Abstract
Background
The detection of high grade dysplasia and cancer in Barrett’s esophagus (BE) can be challenging. Confocal laser endomicroscopy (CLE) allows in vivo visualization of mucosal histology during endoscopy.
Objective
To determine if CLE with optical biopsy and targeted mucosal biopsy (CLE-TB) improves the diagnostic yield of endoscopically inapparent BE-associated neoplasia compared to standard endoscopy with a 4-quadrant random biopsy (SE-RB) protocol
Design
Prospective, double-blind, randomized crossover study
Setting
Single tertiary care academic center
Patients
Patients with BE undergoing routine surveillance or non-localized endoscopically inapparent BE-associated neoplasia referred for treatment
Interventions
All participants underwent both a confocal endomicroscopy with targeted biopsy procedure and standard endoscopy with 4-quadrant biopsy procedure in a randomized order.
Main outcome measurements
Increase in diagnostic yield for neoplasia, reduction in mucosal biopsy number, final pathologic diagnosis.
Results
CLE with targeted biopsy almost doubled the diagnostic yield for neoplasia and was equivalent to the standard protocol for the final diagnosis of neoplasia. 2/3 of patients in the surveillance group did not need any mucosal biopsies at all.
Limitations
Single center study
Conclusions
CLE with targeted biopsy significantly improves the diagnostic yield for endoscopically inapparent BE neoplasia compared to a standard endoscopy with random biopsy protocol. CLE with targeted biopsy also greatly reduces the number of biopsies needed per patient and allows some patients without neoplasia to completely forgo mucosal biopsy.
Keywords: confocal endomicroscopy, Barrett’s esophagus
Introduction
Barrett’s esophagus (BE) increases the risk of esophageal adenocarcinoma, and surveillance for neoplasia is recommended for patients with BE1. Current guidelines for endoscopic surveillance recommend a systematic biopsy protocol involving four-quadrant random biopsies every 1–2 cm for the length of BE2, 3. Despite advances in endoscopic imaging and multiple mucosal biopsies, detection of neoplasia in BE can be difficult1. Rigorous biopsy protocols still miss high grade dysplasia (HGD)4, 5, particularly in flat mucosa without obvious mucosal abnormalities. Furthermore, despite multiple biopsies, the diagnostic yield for neoplasia during surveillance of BE is highly variable depending upon the patient population and prevalence of dysplasia5–7. Hence, during surveillance endoscopy of BE, a large number of biopsies are obtained with a relatively low yield for dysplasia or cancer.
Confocal laser endomicroscopy (CLE) can be used to image the gastrointestinal mucosa during endoscopy, allowing in vivo microscopic examination of tissues throughout the gastrointestinal tract8. CLE has been used to image neoplasia in the colon, stomach, and the esophagus with accurate prediction of mucosal pathology in both normal and abnormal tissues9–11. Using CLE, microscopic images of the mucosa are produced up to 1250× magnification with imaging from the mucosal surface to 250 µm below the surface8. With this level of magnification, the specialized intestinal metaplasia and goblet cells of BE can be identified accurately 11.
The first published study of CLE for BE proposed an endomicroscopy classification system, the Confocal Barrett’s Classification, incorporating vascular structure and cell patterns to distinguish between gastric mucosa, BE, and neoplasia (HGD and cancer)11 (Table 1). Endomicroscopic changes suggesting the presence of HGD or cancer include the presence of irregular black cells with a loss of the normal cellular pattern and distorted subepithelial capillaries with leakage of fluorescein11. In this study, mucosal biopsies were routinely obtained to allow calculation of CLE performance characteristics. Using mucosal pathology as the reference standard, this study showed the classification system had a sensitivity of 92.9%, specificity of 98.4%, and accuracy of 97.4% for predicting BE-associated neoplasia11. These performance characteristics were based upon blinded reading of CLE images after the endoscopic examinations were complete. Furthermore, as the study was designed to develop a CLE classification system for BE, patients with esophageal masses were included. To date, there are no published studies that have validated the Confocal Barrett’s Classification in a prospective, blinded, controlled fashion using in vivo endomicroscopic imaging as the basis for diagnosis. Preliminary data suggest in vivo CLE imaging may be highly accurate and potentially enable targeted mucosal biopsy or selective endoscopic treatment within the same sedated procedure11–16.
Table 1.
Confocal Barrett’s Esophagus Classification11
| Tissue Type | Vessel Pattern | Cell Pattern |
|---|---|---|
| Gastric epithelium | Capillaries of regular shape visible in the deeper mucosa | Regular columnar epithelium with round gland openings and cobblestone pattern |
| Barrett’s esophagus | Capillaries of regular shape seen in deeper and upper mucosal layers | Columnar epithelium with dark mucin-containing goblet cells in the upper mucosal layer. Deeper mucosa shows dark cylindrical cells arranged in a villous pattern. |
| Neoplasia | Irregular capillaries visible throughout the mucosal layer. Vessel leakage leads to heterogeneous and bright lamina propria | Black cells with irregular borders in contrast to surrounding tissue |
Table reprinted from Clinical Gastroenterology and Hepatology, vol 4, issue 9, Kiesslich R et al, In Vivo Histology of Barrett’s Esophagus and Associated Neoplasia by Confocal Laser Endomicroscopy, p. 984, with permission from Elsevier.
The aim of this study was to determine if CLE with optical biopsy and targeted mucosal biopsy (CLE-TB) improves the diagnostic yield of BE-associated neoplasia compared to standard endoscopy with a four-quadrant random biopsy (SE-RB) protocol, in patients referred for suspected non-localized endoscopically inapparent HGD or cancer and in patients undergoing routine surveillance of BE.
