Abstract
BACKGROUND
Reperfusion of penumbral tissue is a promising strategy for treatment of acute cerebral ischemia more than 3 hours from symptom onset. However, there has been only sparse direct evidence that reperfusion after 3 hours prevents infarct growth.
METHODS
We analyzed clinical and serial magnetic resonance imaging (MRI) data on patients who received endovascular recanalization therapy 3–12 hours after last known well time. Multimodal MRIs were acquired pretreatment, early (1–20 hours), and late (2–7 days) after treatment. Degree of recanalization was assessed on end of procedure catheter angiogram, degree of reperfusion on early posttreatment perfusion MRI, and infarct growth by analysis of diffusion lesion volumes on pretreatment and late MRIs.
RESULTS
Twenty-seven (12 men, 15 women) underwent endovascular recanalization procedures at 6.0 ± 2.1 hours (range, 3.0–11.5 hours) after last known well time. Immediate posttreatment perfusion lesion (Tmax ≥4 seconds) volume correlated strongly with infarct growth (r = .951, P < .001), exceeding the correlations of vessel recanalization score (r = −.198, P = .446) and pretreatment diffusion-perfusion mismatch volume (r = .518, P = .033). Without reperfusion, enlargement of DWI lesion volume was observed in all patients, and extent of enlargement depended on volume of immediate posttreatment perfusion defects.
CONCLUSION
Our data indicate that posttreatment reperfusion is the major determinant of threatened tissue outcome, and suggest reperfusion even after 3 hours of symptom onset can alter tissue fate over a wide range of mismatch volumes.
Keywords: Stroke, ischemic, perfusion, thrombolysis, diffusion-weighted imaging, magnetic resonance imaging
Introduction
Recanalization therapy later than 3 hours after onset is a promising strategy for the treatment of late-presenting acute ischemic stroke patients. Treatment with intravenous tissue plasminogen activator in relatively unselected patients appeared beneficial up to at least 4.5 hours after onset in pooled analysis of randomized controlled trials, albeit with a declining benefit with time, attributed to a progressive disappearance of the ischemic penumbra.1 In clinical practice, the proportion of patients who are candidates for thrombolysis within 3 hours is low, and it has been reported that a relevant volume of salvageable tissue is present in >80% of stroke patients 3–6 hours after symptom onset.2,3,4 There have been several studies identifying magnetic resonance imagine (MRI) parameters and thresholds to delineate the ischemic penumbra, especially in stroke patients after 3 hours of symptom onset. The use of multiparametric MRI including diffusion-weighted (DWI) and perfusion-weighted imaging (PWI) to select patients for recanalization therapy in clinical practice is rapidly growing.5,6
Until recently, however, there has been little direct evidence that reperfusion after 3 hours of symptom onset prevents infarct growth. In this study, we performed serial DWI and PWI, MRIs, and assessed correlations of pretreatment mismatch and posttreatment reperfusion with final infarct growth.
Patients and Methods
Study Population
The present analysis was performed as part of a large prospective study of diffusion-perfusion MRI changes in patients receiving recanalization therapy (intravenous and/or intraarterial fibrinolytic therapy and/or mechanical clot retrieval therapy) for acute cerebral ischemia at an academic medical center. This analysis was performed on patients treated from October 2002 through December 2006. Inclusion criteria for this study were: (1) symptoms of acute cerebral ischemia within the middle cerebral artery territory, (2) endovascular recanalization therapy performed more than 3 hours from last known well time, and (3) performance of serial MRIs, including pretreatment, early (target 3–12 hours) posttreatment, and late (target 2–7 days) posttreatment DWI and PWI. Among patients who received endovascular recanalization therapy for acute cerebral ischemia after 3 hours of symptoms within middle cerebral artery territory during the study period, we excluded patients when (1) either pretreatment or follow up PWI at 3–12 hours were not performed, (2) DWI at days 3–5 posttreatment not obtained, (3) technically suboptimal imaging data, and (4) recurrent stroke occurred with newly developed DWI lesions on contralateral side or vertebrobasilar territory during hospitalization. The local Institutional Review Board approved the study.
