Abstract
Melanocortin receptor agonists act in the brain to regulate food intake and body weight and, independently of these actions, affect insulin sensitivity. These experiments investigated the function of novel non-selective melanocortin receptor agonists (BIM-22493, BIM-22511) that cross the blood-brain barrier when administered peripherally. Treatment of diet-induced obese C57BL/6J (B6) mice with melanocortin agonists administered peripherally improved obesity, hyperinsulinemia (∼50%) and fatty liver disease. Specificity of function was determined using B6 melanocortin-3 and melanocortin-4 receptor knockout mice (MC3RKO, MC4RKO). Chow-fed MC4RKO but not MC3RKO used for these tests exhibited obesity, hyperinsulinemia and severe hepatosteatosis associated with increased expression of insulin-stimulated genes involved in lipogenesis. Reduced food intake associated with acute BIM-22493 treatment, and weight loss associated with 14d of treatment with BIM-22511, required functional MC4R but not MC3R. However, while 14d of treatment BIM-22511 did not affect body weight and even increased cumulative food intake in MC4RKO, a significant reduction (∼50%) in fasting insulin was still observed. Despite lowering insulin, chronic treatment with BIM-22511 did not improve hepatosteatosis in MC4RKO, and did not affect hepatic lipogenic gene expression. Together, these results demonstrate that peripherally administered melanocortin receptor agonists regulate body weight, liver metabolism and glucose homeostasis through independent pathways. MC4R are necessary for melanocortin agonist-induced weight loss and improvements in liver metabolism, but are not required for improvements in hyperinsulinemia. Agonists with activity at MC4R improve glucose homeostasis at least partially by causing weight loss, however other melanocortin receptors may have potential for treating aberrations in glucose homeostasis associated with obesity.
Keywords: Obesity, Diabetes, Insulin, Melanocortins, Proopiomelanocortin, melanocyte-stimulating hormones
1. Introduction
Energy homeostasis involves maintaining a balance of caloric intake and energy expenditure, and an imbalance can cause obesity and increased propensity for diabetes (“diabesity”) [17]. Understanding the systems involved in energy homeostasis will aid the development of therapies against obesity and diabetes. The central nervous melanocortin system is involved in energy homeostasis, regulating food intake and energy expenditure [14]. The brain melanocortin system is defined as the primary neurons that express neuropeptide ligands, and secondary neurons expressing the predominant neural melanocortin receptors (MC3R, MC4R) [13]. Mutations in the gene encoding melanocortin receptor agonists, or the genes encoding MC3R or MC4R cause obesity [16]. The other members of the melanocortin receptor family are primarily expressed in the periphery, and are involved in regulating pigmentation (MC1R), adrenal gland function (MC2R), immune function (MC1R/MC3R), and sebaceous gland activity (MC5R) [14].
There are two populations of primary melanocortin neurons that reside in the hypothalamic arcuate nucleus, and which respond to multiple endocrine and neurotransmitter signals of energy balance [13]. Neurons expressing the proopiomelanocortin (Pomc) gene release α-, β- and γ-melanocyte stimulating hormone (MSH), and reside in the arcuate nucleus of the hypothalamus and nucleus tractus solitarius of the brain stem [13]. MSH are melanocortin receptor agonists, and central administration of α- or β-MSH, but not γ-MSH which exhibits agonist activity selectively at the MC3R, reduces food intake and increases energy expenditure [14]. The second population of arcuate neurons expresses the gene encoding agouti-related peptide (AgRP). AgRP exhibits antagonist and inverse agonist properties at the MC3R and MC4R [27]. Central administration of AgRP promotes weight gain by increasing food intake and reducing energy expenditure [35, 39]. The neuropeptides produced by both neuronal populations are considered promising therapeutic targets. Compounds stimulating Pomc neurons or that mimic α-MSH activity may have use for treating obesity and obesity-related metabolic disorders [22, 32].
The regulation of behavioral and metabolic processes associated with energy homeostasis (satiety, energy expenditure) primarily involves MC4R [14]. MC4R are expressed throughout the CNS [13], and regulation of food intake and energy expenditure involves receptors expressed by discrete neuronal populations [3]. In mice, central MC4R also regulate glucose homeostasis and insulin sensitivity. While even modest weight loss may be a factor improving glucose homeostasis, the regulation of glucose metabolism by MC4R is acute and is independent of effects on food intake or weight loss [28, 42]. Together, these properties have led to MC4R being considered as an important target for developing therapies against obesity.
In contrast, the function of the MC3R in energy homeostasis has remained enigmatic. Initial studies of the phenotype of MC3R knockout mice (MC3RKO) indicated a modest obesity [7, 9]. More recent studies have demonstrated that an exaggerated diet-induced obese phenotype in MC3RKO [6, 15, 38]. However, while MC3RKO fed high fat diet (HFD) achieve a level of adiposity comparable to that observed in MC4RKO, the insulin resistant phenotype is still modest and less severe compared to that associated MC4R deficiency [15, 38].
Here we describe the results of studies investigating the function of novel α-MSH derivatives with melanocortin receptor agonist activity. Both agonists improve obesity and diabetes in the rodent. We demonstrate that changes in body weight associated with chronic treatment involve activation of MC4R. MC4R in the paraventricular nucleus of the hypothalamus are necessary for mediating the actions of a-MSH on food intake [3]. These data therefore imply that this agonist acts on sites within the central nervous system to affect appetite. However, a novel finding is that improvement in hyperinsulinemia observed with agonist treatment is retained in MC4RKO. Other melanocortin receptors may therefore have functions affecting insulin sensitivity.
2. Materials and Methods
2.1 Animals
Male diet induced obese (DIO) and leptin-deficient (Lepob/Lepob) mice on the C57BL/6J (B6) background were purchased from the Jackson Laboratories; DIO mice were maintained on a high fat diet (D12492, Research Diets). Female MC4RKO, MC3RKO and wild-type (WT) B6 littermates produced by our laboratory as previously described [38] were maintained on standard rodent chow (Purina 5001). All mice were 10 months of age. For food intake, mice were acclimated to single housing in wire mouse cages for 2 weeks in 12h light and 12h dark cycle. All studies were approved by the Pennington Biomedical Research Centers IACUC.
