Abstract
The lymphatic system is essential for fluid homeostasis, immune responses, and fat absorption, and is involved in many pathological processes, including tumor metastasis and lymphedema. Despite its importance, progress in understanding the origins and early development of this system has been hampered by lack of defining molecular markers and difficulties in observing lymphatic cells in vivo and performing genetic and experimental manipulation of the lymphatic system. Recent identification of new molecular markers, new genes with important functional roles in lymphatic development, and new experimental models for studying lymphangiogenesis has begun to yield important insights into the emergence and assembly of this important tissue. This review focuses on the mechanisms regulating development of the lymphatic vasculature during embryogenesis.
INTRODUCTION
The lymphatic system is an endothelium-lined network of blind-ended capillaries found in nearly all tissues, draining via collecting vessels into large vascular trunks that eventually empty via an evolutionarily conserved drainage point into the blood circulatory system. Other than these conserved drainage connections, the lymphatic system is entirely separate and anatomically distinct from the blood circulatory system, although lymphatic vessels are frequently found in close juxtaposition to veins and arteries. The existence of the lymphatic system has been appreciated since antiquity. Hippocrates first described vessels containing “white blood” around 400 B.C., and Gasparo Aselli re-identified lymphatic vessels in the 1600’s, noting the presence of lipid-filled “milky veins” in the gut of a “well-fed” dog (Aselli, 1627). In the early 20th century, classical vascular anatomists such as Florence Sabin used dye injection and histological methods to characterize the anatomy of the lymphatic system in detail, showing that it innervates and ramifies throughout nearly every part of the body, forming a secondary vascular system arguably as complex as the circulatory system (Sabin, 1902; Sabin, 1909).
The lymphatic system has been shown to have a number of important functions. It transports fluids, plasma macromolecules, and cells extravasated from blood vessels, returning them back into the blood circulation and preventing their build-up in tissues throughout the body. Defects in the lymphatic system, whether congenital (primary lymphedema, relatively uncommon) or acquired (secondary lymphedema, a common complication of surgery and certain parasitic infections), can result in severe, disfiguring edema of affected tissues. The lymphatic system is also a major route for absorption of lipids from the gut (hence the “milky” appearance of the vessels that led to their early identification). Lymphatics are a critical component of the immune system, transporting white blood cells and antigens from distant sites to lymphoid organs. Recent evidence also indicates that the lymphatic system is a major pathway for the dissemination of metastatic cells, making them an important new focus of efforts to develop effective cancer therapies.
Despite its importance, the formation of the lymphatic system has remained relatively obscure in comparison to the blood vasculature (Oliver and Alitalo, 2005). This is partly due to the intense research focus recently placed on blood vessels, but also due to a number of technical challenges in studying lymphatics. Lymphatic vessels are frequently difficult to visualize in histology or electron micrographs because, unlike blood vessels, they are often irregular and collapsed. When they are observed, lymphatic vessels are frequently difficult to distinguish definitively from blood vessels. Defects in the lymphatic system often occur relatively late in postnatal life and are slow in onset, making it challenging to assess the proximal etiology of lymphatic disorders. Molecular identification of lymphatic vessels has only recently become possible. Although most molecular markers of lymphatic endothelial cells are shared with blood endothelial cells, in the past few years a small number of genes have been identified whose expression within lymphatic endothelium is diagnostic for these cells (although none of the genes are expressed exclusively within the lymphatic endothelium). Some of these genes have also been shown to be required for proper formation of the lymphatic system, mostly through murine knockout studies.
This review will focus on the mechanisms that regulate the development of the lymphatic vasculature during embryogenesis. Recently, there has been a relative explosion in the number of studies that focus on various aspects of this process. This is not surprising, given that lymphatics play an integral role in tissue homeostasis, immunity, and cancer metastasis. This review will attempt to examine and highlight some of the most recent advances in understanding the mechanisms that govern lymphatic development.
LYMPHATICS THROUGHOUT THE VERTEBRATES
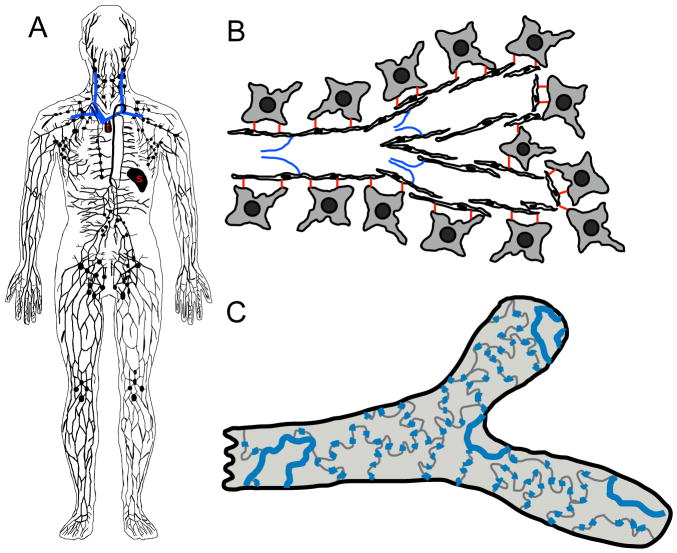
The lymphatic system is a vascular network comprised primarily of thin-walled, blind-ended capillaries (Fig. 1). These capillaries are made up of a single-cell layer of extensively overlapping endothelial cells with endothelial cell leaflets linked by discontinuous button-like endothelial cell-cell junctions which open in response to increased interstitial fluid pressure (Baluk et al., 2007). This structure, together with the lack of a basement membrane and supporting smooth muscle cells or pericytes, makes lymphatic capillaries highly permeable to the protein-rich lymph fluid. Lymphatic capillaries also possess specialized structures called anchoring filaments, extracellular fibrillar structures linking lymphatic endothelial cells to surrounding matrix and tissues (Gerli et al., 1990; Leak and Burke, 1966) (Fig. 1). These anchoring filaments also help to keep the lymphatic capillaries open and increase their permeability as interstitial pressure rises (Rossi et al., 2007). The lymphatic capillaries converge into precollecting lymphatic vessels, which carry lymph to the main collecting trunks (e.g., thoracic duct) for return to the venous circulation via the anastomosis with the cardinal vein. Unlike lymphatic capillaries, pre-collecting and collecting trunks contain smooth muscle cells and pericytes. Collecting lymphatics also have internal valves to prevent retrograde flow of lymph fluid. Lymphatics are generally found in most tissues innervated by the blood vascular system, often in very close association with blood vessels. One notable exception to this is the central nervous system, which lacks lymphatic vessels. Although lymphatic vessels do not contain red blood cells, they do contain lymphoid cells, and depending on the species, the lymphatic system also includes lymphoid organs important for immune responses, such as lymph nodes, tonsils, Peyer’s patches, spleen, and thymus.