All patients underwent complete diagnostic cerebral angiography, including injections of both internal carotid arteries and the dominant vertebral artery. Vessel recanalization was graded employing the Thrombolysis in Myocardial Infarction (TIMI) scale, with assignments of 3 (complete reperfusion), 2 (partial reperfusion), 1 (thrombus reduction without reperfusion), and 0 (no change).7
National Institutes of Health Stroke Scale (NIHSS) points were checked at the time of admission and 3 hours after endovascular recanalization therapy. Dramatic recovery was defined as an improvement of ≥10 NIHSS points or an improvement to ≤3 NIHSS points at 3 hours after treatment, based upon prior studies.8
MRI Methods and Image Analysis
The MRI (1.5T, Siemens Medical System, South Iselin, NJ) protocol included DWI, PWI, gradient-recalled echo examination, and fluid attenuated inversion recovery imaging. Patients underwent MRI prior to treatment, 3–12 hours following treatment, and 2–7 days following treatment. MRI methods have been described in detail elsewhere.9 Maps of Tmax were generated by deconvolution of an arterial input function and tissue concentration curves.10
Image analysis was carried out using an IDL software program designed for analysis of diffusion and perfusion data (Stroke Assessment Visualization Environment [SAVE], version 1.4.2). MRI volume measures were performed by one of the authors (Y.S.R.) who was blinded to the clinical information. We analyzed both Tmax ≥2 second and Tmax ≥4-second criteria for the perfusion defect. Prior studies have found that Tmax ≥2 seconds may tend to overestimate and Tmax ≥4 seconds to underestimate true tissue at risk.11 The results for Tmax ≥4 are shown as main tables and figures, and for Tmax ≥2 as supplemental tables. For each patient, perfusion and DWI lesion volumes were calculated with a computer-assisted volumetric analysis program (Medical Image Processing, Analysis and Visualization, version 2.1, CIT, NIH). Scans were not analyzed if the SAVE program was unable to register diffusion and perfusion sequences (patient motion) or to identify voxels for arterial input demonstrating high-quality tissue concentration time curves.
For dichotomized analyses, the presence of definite diffusion-perfusion mismatch was defined as pretreatment PWI lesion volume exceeding pretreatment DWI lesion volume [(PWI volume-DWI volume)/PWI volume × 100] by 20% or more, marked reperfusion as early posttreatment perfusion defect lesion volume being smaller than pretreated diffusion lesion volume, and occurrence of infarct growth as late (2–7 days) posttreatment diffusion lesion volume exceeding pretreatment by 20% or more.
The relationships of perfusion parameters andmismatch volume to infarct growth during the follow-up period were evaluated using Spearman correlation coefficients and simple linear regression analysis, as appropriate. Probability values less than .05 were accepted as significant.
Results
Among the 27 enrolled patients, 12 were men, with an average age of 63.3 ± 22.9 years. In two patients, the last known well time and the symptoms first observed time differed as onset was during sleep. In the remainder, the last known well time coincided with symptoms of the first observed time. Pretreatment MRIs were completed at 4.2 ± 2.0 hours (range, 1.1–8.0 hours) after last known well time. Posttreatment MRIs were performed 6.2 ± 3.8 hours (range, .9–19.4 hours) and 4.7 ± 2.1 days (range, 1.1–9.2 days) after recanalization therapy. Recanalization treatments were: Merci Clot Retriever alone in 18; intraarterial t-PA alone in 2; combined intravenous and intraarterial t-PA in 1; combined intravenous t-PA and Merci Retriever in 3; and combined intravenous t-PA, intraarterial t-PA, and Merci Retriever in 3. The time interval from last known well time to start of endovascular therapies wasmean 6.0 ± 2.2 hours (range, 3.0–11.5 hours). Partial or complete recanalization (TIMI 2–3) was achieved in 19 of the 27 patients. Patients’ characteristics are shown in Table 1.
Table 1.
