gene product or gene ablation to induce a condition that resembles AI. These models are the least generalizable of the models discussed so far. A key pathway proposed to cause AI is the IL-6 – hepcidin – ferroportin axis 46,49,50. In this model, AI is caused by overproduction of IL-6 inducing the production of hepcidin that then causes the internalization and degradation of the iron export protein ferroportin. According to this model, increase in IL-6 or hepcidin should cause a condition that resembles AI. Mice overexpressing IL-6 develop a disorder that resembles multicentric Castleman’s disease, including anemia that resembles AI51. In another transgenic model, overexpression of hepcidin also induces an iron-restricted anemia similar to AI2. Such models query whether a specific individual mediator can produce AI, or at least an iron-restricted anemia. Clearly, additional or alternative mediators may contribute to AI in more complex models or in human disease. Nevertheless, transgenic models can provide important insights into AI and may be beneficial in studying treatment modalities in the future.
NON-MOUSE MODELS
Other Mammals
Several rat models of AI exist. Although rats are larger, more expensive to purchase and maintain and are more difficult to manipulate genetically than mice, their large size may be beneficial when repeated blood sampling is needed or delicate survival surgeries are planned. CFA-induced anemia in rats has long been used as a model of AI37,38. Peptidoglycan (PG), from the cell wall of gram positive bacteria and carrageenan, isolated from red seeweeds, also cause AI in rats52. The PG model would appear to be a good model of the anemia seen in RA, as a single injection of PG in susceptible rats (Lewis) induces relapsing arthritis and anemia that lasts almost a year 52–54. This model is characterized by elevated hepcidin production and iron restricted anemia 55 but decreased red cell survival may play a role 56. The anemia in this model is partially reversed by treatment with an erythropoiesis stimulating agent 57. PG is a complex mixture of polymers and the specific preparation may be important in determining the ability to cause anemia.
Non-human primates are considered the “gold-standard” for animal models of human diseases. Models using non-human primates are fraught with technical difficulties including cost, slow breeding, technician safety, and ethical and regulatory difficulties. Cynomolgus monkeys develop anemia in response to CIA 58. Multiple infections likely cause anemia in non-human primates but these have not been studied extensively. As targeted therapeutics for AI are developed, primate models will increase in importance.
Non-mammalian (fish) models
Using non-mammalian models of AI increases the risk that the model may not apply to the human condition. However, having access to genetically distant models is important for comparative genomics. Also, some techniques may be particularly suited to non-mammalian organisms. Fish develop anemia in response to infection and non-infectious stress. Catfish exposed to Edwardsiella ictaluri develop anemia with increased hepcidin expression59. Bass with intraperitoneal injections of bacteria or upon exposure in the water to Streptococcus iniae have increased production of hepcidin60; however it has not been reported whether the bass develop anemia. Zebrafish are extremely useful animal models since they are very easy to manipulate genetically and the presence of anemia (if sufficiently severe) can be visualized in the transparent intact animal. Injection of bacteria into zebrafish does cause elevation of hepcidin61 but it has not been determined whether these fish develop anemia.
CONCLUSION
Because AI is not one simple disease but a complex response to diverse inflammatory conditions, it will require many models to study the disease. The choice of models must take into account the applicability to the human disease but should also take account of the practical aspects of cost, reproducibility, and existing information database about the model. Furthermore, when testing pathways that are thought to be necessary for the development of AI, several models may be necessary to assure that the findings are generalizable.
Acknowledgments
The work described in this manuscript was funded in part by the NIH NIDDK/NHLBI 1K08DK074284-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 2.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera S, Liu L, Nemeth E, Gabayan V, Sorensen OE, Ganz T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005;105:1797–1802. doi: 10.1182/blood-2004-08-3375. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen BV, Bota DP, Melot C, Vincent JL. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med. 2003;31:406–410. doi: 10.1097/01.CCM.0000048623.00778.3F. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 6.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 7.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 8.Anosa VO. Postsplenectomy blood values, marrow cytology, erythrocyte life-span, and sequestration in mice. Am J Physiol. 1976;231:1254–1257. doi: 10.1152/ajplegacy.1976.231.4.1254. [DOI] [PubMed] [Google Scholar]
