Abstract
The neural correlates of the perception of faces from different races were investigated. White participants performed a gender identification task in which Asian, Black, and White faces were presented while event-related potentials (ERPs) were recorded. Participants also completed an implicit association task for Black (IAT-Black) and Asian (IAT-Asian) faces. ERPs evoked by Black and White faces differed, with Black faces evoking a larger positive ERP that peaked at 168 ms over the frontal scalp, and White faces evoking a larger negative ERP that peaked at 244 ms. These Black/White ERP differences significantly correlated with participants’ scores on the IAT-Black. ERPs also differentiated White from Asian faces and a significant correlation was obtained between the White-Asian ERP difference waves at ~500 ms and the IAT-Asian. A positive ERP at 116 ms over occipital scalp differentiated all three races, but was not correlated with either IAT. In addition, a late positive component (around 592 ms) was greater for the same race compared to either other race faces, suggesting potentially more extended or deeper processing of the same race faces. Taken together, the ERP/IAT correlations observed for both other races indicate the influence of a race-sensitive evaluative process that may include early more automatic and/or implicit processes and relatively later more controlled processes.
Keywords: Face perception, race, implicit association test (IAT), Event-related potentials (ERP)
Human faces are rich sources of information for guiding interpersonal impressions and interactions (Blair, Judd, Sadler, & Jenkins, 2002; Ekman, 1989; Willis & Todorov, 2006; Young, McWeeny, Hay, & Ellis, 1986). The extraction of information from faces occurs very quickly, and research has shown that an exposure of 100 ms to a face is sufficient to form an impression about a person (Willis & Todorov, 2006). Moreover, characteristics such as race, age, and sex are reliably extracted from facial features. Race is a particularly important characteristic and can elicit spontaneous activation of stereotypes and prejudices (Blair, Judd, & Fallman, 2004; Smith-McLallen, Johnson, Dovidio & Pearson, 2006). Here, we investigated the relation between implicit racial attitudes and the perception of faces from different races, focusing on the time course of this relationship.
Several prior studies have investigated the timeline of attending to racial cues from human faces (Ito, Thompson, & Cacioppo, 2004; Ito & Urland, 2003, 2005). Recently, studies of cross-race face processing have attempted to determine when in time facial features that differentiate racial groups and racial attitudes held by an observer influence the neural processing of faces and what brain structures are sensitive to these variables. These studies have used both functional magnetic resonance imaging (fMRI) and event-related potential (ERP) techniques (Caldara, Rossion, Bovet, & Hauert, 2004; Caldara, Thut, Servoir, Michel, Bovet, & Renault, 2003; Cunningham, Johnson, Raye, Gatenby, Gore, & Banaji, 2004; Golby, Gabrieli, Chiao, & Eberhardt, 2001; Hart, Whalen, Shin, McInerney, Fischer, & Rauch, 2000; Ito & Urland, 2003; James, Johnstone, & Hayward, 2001; Lieberman, Hariri, Jarcho, Eisenberger, & Bookheimer, 2005; Phelps, O’Connor, Cunningham, Funayama, Gatenby, Gore, & Banaji, 2000). fMRI studies can localize activity to specific brain regions at the millimeter level, while ERP studies are sensitive to the time course of neural processes at the millisecond level.
Functional MRI studies of cross-race face perception in White participants have shown that the amygdala, a medial temporal lobe structure associated with attention to and memory for emotional information (LeDoux, 2000), is activated more by unfamiliar Black faces than by unfamiliar White faces (Cunningham et al., 2004; Hart et al., 2000). Other fMRI studies have examined cross-race face processing in the fusiform gyrus, a ventral occipitotemporal brain region within which selective responses to face stimuli have been reliably demonstrated by both subdural ERP recording (Allison, Ginter, McCarthy, Nobre, Puce, Luby & Spencer, 1994) and fMRI (Puce, Allison, Gore, & McCarthy, 1995). Golby et al. (2001) and Kim and colleagues (Kim, Yoon, Kim, Jeun, Jung, & Choe, 2006) reported that for White participants the fusiform gyrus was more activated by unfamiliar White faces than by unfamiliar Black or Asian faces. In addition, Cunningham et al. (2004) reported that greater activation among White participants in bilateral fusiform gyrus to White faces compared to Black faces was significantly correlated with scores from a test assessing negative associations to Black faces when each face was only presented for 30 ms.