2.2 Osmotic Mini-pump Implantation
Mice were surgically implanted with 14d osmotic mini pumps (Alzet). Pumps contained either 0.9% saline, or agonist dissolved in 0.9% saline plus 0.1% BSA. The α-MSH analogs (BIM-22493, BIM-22511) were synthesized by Biomeasure Inc., IPSEN (Milford, MA). Mice were anesthetized with isoflurane gas, an area on the dorsal surface in the interscapular region shaved and sterilized for surgery. A small incision was then made above the scapula and blunt forceps used to make a small space in the interscapular region. After insertion of the pumps, the incision was closed using a metal clip.
2.3 Glucose Tolerance Test
After fasting overnight, mice were weighed and pre-injected with the melanocortin agonist BIM-22493. Baseline blood glucose was measured preinjection using a small sample of blood taken from a tail nick using a OneTouch Glucometer, and 2 g/kg body weight of D-glucose injected by i.p. Blood glucose was measured at 15, 30, 60, and 120 minutes post injection. Mice were not restrained during the test.
2.4 Fasting Blood Chemistries
Trunk blood was collected from mice after a 4 h fast. Serum glucose, triglyceride (TG), nonesterified free fatty acids (NEFA) and total cholesterol (TC) were measured by the Clinical Chemistry laboratory of Pennington Biomedical Research Center. Serum levels of insulin, leptin, and resistin were measured using a multiplex ELISA kit (Millipore). Homeostasis model assessment of insulin resistance (HOMA-IR) values were calculated using the following equation: [(fasting insulin in mU/ml) × (fasting glucose in mmol/l)]÷22.5. Note that, for the remainder of this manuscript, insulin levels measured in serum samples from fasted mice are expressed as pg/ml.
2.5 Liver Lipid Measurements
Liver samples were frozen immediately at termination. A piece of liver was fixed in formaldehyde solution and processed for histological examination in department of Pathobiological sciences, School of Veterinary medicine, Lousiana State University, Baton Rouge, LA. Total liver lipid was measured by Folch extraction method, and liver TG from the extracted liver lipid pool was measured by using the Wako diagnostic kit (Wako).
2.6 Gene Expression Analysis
RNA was extracted from liver samples using RNA extraction kit (Qiagen). Gene expression analysis was performed in 384 well plates using SYBR green-based or TaqMan-based detection methods in an ABI Prism 7900 HT system (Applied Biosystems) as described previously [37]. Gene expression data are expressed as a percent of control values.
2.7 Radioligand Binding Assay to human MC1R, MC3R, MC4R and MC5R
Cell membranes were prepared from CHO-K1 cells stably expressing the human melanocortin receptor subtypes (MC1R, MC3R, MC4R and MC5R). They were incubated at 1-10 μg protein/well in 50 mM Tris-HCl, pH 7.4, containing 0.2% BSA, 5 mM MgCl2, 1 mM CaCl2 and 0.1 mg/mL bacitracin, with increasing concentrations of compound to be tested and 0.1-0.3 nM [125I]-NDP-α-MSH (Amersham Biosciences) for 90-120 min at 37°C, depending on the receptor subtype. Bound from free [125I]-NDP-α-MSH was separated by filtration through GF/C glass fiber filters presoaked with 0.1 % (w/v) PEI. Filters were washed three times with 50 mM Tris-HCl, pH 7.4, at 0-4°C and assayed for radioactivity using Perkin Elmer Topcount scintillation counter.
2.8 Intracellular cAMP Assay
Intracellular cAMP levels were determined by an electrochemiluminescence (ECL) assay (Meso Scale Discovery, MSD). CHO-K1 cells stably expressing the human MC1R, MC3R, MC4R and MC5R were suspended in RMPI 1640 containing 0.5 mM IBMX, and 0.2% BSA. They were dispensed (7,000 cells per well) in Multi-Array plates (MSD) containing integrated carbon electrodes and coated with anti-cAMP antibody. Increasing concentrations of compound were added for 40 min incubation at 37°C. Then, the cells were lysed and 2.5 nM TAG ruthenium-labeled cyclic AMP (MSD) was added. After 90 minutes, cyclic AMP levels were determined by ECL detection using Sector Imager 6000 reader (MSD). NDP-MSH was used as the reference compound. Maximal cAMP stimulation level was 12 to 20 folds higher than basal level, depending on receptor subtype.
2.9 Data analysis
Data from animal studies are presented as mean ± standard error. The effect of treatment on food intake and body weight phenotypes was determined by ANOVA for each genotype. Food intake (g) and body weight gain/loss (g and % gain) were analyzed by ANOVA with repeated measures using a mixed model, with treatment as the between-group factor and day as the within-group factor. Individual comparisons were evaluated using Tukey's modified t-test.
Binding and cAMP experiments were analyzed by computer-assisted non-linear regression analysis (XL fit; IDBS) with the determination of 50% inhibitory concentration (IC 50, binding data) and 50% effective concentration (EC50, cAMP data) values. For the binding experiments, inhibition constant (Ki) values were calculated using the formula of Cheng and Prusoff: Ki = IC 50 /(1 + A/Kd), where A and Kd are the concentration and the dissociation constant of [125I]-NDP-α-MSH, respectively. For MC4 receptors, results are expressed as the logarithmic mean of 3 separate experiments. For MC1R, MC3R and MC5R, results are from a single experiment. For cAMP data, MC4R results are expressed as the logarithmic mean of 3 separate experiments. For MC1R, MC3R and MC5R, the results are from a single experiment.
3. Results
3.1 In vitro binding and receptor activation data
BIM-22493 and BIM-22511 exhibit agonist activity at the MC1R, MC3R and MC4R (Table 1). Receptor affinity (Ki) and activity (EC50) data of both ligands at human MC1R, MC3R, MC4R and MC5R are shown in Table 1. Both compounds exhibit activity at the MC1R-MC4R, but exhibit weak activity at MC5R (EC50>1 μM) and are inactive at the mouse MC2R (EC50>10 μM). To determine whether agonist activity is also observed in the rodent, we assessed Ki and EC50 in rat MC4R Ki and EC50 data for BIM-22493 and BIM-22511 were comparable in rat and human MC4R (Table 1).