Patients’ Characteristics
| Age/Sex | Stroke Mechanism | NIHSS | Mode of Treatment | TIMI grade |
DWI Lesion Volume (mL) |
PWI Lesion Volume (mL) |
||
|---|---|---|---|---|---|---|---|---|
| PreTx | Final | PreTx | PostTx | |||||
| M/85 | Cardioembolic | 19 | CR | 1 | 118 | 338 | 308.9 | 186.2 |
| F/51 | Cardioembolic | 21 | CR | 2 | 23 | 89 | 125.4 | 51.8 |
| M/71 | Cardioembolic | 17 | IV tPA + CR | 2 | 76 | 130 | 82.9 | 88.8 |
| M/76 | Cardioembolic | 19 | CR | 0 | 42 | 107 | 130.5 | 23.1 |
| M/57 | Cardioembolic | 6 | IV + IA tPA + CR | 2 | 2.2 | 31.3 | 31.1 | .0 |
| M/57 | Atherothromboembolix | 18 | CR | 2 | 18 | 179 | 153.3 | 243.8 |
| F/46 | Atherothromboembolix | 8 | IV tPA + CR | 1 | 5 | 25 | 29.6 | 9.6 |
| F/95 | Cardioembolic | 22 | CR | 2 | 27 | 52 | 102.7 | 75.6 |
| F/26 | Cardioembolic | 18 | CR | 2 | 97 | 133 | 81.5 | 22.7 |
| F/86 | Cardioembolic | 14 | IV + IA tPA + CR | 2 | 6.2 | 14.6 | 89.5 | .0 |
| M/62 | Cardioembolic | 10 | CR | 2 | 20 | 29 | 6.3 | 6.5 |
| M/78 | Atherothromboembolix | 11 | CR | 2 | 1 | 4 | 86.8 | 11.5 |
| M/41 | Atherothromboembolix | 27 | IV t-PA + CR | 3 | 230 | 265 | 149.7 | 5.0 |
| F/88 | Cardioembolic | 22 | CR | 3 | 13 | 6 | 137.7 | .6 |
| F/89 | Cardioembolic | 14 | CR | 2 | 7 | 11 | 2.6 | .0 |
| M/53 | Cardioembolic | 10 | IV +IA t-PA | 3 | 2 | 2 | 85.9 | .0 |
| M/64 | Atherothromboembolix | 7 | IA t-PA | 0 | 9.1 | 4.0 | 70.6 | 8.2 |
| M/15 | Cardioembolic | 11 | CR | 3 | 12.8 | 15.4 | 142.6 | .5 |
| F/23 | Dissection | 13 | IA tPA | 1 | 13.8 | 41.9 | 7.2 | 6.9 |
| F/76 | Cardioembolic | 18 | CR | 3 | 45 | 51.3 | 189.8 | 9.2 |
| F/95 | Cardioembolic | 8 | CR | 3 | .5 | .0 | .2 | 1.1 |
| F/47 | Cardioembolic | 19 | CR | 1 | 68.9 | 126.3 | 109.6 | 22.2 |
| F/82 | Cardioembolic | 20 | IV tPA + CR | 2 | 43 | 29 | 88.7 | 48.3 |
| F/71 | Atherothromboembolix | 22 | CR | 0 | 68 | 102 | 136.5 | 68.4 |
| F/84 | Cardioembolic | 23 | CR | 2 | 35.6 | 120 | 22.2 | 65.3 |
| M/29 | Cardioembolic | 9 | CR | 2 | 62.2 | 144.5 | 144.2 | 130.7 |
| F/61 | Cardioembolic | 10 | CR | 1 | 2.2 | 19.2 | 65.6 | 25.8 |
NIHSS = The National Institutes of Health Stroke Scale on admission; PreTx = pretreatment; postTx = immediate posttreatment; CR = Mechanical clot retrieval therapy; IV t-PA = intravenous tissue plasminogen activator; IA t-PA = intraarterial tissue plasminogen activator.