- 9.Wallner SF, Vautrin R, Katz J. The haematopoietic response to burning: studies in a splenectomized animal model. Burns Incl Therm Inj. 1987;13:15–21. doi: 10.1016/0305-4179(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 10.Schubert TE, Obermaier F, Ugocsai P, Mannel DN, Echtenacher B, Hofstadter F, et al. Murine models of anaemia of inflammation: extramedullary haematopoiesis represents a species specific difference to human anaemia of inflammation that can be eliminated by splenectomy. Int J Immunopathol Pharmacol. 2008;21:577–584. doi: 10.1177/039463200802100310. [DOI] [PubMed] [Google Scholar]
- 11.Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25:888–895. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- 12.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30 (Suppl 1):53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 13.Freise H, Bruckner UB, Spiegel HU. Animal models of sepsis. J Invest Surg. 2001;14:195–212. doi: 10.1080/089419301750420232. [DOI] [PubMed] [Google Scholar]
- 14.Schubert T, Echtenacher B, Hofstadter F, Mannel DN. TNF-independent development of transient anemia of chronic disease in a mouse model of protracted septic peritonitis. Lab Invest. 2003;83:1743–1750. doi: 10.1097/01.lab.0000101693.12149.2c. [DOI] [PubMed] [Google Scholar]
- 15.Schubert TE, Echtenacher B, Hofstadter F, Mannel DN. Failure of interferon-gamma and tumor necrosis factor in mediating anemia of chronic disease in a mouse model of protracted septic peritonitis. Int J Mol Med. 2005;16:753–758. [PubMed] [Google Scholar]
- 16.Gallimore B, Gagnon RF, Subang R, Richards GK. Natural history of chronic Staphylococcus epidermidis foreign body infection in a mouse model. J Infect Dis. 1991;164:1220–1223. doi: 10.1093/infdis/164.6.1220. [DOI] [PubMed] [Google Scholar]
- 17.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–2640. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SW, Kang YA, Yoon YS, Urn SW, Lee SM, Yoo CG, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21:1028–1032. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–1753. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 20.Lounis N, Truffot-Pernot C, Grosset J, Gordeuk VR, Boelaert JR. Iron and Mycobacterium tuberculosis infection. J Clin Virol. 2001;20:123–126. doi: 10.1016/s1386-6532(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 21.Marquis JF, Lacourse R, Ryan L, North RJ, Gros P. Genetic and functional characterization of the mouse Trl3 locus in defense against tuberculosis. J Immunol. 2009;182:3757–3767. doi: 10.4049/jimmunol.0802094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ihedioha JI, Ochiogu IS, Ihedioha TE. Co-administration of Na-EDTA and diminazene aceturate (DA) to mice infected with DA-resistant Trypanosoma brucei. J Comp Pathol. 2007;136:206–211. doi: 10.1016/j.jcpa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Amole BO, Clarkson AB, Jr, Shear HL. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1982;36:1060–1068. doi: 10.1128/iai.36.3.1060-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emeribe AO, Anosa VO. Haematology of experimental Trypanosoma brucei gambiense infection. II. Erythrocyte and leucocyte changes. Rev Elev Med Vet Pays Trop. 1991;44:53–57. [PubMed] [Google Scholar]
- 25.Stijlemans B, Vankrunkelsven A, Brys L, Magez S, De BP. Role of iron homeostasis in trypanosomiasis-associated anemia. Immunobiology. 2008;213:823–835. doi: 10.1016/j.imbio.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 26.de MQ, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis. 2009;199:253–262. doi: 10.1086/595790. [DOI] [PubMed] [Google Scholar]
- 27.Howard CT, McKakpo US, Quakyi IA, Bosompem KM, Addison EA, Sun K, et al. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg. 2007;77:623–626. [PubMed] [Google Scholar]
- 28.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006 doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol Res. 2006 doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]
- 31.Thawani N, Tarn M, Stevenson MM. STAT6-mediated suppression of erythropoiesis in an experimental model of malarial anemia. Haematologica. 2009;94:195–204. doi: 10.3324/haematol.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb TJ, Langhorne J. The severity of malarial anaemia in Plasmodium chabaudi infections of BALB/c mice is determined independently of the number of circulating parasites. Malar J. 2008;7:68. doi: 10.1186/1475-2875-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes JK, Starnbach MN, Wirth DF. Secreted antibody is required for immunity to Plasmodium berghei. Infect Immun. 2009;77:414–418. doi: 10.1128/IAI.00982-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigario AM, Belnoue E, Gruner AC, Mauduit M, Kayibanda M, Deschemin JC, et al. Recombinant human IFN-alpha inhibits cerebral malaria and reduces parasite burden in mice. J Immunol. 2007;178:6416–6425. doi: 10.4049/jimmunol.178.10.6416. [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves J, Yoeli M, Nussenzweig RS. Immunological studies in rodent malaria. I: Protective immunity induced in mice by mild strains of Plasmodium berghei yoelii against a virulent and fatal line of this plasmodium. Ann Trop Med Parasitol. 1975;69:289–299. [PubMed] [Google Scholar]