Patients and Methods
Study Design and Setting
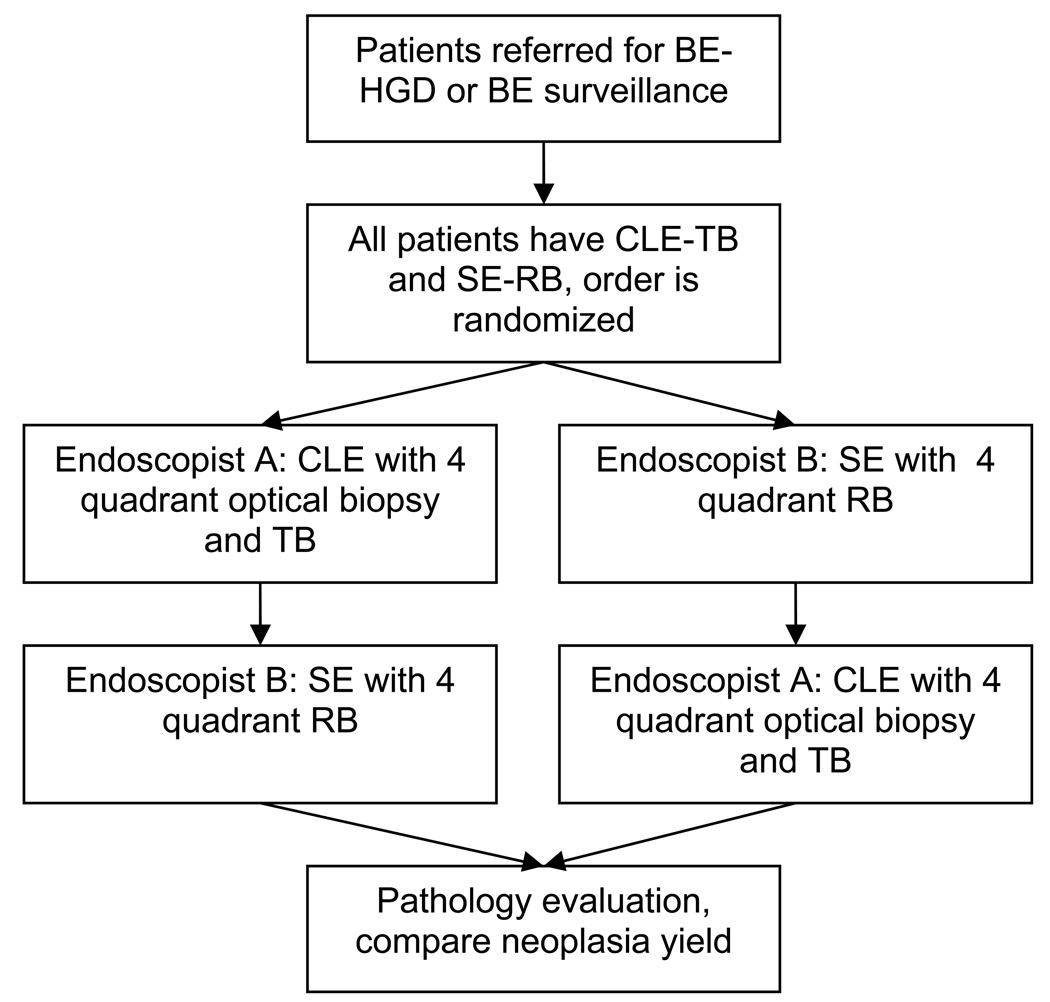
This prospective, controlled, double-blind (endoscopists and pathologists), crossover trial was conducted at a single, tertiary care, academic medical center. Each patient underwent confocal endomicroscopy and standard endoscopic exams, but the order in which CLE-TB and SE-RB were performed was randomized (Figure 1). Randomization was 1:1 in blocks of four according to a computer-generated list.
Figure 1.
Overall study design. Patients are referred for Barrett’s esophagus (BE) or BE with high grade dysplasia (HGD) BE-HGD. All patients have confocal laser endomicroscopy (CLE) with targeted biopsy (TB) and standard endoscopy (SE) with random biopsy (RB) in a randomized order. For patients randomized to CLE first, endoscopist A performed CLE - TB. Two to 6 weeks later, the patient has SE with 4 quadrant random biopsy. All biopsies undergo histopathologic examination and the yield for neoplasia between CLE and SE is compared.
One endoscopist (MC) performed all CLE procedures, while a second endoscopist (PO) performed all SE procedures. Both endoscopists had more than ten years of endoscopic experience and clinical practices including BE and BE-neoplasia patients. The endoscopist performing CLE had completed 30 supervised and 85 independent endomicroscopy procedures. Both endoscopists were aware of the indication for the procedure (routine surveillance or suspected neoplasia), but were blinded to the prior endoscopy and pathology results. The second procedure was performed two to six weeks after the first, to allow healing of prior biopsy sites and minimize bias during the second procedure.
Patients
Patients with BE or BE with suspected, non-localized, endoscopically inapparent HGD were recruited from the gastroenterology clinics at Johns Hopkins University in Baltimore, Maryland, USA from April 2007 though May 2008. The study was approved by the Johns Hopkins University Institutional Review Board (clinicaltrials.gov NCT00487695).
The inclusion criteria were adults with: 1) biopsy-proven BE or 2) biopsy-proven BE with suspected non-localized, endoscopically inapparent HGD. Exclusion criteria for the study were known esophageal adenocarcinoma, BE with a biopsy-proven malignant lesion, allergy to fluorescein sodium, coagulopathy, cardiopulmonary instability, active wheezing, or a history of anaphylaxis.
All patients enrolled in the study completed a standardized questionnaire used for the Johns Hopkins Barrett’s Esophagus Registry that recorded patient demographics, gastrointestinal symptoms, duration of BE, and other relevant medical history.