Figure 1.
Characteristics of the lymphatic vasculature. (A) An overview of the human lymphatic system, including lymphatic vessels, lymph nodes, and lymphoid tissue (s-spleen, t-thymus). Major veins into which the lymphatics drain are shown in blue. (B) The lymphatic endothelial cells attach directly to the extracellular matrix and surrounding cells via anchoring filaments (red). Valves (blue) prevent lymph reflux to promote unidirectional lymph propulsion. Note the extensive overlap of adjacent endothelial cells in lymphatic capillaries. (C) Surface view of a lymphatic capillary emphasizing the loose, button-like intercellular junctions (blue).
Comparative phylogenetic analysis has shown that a true lymphatic vascular system is present only in the vertebrates (Isogai et al., 2009; Kampmeier, 1969). A lymphatic-like secondary vascular system is found in primitive fish, but it contains blood and is still considered to be part of the circulatory system. Teleost fish are arguably the first vertebrates with an anatomically distinct lymphatic system (Isogai et al., 2009; Yaniv et al., 2006). The lymphatics of amphibians and reptiles are similar to those of fish, although they often have specialized propulsive organs for the lymph called “lymph hearts”, small contractile structures containing striated muscle fibers (Rusznyak et al., 1960). Lymph hearts are absent in mammals and adult birds, and are only exceptionally found in fish (Rusznyak et al., 1960). The lymphatic system of amphibians, reptiles, and fish often appears as a large system of interconnected sacs or sinuses, rather than a defined network of tubular vessels. Unlike fish and frogs, lymphoid tissues are connected to the lymphatic vascular system in birds, although there are only a very small number of lymph nodes. Unlike most lower vertebrates, birds and mammals have lymphatic networks consisting of distinct, narrow, thin-walled tubular lymphatic vessels. Mammalian lymphatics generally contain large numbers of valves and many lymph nodes.
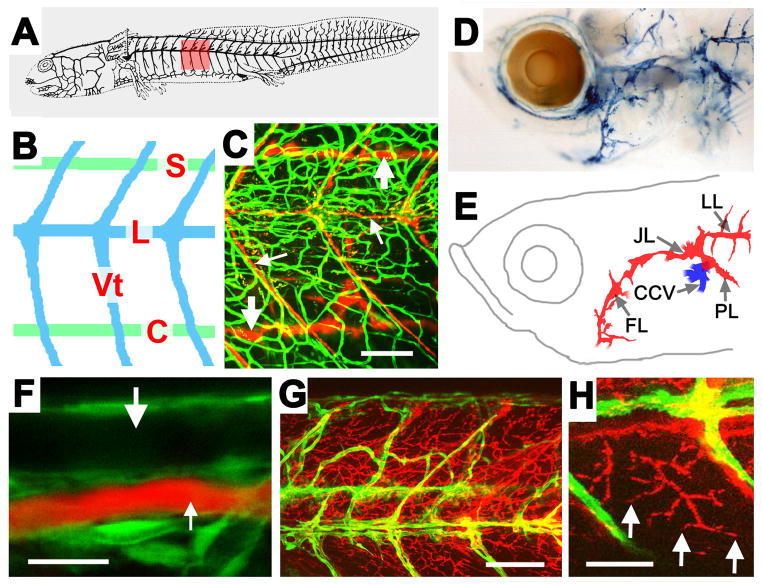
The lymphatic system has recently been characterized in a number of important developmental model organisms. The emergence of lymphatics and their requirement for lymphatic regulatory genes has been examined in Xenopus embryos (Ny et al., 2005). Recent reports have also demonstrated that the zebrafish has a well-defined lymphatic vascular system that shares many of the morphological, molecular, and functional characteristics of the lymphatic vessels found in other vertebrates (Kuchler et al., 2006; Yaniv et al., 2006). A distinct, anatomically conserved system of lymphatics is present with an overall pattern and morphology resembling that found in other developing vertebrates (Fig. 2). Zebrafish lymphatics express known markers of lymphatic endothelial cell fate, and require the function of genes known to be important for lymphangiogenesis in mice and other species. Importantly, fish lymphatics are also able to drain and collect subcutaneously injected dyes, as reported for the lymphatics of other species (Ny et al., 2005; Tilney, 1971) (Fig. 2). The identification of a lymphatic system in the zebrafish is an important finding, given the accessibility of the fish to forward-genetic analysis and high-resolution in vivo imaging of the vasculature.
Figure 2.
Anatomical and functional characterization of zebrafish lymphatic vessels. (A, B) Diagram of the superficial (A) and major conserved superficial (blue) and deeper (green) lymphatics (B) of the salamander, including lateral (L) and vertebral (Vt) superficial, and spinal (S) and collateral cardinal (C) deeper lymphatics. (C) 5 week old Tg(fli1:EGFP)yl zebrafish (green vessels) with fluorescent microspheres injected into the lymphatic vascular system (red lymphatics), showing major trunk superficial (small arrows) and deeper (large arrows) lymphatics similar to those in panel A. (D, E) Berlin blue dye injected into the lymphatic vascular system of a 5 week zebrafish (D) with explanatory diagram (E). As in other vertebrates, longitudinal lateral lymphatic (LL), pectoral lymphatic (PL), and facial lymphatic (FL) vessels come together to drain into the common cardinal vein (CCV). (F–H) Confocal imaging of an 18 dpf Tg(fli1:EGFP)yl zebrafish (green) injected subcutaneously with 2 Md rhodamine-dextran (red). (F) Subcutaneously injected rhodamine-dextran is taken up by lymphatic vessels and drains into the thoracic duct (small arrow), but does not label the adjacent dorsal aorta (large arrow). (G) Numerous rhodamine-dextran labeled vessels (red) are visible between the blood vessels (green). (H) Higher magnification image of blind-ended (arrows) rhodamine-dextran labeled vessels. Scale bars = 100 μm (C), 20 μm (F), 50 μm (G), or 100 μm (H). Images from Yaniv et al., Nature Medicine 12,711–716 (2006).