Pretreatment diffusion lesions were 38.8 ± 49.7 mL (range, .5–230.1 mL), pretreatment perfusion lesions 95.6 ± 68.1 mL (range, .2–308.9 mL), and pretreatment mismatch volumes (PWI volume-DWI volume) 61.8 ± 53.7 mL (range, 0– 190.9 mL). Definite pretreatment mismatch was present in 19 patients (70.4%). Early (3–12 hours) posttreatment diffusion lesion volumes were 53.8 ± 64.5 mL (range, 0–256.5mL), and perfusion lesion volume 41.2 ± 60.7 mL (range, .1–243.8 mL). Marked reperfusion was achieved in 13 (48.1%) patients. Late (2–7 day) diffusion lesion volumes were 80.7 ± 91.6 mL (range, 0–338.0 mL). Infarct growth across all patients averaged 222.3 ± 390.4% (range, −100.0–1516.2%). Infarct growth greater than 20% of pretreatment diffusion lesion volume occurred in 21 patients (77.8%). The proportion of mismatch regions that evolved to infarct (days 2–7) averaged 86.5 ± 170.6% (range, −30.4– 766.8%).
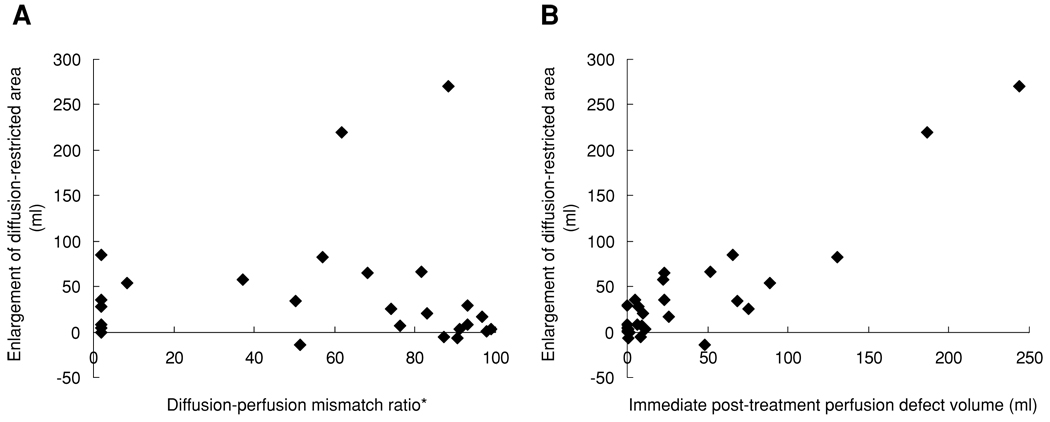
Table 2 shows the correlation of infarct growth with various potential clinical and imaging predictors. A Kolmogorov-Smirnov test showed that the distribution of volumes of infarct growth was normally distributed. Infarct growth correlated very highly with early (3–12 hours) posttreatment perfusion lesion volume (r = .907, P < .001), substantially more closely than with pretreatment diffusion-perfusion mismatch volume (r = .417, P = .031) (Fig 1). Among pretreatment MRI parameters, perfusion lesion volume (r = .529, P = .005) correlated more closely with infarct growth than diffusion-perfusion mismatch volume (r = .417, P = .031) or diffusion lesion volume (r = .265, P = .182). The importance of early posttreatment perfusion lesion volume was robust across alternative thresholds for definition of perfusion defect, employing Tmax ≥2 seconds and Tmax ≥6 seconds. With these cutoffs, early posttreatment perfusion defect volume also was a better predictor for infarct growth than pretreatment diffusion-perfusion mismatch or pretreatment DWI lesion volume (supporting table).
Table 2.
Predictors of Infarct Growth
| Predictor Variable | Correlation Coefficient |
P |
|---|---|---|
| Age | −.035 | .861 |
| NIHSS score on admission | .271 | .171 |
| Glucose | .152 | .449 |
| Pretreatment DWI lesion volume | .265 | .182 |
| Pretreatment PWI lesion volume | .529 | .005 |
| Pretreatment PWI-DWI Mismatch volume |
.417 | .031 |
| TIMI vessel grading | −.256 | .198 |
| Early posttreatment PWI Lesion volume | .907 | <.001 |
Fig 1.