Prior ERP studies focused upon the time-course of differential neural activity evoked by same race and other race faces. Ito and colleagues (Ito et al., 2004; Ito & Urland, 2003, 2005) reported that for White participants, Black faces evoked larger N100s (peaking at around 120 ms post-face onset) and P200s (peaking at around 180 ms) than did White faces, and White faces evoked larger N200s (peaking at around 250 ms) at frontal electrode sites than did Black faces. Other ERP studies with White participants reported larger P200s to Asian compared to White faces, and larger N200s to White faces (James et al., 2001; Willadsen-Jensen & Ito, 2006). Some investigators (Ito et al., 2004; Ito & Urland, 2003, 2005; Willadsen-Jensen & Ito, 2006) have interpreted these timing sequences to suggest that attention is initially captured by other-race faces (N100 and P200), but then is redeployed to same-race faces (N200), facilitating the encoding of same-race faces. This more extended attention to same-race faces is presumed to underlie the better recognition memory typically obtained for same-race than other-race faces (Meissner & Brigham, 2001).
N170 is an ERP component recorded over the posterior lateral temporal scalp that exhibits a strong sensitivity to faces (Bentin, Allison, Puce, Perez, & McCarthy, 1996; George, Evans, Fiori, Davidoff, & Renault, 1996) and thus is a natural focus of cross-race studies. The findings to date concerning the N170 elicited by faces from different races have been inconsistent. Some ERP studies reported that the N170 is not sensitive to the race of the faces when comparing N170 responses in White participants evoked by White and Asian faces (Caldara et al., 2003, 2004), or by White and Black faces (Ito et al, 2004). However, the reported insensitivity of N170 to race has recently been challenged by Ito and Urland (2005), who observed a greater N170 to White faces than to Black faces, but only in tasks in which race was an attended face feature. The N170 to same-race (White) and other-race (Black) faces did not differ in nonracial categorization tasks in these same participants. While Ito and Urland (2005) reported larger N170 to same-race faces, Herrmann and colleagues (Herrmann, Schreppel, Jager, Koehler, Ehlis, & Fallgatter, 2007) reported the opposite result in a study with Whites in which a larger N170 was obtained for Asian than White faces using a perceptual priming task in which race was not an attended face feature. Thus, the relationship between N170 and responses to faces of people of different races appears to be moderated by the nature of the task and the particular race observed
These previous studies used only one other race, and the results from a single “other race” may not generalize to different races. For instance, Asian and Black are both “other races” for White participants, but because of potential differences in associated racial attitudes and race-related physical differences, the neural activations elicited by these different other races may not be the same. Thus, we attempted to discriminate the neural differences due to same-race versus other-race responses and the differences that are associated with specific races by using two different other-race (Asian and Black) stimuli for White participants.
Perception of race elicits complex responses in observers. Differentiating sources of variation in the neural responses evoked by faces have been the focus of several studies. Some have correlated individual differences in both indirect and implicit measures of racial attitudes with neural responses (Cunningham et al., 2004; Ito et al., 2004; Phelps et al., 2000). The Modern Racism Scale (MRS, McConahay, 1986) was originally designed as an indirect measure of racial attitudes but it is now conceived of as more explicit/deliberative by many (Fazio & Dunton; Fazio, Williams, et al., 2005). It asks people about issues related to race (e.g., Discrimination against Blacks is no longer a problem in the United States). The Implicit Association Test (IAT, Greenwald & Banaji, 1995; Greenwald, McGhee, & Schwartz, 1998) is a response time measure that assesses how easily participants can associate positively valenced words and faces of a particular race with the same response. Measures using self-reports, are assumed to reflect the deliberative and controlled expression of attitudes; implicit measures are assumed to reflect spontaneous and often unconscious expressions of associations or attitudes. People typically show lower levels of racial prejudice on indirect than implicit measures, in part because implicit measures are less influenced by social desirability concerns (Fazio & Olson, 2003).
Functional MRI studies with White participants that studied unfamiliar Black faces and White faces have found that greater race-related differential amygdala activation was associated with greater implicit racial associations, as assessed by the IAT (Cunningham et al., 2004; Phelps et al., 2000). To date, to our knowledge, only one ERP study has explored the relationship between ERP differences and racial attitudes. Ito et al. (2004) reported that the contrast between ERPs evoked by White and Black faces for a late-positive potential (LPP, peaking at approximately 520 ms) was correlated with greater MRS scores. Ito et al. did not investigate the correlation between race-associated ERP differences and response time measures assessing implicit racial associations (e.g., the IAT).