Table 1. Affinity and EC50 data for the BIM22492 and BIM-22511 peptides in CHO-K1 cells expressing the human melanocortin receptors (hMC1R, hMC3R-hMC5R), or expressing the rat MC4R (rMC4R).
| Binding, Ki (nM) | cAMP, EC50 (nM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | hMC1R | hMC3R | hMC4R | rMC4R | hMC5R | hMC1R | hMC3R | hMC4R | rMC4R | hMC5R |
| BIM-22493 | 3.9 | 10 | 2.1 | 2.7 | 430 | 5.8 | 5.3 | 0.27 | 0.28 | 1600 |
| BIM-22511 | 8.6 | 94 | 1.3 | 2.4 | 7800 | 8.8 | 7.9 | 0.091 | 0.17 | 4000 |
3.2 Acute effects of BIM-22493 on Food Intake and glucose homeostasis
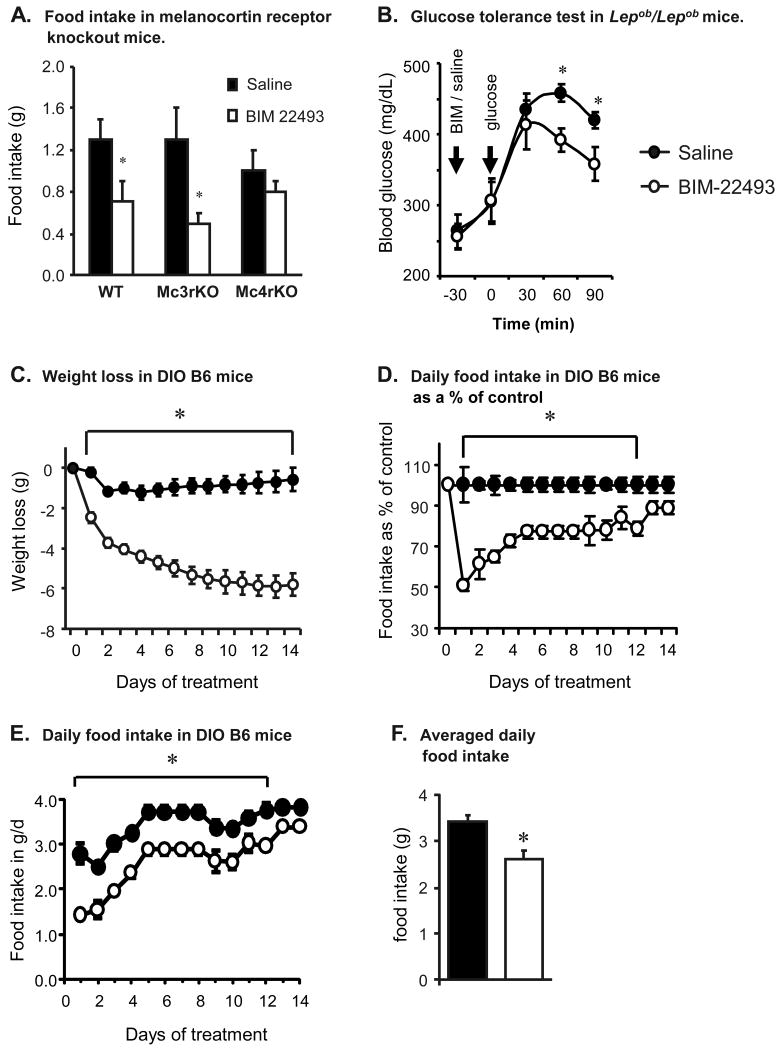
BIM-22493 inhibited food intake in mice during refeeding after overnight fasting when administered peripherally as a single ip. injection (Fig. 1 and data not shown). The reduction of food intake by MTII, another potent non-selective peptide melanocortin agonist, is dependent on MC4R [3, 12, 25]. To test specificity, we injected WT (n=4/group), MC3RKO (n=6) or MC4RKO (n=6) after an overnight fast with a single ip. injection of BIM-22493 (6.4μmol) or saline, and then returned food. Inhibition of refeeding after an overnight fast by BIM-22493 was dependent on functional MC4R, and did not require MC3R (Fig. 1A). Studies using male Sprague-Dawley rats indicated that reduced food intake during refeeding after overnight fast was similar with BIM-22493 and BIM-22511 at either 100 or 500 nmol/kg given sc. (data not shown).
Figure 1. Response of melanocortin receptor knockout and diet-induced obese mice to BIM-22493 treatment.
(A) Reduced food intake by BIM-22493 is dependent on MC4R, and not MC3R. Mice were fasted overnight and then given a single ip. injection of BIM-22493 (6.4 μm) 30 min. prior to food presentation. (B) A single injection of BIM-22493 improves glucose tolerance of obese Lepob/Lepob mice. Mice were fasted overnight, and injected with either BIM-22493 (6.4 μm) or saline followed by glucose. *P<0.05 vs control; n=6/group for panels A and B. (C) Weight loss in diet-induced obese B6 mice treated with BIM-22493 (300 nmol/kg/d by subcutaneous osmotic pump for 14 days). (D, E) Food intake expressed as a percent of control (D) or grams per mouse per day (E). Reduced food intake associated with BIM-22493 was most severe prior to day 3. (E) Average daily food intake of controls and treatment group during the treatment period.* P<0.05 vs. control. N=6/group.
CNS Melanocortins acutely regulate glucose tolerance and insulin signaling [28, 42]. To test whether a BIM-22493 acutely improves glucose homeostasis, obese glucose intolerant Lepob/Lepob mice were administered a single ip. injection of 6.4 μmol of BIM 22493 or saline 30 min. prior to a bolus of glucose (2g/Kg, n=6/group). Lepob/Lepob mice treated with BIM-22493 exhibited significantly improved glucose clearance when compared to saline treated controls (Fig. 1B).
3.3 Chronic Treatment Of Diet Induced Obese Mice With BIM-22493 Causes Weight Loss And Improves Hyperinsulinemia And Fatty Liver
Having tested the efficacy of single injections of BIM-22493 to affect food intake and glucose homeostasis, we next assessed the long-term efficacy of this peptide at improving obesity and diabetes in male DIO B6 mice. BIM-22493 was administered chronically at a dose of 300 nmol/kg/day for 14 days by sc. osmotic pump. Controls were administered with 0.9% saline during the same period. The two groups of male DIO mice were matched for body weight at the start of the study (47.6 ± 1.08 vs. 46.2 ± 0.88 g, p=0.3517).
Weight loss associated with BIM-22493 treatment was due to reduced food intake (Fig 1C-F). Analysis of the food intake data using repeated measures ANOVA showed a significant effect of treatment (P <0.05), and a significant interaction with time (P < 0.05). When assessed as a percent of control values, suppression of food intake and weight loss was most pronounced during days 1 to 4 of treatment (Fig. 1C, D). When assessed as grams of food ingested per day, it was evident that stress associated with surgery may have resulted in low consumption on day 1 and 2, with recovery occurring thereafter (Fig. 1E). Note that the data for days 5-8 represent an averaged mean, as no daily data was collected during this period. For mice treated with BIM-22493, food intake was ∼50 % of controls on day 1, increasing to 75 % of control levels by day 4. By day 12, the effect on food intake was no longer significant although consumption still appeared lower compared to controls.