ORIGINS OF LYMPHATIC ENDOTHELIAL CELLS
The ontogeny of the lymphatic vascular system and the source of the lymphatic endothelial cells (LEC) of which it is comprised have been controversial. Classical pre-molecular era studies examining the origins of the lymphatics relied primarily on either injection (dyes, plastic resins) or serial sectioning methods to visualize and characterize the emergence of the earliest lymphatic vessels and lymph sacs. Use of these alternate methods early in the past century led to the formulation of two major types of models to explain the origins of lymphatics. Anatomists and embryologists using injection methods favored the view that the primary lymph sacs bud off from primitive veins, and that the lymphatic vessels grow out “centrifugally” from these sacs by endothelial budding and lymphangiogenesis. Florence Sabin was a major protagonist of this view (Sabin, 1902). Other investigators objected to these findings, noting that injections can only be made into an uninterrupted system of tubes, so that failure to demonstrate lymph vessels by way of injections does not necessarily mean that the injected area is devoid of lymphatics existing in the form of mesodermal clefts. In contrast, researchers using serial sectioning methods developed the alternative view that lymphatic vessels arise from mesenchymal spaces. The primary sacs arise, and the general systemic lymphatic vessels initially develop, along the course of the embryonic veins. Centripetal extensions subsequently make connections with the lymph sacs, which make secondary connections with the venous system. The “centripetal” model was championed by Huntington and McClure (1910). The methods of investigation leading to this concept were also criticized, on the grounds that any study of growing lymphatics in serial sections is unreliable because not all of the lymphatic endothelium present at any period of development can be seen in stained cross-sections and the identification of lymphatics is problematic.
The recent advent of molecular markers (see below) and methods for analyzing the formation of the lymphatics has permitted a more conclusive re-examination of the origins of LEC in a number of different developmental model organisms. The origins of avian lymphatics were studied using quail-chick embryo chimera grafting experiments (Wilting et al., 2006; Wilting et al., 2000). Endothelium of quail (but not chick) blood and lymphatic vessels can be marked using a specific antibody, QH1. Quail somites grafted into the wing level in chick embryo hosts contributed QH1/VEGFR3-positive lymphatic endothelial cells to the wing, suggesting that at least some peripheral lymphatic endothelial cells originate in paraxial/somitic mesoderm (Wilting et al., 2000). A subsequent study using quail-chick-chimeras and DiI-conjugated LDL labeling of blood vascular endothelium suggested that the deeper lymphatics of the jugular sac have a dual origin, arising both from adjacent veins and from lymphangioblasts derived from local mesoderm (Wilting et al., 2006). Analysis of staged expression of the lymphangioblast-LEC homeobox gene, Prox1, in Xenopus tadpoles suggests that LEC in this species also emerge from both veins and mesenchyme, although in this case the conclusions are based solely on marker expression patterns and not on grafting, lineage tracing, or other direct methods for examining the lymphangiogenic potential of specific tissues (Ny et al., 2005).
In mice, Prox1 is expressed beginning at approximately embryonic day 9.5 (E9.5) in a subpopulation of endothelial cells in the anterior cardinal vein. It is subsequently found in isolated cells that appear to be emerging from the vein, and then in adjacent lymph sacs, suggesting that the first LEC in mice have a venous origin (Wigle and Oliver, 1999). Recently, tamoxifen (TM)-inducible Cre/LoxP-based tracing was used to genetically mark early Prox1-expressing murine LEC progenitors, venous endothelial cells, and hematopoietic cells, examining the lymphangiogenic potential of each. The results of this study further substantiated the idea that venous endothelial cells are the major source of LEC during murine development (Srinivasan et al., 2007). Other reports have suggested alternative sources for LEC in mice. Scattered mesenchymal cells with leukocyte and lymphoendothelial characteristics first detected after E10.5 appear to eventually integrate into the lymphatics at later stages (Buttler et al., 2008; Buttler et al., 2006). In addition, a subpopulation of Syk- and Slp-76-expressing hematopoietic-derived cells has been found to be required cell-autonomously to maintain the separation between the blood and lymphatic vascular systems, although it is not clear whether these cells are actually contributing to lymphatic endothelium (Sebzda et al., 2006). Other reports have not found evidence for a hematopoietic contribution to murine LEC, however (Samokhvalov et al., 2007; Srinivasan et al., 2007; Stadtfeld and Graf, 2005). Thus, it seems likely that if there is a hematopoietic contribution to LEC during murine development, it is a minor component that appears relatively late and/or peripherally in the lymphatics. However, other reports do suggest that alternative sources of LEC can contribute to normal or pathologic lymphangiogenesis during postnatal life.
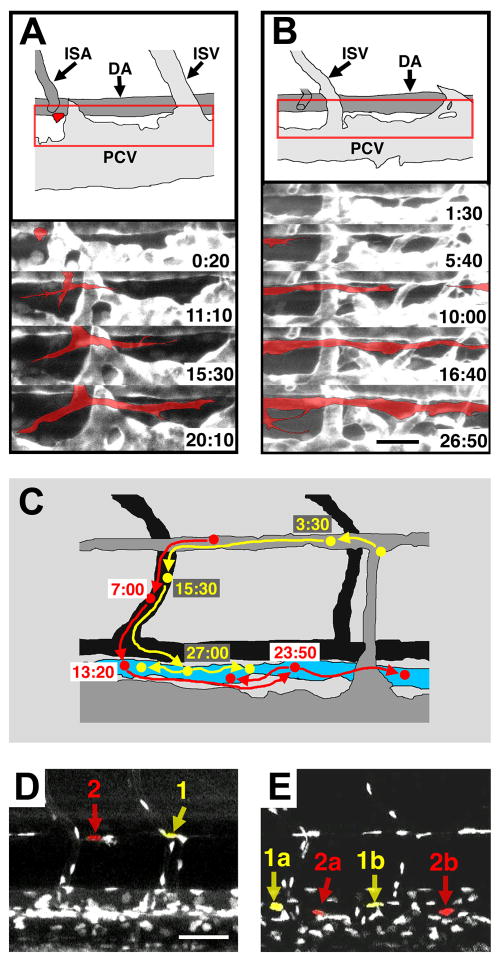
Perhaps the most definitive evidence for a venous origin for early lymphatic endothelial cells has come from the zebrafish (Yaniv et al., 2006). As noted above, recent studies have shown that the zebrafish possesses a lymphatic vascular system with many of the morphological, molecular, and functional characteristics of the lymphatics of other vertebrates. The molecular mechanisms that govern lymphatic development are also conserved, as exemplified by the absence of lymphatics in vegfC- (Kuchler et al., 2006; Yaniv et al., 2006) or prox1- (Yaniv et al., 2006) deficient embryos. The optical clarity of zebrafish embryos and larvae, the availability of transgenic zebrafish with fluorescently “tagged” vessels (Lawson and Weinstein, 2002), and the development of sophisticated methods for long-term time-lapse two-photon imaging (Kamei and Weinstein, 2005) make this a superb model for high-resolution dynamic optical imaging of vascular development in living animals (Cha and Weinstein, 2007; Kamei et al., 2004; Weinstein, 2002). Lineage tracing was performed on individual transgenically marked thoracic duct LECs using two-photon time-lapse imaging in order to trace precisely from where each cell came (Fig. 3). All of the cells examined (18 out of 18) arose from progenitors in the parachordal vessel, itself a derivative of the posterior cardinal vein. These results provide direct, conclusive evidence that the vast majority of cells contributing to the LEC of thoracic duct in the zebrafish arise from primitive veins (Yaniv et al., 2006).