Standard Endoscopy Procedure
During the standard endoscopy procedure, the endoscopist performed a videoendoscopic examination using the Olympus video upper endoscope (GIF 160, Olympus Corporation, Tokyo, Japan). Endoscopic landmarks, including the level of the GE junction, Z line, and BE length and pattern (circumferential, tongues, and islands) were recorded. The presence of esophagitis was described using the Los Angeles classification17. The endoscopist evaluated the size, morphology, and location of any visible lesions which were described using the Japanese Classification of esophageal cancer18. Then, biopsies of any discrete lesions were obtained, followed by four-quadrant random biopsies of the flat BE mucosa every 1 centimeter (for suspected neoplasia) or 2 centimeters (for BE surveillance) beginning at the GE junction, moving proximally to the Z line.
Confocal Endomicroscopy Procedure
The Pentax endomicroscope (EC3870KCILK, joint venture between Pentax, Tokyo, Japan and Optiscan Pty Ltd, Notting Hill, Melbourne, Australia) was used for all endomicroscopy exams. The endomicroscope is the length of a standard gastroscope, with a 12.8 mm diameter and 2.8 mm channel. The shaft of the endomicroscope is labeled in 1 cm increments to allow accurate measurement of location. The endomicroscope laser has a wavelength of 488 nm, with maximum laser output of <1 mW at the mucosal surface. All images were collected at a scan rate of 0.8 frames/second, giving a resolution of 1024×1024 pixels. The field of view is 500×500 µm, with lateral resolution of 0.7 um and an optical slice thickness of 7 µm. At each imaging site, multiple images were collected from the surface down to the maximum imaging depth of 250 µm.
The standard videoendoscope built into the confocal endomicroscope was used to examine the esophagus, similar to that described for the standard endoscopy procedure above, recording endoscopic landmarks, BE characteristics, and lesions.
Then confocal endomicroscopy was performed. Five milliliters of 10% fluorescein sodium (Ak-fluor, Akorn Pharmaceuticals, Lake Forest, Illinois, USA) was administered intravenously and images were acquired by placing the tip of the endomicroscope against the mucosal surface, using suction to stabilize the tip for image acquisition. Discrete lesions were imaged first and then four-quadrant optical biopsies of the flat BE mucosa were acquired every 1 centimeter (for suspected neoplasia) or 2 cm (for BE surveillance). Optical biopsies were obtained by imaging from the mucosal surface to a depth of 250 um to visualize the epithelial cells, lamina propria, and blood vessels. At each optical biopsy site, the endoscopist used the CLE images to predict the histology expected on mucosal biopsy, interpreting each image according to the Confocal Barrett’s Classification11, differentiating neoplasia from BE and gastric epithelium. For CLE imaging sites suspicious for neoplasia, targeted mucosal biopsies were acquired, guided by the suction polyp created by endomicroscopic imaging. For CLE imaging sites that did not suggest neoplasia, no biopsies were taken. No random mucosal biopsies were acquired during the endomicroscopy procedure.
Endoscopic Mucosal Resection
Informed consent was obtained on all patients prior to endoscopy for possible endoscopic mucosal resection (EMR) at the end of the second procedure, if there was endoscopic or CLE evidence of HGD. At the end of the second endoscopic procedure, the study co-investigator (KD) was allowed to unblind the endoscopist and disclose the prior pathologic diagnoses and the location of any areas of biopsy-proven HGD. If an area of localized HGD was detected by endomicroscopy, or if the specific location of HGD was known from prior endoscopy, then EMR was performed using the Duette multiband ligation device (Cook Medical, Bloomington, Indiana, USA). Alternatively, if the second endoscopist felt that a mucosal lesion was highly suspicious for HGD or early cancer, EMR could be performed.
Pathology
Mucosal biopsies were placed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, as well as periodic acid-Schiff/Alcian blue (PAS/AB) stain for identification of goblet cells. Mucosal biopsies were reviewed by the gastrointestinal pathology service and were re-reviewed by an expert BE pathologist (EM), who was blinded to the outside pathology results, endoscopic procedure type, endoscopic findings, and CLE diagnoses. Each individual biopsy was graded according to the Vienna classification of gastrointestinal epithelial neoplasia19. The individual mucosal biopsies were counted for each procedure for a given patient.
Statistical Analysis
Results for each patient were analyzed in a paired fashion, comparing each patient’s CLE procedure with their standard endoscopic procedure. The final per patient histopathologic diagnosis for CLE-TB and SE-RB were compared using McNemar’s test. The primary endpoint, the diagnostic yield (the number of mucosal biopsies showing HGD or cancer divided by the total number of mucosal biopsies) was calculated per patient for each procedure and compared using the Wilcoxon signed-rank test. The secondary endpoints of the mean number of biopsies obtained per patient and mean number of biopsies with HGD were compared by procedure type using the signed-rank test. The prevalence of HGD in lesions and flat mucosa was calculated. Two-tailed P-values of <0.05 were considered statistically significant.
Sample Size Calculation
The sample size calculation was based on the predicted yield for neoplasia using standard endoscopy and CLE. Based on published studies and prior data collected at Johns Hopkins, the yield for neoplasia of SE-RB was estimated to be 10% and the neoplasia yield for CLE-TB was estimated to be 40%6, 11. Using an alpha of 0.05 and power of 90%, 37 patients were needed using a paired design. To allow for dropouts, we planned to enroll 48 patients. Statistical analysis was performed using Stata 9.0 (Stata Corporation, College Station, Texas, USA).