Correlation between (A) pretreatment diffusion-perfusion mismatch volume and infarct growth, and (B) immediate posttreatment perfusion defect and infarct growth.
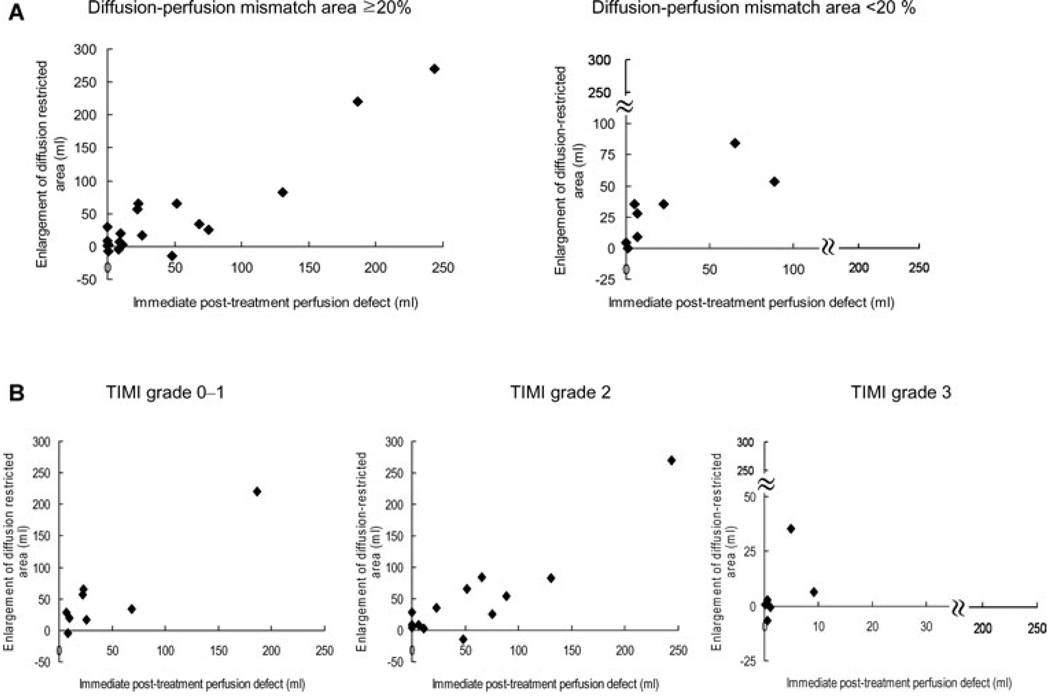
Infarct growth less than 20% was numerically more frequently observed in patients with marked reperfusion than in patients with absence of it (38.5% vs. 14.3%), although this difference did not reach statistically significant due to small numbers of patients (P = .209). Such association was consistently found regardless of treatment time (3.0–11.5 hours) (Fig 2). The close association of early posttherapy perfusion lesion volume and infarct growth was observed both in patients with and without definite pretreatment diffusion-perfusion mismatch (r = .918, P < .001 for patients with mismatch and r = .807, P = .016 for no definite mismatch) (Fig 3A). However, the degree of infarct growth was not different between patients without and with definite pretreatment diffusion-perfusion mismatch (31.3 ± 26.4 mL vs. 46.4 ± 75.6 mL, P = .696).