Figure 3.
Assembly and origins of the zebrafish lymphatic thoracic duct visualized directly in the zebrafish. (A, B) Two-photon time-lapse imaging of formation of the trunk thoracic duct, collected from 2–4 dpf Tg(fli1:EGFP)yl zebrafish. Diagrams of the areas imaged (red box) at time zero are shown at top. Selected frames from the time-lapse sequences are shown below, with the lymphatic sprouts highlighted in red. (A) A lymphatic sprout for the thoracic duct emerging and then growing rostrally and caudally ventral to the dorsal aorta. (B) Lymphatic vessel sprouts growing across the trunk and merging ventral to the dorsal aorta. (C–E) Two-photon time-lapse imaging of EGFP-positive endothelial cell nuclei migrating to and incorporating into the thoracic duct in the trunk of a 2–4 dpf Tg(fli1:nEGFP)y7 zebrafish. (C) Explanatory diagram showing that two cells (yellow and red) migrate ventrally from the parachordal vessel to contribute to the lymphatic thoracic duct. The diagram shows the location of the cells and their daughters at different time points (in hours). (D, E) Actual images from the time-lapse sequence showing the positions of lymphatic progenitor cells (1, 2) and their daughters (1a, 1b, 2a, 2b) at time zero (D) and 30.5 hr after the start of the time lapse sequence (E). Scale bars = 25 μm (A, B), 50 μm (D, E). Images from Yaniv et al., Nature Medicine 12,711–716 (2006).
MOLECULAR REGULATION OF LYMPHATIC DEVELOPMENT
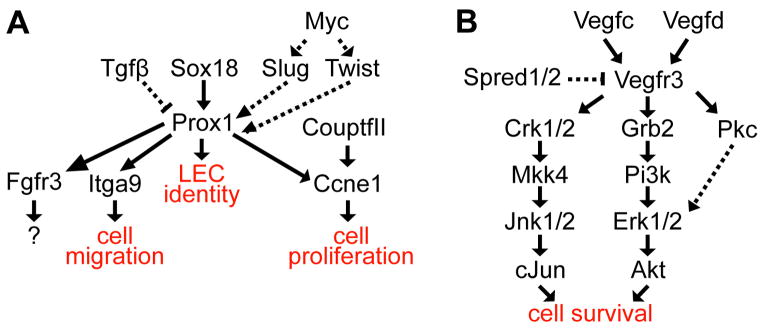
As alluded to above, application of molecular methods to lymphangiogenesis research has resulted in dramatic progress in the study of this traditionally somewhat elusive tissue. Many new molecular markers of the lymphatic endothelium have been identified (although few if any are absolutely specific to lymphatic vessels), and these markers have greatly facilitated experimental analysis of lymphatic vessel formation. Functional analysis of some of these genes has led to important new insights into the molecular regulation of lymphatic endothelial specification and lymphangiogenesis (Fig. 4), some of which are described below. The realization that some of these functionally important genes correspond to human lymphatic disease loci has begun to lead to advances in our understanding of and ability to treat lymphatic disorders.
Figure 4.
Simplified speculative upstream and downstream Prox1 (A) and Vegfr3 (B) signaling pathways in lymphatic endothelium. Solid arrows show activation and perpendicular lines show inhibition. Solid arrows show direct interactions, whereas dashed arrows show undefined interactions. The pathways shown are not comprehensive, nor are their functional outputs as discrete as presented (e.g., VegfC promotes both survival and migration of Vegfr3 expressing LRC).
Regulation of lymphatic endothelial cell specification
As described above, the use of lymphatic endothelial-specific markers has permitted a detailed assessment of the emergence of the initial LECs in murine embryos. Lyve1 (lymphatic vessel endothelial hyaluronan receptor 1), a gene identified in a search for new hyaluronan receptors, exhibited specific expression in LECs (Banerji et al., 1999). The first indication that lymphatic development is commencing is Lyve1 expression in a subpopulation of venous endothelial cells located in the cardinal vein (Wigle et al., 2002). Despite its early expression in these lymphatic endothelial progenitor cells, a normal lymphatic vasculature is formed in the absence of Lyve1, arguing against its involvement in establishing endothelial cell identity (Gale et al., 2007). Lymphatic development is also normal in mice lacking both Lyve1 and its homolog, Cd44 (both of which are expressed in LEC), indicating hyaluronin receptor function is not essential for LEC specification and lymphangiogenesis (Luong et al., 2009).
In contrast to Lyve1, the homeobox transcription factor, Prox1 (prospero-related homeobox 1), is required for specification of LECs. The requirement for Prox1 in lymphatic development was originally demonstrated by the absence of lymphatic vessels in Prox1 knockout mouse embryos (Wigle and Oliver, 1999). The absence of lymphatic vessels was determined to result from failure to specify LECs (Wigle et al., 2002). Misexpression of Prox1 in blood endothelial cells showed that Prox1 is sufficient to induce expression of LEC-specific genes, such as Pdpn/Gp38/T1alpha (podoplanin) and Vegfr3, while reducing expression of genes associated with BECs (Hong et al., 2002; Petrova et al., 2002). Prox1 expression is initiated in a subset of Lyve1+ venous endothelial cells in the anterior cardinal vein (Wigle and Oliver, 1999). Prox1 function in these venous endothelial precursors is required for lymphatic development, since conditional deletion of Prox1 in blood vessels causes lymphatic hypoplasia (Srinivasan et al., 2007). Not only is Prox1 required for the initial specification of LEC identity, but temporal inactivation of Prox1 has demonstrated the necessity of maintaining Prox1 in mature LECs (Johnson et al., 2008). Temporal inactivation of Prox1 during various points of embryonic and postnatal lymphangiogenesis resulted in loss of LEC identity and a reversion to BEC identity (Johnson et al., 2008). The requirement of Prox1 in mice, zebrafish, frogs, and chicks for LEC specification demonstrates that Prox1 function in the lymphatics has been conserved throughout evolution (Wigle, 1999; Yaniv, 2006; Ny, 2005).