Results
Patient Characteristics
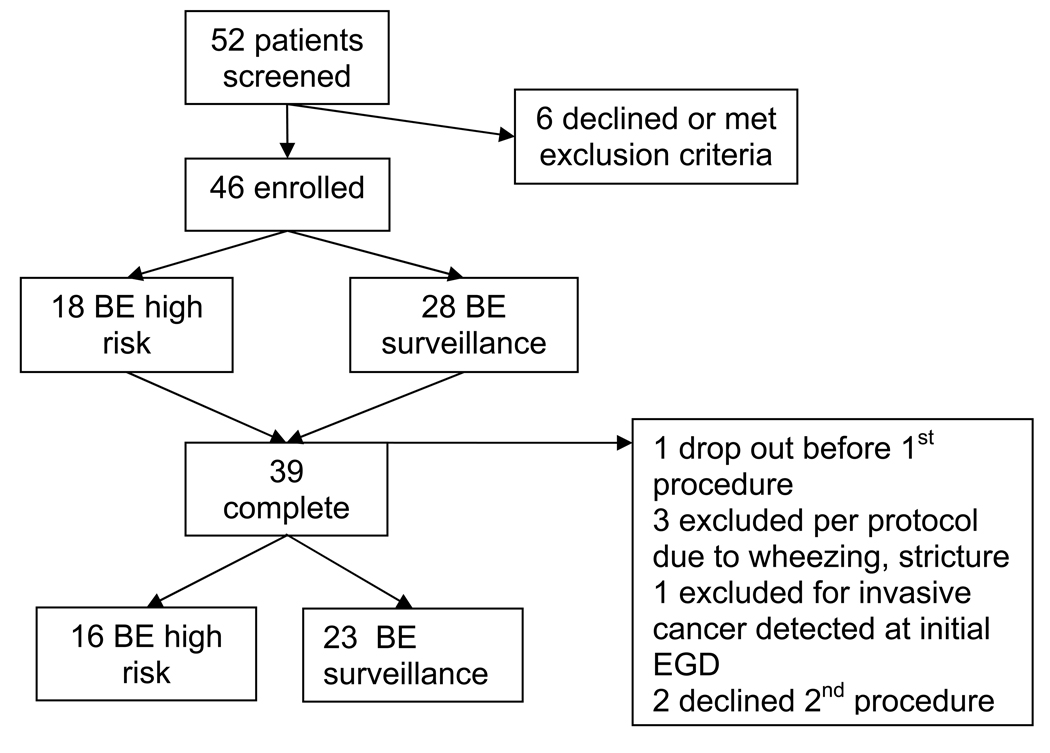
Fifty-two patients with BE or suspected BE neoplasia were screened for participation. Six of the screened patients declined to participate, leaving 46 patients who enrolled in the study (18 with suspected HGD and 28 for BE surveillance). A total of 39 patients completed the study, including 16 patients with suspected neoplasia and 23 BE surveillance patients (Figure 2). The characteristics of the study participants are listed in Table 2. The mean length of BE was longer in the high risk group (mean=6 cm, range 1–11 cm) than the surveillance group (mean=4 cm, range 1–10 cm) (Table 3). Half of the high risk patients and one-third of the surveillance patients had circumferential BE. Esophagitis (LA grade B) was identified in only two of the study participants who were enrolled in the surveillance group. No patients were found to have masses or nodules greater than 0.5 cm. In the high risk group, nine patients were found to have subtle lesions. There were no lesions in the routine surveillance group.
Figure 2.
Study Participants
Table 2.
Participant Characteristics (N = 39)
| Mean age in years (range) | 64 (43–80) |
| Gender Number (%) | 30 Male (77%), Female 9 (23%) |
| Suspected neoplasia Number (%) | 16 (41%) |
| History of esophageal reflux Number (%) | 36 (92%) |
| Mean duration of symptomatic esophageal reflux in years (range) | 21 (0–50) |
| Daily PPI use Number (%) | 36 (92%) |
| Final pathologic diagnosis by patient Number (%) | 0 |
| Cancer | 13 (33%) |
| HGD | 26 (66%) |
| LGD/indefinite/no dysplasia | |
| Patients with lesions identified during endoscopy | 9 (23%) |
| Number (%) | |
| Total number of lesions identified | 17 |
| Japanese Classification of Lesions (n=17) Number (%) | Lesions Containing BE with High Grade Dysplasia * |
| 0-IIa: superficial flat, slightly elevated ( n=9) | 6 (53%) |
| 0-IIc: superficial flat, slightly depressed (n=2) | 2 (100%) |
| 0-I: superficial protruding (n=2) | 1 (50%) |
| I: polypoid (n=4) | 0 (0%) |
Histopathology of the remaining lesions – LGD, indefinite, nondysplastic BE, gastric cardia.
Table 3.
Description of Barrett’s Esophagus
| Characteristic | Total Group n=39 | High Risk Group n=16 | Surveillance Group n=23 |
|---|---|---|---|
| Mean BE length (cm), (SD) Range (cm) | 4.8 (3.4) 1–11 |
6.0 (3.7) 1–11 |
4.0 (2.9) 1–10 |
| Circumferential BE N (%) | 16 (41%) | 8 (50%) | 8 (34.8%) |
| Tongues or Islands only N (%) | 23 (59%) | 8 (50%) | 15 (65%) |
| Esophagitis present | 2 (5.3%) | 0 | 2 (8.7%) |
Final Pathologic Diagnosis
The final pathologic diagnosis was established by recording the highest grade of neoplasia from the blinded reading of the two sets of mucosal biopsies from the SE-RB and CLE-TB procedures (Table 2).
In the high risk group referred for suspected non-localized neoplasia, 13 cases of HGD and no cancers were identified. CLE-TB and SE-RB each detected 11 cases of HGD, and there was no statistically significant difference in neoplasia detection by the two methods (p=1.0). Four patients in the study had discordant final diagnoses. For the two cases of neoplasia detected by only by SE-RB, the random biopsy protocol found a single biopsy showing focal HGD. For the two cases of neoplasia detected by CLE-TB alone, CLE found areas of HGD not detected by standard endoscopy: one area of HGD in one patient and two areas of HGD in the second case.