Blood chemistries from sera collected after a 4 h fast are shown in Table 2. BIM-22493 treatment was associated with significantly lower levels of serum insulin, glucose and HOMA-IR values, suggesting an improvement in insulin sensitivity. Serum leptin levels were also lower in the BIM-22493 treatment group, suggesting reduced adiposity. Analysis of serum lipids revealed significantly lower levels of total cholesterol, but there were no significant differences in TG or NEFA. Serum levels of resistin, implicated in insulin resistance in mice [33, 34, 36], were also not significantly different from controls.
Table 2. Blood chemistries of DIO mice after treatment with saline or BIM-22493 for 14d.
| Parameter | Control (n=6) | BIM-22493 (n=6) | Statistical analysis |
|---|---|---|---|
| Serum glucose | 219 ± 16 | 177 ± 11 | < 0.05 |
| Serum insulin (pg/ml) | 1004 ± 162 | 552 ± 89 | < 0.05 |
| HOMA-IR | 13.8 ± 2.7 | 6.0 ± 1.0 | < 0.05 |
| Triglycerides (mg/dL) | 71 ± 6 | 60 ± 7 | 0.126 |
| Total Cholesterol (mg/dL) | 173 ± 10 | 133 ± 5 | < 0.01 |
| NEFA (mEq/L) | 1.57 ± 0.10 | 1.38 ± 0.10 | 0.104 |
| Leptin (pg/ml) | 6968 ± 664 | 4866 ± 231 | <0.05 |
| Resistin (pg/ml) | 1764 ± 111 | 1525 ± 297 | 0.240 |
Treatment with BIM-22493 was associated with improved liver steatosis (P < 0.05, Fig 2A, 3B). To explore the underlying molecular mechanism, we analyzed expression key genes involved in lipogenesis and fat oxidation (Fig. 2C). Surprisingly, no significant differences in the expression of genes encoding lipogenic enzymes were observed. However, a significant but modest increase in liver specific Cpt1a expression may indicate increased fatty acid oxidation in liver.
Figure 2. BIM-22493 significantly improves hepatic steatosis in diet-induced obese mice.
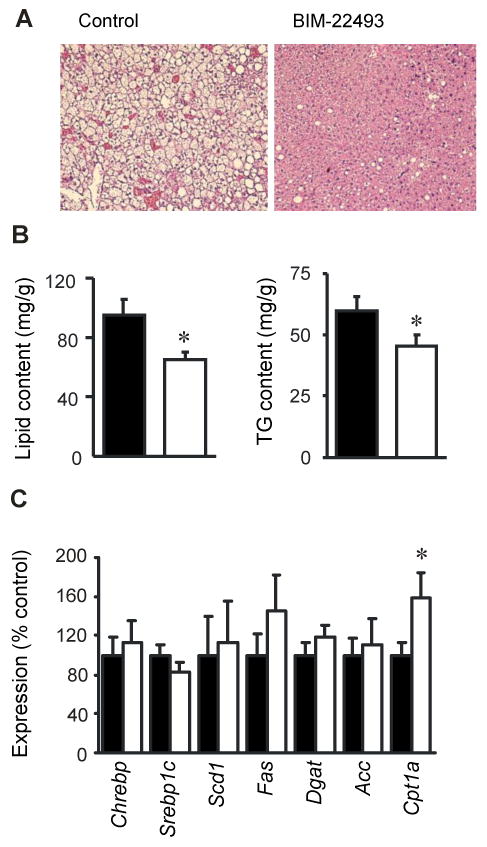
(A) Represented H&E stained section from diet-induced mice treated with saline (left panel) or BIM-22493 (right panel) showing severe hepatic steatosis in DIO mice treated with saline. (B) BIM-22493 significantly reduced liver lipid (left panel) and TG content (right panel). * P<0.01 vs. saline. (C) The effect of BIM-22493 treatment on the expression of liver genes involved in lipogenesis and fat oxidation. * P<0.05 vs. saline.
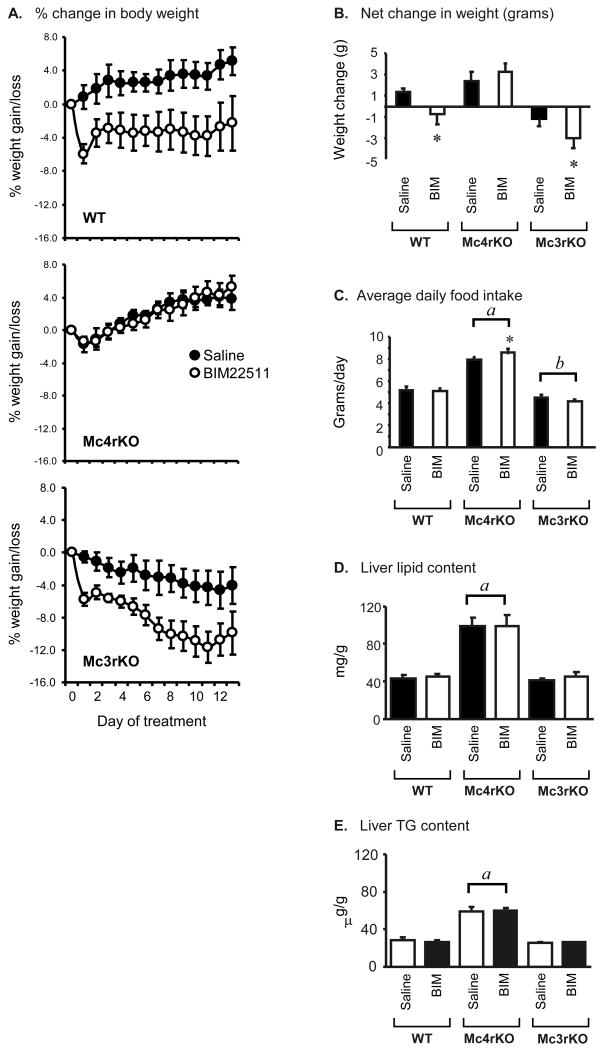
Figure 3. Response of WT, MC3RKO and MC4RKO treated with BIM-22511 for 14d.