The direct downstream targets regulated by Prox1 are mostly unknown. Cell culture studies in which Prox1 was expressed in BECs demonstrated strong induction of fibroblast growth receptor 3 (Fgfr3) (Shin et al., 2006). Prox1 demonstrated the ability to bind to the Fgfr3 promoter in culture and in vitro, suggesting it maybe a direct downstream target of Prox1 (Shin et al., 2006). Similar experiments showed that misexpression of Prox1 in differentiated embryonic stem (ES) cells and human umbilical vein endothelial cells (HUVECs) inhibited endothelial sheet formation, increased cell motility, and increased migration towards Vegfc (Mishima et al., 2007). Modest induction of integrin α-9 and inhibition of Prox1-induced cell migration by integrin α-9 antibody suggest it may be a target of Prox1 (Mishima et al., 2007). The future study of the genes regulated by Prox1 should yield additional genes necessary for lymphatic specification.
Recent studies indicate that Sox18 controls expression of Prox1 (Francois et al., 2008). SOX18, an SRY-related HMG domain transcription factor, was implicated in lymphatic development by the identification of SOX18 mutations in individuals with hypotrichosis-lymphedema-telangiectasia syndrome (Irrthum et al., 2003). Mice homozygous for both the Sox18 ragged-opossum and knockout alleles developed gross edema and failed to express Prox1 in endothelial cells of the cardinal vein (Francois et al., 2008). Lentiviral expression of Sox18 in both differentiating ES cells and blood vascular endothelial cells induced expression of Prox1 and Podoplanin (Francois et al., 2008). Further investigation determined that Sox18 directly controlled Prox1 expression by binding to its promoter both in vitro and in vivo (Francois et al., 2008). In addition to Sox18, the transcription factor Myc was shown to be required for Prox1 expression in Xenopus (Rodrigues et al., 2008). Myc morphants developed edema, which could be rescued with Slug and Twist mRNA (Rodrigues et al., 2008). Transforming growth factor-β (TGF-β) was shown to inhibit Prox1 expression and, ultimately, LEC identity by an undefined mechanism (Oka et al., 2008).
As described further below, vascular endothelial growth factor C (Vegfc) plays an integral role in the proliferation and migration of LECs. However, at least one report suggests that Vegfc may also play a role in LEC specification of cultured embryoid bodies (Kreuger et al., 2006). However, LECs are correctly specified in mice lacking Vegfc, making its in vivo role unclear, although another Vegfr3 ligand, Vegfd, could be compensating in this context (see below) (Karkkainen et al., 2004). In any case, further in vivo evidence is need to validate a specific role for Vegfc in lymphatic specification.
Proliferation and Migration of Lymphatic Endothelial Cells
Once endothelial cells have been specified from primitive veins, they migrate and proliferate to establish the initial lymph sacs and early lymphatic vessels such as the thoracic duct. The molecular mechanisms controlling budding and migration of LECs from embryonic veins to form lymph sacs have also been intensively studied. Vascular endothelial growth factor 3 (VEGFR3), a receptor tyrosine kinase, controls both cell proliferation and migration of LECs (recently reviewed in Lohela et al., 2009). Although initially expressed in both blood and lymphatic endothelial cells, Vegfr3 expression becomes restricted to LECs at E12.5 during mouse embryogenesis (Kaipainen et al., 1995). In humans, VEGFR3 mutations cause Milroy Disease, a form of congenital lymphedema resulting from hypoplasia of the lymphatic vessels (Karkkainen et al., 2000). Functional studies of mutant VEGFR3 demonstrated that ligand-induced autophosphorylation was decreased and acted in a dominant negative manner in the presence of wild type receptor (Karkkainen et al., 2000). All of the reported VEGFR3 mutations are located in tyrosine kinase domains disrupting autophosphorylation (listed in Butler et al., 2007). Surprisingly, mutations have not been identified in the VEGFR3 ligand binding domains or in VEGFC in Milroy patients (Connell et al., 2009). Like humans, mice with Vegfr3 mutations exhibit lymphatic hypoplasia and hindlimb edema (Karkkainen et al., 2001). Two growth factor ligands, Vegfc and Vegfd, bind to and activate signaling by Vegfr3 (Achen et al., 1998; Joukov et al., 1996; Joukov et al., 1997). Both transgenic and retroviral expression of Vegfc and Vegfd in mice are sufficient to stimulate lymphangiogenesis in the areas of misexpression (Byzova et al., 2002; Enholm et al., 2001; Jeltsch et al., 1997). Vegfc performs an indispensable function in LEC proliferation and migration, as demonstrated by the absence of LEC budding and formation of jugular lymph sacs in Vegfc knockout mice (Karkkainen et al., 2004). Unlike Prox1 knockout mice, LECs are correctly specified, as demonstrated by the normal expression of Lyve1, Prox1, and Vegfr3 in LEC. Treatment of embryoid bodies with a combination of Vegfc and Vegf is sufficient to generate LEC in venous endothelial cells, but the absence of Vegfc in the surrounding mesenchyme causes an arrest in LEC proliferation and migration (Karkkainen et al., 2004). In contrast to Vegfc, Vegfd knockout mice have no discernible lymphatic phenotype, suggesting that it plays only a minor role in developmental lymphangiogenesis (Baldwin et al., 2005). Vegfc/d morphant Xenopus embryos exhibit defective migration of LECs resulting in edema, a phenotype not present in either of the single morphants, suggesting at least partial functional overlap between these genes (Ny et al., 2008).
Upon receptor activation, Vegfr3 forms homodimers or heterodimers with Vegfr2, leading to receptor autophosphorylation (Dixelius et al., 2003). Vegfr3 signaling promotes cell survival, proliferation, and migration via both the PI3K and MAPK pathways (Makinen et al., 2001). The use of a chimeric EGFR/VEGFR3 receptor in HUVECs demonstrated that VEGFR3 activation inhibits growth factor starvation-induced apoptosis (Salameh et al., 2005). VEGFR3 activation recruited both CRK1/2 and GRB2 to different tyrosine residues, initiating CRK1/2-MKK4-JNK1/2-cJUN and GRB2-PI3K-ERK1/2-AKT signal transduction cascades to inhibit apoptosis (Salameh et al., 2005). Integrins, heterodimeric transmembrane receptors that interact with extracellular matrix ligands, contribute to VEGFC/D/VEGFR3 signaling. Cultured LECs had increased VEGFR3 phosphorylation and reduced apoptosis in response to Vegfc when cultured in fibronectin coated dishes, which could be blocked by Integrinα5β1antibody (Zhang et al., 2005). VEGFC and VEGFD are also bound by integrinα9β1 and activate phosphorylation of ERK1/2 and PAXILLIN in cultured endothelial cells, suggesting integrin activation is involved in LEC migration (Vlahakis et al., 2005).