In the surveillance endoscopy group, one patient had a single biopsy obtained in the setting of esophagitis during SE-RB interpreted as focal HGD which could not be confirmed on three subsequent endoscopic procedures performed after treatment with double dose proton pump inhibitors. Otherwise, no cases of neoplasia were identified in the surveillance group by SE-RB or CLE-TB.
Mucosal Biopsy Number per Procedure and the Diagnostic Yield for Neoplasia
In the 16 patients with suspected high grade dysplasia, CLE-TB led to a significant 59% decrease in the number of mucosal biopsies taken per patient during endomicroscopy compared to SE-RB (9.8 biopsies versus 23.8 biopsies, p=0.002) (Table 4). Furthermore, despite fewer total biopsies, the mean number of mucosal biopsies per patient showing HGD or cancer was not significantly different between the groups, with 3.1 and 3.7 neoplastic biopsies obtained during CLE-TB and SE-RB, respectively (p=0.89). The diagnostic yield for neoplasia with CLE-TB was 33.7% (95% CI 15.2%–52.2%) while the diagnostic yield for neoplasia during SE-RB was 17.2% (95% CI 6.2%–28.2%), giving a difference in yield of 16.5% (95% CI 5.2%–27.8%, p=0.01) (Table 4).
Table 4.
Diagnostic Yield for Neoplasia - Per Patient Analysis
| High Risk Group n = 16 (Suspected HGD or CA) | Surveillance Group n = 23 | |||||
|---|---|---|---|---|---|---|
| CLE-TB | SE-RB | p | CLE-TB | SE-RB | p | |
| Mean number of biopsies with HGD or CA (range) | 3.1 (0–15) |
3.7 (0–19) |
0.89 | 0 | 0 | 1.0 |
| Mean number of mucosal biopsies obtained (range) | 9.8 (1–22) |
23.7 (3–41) |
0.002 | 1.7 (0–12) |
12.6 (1–28) |
<0.0001 |
| Mean diagnostic yield (% positive biopsies for HGD or CA) | 33.7% | 17.2% | 0.01 | 0 | 0 | - |
SE-RB = standard endoscopy with random mucosal biopsy
CLE-TB = confocal laser endomicroscopy with targeted mucosal biopsy
HGD = high grade dysplasia
CA= esophageal adenocarcinoma
In BE patients undergoing surveillance, the mean number of mucosal biopsies was 87% lower during CLE-TB than SE-RB (1.7 versus 12.6, p<0.0001) (Table 4). Sixty-five percent of the 23 patients undergoing surveillance endoscopy did not need any mucosal biopsies during CLE-TB, as the in vivo endomicroscopic imaging did not suggest BE with neoplasia. No patient in the surveillance group was found to have HGD. Hence, the diagnostic yield for HGD for both CLE-TB and SE-RB was zero.
Prevalence of HGD or Cancer in Lesions
No patients in the surveillance group were found to have lesions during the study. Nine of 16 patients (56%) in the high risk group had 17 subtle mucosal lesions. One patient had four lesions, one patient had three lesions, three patients had two lesions, and four patients had one lesion. Of the 17 lesions identified, nine were Japanese classification Type 0-IIa (superficial flat, slightly elevated), two were Type 0-IIc (superficial flat, slightly depressed), two were Type 0-I lesions (superficial protruding), and four were Type I (small polypoid lesions) (Table 2). Only nine out of 17 (53%) of the mucosal lesions contained HGD or cancer by mucosal biopsy or EMR. The other eight lesions in the high risk patients contained non-dysplastic BE, BE with low-grade dysplasia (LGD), BE with indefinite dysplasia, or gastric cardia.