- 36.Frazer DM, Wilkins SJ, Millard KN, McKie AT, Vulpe CD, Anderson GJ. Increased hepcidin expression and hypoferraemia associated with an acute phase response are not affected by inactivation of HFE. Br J Haematol. 2004;126:434–436. doi: 10.1111/j.1365-2141.2004.05044.x. [DOI] [PubMed] [Google Scholar]
- 37.Mikolajew M, Stachurska J, Kalczak M, Lewicka S, Kossakowska M. Hematologic changes in rats with adjuvant-induced disease. Intravascular clotting and fibrinolysis as a possible factor in the pathogenesis of anemia. Reumatologia. 1975;13:47–56. [PubMed] [Google Scholar]
- 38.Wang Q, Liao QK. Effect of nitric oxide on iron metabolism in rats with anemia of chronic disease. Zhongguo Shi Van Xue Ye Xue Za Zhi. 2003;11:385–389. [PubMed] [Google Scholar]
- 39.Asai F, Oshima T. Recombinant human erythropoietin, but not iron supplementation, improves anemia in rats with adjuvant-induced arthritis. Jpn J Pharmacol. 1991;57:291–298. doi: 10.1254/jjp.57.291. [DOI] [PubMed] [Google Scholar]
- 40.Feldman BF, Kaneko JJ, Farver TB. Anemia of inflammatory disease in the dog: ferrokinetics of adjuvant-induced anemia. Am J Vet Res. 1981;42:583–585. [PubMed] [Google Scholar]
- 41.Sasu B, Hainu M, Boone TC, Bi X-J, Lee KJ, Arvedson T, Winters A, Cooke K, Sheng Z. [1-30-2008];Hepcidin, hepcidin antagonists and methods of use. [Google Scholar]
- 42.Kemna EH, Kartikasari AE, van Tits LJ, Pickkers P, Tjalsma H, Swinkels DW. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis. 2008;40:339–346. doi: 10.1016/j.bcmd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Montosi G, Corradini E, Garuti C, Barelli S, Recalcati S, Cairo G, et al. Kupffer cells and macrophages are not required for hepatic hepcidin activation during iron overload. Hepatology. 2005;41:545–552. doi: 10.1002/hep.20620. [DOI] [PubMed] [Google Scholar]
- 44.Lasocki S, Millot S, Andrieu V, Letteron P, Pilard N, Muzeau F, et al. Phlebotomies or erythropoietin injections allow mobilization of iron stores in a mouse model mimicking intensive care anemia. Crit Care Med. 2008;36:2388–2394. doi: 10.1097/CCM.0b013e31818103b9. [DOI] [PubMed] [Google Scholar]
- 45.Sun XF, Zhou DB, Zhao YQ. An iron regulator hepcidin is affected by EPO. Zhongguo Shi Van Xue Ye Xue Za Zhi. 2006;14:778–782. [PubMed] [Google Scholar]
- 46.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raja KB, Duane P, Peters TJ. Effects of turpentine-induced inflammation on the hypoxic stimulation of intestinal Fe3+ absorption in mice. Int J Exp Pathol. 1990;71:785–789. [PMC free article] [PubMed] [Google Scholar]
- 48.Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. doi: 10.1385/1-59259-771-8:207. [DOI] [PubMed] [Google Scholar]
- 49.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 50.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 51.Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–311. doi: 10.1006/cyto.2002.2012. [DOI] [PubMed] [Google Scholar]
- 52.Sartor RB, Anderle SK, Rifai N, Goo DA, Cromartie WJ, Schwab JH. Protracted anemia associated with chronic, relapsing systemic inflammation induced by arthropathic peptidoglycan-polysaccharide polymers in rats. Infect Immun. 1989;57:1177–1185. doi: 10.1128/iai.57.4.1177-1185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009 doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 54.Coccia MA, Cooke K, Stoney G, Pistillo J, Del CJ, Duryea D, et al. Novel erythropoiesis stimulating protein (darbepoetin alfa) alleviates anemia associated with chronic inflammatory disease in a rodent model. Exp Hematol. 2001;29:1201–1209. doi: 10.1016/s0301-472x(01)00723-8. [DOI] [PubMed] [Google Scholar]
- 55.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009 doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 56.Foller M, Biswas R, Mahmud H, Akel A, Shumilina E, Wieder T, et al. Effect of peptidoglycans on erythrocyte survival. Int J Med Microbiol. 2009;299:75–85. doi: 10.1016/j.ijmm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Coccia MA, Cooke K, Stoney G, Pistillo J, Del CJ, Duryea D, et al. Novel erythropoiesis stimulating protein (darbepoetin alfa) alleviates anemia associated with chronic inflammatory disease in a rodent model. Exp Hematol. 2001;29:1201–1209. doi: 10.1016/s0301-472x(01)00723-8. [DOI] [PubMed] [Google Scholar]
- 58.Uchiyama Y, Koike N, Mihara M. Anemia in monkey collagen-induced arthritis is correlated with serum IL-6, but not TNFalpha. Rheumatol Int. 2008;28:879–883. doi: 10.1007/s00296-008-0547-2. [DOI] [PubMed] [Google Scholar]
- 59.Hu X, Camus AC, Aono S, Morrison EE, Dennis J, Nusbaum KE, et al. Channel catfish hepcidin expression in infection and anemia. Comp Immunol Microbiol Infect Dis. 2007;30:55–69. doi: 10.1016/j.cimid.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Shike H, Lauth X, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, et al. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. Eur J Biochem. 2002;269:2232–2237. doi: 10.1046/j.1432-1033.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 61.Shike H, Shimizu C, Lauth X, Burns JC. Organization and expression analysis of the zebrafish hepcidin gene, an antimicrobial peptide gene conserved among vertebrates. Dev Comp Immunol. 2004;28:747–754. doi: 10.1016/j.dci.2003.11.009. [DOI] [PubMed] [Google Scholar]