One way to limit lymphatic growth is to disrupt Vegfc signaling. Neuropilin 2, a non-signaling transmembrane receptor, has been determined to act as a co-receptor for Vegfr3. Neuropilin2−/− mice fail to form normal lymphatic vessels and capillaries (Yuan et al., 2002). The membrane-associated proteins, Spred1 and Spred2 (Sprouty-related Ena/VASP homology1 domain-containing proteins), both suppress Erk activation by Vegfr3 (Taniguchi et al., 2007). Deletion of both Spred1 and Spred2 in Spred1−/−;Spread2−/− compound homozygotes results in edema, as well as an increase in the number of lymphatic vessels (Taniguchi et al., 2007).
In addition to Vegfc and Vegfd signaling, adrenomedullin (Amd) signaling is required for normal proliferation of LEC. Disruption of adrenomedullin signaling results in edema and embryonic lethality, as demonstrated in mice lacking Amd, Calcrl (calcitonin receptor-like), or Ramp2 (receptor activity modifying protein 2) (Fritz-Six et al., 2008). Conditional deletion of Calcrl in endothelium was sufficient to phenocopy the edema observed in the Calcrl−/− mice (Fritz-Six et al., 2008). Lymphatic vessels were normally specified in both Amd−/− and Ramp2−/−, but the lymph sacs were hypoplastic and lined with thin LECs (Fritz-Six et al., 2008). Studies with cultured LECs suggest that lymph sac hypoplasia might result from decreased proliferation of LECs due to the absence of Amd-mediated ERK phosphorylation (Fritz-Six et al., 2008). Interestingly, Prox1 overexpression in LECs induced both Calcrl and Ramp2, suggesting that Prox1 makes LECs responsive to Amd-mediated signaling (Fritz-Six et al., 2008). Whether Amd acts with or in addition to Vegfc is currently unknown.
Recent studies have suggested that CoupTFII, a transcription factor required for venous endothelial cell fate, has been found in LEC (Yamazaki et al., 2009; You et al., 2005). COUPTFII was shown to physically interact with PROX1 to control transcription of CCNE1 (CYCLIN E1), and was required to maintain expression of VEGFR3 (Yamazaki, 2009). CoupTFII also regulates lymphatic proliferation and migration by modulating Vegfc signaling. Misexpression of both PROX1 and COUPTFII in LECs causes a reduction in PROX1-mediated cell proliferation by reducing CCNE1 (CYCLINE1) expression (Yamazaki et al., 2009). The reduced expression of CCNE1 was shown to result from physical interaction of PROX1 and COUPTFII with the CCNE1 promoter (Yamazaki et al., 2009). COUPTFII also limited migration towards VEGFC by reducing expression of VEGFR3 (Yamazaki et al., 2009). Interesting, knockdown of endogenous COUPTFII caused a reduction in migration towards VEGFC and a reduction in VEGFR3 expression (Yamazaki et al., 2009). Therefore, precise dosage of COUPTFII seems to be required in LECs.
In the zebrafish, characterization of the full of fluid mutant has implicated ccbe1 (collagen and calcium binding EGF domain 1), a matrix-associated protein, in sprouting and migration of LECs (Hogan et al., 2009). A conservative amino acid change in the calcium EGF binding domain of the secreted (predicted) ccbe1 protein leads to the absence of the thoracic duct (Hogan et al., 2009). The ccbe1 gene is not expressed in endothelium, indicating that it functions non-autonomously (Hogan et al., 2009). The mechanism by which ccbe1 promotes migration of LECs remains to be determined.
Remodeling of the Nascent Lymphatic Plexus
The initial lymphatic vascular plexus undergoes extensive expansion and remodeling prior to and during postnatal life. Various genes have been shown to play important roles in this remodeling. The forkhead transcription factor, FOXC2 (formerly MFH1), was implicated in lymphatic vessel formation by the identification of FOXC2 mutations in humans as the cause of lymphedema-distichiasis syndrome (LD) (Fang et al., 2000). Unlike in Milroy disease, LD syndrome is characterized by onset of lymphedema at puberty, resulting from lymphatic reflux as indicated by lymphangioscintigraphy. Most FOXC2 mutations are frameshift mutations that likely result in null alleles showing that haploinsufficiency of FOXC2 causes lymphedema in humans (Bell et al., 2001; Erickson et al., 2001; Fang et al., 2000; Finegold et al., 2001). Like in humans, Foxc2+/− mice have abnormal lymphatic function indicated by lymphatic reflux of Evans blue dye (Kriederman et al., 2003). Despite being expressed in LECs in the lymph sac, lymphatic vessels are specified correctly in Foxc2−/− mice (Dagenais et al., 2004). Further study demonstrated that the absence of Foxc2 causes abnormal recruitment of smooth muscle cells to lymphatic capillaries as well as agenesis of lymphatic valves (Petrova et al., 2004). Recently, Foxc2 has been shown to play an integral role in the formation of mature lymphatic collectors (Norrmen et al., 2009). Using murine mesenteric lymphatic vessels as a model, a primitive capillary-like network of lymphatic vessels was shown to undergo morphological changes including the formation of valves, recruitment of mural cells and smooth muscle, and pruning of branches to form colleting lymphatics (Norrmen et al., 2009). These morphological changes were absent in Foxc2−/− mice, which maintained a primitive capillary-like plexus (Norrmen et al., 2009). Furthermore, analysis of genes involved in cardiac valve formation found that the transcription factor, Nfatc1, played a synergistic role with Foxc2 in this process (Norrmen et al., 2009). Nfatc1 is expressed in LECs throughout lymphatic development, and reduction of Nfatc1 nuclear localization due to cyclosporin A treatment caused poor organization of jugular lymph sacs and reduction of some LEC markers like podoplanin (Kulkarni et al., 2009).
A number of other genes have also been implicated in lymphatic remodeling and maturation. Like Foxc2, EphrinB2 (Efnb2) ligand is necessary for the maturation of lymphatic vessels (Makinen et al., 2005). EphrinB2 is differentially expressed in lymphatic collectors compared to lymphatic capillaries (Makinen et al., 2005). Efnb2 PDZ domain mutant mice have defects in lymphatic remodeling, unlike mice with complete deletion of Efnb2. Deletion of Aspp51 (apoptosis stimulating protein of p53) in mice caused subcutaneous edema resulting from impaired lymph drainage caused by dysmorphic lymphatic vessels (Hirashima et al., 2008). Proliferation and cell fate specification were normal, but the cell morphology was markedly different (Hirashima et al., 2008). Interestingly, absence of p53 had no effect on Aspp51 mutants, suggesting a yet unknown function for Aspp51 (Hirashima et al., 2008). With the exception of larger lymphatic channels, like the thoracic duct, LECs normally make direct contact with the extracellular matrix. Analysis of Emilin1, an extracellular matrix glycoprotein associated with elastic fibers, demonstrated that disrupting the attachments of LECs to the extracellular matrix leads to abnormal lymphatic vessels and lymphatic function (Danussi et al., 2008). Emilin1 knockout mice had more lymphatic vessels and abnormal vessel morphology that ultimately caused hindlimb edema (Danussi et al., 2008).