(A) Change in body weight as a percent of initial weight in WT (top panel), MC4RKO (middle panel) or MC3RKO (bottom panel) treated with BIM-22511 (solid circles) or saline (open circles). Note the absence of a response in MC4RKO. (B) Net weight gain or loss in mice treated with saline or BIM-22511. The effects of BIM-22511 on body weight are dependent on MC4R activation. (C) Average daily food intake of mice treated with saline or BIM-22511. MC4RKO were hyperphagic, while MC3RKO exhibited reduced food intake relative to WT (a, P<0.001 vs WT, MC3RKO; b, P<0.05 vs WT). Note that the bars with letters indicate within genotype comparisons (i.e., LSM of saline and BIM treatment groups combined for MC4RKO, compared to LSM of saline and BIM treatment groups for WT). Repeated measures ANOVA indicated a significant increase in MC4RKO treated with BIM-22511. * P<0.01 vs saline within treatment. (D, E) MC4RKO exhibit hepatic steatosis (elevated liver lipid and triglyceride) relative to WT and MC3RKO. There was no significant effect of treatment with BIM22511 on hepatic lipid content, irrespective of genotype.
3.4 Chronic Treatment of Chow Fed C57BL/6J Mice Lacking MC3R or MC4R: Effect Of Genotype
We next examined the effect of chronic treatment with BIM-2511 on energy homeostasis using MC3KO and MC4RKO mice. We used the BIM-22511 compound as it exhibited more selectivity in binding assays for the MC4R over the MC3R (∼70-fold selectivity, compared to ∼5-fold for BIM-22493). An experiment examining the response to chronic administration was performed using chow-fed female WT, MC3RKO and M4rKO administered 100 nmol/kg/day BIM-22511 or saline by osmotic pump for 14 days (n=4-6/group). The data from this study was analyzed using a 2-way ANOVA testing for effects of genotype and treatment. The data will be presented in two sections, with the first section focusing on the effect of genotype and the second section focusing on treatment effects.
MC4RKO exhibited the expected obese phenotype, with a significant marked increase in body weight compared to both MC3RKO and WT (weight in grams for WT, 24.6 ± 0.6; for MC3RKO, 25.6 ± 0.9; for MC4RKO, 60.0 ± 1.4, p<0.001 MC4RKO vs WT, MC3RKO). MC4RKO were hyperphagic, consuming 60% more per day relative to WT. In contrast, MC3RKO consumed 15% less food per day compared to WT (average food intake in g/d for WT, 5.2 ± 0.2; MC3RKO, 4.4 ± 0.2; MC4RKO, 8.2 ± 0.2; p<0.001 MC4RKO vs WT, MC3RKO; p<0.05 Mc3KO vs WT).
MC4RKO were hyperinsulinemic relative to WT and MC3RKO [least square means (LSM) for serum insulin in pg/ml for WT, 141 ± 148; MC3RKO, 217 ± 157; MC4RKO, 2755 ± 179; P<0.001 MC4RKO vs WT and MC3RKO. Significant effects of genotype were also observed on serum glucose (P<0.01). However, fasting serum glucose levels of MC4RKO were not significantly different from WT, suggesting β-cell compensation sufficient to counter insulin resistance. In contrast, MC3RKO exhibited significant fasting hyperglycemia relative to controls (LSM for serum glucose in mg/dL for WT, 115 ± 13; MC3RKO, 178 ± 14; MC4RKO, 136 ± 15; P<0.01 MC3RKO vs. WT).
Hyperresistinemia is associated with insulin resistance in mice [33, 34, 36]. However, circulating levels of resistin were not significantly affected by genotype in saline-treated controls (Table 3). Serum leptin levels correlate with adiposity [40], and in this study hyperleptinemia was observed in MC4RKO but not MC3RKO, correlating with a more severe obesity (LSM for serum leptin in pg/ml for WT, 1815 ± 1659; MC3RKO, 2541 ± 1760; MC4RKO, 15265 ± 1916; P<0.001 MC4RKO vs WT and MC3RKO).
Table 3. Blood chemistries of WT, Mc3rKO and Mc4rKO treated with BIM-22511 compared to controls.
* P<0.01 compared to saline treatment within genotype. Serum was collected after a 4h fast.
| Genotype: Treatment: |
WT | Mc3rKO | Mc4rKO | |||
|---|---|---|---|---|---|---|
| Saline | BIM-22511 | Saline | BIM-22511 | Saline | BIM-22511 | |
|
Glucose (mg/dL) |
100 ± 7 | 129 ± 20 | 164 ± 22 | 193 ± 28 | 153 ± 10 | 119 ± 15 |
|
Insulin (pg/ml) |
138 ± 30 | 145 ± 40 | 124 ± 21 | 310 ± 55 | 3588 ± 812 | 1899 ± 160 * |
| HOMA-IR | 0.9 ± 0.2 | 1.3 ± 0.5 | 1.3 ± 0.3 | 3.5 ± 0.5 | 33.5 ± 7.0 | 14.2 ± 3.7 * |
|
TG (mg/dL) |
34 ± 5 | 55 ± 10 | 59 ± 6 | 109 ± 12 * | 61 ± 9 | 70 ± 6 |
|
TC (mg/dL) |
74 ± 9 | 93 ± 11 | 100 ± 2 | 107 ± 7 | 90 ± 9 | 107 ± 1 |
|
NEFA (mEq/L) |
1.12 ± 0.11 | 1.57 ± 0.28 | 1.70 ± 0.11 | 1.92 ± 0.07 | 1.56 ± 0.16 | 1.71 ± 0.15 |
|
Leptin (pg/ml) |
1985 ± 434 | 1645 ± 432 | 2247 ± 739 | 2834 ± 595 | 15582 ± 2294 | 14948 ± 5125 |
|
Resistin (pg/ml) |
1798 ± 127 | 1015 ± 175 * | 1395 ± 209 | 1330 ± 83 | 1749 ± 258 | 2227 ± 202 |
Obesity and insulin resistance are associated with increased hepatic lipogenesis, atherogenic dyslipidemia and nonalcoholic fatty liver disease [29]. A strong trend for an increase in fasting TG was observed for MC4RKO compared to WT (LSM for TG in mg/dL for MC4RKO, 64 ± 6.1 mg/dL; for WT, 44 ± 5.7, p=0.077). Obese MC4RKO also exhibited hepatic steatosis (increased liver content of lipid and TG) compared to WT and MC3RKO (Fig. 5).