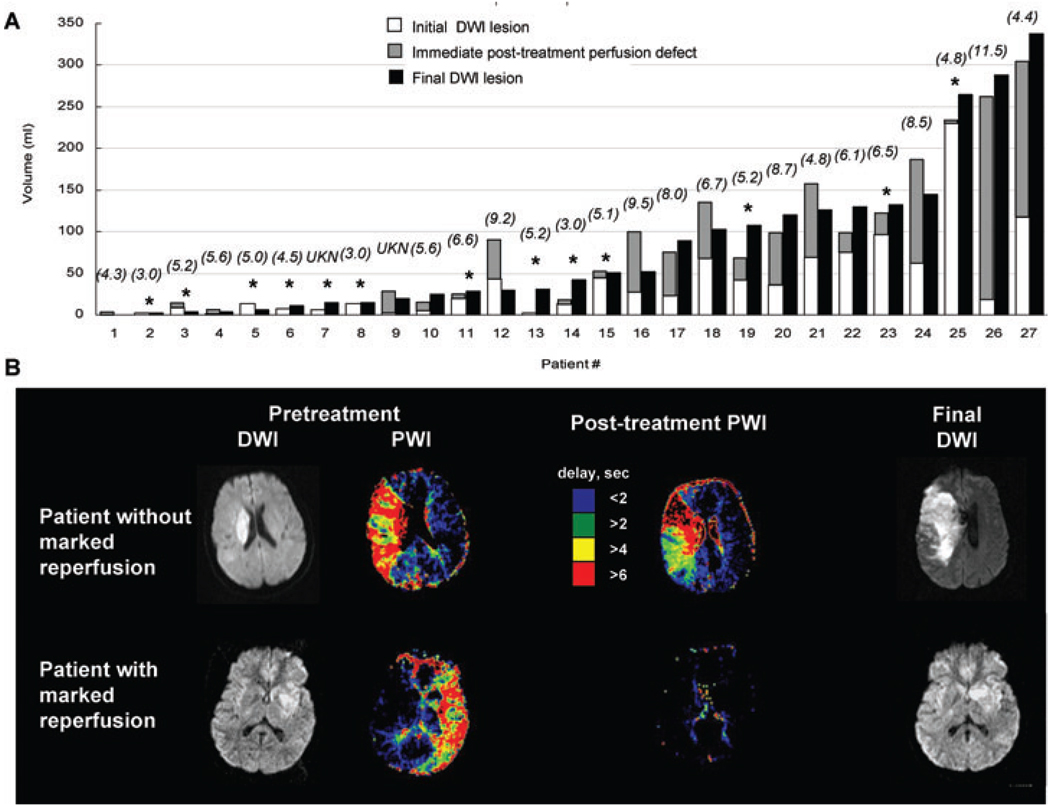
Fig 2.
(A) Volume of initial and final DWI lesions and posttreatment perfusion defect. (B) MRI findings that show the DWI changes in patients (upper) without and (lower) with marked improvement in perfusion defect on immediate posttreatment PWI. Numbers in parenthesis represent time from last known well time to start of endovascular therapies. *Marked reperfusion. UKN = patient who developed symptoms during sleep.
Fig 3.
Enlargement of DWI lesion volumes depending on (A) the presence and absence of definite diffusion-perfusion mismatch, and (B) the degree of recanalization.
No or poor reperfusion (TIMI 0–1) was observed in 8 patients, whereas partial (TIMI 2) or complete (TIMI 3) reperfusion was observed in 13 and 6 patients, respectively. Infarct growth was lower in patients with complete recanalization (6.2 ± 14.8 mL) than in those with partial (50.6 ± 73.0 mL) or no recanalization (54.6 ± 70.5 mL) (P = .009), with overall correlation r = −.256 (P = .198). Infarct growth correlated strongly with posttreatment perfusion lesion volumes regardless of whether or not recanalization was achieved (r = .924, P = .001 for patients with TIMI 0–1, and r = .908, P < .001 for those with TIMI 2–3) (Fig 3B).
Pretreatment NIHSS was evaluated in all patients (median 17, inter-quartile range 10–20, full range 6–27), and 3 hours posttreatment NIHSS was measured in 24 patients (median 14, IQR 4–19, full range 0–28). Dramatic recovery was observed in 5 (20.8%) of 24 patients. Most of the patients with dramatic recovery (4, 80%) had marked reperfusion, whereas marked reperfusion was observed in 9 (47.4%) of 19 patients without dramatic recovery. The occurrence of dramatic recovery correlated with TIMI grade (0–1 vs. 2 vs. 3) (r = .586, P = .003) and infarct growth (r = −.452, P = .027). The degree of reduction in 3 hours posttreatment NIHSS correlated with pretreatment DWI volume (r = −.475, P = .019), rather than with the presence of marked reperfusion (r = .270, P = .202) (Supporting figure).