Separation of the Blood Vessels and Lymphatics
The lymphatic vasculature returns lymph into the blood vasculature via anastamoses between the thoracic duct and right lymphatic duct with the left and right subclavian veins, respectively. The study of Slp76 (SRC homology 2-domain–containing leukocyte protein of 76 kDa) and Syk (spleen tyrosine kinase) mutant mice provided the first insight into the mechanisms that prevent abnormal shunting between the lymphatics and blood vessels (Abtahian et al., 2003). In both Slp76 and Syk knockout mice, abnormal connections between blood vessels and lymphatics cause lymphatic vessels to fill with blood as early as E11.5, as demonstrated by blood-filled jugular lymph sacs (Abtahian et al., 2003). Using bone marrow transplant rescue experiments, gata promoter–driven transgenic hematopoietic expression of Slp76, and other methods, a number of studies have suggested that the absence of Slp76/Syk signaling, in a yet to be identified circulating cell type, is responsible for the lymphangiogenic defects (Abtahian et al., 2003) More recently, abnormal blood-filled lymphatics were observed in a spontaneous mutant of Plcg2 (Phospholipase Cγ2), termed abnormal lymphatics (ab) (Ichise et al., 2009). A Plcg2EGFP/EGFP knockin mouse model was utilized to identify abnormal anastomoses between the lymphatic and blood vessels causing the lymphatics to fill with blood (Ichise et al., 2009). Bone marrow transplantation from Plcg2EGFP/EGFP to lethally irradiated wild type mice was sufficient to generate shunting between lymphatics and blood vessels, but wild type bone marrow did not rescue all shunting when transferred to Plcg2EGFP/EGFP mice (Ichise et al., 2009). Consequently, the cell type requirement for Plcg2 function remains to be fully elucidated.
CONCLUSIONS
The application of modern molecular techniques has reinvigorated the investigation of the lymphatic system. Classic studies of the ontogenesis of lymphatic vessels proposed the centrifugal and centripetal models. Roughly a century later, the use of lymphatic-specific markers in model organisms has provided evidence for both models. Definitive evidence for the centrifugal model has been provided by the study of Prox1, as well as live imaging in zebrafish of the establishment of the thoracic duct by lymphatic endothelial precursors from the cardinal vein. Studies of lymphatic development in chick and frog support both the centrifugal and centripetal models. Likewise, the study of human genetic disease and murine knockout models has provided insight into some of the molecular mechanisms required for lymphatic specification, lymphangiogenesis, and lymphatic maturation. The establishment of zebrafish as a model for lymphatic development will allow forward genetic screens to identify additional genes involved in each of these processes. This will increase the number of candidate genes for genetic testing in cases of idiopathic forms of lymphedema, such as Meige disease, in addition to sporadic cases of lymphedema. Similarly, a better understanding of each of these processes will eventually lead to effective treatments for disorders of the lymphatic system.
References
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science (New York, NY. 2003;299(5604):247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aselli G. De Lacteibus sive Lacteis Venis, Quarto Vasorum Mesarai corum Genere novo invento. Milan: Mediolani; 1627. [Google Scholar]
- Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Molecular and cellular biology. 2005;25(6):2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of experimental medicine. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. The Journal of cell biology. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Brice G, Child AH, Murday VA, Mansour S, Sandy CJ, Collin JR, Brady AF, Callen DF, Burnand K, Mortimer P, Jeffery S. Analysis of lymphoedema-distichiasis families for FOXC2 mutations reveals small insertions and deletions throughout the gene. Human genetics. 2001;108(6):546–551. doi: 10.1007/s004390100528. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dagenais SL, Rockson SG, Glover TW. A novel VEGFR3 mutation causes Milroy disease. American journal of medical genetics. 2007;143A(11):1212–1217. doi: 10.1002/ajmg.a.31703. [DOI] [PubMed] [Google Scholar]
- Buttler K, Ezaki T, Wilting J. Proliferating mesodermal cells in murine embryos exhibiting macrophage and lymphendothelial characteristics. BMC developmental biology. 2008;8:43. doi: 10.1186/1471-213X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn. 2006;235(6):1554–1562. doi: 10.1002/dvdy.20737. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Goldman CK, Jankau J, Chen J, Cabrera G, Achen MG, Stacker SA, Carnevale KA, Siemionow M, Deitcher SR, DiCorleto PE. Adenovirus encoding vascular endothelial growth factor-D induces tissue-specific vascular patterns in vivo. Blood. 2002;99(12):4434–4442. doi: 10.1182/blood.v99.12.4434. [DOI] [PubMed] [Google Scholar]
- Cha YR, Weinstein BM. Visualization and experimental analysis of blood vessel formation using transgenic zebrafish. Birth Defects Res C Embryo Today. 2007;81(4):286–296. doi: 10.1002/bdrc.20103. [DOI] [PubMed] [Google Scholar]
- Connell FC, Ostergaard P, Carver C, Brice G, Williams N, Mansour S, Mortimer PS, Jeffery S. Analysis of the coding regions of VEGFR3 and VEGFC in Milroy disease and other primary lymphoedemas. Human genetics. 2009;124(6):625–631. doi: 10.1007/s00439-008-0586-5. [DOI] [PubMed] [Google Scholar]
- Dagenais SL, Hartsough RL, Erickson RP, Witte MH, Butler MG, Glover TW. Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr Patterns. 2004;4(6):611–619. doi: 10.1016/j.modgep.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Danussi C, Spessotto P, Petrucco A, Wassermann B, Sabatelli P, Montesi M, Doliana R, Bressan GM, Colombatti A. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Molecular and cellular biology. 2008;28(12):4026–4039. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. The Journal of biological chemistry. 2003;278(42):40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, Jackson DG, Yla-Herttuala S, Alitalo K. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circulation research. 2001;88(6):623–629. doi: 10.1161/01.res.88.6.623. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Dagenais SL, Caulder MS, Downs CA, Herman G, Jones MC, Kerstjens-Frederikse WS, Lidral AC, McDonald M, Nelson CC, Witte M, Glover TW. Clinical heterogeneity in lymphoedema-distichiasis with FOXC2 truncating mutations. Journal of medical genetics. 2001;38(11):761–766. doi: 10.1136/jmg.38.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. American journal of human genetics. 2000;67(6):1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Kimak MA, Lawrence EC, Levinson KL, Cherniske EM, Pober BR, Dunlap JW, Ferrell RE. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Human molecular genetics. 2001;10(11):1185–1189. doi: 10.1093/hmg/10.11.1185. [DOI] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. The Journal of clinical investigation. 2008;118(1):40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Molecular and cellular biology. 2007;27(2):595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R, Ibba L, Fruschelli C. A fibrillar elastic apparatus around human lymph capillaries. Anatomy and embryology. 1990;181(3):281–286. doi: 10.1007/BF00174621. [DOI] [PubMed] [Google Scholar]