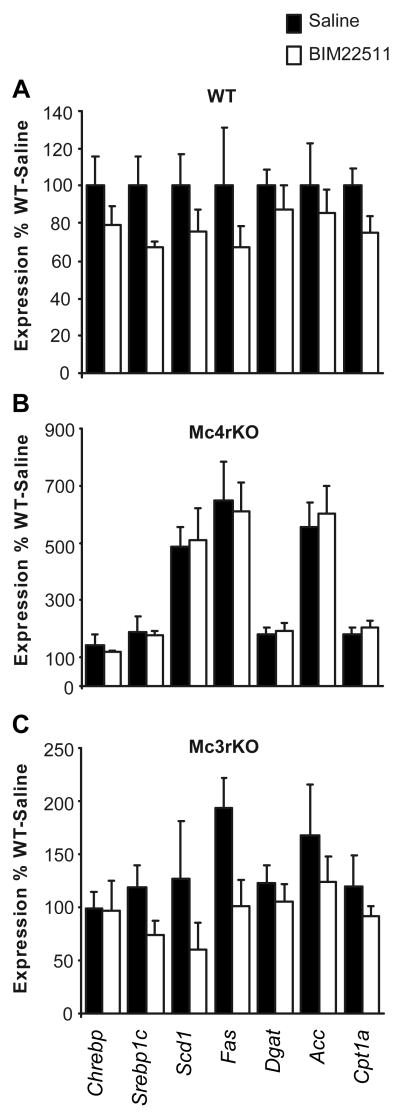
Increased hepatic steatosis in insulin resistant mice involves stimulation of sterol-regulatory element binding transcription factor 1 (Srebf1), a transcription factor that regulates the expression of lipogenic genes including fatty acid synthase (Fas), stearoyl-Coenzyme A desaturase 1 (Scd1), and acetyl-CoA carboxylase (Acc) [4]. MC4RKO exhibited a significant ∼2-fold increase in the expression of Srebf1 in liver, while MC3RKO exhibited normal expression (LSM for Srebf1 expression in WT, 0.68 ± 0.11; MC3RKO, 0.78 ± 0.11; MC4RKO 1.49 ± 0.12; p<0.001 MC4RKO vs. WT, MC3RKO). The expression of key lipogenic enzymes was also increased in MC4RKO relative to WT and MC3RKO (LSM for Fas expression in WT, 0.35 ± 0.20; MC3RKO, 0.61 ± 0.20; MC4RKO, 2.621 ± 0.23; Scd1 expression in WT, 0.37 ± 0.17; MC3RKO, 0.39 ± 0.17; MC4RKO, 2.07 ± 0.19; Acc expression for WT, 0.44 ± 0.17; MC3RKO, 0.68 ± 0.17; MC4RKO, 2.73 ± 0.20; all genes p<0.001 MC4RKO vs MC3RKO, WT). Expression of carbohydrate-response element binding protein 1 (Chrebp1), which is involved in stimulation of lipogenesis by glucose [41], was not significantly affected by genotype (LSM for Chrebp1 expression in WT, 0.76 ± 0.11; MC3RKO, 0.83 ± 0.11; MC4RKO 1.10 ± 0.13).
3.5 Chronic Treatment Of Chow Fed C57BL/6J Mice Lacking MC3R Or MC4R: Effects Of BIM-22511 Treatment
In WT mice, administration of BIM-22511 resulted in a modest but significant reduction in weight gain compared to saline treated controls (Fig. 3A, top panel, Fig. 3b). This was not due to reduced food intake (Fig 3C) (average daily food intake in grams for controls, 5.2 ± 0.3; for BIM-22511 treatment, 5.1 ± 0.2). There was no significant effect of treatment on serum glucose, insulin, TG, cholesterol, NEFA, or leptin (Table 3). However, lower levels of resistin were observed with BIM-22511 treatment (Table 3). Total liver lipid and liver triglyceride level and hepatic expression of genes involved in lipid metabolism were also not affected by treatment with BIM-22511 (Fig 4A).
Figure 4. Expression of liver genes involved in lipid metabolism in WT (A), MC4RKO (B) and MC3RKO (C) treated with saline or BIM-22511.
MC4RKO exhibit an increase in expression of key lipogenic genes (Scd1, Fas, Acc). However, treatment with BIM-22511 for 14d had no effect on expression. The effect of treatment on gene expression observed in MC3RKO was not statistically significant. Note the difference in scale of the y-axis; all data are expressed as a percent of saline treated WT.
The reduction of weight associated with BIM-22511 treatment of WT mice was not observed in MC4RKO (Fig. 3A, middle panel, 3B). Repeated measures ANOVA indicated that food intake of MC4RKO mice treated with BIM-22511 was increased by approximately 10% when compared to saline treated MC4RKO (Fig. 3C) (Saline treated = 7.83±0.15 vs. BIM-22511 treated = 8.55±0.15; P=0.0061),. A similar analysis of food intake data in WT and MC3RKO indicated no significant effect of treatment within genotype. A significant improvement in hyperinsulinemia and HOMA-IR was observed in MC4RKO treated with BIM-22511 (Table 3), however the treatment did not affect hepatic steatosis (Fig. 4D, E). Treatment with BIM-22511 also did not affect the expression of lipogenic genes in liver, irrespective of genotype (Fig. 5).
BIM-22511 treatment significantly reduced body weight in MC3RKO (Fig 3A, bottom panel). This was due at least in part to a significant reduction of food intake (intake in g/d for saline controls: 4.5 ± 0.1; BIM treated, 4.2 ± 0.1, p=0.56). There was no significant difference in serum glucose or insulin levels in saline and BIM-22511 treated MC3RKO (Table 3). Indeed, while not statistically significant if anything the levels of both appeared elevated with BIM-22511 treatment. Serum cholesterol, leptin, and resistin levels were also not significantly affected by treatment (Table 3). However, a modest but significant difference in TG was observed, with levels being higher in the BIM-22511 treatment group (Table 3). Total liver lipid and liver triglyceride were also similar between treatment and control groups (total liver lipid: Saline: 40.5 ± 1.9 vs BIM: 44.2 ± 5.2, p=0.495).