CLE-Guided Endoscopic Mucosal Resection
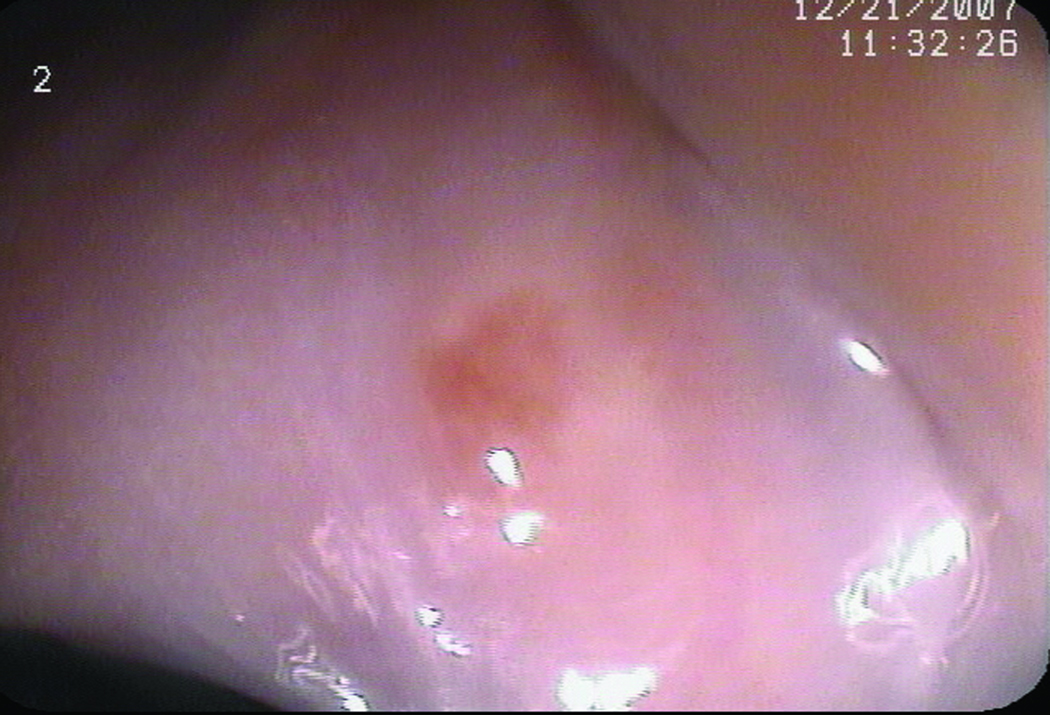
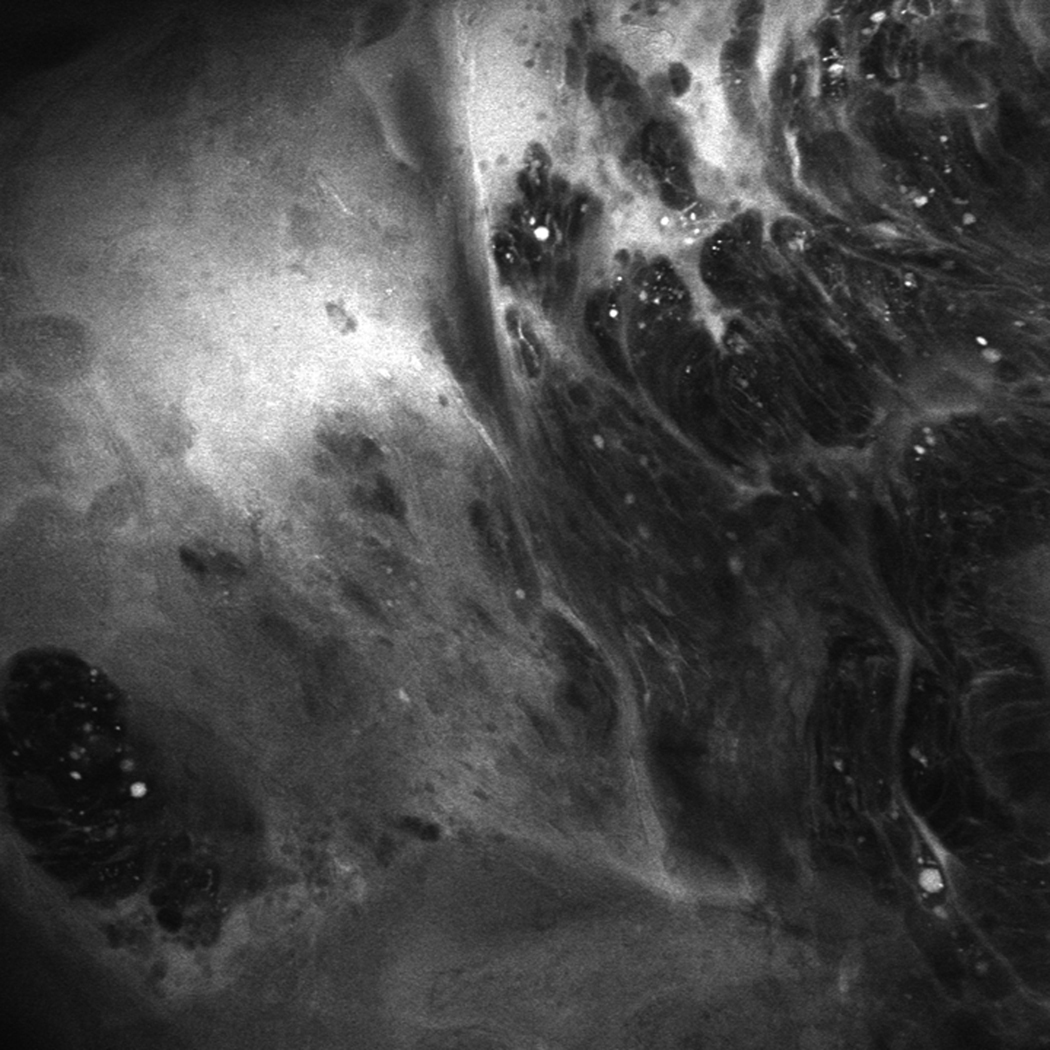
Two patients had EMR during their CLE-TB procedures. The decision to perform EMR was based on CLE images of flat mucosa suggesting HGD. In one patient, a small flat BE island was found to have changes suggestive of HGD using CLE: dark irregular cells and loss of the normal BE glandular pattern (Figure 3). EMR was performed, and subsequent histopathologic examination confirmed the presence of HGD (Figure 3). The second patient with flat, endoscopically inapparent HGD also underwent EMR based on endomicroscopic imaging, and histopathology confirmed the presence of HGD.
Figure 3.
(A) Unmagnified standard white light endoscopic image of a tiny island of Barrett’s esophagus obtained with the endomicroscope prior to endomicroscopic imaging. (B and C) Confocal endomicroscopy images of the island shows glands with irregularly-shaped, distorted dark cells, indistinct cell borders, and loss of normal crypt architecture suggestive of high grade dysplasia (D) Histopathology confirmed Barrett’s esophagus with HGD in the endoscopic mucosal resection specimen
Four endoscopic mucosal resections were performed at the end of the SE-RB procedure. Of these, two were based on endoscopic appearance of the mucosa suspicious for neoplasia, as lesions were present. One EMR showed LGD, while the other had nondysplastic BE on histopathology. The other two EMRs were performed per protocol when the endoscopist was unblinded and informed by the study coordinator about the location of biopsy-confirmed HGD found during prior CLE. The results of these EMRs showed HGD for one patient and LGD for the other.
CLE Imaging Time
Procedure times were not a major endpoint of the study. The study protocol did not allow complete recording of the time needed to perform CLE and acquire mucosal biopsies. However, approximate times were available for a subset of the study sample. Esophageal imaging with CLE added a median of 18 minutes to the procedure time (interquartile range (IQR) 12–22 minutes). In contrast, the time spent acquiring targeted mucosal biopsies during CLE (median 1.5 minutes, IQR 0–5 minutes) was shorter than the time needed for biopsies during SE-RB (median 9 minutes, IQR 5–15 minutes) (p<0.001). For patients with long BE (>6 cm), the median imaging time was 23.5 minutes (IQR 20–32.5 minutes). The median time spent acquiring mucosal biopsies during CLE in patients with long BE was 5 minutes (IQR 1–6 minutes) compared to 14.5 minutes (IQR 10–18.5 minutes) during SE-RB (p<0.001). For patients with short BE (<3 cm), the median time spent imaging was 10 minutes (IQR 7–16 minutes). The median time spent acquiring targeted mucosal biopsies in short BE was 0 minutes (IQR 0–2 minutes) during CLE compared to 4 minutes (IQR 3–6 minutes) during SE-RB (p<0.02).