- Hirashima M, Sano K, Morisada T, Murakami K, Rossant J, Suda T. Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Developmental biology. 2008;316(1):149–159. doi: 10.1016/j.ydbio.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nature genetics. 2009;41(4):396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225(3):351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Huntington G, McClure C. The anatomy and development of the jugular lymph sac in the domestic cat (Felis domestica) Am J Anat. 1910;10:177–311. [Google Scholar]
- Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development (Cambridge, England) 2009;136(2):191–195. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. American journal of human genetics. 2003;72(6):1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S, Hitomi J, Yaniv K, Weinstein BM. Zebrafish as a new animal model to study lymphangiogenesis. Anatomical science international/Japanese Association of Anatomists. 2009 doi: 10.1007/s12565-009-0024-3. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science (New York, NY. 1997;276(5317):1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes & development. 2008;22(23):3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. The EMBO journal. 1996;15(7):1751. [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. The EMBO journal. 1997;16(13):3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Isogai S, Weinstein BM. Imaging blood vessels in the zebrafish. Methods in cell biology. 2004;76:51–74. doi: 10.1016/s0091-679x(04)76004-5. [DOI] [PubMed] [Google Scholar]
- Kamei M, Weinstein BM. Long-term time-lapse fluorescence imaging of developing zebrafish. Zebrafish. 2005;2(2):113–123. doi: 10.1089/zeb.2005.2.113. [DOI] [PubMed] [Google Scholar]
- Kampmeier OF. Evolution and comparative morphology of the lymphatic system. Springfield: Charles C. Thomas Publisher; 1969. [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nature genetics. 2000;25(2):153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature immunology. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Nilsson I, Kerjaschki D, Petrova T, Alitalo K, Claesson-Welsh L. Early lymph vessel development from embryonic stem cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(5):1073–1078. doi: 10.1161/01.ATV.0000217610.58032.b7. [DOI] [PubMed] [Google Scholar]
- Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Human molecular genetics. 2003;12(10):1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16(12):1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kulkarni RM, Greenberg JM, Akeson AL. NFATc1 regulates lymphatic endothelial development. Mechanisms of development. 2009;126(5–6):350–365. doi: 10.1016/j.mod.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology. 2002;248(2):307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. The American journal of anatomy. 1966;118(3):785–809. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Current opinion in cell biology. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Luong MX, Tam J, Lin Q, Hagendoorn J, Moore KJ, Padera TP, Seed B, Fukumura D, Kucherlapati R, Jain RK. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. Journal of cellular physiology. 2009;219(2):430–437. doi: 10.1002/jcp.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes & development. 2005;19(3):397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. The EMBO journal. 2001;20(17):4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T, Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Molecular biology of the cell. 2007;18(4):1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, Augustin HG, Yla-Herttuala S, Alitalo K, Petrova TV. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. The Journal of cell biology. 2009;185(3):439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, Terclavers S, Ciesiolka M, Kalin R, Man WY, Senn I, Wyns S, Lupu F, Brandli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nature medicine. 2005;11(9):998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Ny A, Koch M, Vandevelde W, Schneider M, Fischer C, Diez-Juan A, Neven E, Geudens I, Maity S, Moons L, Plaisance S, Lambrechts D, Carmeliet P, Dewerchin M. Role of VEGF-D and VEGFR-3 in developmental lymphangiogenesis, a chemicogenetic study in Xenopus tadpoles. Blood. 2008;112(5):1740–1749. doi: 10.1182/blood-2007-08-106302. [DOI] [PubMed] [Google Scholar]
- Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111(9):4571–4579. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annual review of cell and developmental biology. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nature medicine. 2004;10(9):974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. The EMBO journal. 2002;21(17):4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CO, Nerlick ST, White EL, Cleveland JL, King ML. A Myc-Slug (Snail2)/Twist regulatory circuit directs vascular development. Development (Cambridge, England) 2008;135(11):1903–1911. doi: 10.1242/dev.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Weber E, Sacchi G, Maestrini D, Di Cintio F, Gerli R. Mechanotransduction in lymphatic endothelial cells. Lymphology. 2007;40(3):102–113. [PubMed] [Google Scholar]
- Rusznyak I, Foldi M, GS . Lymphatics and the lymph circulation. Oxford: Pergamon Press; 1960. [Google Scholar]
- Sabin F. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1:367–389. [Google Scholar]
- Sabin F. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. The American journal of anatomy. 1909;9:43–91. [Google Scholar]
- Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106(10):3423–3431. doi: 10.1182/blood-2005-04-1388. [DOI] [PubMed] [Google Scholar]
- Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- Sebzda E, Hibbard C, Sweeney S, Abtahian F, Bezman N, Clemens G, Maltzman JS, Cheng L, Liu F, Turner M, Tybulewicz V, Koretzky GA, Kahn ML. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Developmental cell. 2006;11(3):349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Molecular biology of the cell. 2006;17(2):576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & development. 2007;21(19):2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development (Cambridge, England) 2005;132(1):203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kohno R, Ayada T, Kato R, Ichiyama K, Morisada T, Oike Y, Yonemitsu Y, Maehara Y, Yoshimura A. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Molecular and cellular biology. 2007;27(12):4541–4550. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. Journal of anatomy. 1971;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. The Journal of biological chemistry. 2005;280(6):4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B. Vascular cell biology in vivo: a new piscine paradigm? Trends in cell biology. 2002;12(9):439–445. doi: 10.1016/s0962-8924(02)02358-9. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. The EMBO journal. 2002;21(7):1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M. Dual origin of avian lymphatics. Developmental biology. 2006;292(1):165–173. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Wilting J, Papoutsi M, Schneider M, Christ B. The lymphatic endothelium of the avian wing is of somitic origin. Dev Dyn. 2000;217(3):271–278. doi: 10.1002/(SICI)1097-0177(200003)217:3<271::AID-DVDY5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells. 2009;14(3):425–434. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nature medicine. 2006;12(6):711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435(7038):98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development (Cambridge, England) 2002;129(20):4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. Journal of cellular physiology. 2005;202(1):205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]