Patients experiencing dramatic early recovery tended to have lower pretreatment NIHSS scores (median 10 vs. 18, P = .139) and exhibited smaller pretreatment DWI lesion volumes (48.1 ± 55.5 vs. 6.1 ± 6.4 mL, P = .009) than patients not experiencing dramatic early recovery.
Discussion
In this study, the extent of the early posttreatment perfusion lesion volume predicted infarct growth with high accuracy. The correlation of early reperfusion with infarct growth substantially exceeded that of other analyzed clinical, angiographic, and imaging potential predictor variables, including age, pretreatment neurologic deficit, serum glucose, pretreatment perfusion-diffusion mismatch volume, and end of procedure vessel recanalization status. These findings confirm that the extent of achieved reperfusion is a dominant factor determining threatened tissue fate in the 3- to 12-hours time window, as in the under 3-hours time window.12
Our findings accord with and extend results from recent MRI monitored >3 hours intravenous fibrinolysis trials.13–15 These studies have all suggested that inhibition of infarct growth is related to early reperfusion. However, several of these studies assessed reperfusion late after therapy. Earlier posttreatment assessment better distinguishes patients who reperfuse early enough to salvage threatened tissue from patients who reperfuse late, after infarction is already completed. Better capture of clinically relevant reperfusion likely accounts for the substantially stronger correlation between reperfusion and tissue outcome in our study (r = .95) than in the EPITHET cohort (r = .51). Also, our study of endovascular therapies uniquely permitted assessment of vessel status at the end of the therapeutic procedure and direct demonstration that infarct growth correlated with posttreatment perfusion lesion volumes, regardless of the degree of recanalization.
As expected, infarct growth was much greater among patients with perfusion-diffusion mismatch evidence of threatened penumbra prior to treatment than in patients with perfusiondiffusion match evidence of largely completed infarctions. However, a modest amount of infarct growth did occur in the matched lesion patients, and within this reduced range the degree of infarct growth correlated strongly with extent of persisting perfusion impairment. Pretreatment diffusion-perfusion mismatch is a useful index of salvageable tissue that can guide decision making in treating patients with acute ischemic stroke. However, mismatch lesions are only an approximation of the true biologic penumbra.16 Early diffusion lesions are in part reversible and often include both irreversibly infarcted tissue and penumbra. The visible zone of perfusion abnormality overestimates the penumbra by including regions of benign oligemia.16 Moreover, diffusion-perfusion mismatch is a dynamic process, changing over time and with spontaneous and therapeutic recanalization. 11,17 Our results suggest that an effort to reperfuse may beworthwhile in select patients without diffusion-perfusion mismatch, especially if the intensity of diffusion changes are modest.
Our results are in strong agreement with previous reports of early dramatic recovery associated with recanalization,8,18 and additionally show that early dramatic recovery is associated with marked reperfusion after recanalization therapy and reduced final infarct volumes. Our finding that patients were more likely to experience dramatic early recovery after reperfusion if they had smaller pretreatment diffusion lesion volumes suggests that patients who have already experienced substantial tissue injury prior to treatment are capable of only moderate, not spectacular, improvement. An additional factor likely accounting for only modest early improvement in neurologic deficits among some marked reperfusion patients is that some brain tissue that has been reperfused is still “stunned” and not yet functional.19 Thus, early imaging of posttreatment reperfusion will often provide a better guide to the success of therapy than early changes in clinical neurologic deficit, particularly in patients without dramatic recovery.
This study has several limitations. First, although our data include serial MRI including pretreatment and immediate posttreatment PWI as well as angiographic results, the sample size was modest, the results achieved at a single center, and a variety of recanalization interventions were employed. Further studies are needed with larger and multicenter cohorts. Second, in the present study follow-up MRI was performed at 2 to 7 days after the onset. Ischemic edema can be prominent at this period, which may affect the results of volumetric analysis. In addition, late second ischemic injury might be not evaluated in some patients who underwent follow up MRI at earlier time points.20 Further studies with the data of 30–90th-day MRI are needed.