Complications
All study-related procedures were performed with intravenous propofol administered by anesthesiology. There were no serious complications related to SE-RB or intravenous fluorescein sodium during CLE. One post-procedure pneumonia occurred after a CLE procedure. This resulted in a two-day hospitalization and complete resolution of the infection following antibiotics.
Discussion
This is the first prospective, randomized, controlled blinded trial that validates the Confocal Barrett’s Classification for in vivo prediction of mucosal histopathology. It also demonstrates the potential role of in vivo endoscopic diagnosis with CLE for the surveillance of BE. By combining in vivo CLE diagnosis with targeted mucosal biopsy, we demonstrated significant reduction in the number of mucosal biopsies required for surveillance of BE in patients undergoing routine surveillance and those referred for suspected endoscopically inapparent non-localized neoplasia. There was almost 60% reduction in the number of mucosal biopsies required to make a diagnosis of neoplasia comparing CLE-TB to SE-RB. Importantly, the detection of HGD using CLE-TB and SE-RB was comparable, despite CLE-TB obtaining significantly fewer biopsies. Hence, CLE-TB almost doubled the diagnostic yield of mucosal biopsies for neoplasia compared to SE-RB (33.7% versus 17.2%). In addition, almost two-thirds of patients in the routine surveillance group did not need any mucosal biopsies during CLE due to absence of neoplasia during in vivo imaging. The biopsy reduction in these patients without suspected neoplasia was even greater, with an 86% reduction in the number of biopsies needed during CLE-TB compared to SE-RB.
The first CLE study by Kiesslich et al in BE reported a potential reduction in the number of mucosal biopsies needed, as only 30 of 156 (19.2%) CLE sites in 63 patients examined would have required a mucosal biopsy for confirmation of the diagnosis of neoplasia11. However, this potential biopsy reduction was calculated based on the study data but not prospectively studied. Our study demonstrates that CLE led to a reduction in the number of biopsies needed. Furthermore, all CLE interpretation in this study was performed in real time, which differs from other studies of endomicroscopy in BE11.
The performance characteristics for the in vivo diagnosis of BE and associated neoplasia are not well characterized and require further investigation. This study could not assess accuracy because mucosal biopsy was not routinely performed during the CLE procedure if in vivo CLE imaging did not show high grade dysplasia or cancer. The final pathologic diagnoses suggest comparable detection of neoplasia between SE-RB and CLE-TB.
We chose a crossover design for this study to help ensure a fair comparison between CLE and standard endoscopy. To reduce the potential for bias and to minimize interobserver variability in CLE image interpretation, one endoscopist performed all CLE-TB procedures and a second endoscopist performed all SE-RB procedures. Both endoscopists were blinded to the details of prior endoscopies and pathology results to reduce the potential for bias. In addition, the study gastrointestinal pathologist read all the study biopsies and was blinded to the suspected diagnosis, endoscopy findings, and CLE findings.
We excluded patients with known masses and lesions from this study and focused on patients with non-localized, endoscopically inapparent neoplasia. Some subtle lesions were identified during the study, but only 53% of the lesions showed HGD, and none showed cancer. This is comparable to other published reports. In one study of high-resolution endoscopy, only 17 of 30 (57%) suspicious lesions were shown to have HGD or cancer on biopsy7. Thus, even when mucosal lesions are present in BE, accurate endoscopic identification of HGD and cancer is still challenging. Our study demonstrates the potential clinical utility of in vivo CLE for localization and detection of HGD in flat BE mucosa. In our study, EMR was possible in two high risk patients with endoscopically inapparent HGD who had CLE as the second procedure. By comparison, three of the four EMRs performed on endoscopically-suspicious mucosal lesions at the end of the standard endoscopy procedure were unnecessary. The use of CLE could potentially impact in vivo decision-making for the treatment of localized HGD or early cancer by allowing EMR during the same procedure, potentially reducing the difficulty of relocating the precise site of HGD at a later time. Our study was not designed to compare the potential clinical impact of the CLE diagnosis on the decision to perform EMR. Future studies should examine how in vivo endomicroscopic diagnosis might allow selective and immediate application of EMR.
The additional time needed to perform CLE after SE was not a major endpoint of this study, but it is an important issue. This would be best addressed by a study comparing the time difference of SE alone versus SE + CLE with the endomicroscopist performing both procedures on the same patient. This was not feasible with the design of this study. With current CLE imaging technology, the additional time needed to perform CLE beyond SE is influenced by BE length, prevalence of neoplasia, and operator experience. From our study data, the time needed for acquisition of mucosal biopsies was shorter with CLE than SE, particularly in patients with long BE. A multicenter study of CLE in Barrett’s esophagus is also planned to address this issue.
There are several limitations to our study. Our study is relatively small and based in a single-center tertiary referral academic center. However, despite the small sample size, the crossover, paired study design provided significant power (90%) for our study results. The participants in this study may not be representative of the general American population, as our hospital is a referral center for BE and endoscopic therapy of neoplasia. However, our study population included both an enriched population of patients with suspected non-localized neoplasia as well as patients undergoing routine surveillance. In addition, we excluded patients with obvious cancers and lesions. which differs from prior studies11.