4. Discussion
The first group of studies presented here examined the acute effect of BIM-22493 on food intake and glucose homeostasis. We have demonstrated that BIM-22493 is a melanocortin receptor agonist that efficiently reduce food intake, body weight and reverse some aspects of the metabolic syndrome (hyperinsulinemia, dyslipidemia, and fatty liver disease) when administered peripherally to DIO mice. The effect of BIM-22493 on food intake is attenuated with time, but is not completely lost. Tachyphylaxis in response to continued administration of anorectic compounds such as melanocortin agonists is frequently observed in mice, and may reflect feedback from counter regulatory mechanisms to prevent severe weight loss. We have determined that the inhibition of food intake and weight loss by BIM-22493 involves MC4R. These findings are consistent with previous results from studies using MTII, the most frequently used melanocortin agonist [12, 25].
We observed that BIM-22493 reduces food intake, and that this effect is dependent on MC4R. This result is similar to that reported by others examining the effect of MTII on food intake in MC3RKO and MC4RKO [10, 11, 24]. We also demonstrated that BIM-22493 rapidly improves glucose homeostasis in severely obese Lepob/Lepob mice. Again, this result is similar to recent data showing that stimulation of CNS melanocortin receptors rapidly improves glucose homeostasis independently of reducing food intake or causing weight loss [42]. Treatment of DIO B6 mice with BIM-22493 for 2 wk reduced body weight, improved glucose homeostasis and reduced serum NEFA and cholesterol levels suggesting improvements in dyslipidemia. Collectively, these data suggest that BIM-22493 is effective at reversing diabesity in the DIO B6 mouse when administered peripherally.
The second group of experiments investigated the response of MC3RKO and MC4RKO to a second compound, BIM-22511, which was also administered for 14d by osmotic pump. This peptide exhibits an EC50 at the MC3R and MC4R similar to that of BIM-22493, although receptor affinity data suggest increased selectivity at MC4R over MC3R. The body weight and insulin resistant phenotype data observed in the chow-fed MC3RKO and MC4RKO used for these studies are consistent with previous data (2, 13, 14, 15). Chow fed MC4RKO on the B6 background exhibit a deterioration in glucose homeostasis that become severe between 2- and 3-months of age [1, 38]. In the current study using chow fed mice, the obese phenotype associated with loss of MC4R was far more severe than that associated with loss of MC3R. A modest hyperglycemia in MC3RKO compared to WT and MC4RKO may suggest insulin resistance and altered β-cell function, although historically insulin resistance associated with even severe obesity in this strain is mild [15, 38].
In this study, we observed that BIM-22511 agonist-induced weight loss was dependent on MC4R. However, BIM-22511 treatment significantly improved hyperinsulinemia in obese MC4RKO. This result suggests that increased activity of another melanocortin receptor, presumably MC1R and/or MC3R, can improve insulin sensitivity independently of weight loss or reduced food intake. MC5R have been reported to regulate fat oxidation in muscle [2]. However, BIM-22511 is a very weak agonist at the MC5R (Ki 7.8 μm, EC50 4 μm, compared to Ki and EC50 of 10-100 nm at MC1R and MC3R).
We recently demonstrated that indirect stimulation of MC4R, but not MC3R, can improve insulin sensitivity in obese mice [42]. In that study, activation of MC4R was achieved through stimulation of Pomc neurons by meta-chlorophenylpiperazine (mCPP), a piperazine-based serotonin (5-HT) receptor agonist. Reduced food intake and improved insulin sensitivity associated with administration of 5-HT compounds involves 5HT2CR expressed on Pomc neurons [19]. Therefore, the effects of mCPP administered peripherally are probably mediated via increasing the activity of melanocortin receptors expressed in secondary neurons, and that are downstream of Pomc and AgRP neurons. In contrast, the current study observed improved hyperinsulinemia in MC4RKO treated peripherally with BIM-22511. Together, these observations indicate that MC1R and/or MC3R in peripheral tissues may mediate the improvements in insulin sensitivity in MC4RKO treated with BIM-22511.
Further evidence suggesting that the effects of BIM-22511 on hyperinsulinemia in MC4RKO involve peripheral receptors comes from the study by Obici et al. [28]. In that study, improvements in hepatic insulin action associated with central administration of α-MSH were blocked by intracerebroventricular infusion of antisense oligonucleotides targeting MC4R. The results of that study suggested that central MC3R are not able to compensate for loss of MC4R in metabolic regulation by hypothalamic α-MSH. Further indirect evidence for MC4R-independent regulation of insulin sensitivity has come from analysis of mice carrying the Mahoganoid (Mgrn1md) mutation [30]. Mgrn1 is an E3 ubiquitin ligase widely expressed in the brain, liver, skeletal and cardiac muscle, spleen, kidneys and adipose tissue [31]. Mgrn1md is a negative modifier of the obese, diabetic and coat pigmentation phenotype observed in lethal yellow (Ay/a) mice which over express agouti, an MC1R/MC4R antagonist [26, 30]. While inhibition of obesity by Mgrn1md is dependent on functional MC4R, improvements in diabetes do not require functional MC4R [30]. Phan and colleagues speculated that increased hypothalamic MC3R activity was involved in improving hyperinsulinemia in mice homozygous for the Mgrn1md and MC4R mutations. However, as the Mgrn1 gene is expressed outside the central nervous system, involvement of peripheral melanocortin recepors may also be involved.
The mechanisms involved in the MC4R-independent regulation of insulin resistance remain to be determined. However, it is of interest to note that melanocortin receptors are expressed in the immune system, and have been implicated in regulating inflammatory processes [5]. A link has been established between inflammation and the development insulin resistance with obesity [18]. Whether the anti-ant-inflammatory actions of melanocortin agonists are involved in improving insulin sensitivity remains to be investigated.
Nonalcoholic fatty liver disease is frequently observed with obesity and insulin resistance [29]. Insulin resistance is associated with reduced capacity for suppression of hepatic glucose production, however the ability of insulin to stimulation lipogenic enzymes is retained [4]. We observed that hepatic insulin resistance and severe steatosis is always observed in obese female MC4RKO, irrespective of diet [1, 38]. In this study, the MC4R-independent improvements in insulin sensitivity associated with BIM-22511 treatment were not associated with improvements in fatty liver in obese MC4RKO. These results suggest that while MC4R-independent pathways can improve in insulin sensitivity, this may not involve the liver. One possible interpretation is that activation of neural MC4R is required for reversal of hepatic steatosis by non-selective melanocortin agonists. This may involve a specific role for MC4R in regulating liver lipid metabolism. Indeed, previous studies have demonstrated regulation of genes involved in lipid metabolism by MTII in obese rodents [8, 21]. On the other hand, it may be that reducing food intake and weight loss associated with the activation of MC4R are important for improving hepatic steatosis.