Our findings confirm that early reperfusion has a marked ameliorative effect on tissue outcome in acute cerebral ischemia. These observations lend support to both of the early treatment strategies that achieve reperfusion, recanalization, and collateral enhancement. Monitoring of the achievement of early reperfusion with early parenchymal perfusion imaging to allow intensification of therapy in patients with no or subtotal reperfusion is a promising approach to acute stroke management.
Acknowledgments
This work was supported by UCLA MRI Investigators: Oh Young Bang, MD PhD; David S. Liebeskind, MD; Brian H. Buck, MD; Jeffry R Alger, PhD; Sidney Starkman, MD; Bruce Ovbiagele, MD; Doojin Kim, MD; Latisha K. Ali, MD; Nerses Sanossian, MD; Paul M Vespa, MD; Sa Rah Yoon, MD; Reza Jahan, MD; Noriko Salamon, MD; Gary R. Duckwiler, MD; J Pablo Villablanca; Fernando Viñuela, MD; and Jeffrey L. Saver, MD. It was funded in part by NIH/NINDS Award P50 NS044378, 1 K23 NS054084 – 01A1.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article
Table S1. Univariate Predictors of DWI Expansion
Figure S1. Relationship between the reduction in 3 h post-treatment NIHSS and (A) infarct growth, (B) pretreatment of NIHSS score and pretreatment DWI lesion volume (the sizes of diamonds represent the DWI volume), (C) recanalization, and (D) marked reperfusion. Gray area indicates patients with early dramatic recovery. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of atlantis, ecass, and ninds rt-pa stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Rother J, Schellinger PD, Gass A, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke. 2002;33:2438–2445. doi: 10.1161/01.str.0000030109.12281.23. [DOI] [PubMed] [Google Scholar]
- 3.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 4.Schellinger PD, Fiebach JB, Jansen O, et al. Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol. 2001;49:460–469. [PubMed] [Google Scholar]
- 5.Hjort N, Butcher K, Davis SM, et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- 6.Kohrmann M, Juttler E, Fiebach JB, et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 hours time window after stroke onset: a cohort study. Lancet Neurol. 2006;5:661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- 7.Timi study group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase 1 findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 8.Felberg RA, Okon NJ, El-Mitwalli A, et al. Early dramatic recovery during intravenous tissue plasminogen activator infusion: clinical pattern and outcome in acute middle cerebral artery stroke. Stroke. 2002;33:1301–1307. doi: 10.1161/01.str.0000015556.48283.74. [DOI] [PubMed] [Google Scholar]
- 9.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–1430. doi: 10.1161/01.STR.0000072998.70087.E9. [DOI] [PubMed] [Google Scholar]
- 10.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 11.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 12.Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36:2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Albers G, Al-Rawi Y, et al. The desmoteplase in acute ischemic stroke trial (dias): a phase ii MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 14.Furlan AJ, Eyding D, Albers GW, et al. Dose escalation of desmoteplase for acute ischemic stroke (dedas): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 16.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 17.Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, et al. Effect of incomplete (spontaneous and postthrombolytic) recanalization aftermiddle cerebral artery occlusion: a magnetic resonance imaging study. Stroke. 2004;35:109–114. doi: 10.1161/01.STR.0000106482.31425.D1. [DOI] [PubMed] [Google Scholar]
- 18.Minematsu K, Yamaguchi T, Omae T. ‘pectacular shrinking deficit’: rapid recovery from a major hemispheric syndromeby migration of an embolus. Neurology. 1992;42:157–162. doi: 10.1212/wnl.42.1.157. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrov AV, Hall CE, Labiche LA, et al. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]
- 20.Kidwell CS, Saver JL, Starkman S, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol. 2002;52:698–703. doi: 10.1002/ana.10380. [DOI] [PubMed] [Google Scholar]