The interobserver variability for the in vivo endomicroscopic findings is unknown, and we were unable to evaluate in vivo interobserver agreement as only one endoscopist performed CLE. This was not a primary endpoint of our study, but is an important issue that needs be addressed by future studies of CLE. Preliminary data on the interobserver agreement of the interpretation of selected CLE images appears to be moderate to substantial particularly with respect to gastrointestinal neoplasia20, 21. The in vivo interpretation with CLE is likely to be influenced by technical factors, operator experience, and disease prevalence.
Finally, the only contrast agent available at our institution for CLE is fluorescein sodium, which does not stain the nuclei of cells. However, high grade neoplasia and cancer can still be distinguished from nondysplastic BE with pattern recognition using the published Confocal Barrett’s Classification11. In the future, improvements in endomicroscopic imaging technology and molecular markers25 may obviate the need for imaging with nonspecific agents such as fluorescein. This may enhance the application of CLE to BE surveillance. The current Pentax endomicroscope is equipped only with standard videoendoscopic resolution without mucosal enhancement features. The diagnosis of BE-associated neoplasia might be significantly altered if high resolution endoscopy is used with other mucosal enhancement techniques, such as narrow band imaging, autofluorescence, or chromoendoscopy7, 22–24. However, at this time, none of the newer imaging modalities have been clearly shown to be significantly better than endoscopy with a four-quadrant random biopsy protocol5 or high resolution endoscopy alone23. Hence, the current standard of practice for detection of neoplasia in BE involves a systematic biopsy protocol after careful white light endoscopy.
Conclusions
In summary, our study shows that in vivo imaging with CLE and targeted mucosal biopsy of imaging abnormalities is superior to standard endoscopy with four-quadrant random biopsy for detection of endoscopically inapparent neoplasia in Barrett’s esophagus. The number of biopsies required to make a diagnosis was significantly lower and the diagnostic yield for neoplasia was higher, suggesting that CLE-TB may assist the endoscopist with taking “smarter” biopsies. Our study demonstrates how CLE can enable more selective sampling of the mucosa, without the need for the gastroenterologist to replace the pathologist. Future studies will need to evaluate the clinical impact of in vivo diagnosis with CLE on the immediate endoscopic treatment of BE with associated neoplasia.
Acknowledgments
Support:
American Society of Gastrointestinal Endoscopy Endoscopic Research Award 2007, NIH Roadmap Johns Hopkins Multidisciplinary Clinical Research Career Development Award Grant (K12 RR023266), and an unrestricted educational grant from Pentax Medical Corporation. This work has also been made possible in part through the generosity of the Jerry D’Amato Foundation and the Roy L Jeannotte Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falk GW. Barrett's esophagus. Gastroenterology. 2002;122:1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 2.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 4.Reid BJ, Blount PL, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:3089–3096. doi: 10.1111/j.1572-0241.2000.03182.x. [DOI] [PubMed] [Google Scholar]
- 5.Egger K, Werner M, Meining A, et al. Biopsy surveillance is still necessary in patients with Barrett's oesophagus despite new endoscopic imaging techniques. Gut. 2003;52:18–23. doi: 10.1136/gut.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett's esophagus. Gastrointest Endosc. 2000;51:560–568. doi: 10.1016/s0016-5107(00)70290-2. [DOI] [PubMed] [Google Scholar]
- 7.Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett's esophagus: a prospective randomized crossover study. Endoscopy. 2005;37:929–936. doi: 10.1055/s-2005-870433. [DOI] [PubMed] [Google Scholar]
- 8.Kiesslich R, Goetz M, Vieth M, et al. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715–731. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JN, Li YQ, Zhao YA, et al. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843–853. doi: 10.1016/j.gie.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Canto MI, Dunbar KB, Kiesslich R, et al. In vivo real time confocal laser endomicroscopic diagnosis of Barrett's esophagus and associated neoplasia. Gastrointest Endosc. 2007;65:AB346. [Google Scholar]
- 13.Meining A, Saur D, Bajbouj M, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007;5:1261–1267. doi: 10.1016/j.cgh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Badreddine RJ, Wang KK, Prasad GA, et al. Confocal Laser Microscopy (CLM) Guided Endoscopic Mucosal Resection in Barrett's Esophagus with High Grade Dysplasia. Gastrointest Endosc. 2008;67:AB177. [Google Scholar]
- 15.Leung KK, Maru D, Abraham S, et al. Optical EMR: confocal endomicroscopy-targeted EMR of focal high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc. 2008 doi: 10.1016/j.gie.2008.03.1068. in press, available online June 25, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Pohl H, Rosch T, Vieth M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's esophagus. Gut. 2008 doi: 10.1136/gut.2008.157461. [DOI] [PubMed] [Google Scholar]
- 17.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo M, Kawano T. Detection and classification of early squamous cell esophageal cancer. Dis Esophagus. 1997;10:155–158. doi: 10.1093/dote/10.3.155. [DOI] [PubMed] [Google Scholar]
- 19.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunbar KB, Kiesslich R, Wirths K, Goetz M, Maitra A, Montgomery E, Vieth M, Zhang Z, Canto MI. Confocal laser endomicroscopy image interpretation: interobserver agreement among gastroenterologists and pathologists. Gastrointest Endosc 2007. 2007;65:AB348. [Google Scholar]
- 21.Kiesslich R, Anagnostopoulos GK, Axon A, et al. Interobserver variation and standardized training for confocal laser endomicroscopy image interpretation in the upper and lower GI tract. Gastrointest Endosc. 2007;65:AB354. [Google Scholar]
- 22.Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett's oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–172. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 23.Curvers W, Baak L, Kiesslich R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett's esophagus. Gastroenterology. 2008;134:670–679. doi: 10.1053/j.gastro.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Kara MA, Peters FP, Fockens P, et al. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett's esophagus. Gastrointest Endosc. 2006;64:176–185. doi: 10.1016/j.gie.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]