Another interesting observation was the failure of BIM-22511 to reduce fasting glucose in MC3RKO. MC3RKO exhibited fasting hyperglycemia compared to WT, with glucose levels increase by 60% (164-193 vs. 100-129 mg/dL). Fasting insulin levels were not significantly different, suggesting a failure to increase insulin production to compensate for insulin resistance. If stimulation of MC4R (and, based on the response of MC4RKO, other melanocortin receptors) can improve insulin action, a reduction in fasting glucose with BIM-22511 treatment should have occurred. Remarkably, while not significant both insulin and glucose were higher in MC3RKO treated with BIM-22511. This observation may suggest that activation of MC4R is not able to reverse fasting hyperglycemia in the absence of MC3R. However, the MC3RKO used for this study were not obese, and it is notable that the lean WT controls also showed no significant reductions in insulin or glucose. Future studies will be required examining how MC3RKO with severe obesity and hyperinsulinemia due to ingestion of high fat diets (11, 15) respond to melanocortin receptor agonists.
A significant increase in fasting blood glucose of MC3RKO was not reported in the original studies investigating the phenotype of MC3RKO on out bred backgrounds [7, 9]. However, we have previously observed fasting hyperglycemia in the absence of significant hyperinsulinemia in male MC3RKO backcrossed onto the B6 background fed HFD [38]. That study observed no difference in 6-7 month old female MC3RKO. Again, it is important to note that the mice used for the current study were significantly older (>10 months). It is also important to consider that, in previous experiments glucose was measured using a one-touch glucometer. in the current study, serum glucose was measured by the Clinical Chemistry laboratory which provides more accurate measurements.
MC4RKO do not exhibit a significant feeding response to a single injection of a melanocortin agonist. However, the results of the current study suggest a modest increase of food intake in MC4RKO chronically treated with a melanocortin agonist. This may involve increased activity of hypothalamic MC3R, which are thought to act as an autoreceptor in the hypothalamic melanocortin system and reduce activity of Pomc neurons [14]. Treatment of mice with low doses of d-Trp8-γ-MSH, which is a moderately selective MC3R agonist, has been reported to increase food intake in mice [23]. A similar response has been reported in rats, which also exhibit a reduction in hypothalamic Pomc mRNA expression [20].
In summary, these experiments have demonstrated the use of two novel melanocortin receptor agonists. Our data suggest that MC4Rare necessary for weight loss associated with the use of these compounds. However, activity at other melanocortin receptors may be responsible for significant improvements insulin sensitivity independently of the MC4R and of weight loss.
Acknowledgments
This work was supported by grants from the NIH (R01 DK073189) and the Pennington Medical Research Foundation to AAB. AAB is also supported in part by CNRU Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by the NIDDK. HAH, JZD and MDC are employees of Biomeasure Incorporated, IPSEN; AAB has also been a paid consultant for Biomeasure, Inc. The authors thank Dr Nathan Markward for assistance with the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–52. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- 2.An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007;282:2862–70. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- 3.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- 6.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–90. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 8.Cettour-Rose P, Rohner-Jeanrenaud F. The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology. 2002;143:2277–83. doi: 10.1210/endo.143.6.8871. [DOI] [PubMed] [Google Scholar]
- 9.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 10.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 11.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 12.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 13.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 14.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–49. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 15.Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148:6186–94. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–77. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 17.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 19.Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000245. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Kim A, Conwell IM, Hruby V, Mayorov A, Cai M, et al. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides. 2008;29:440–7. doi: 10.1016/j.peptides.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Choi YH, Hartzell DL, Li C, Della-Fera MA, Baile CA. CNS melanocortin and leptin effects on stearoyl-CoA desaturase-1 and resistin expression. Biochem Biophys Res Commun. 2003;311:324–8. doi: 10.1016/j.bbrc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.MacNeil DJ, Howard AD, Guan X, Fong TM, Nargund RP, Bednarek MA, et al. The role of melanocortins in body weight regulation: opportunities for the treatment of obesity. Eur J Pharmacol. 2002;440:141–57. doi: 10.1016/s0014-2999(02)01425-5. [DOI] [PubMed] [Google Scholar]
- 23.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–64. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 25.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 26.Miller KA, Gunn TM, Carrasquillo MM, Lamoreux ML, Galbraith DB, Barsh GS. Genetic studies of the mouse mutations mahogany and mahoganoid. Genetics. 1997;146:1407–15. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijenhuis WA, Oosterom J, Adan RA. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–71. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 28.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–85. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–69. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 30.Phan LK, Chung WK, Leibel RL. The mahoganoid mutation (Mgrn1md) improves insulin sensitivity in mice with mutations in the melanocortin signaling pathway independently of effects on adiposity. Am J Physiol Endocrinol Metab. 2006;291:E611–20. doi: 10.1152/ajpendo.00034.2006. [DOI] [PubMed] [Google Scholar]
- 31.Phan LK, Lin F, LeDuc CA, Chung WK, Leibel RL. The mouse mahoganoid coat color mutation disrupts a novel C3HC4 RING domain protein. J Clin Invest. 2002;110:1449–59. doi: 10.1172/JCI16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pissios P, Maratos-Flier E. More than satiety: central serotonin signaling and glucose homeostasis. Cell Metab. 2007;6:345–7. doi: 10.1016/j.cmet.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, et al. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083–90. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- 34.Rangwala SM, Rich AS, Rhoades B, Shapiro JS, Obici S, Rossetti L, et al. Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes. 2004;53:1937–41. doi: 10.2337/diabetes.53.8.1937. [DOI] [PubMed] [Google Scholar]
- 35.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–31. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 36.Singhal NS, Patel RT, Qi Y, Lee YS, Ahima RS. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am J Physiol Endocrinol Metab. 2008;295:E331–8. doi: 10.1152/ajpendo.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, et al. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–55. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–96. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolle V, Low MJ. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57:86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 40.Trevaskis JL, Meyer EA, Galgani JE, Butler AA. Counterintuitive effects of double-heterozygous null melanocortin-4 receptor and leptin genes on diet-induced obesity and insulin resistance in C57BL/6J mice. Endocrinology. 2008;149:174–84. doi: 10.1210/en.2007-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–10. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, et al. Serotonin 2C Receptor Agonists Improve Type 2 Diabetes via Melanocortin-4 Receptor Signaling Pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]