In the present study, we attempted to identify the time-course of cross-race face perception ERP differences related to implicit racial associations. Of course, ERP differences among faces of different races that could reflect differences in processing lower-level visual features among faces from different races (e.g., luminance, contrast), likely also should occur, but would not necessarily be correlated with implicit racial associations. In this study with White participants, we used faces from two other racial groups in order to determine whether faces from these two other racial groups show the same ERP differences despite anticipated intra-participant differences in IAT scores. Such a result would suggest that a general “in-group/out-group” distinction may be more important than attitudes toward specific races. Alternatively, ERP differences between White versus Black comparisons and White versus Asian comparisons would suggest race-specific effects. Our focus was upon the N170, P200, N200, and LPP ERP components previously implicated in cross-race perception as reviewed above. We also investigated short-latency ERPs (e.g., P100) previously implicated in the allocation of visual attention (Linkenkaer-Hansen, Palva, Sams, Hietanen, Aronen, & Ilmoniemi, 1998; Rossion, Joyce, Cottrell, & Tarr, 2003; Taylor, 2002). In short, we expected that the earliest component(s) might be sensitive to differences in faces from different races but would not necessarily be associated with individual differences in IAT scores while later component(s) would be associated with IAT scores. Furthermore, similarities and differences in ERP responses to Black and Asian faces (relative to White faces) provides information regarding whether “other race” effects are general or more specific to race.
METHODS
Participants
Participants were 21 White young adults (mean age 20 years, 7 males, 2 left-handed) who participated, after completing a certificate of informed consent, for course credit or for monetary payment. Six additional participants were excluded for excessive eye movement artifacts as detailed below.
Stimuli
Color pictures of 150 neutral faces from 150 unique individuals were used. These included 50 Asian faces, 50 Black faces, and 50 White faces (half male and half female). Full front views of faces with neutral expressions were obtained from the FERET face database (Phillips, Moon, Rizvi, & Rauss, 2000; Phillips, Wechsler, Huang, & Rauss, 1998), the Nimstim faces (Tottenham et al., in press), and lab collections. All faces were resized to a common height and cropped with an oval-shaped mask to remove most hair. Distinctive non-face features such as jewelry were either cropped or removed with image processing software (Photoshop CS2). Each face was then superimposed onto a white canvas. The overall luminance and contrast of pictures were then modified in order to make the pictures as uniform as possible. The CIGAL presentation software (Voyvodic, 1999) was used to display the faces on a LCD positioned approximately 60 centimeters away from the participant.
Procedure
Participants viewed a series of faces presented singly (see Figure 1). A crosshair appeared in the middle of the screen for 800-1200 ms and was followed by a picture of a face for 1000 ms. To obscure the true purpose of the study, the question “Male or Female?” appeared on the screen following the presentation of the face. Participants were instructed to withhold their response until this question appeared and then press a key corresponding to their choice. After the key press, the question disappeared and the next fixation cross was presented followed by the next face. Participants completed two runs, each lasting for 3-4 minutes and separated by a 1-minute-break. Each run contained an equal number of male and female faces and an equal number of faces from each race. The race and sex of the faces within each block were randomized and no picture was repeated. After the experiment, to obtain a measure of cross-race contact, the participants were asked “if you list ten of your best friends and most important people to you, how many of them are from your own race?” The number of the other-race friends reported represented a measure of cross-race contact that was used in subsequent correlational analyses.
Figure 1.
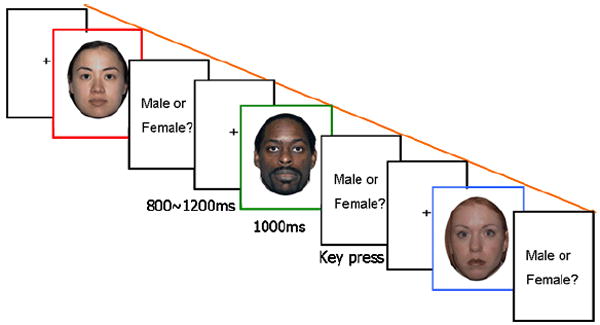
Experimental procedure. Three faces from NimStim database (Tottenham et al., in press) with permission to publish are shown in the figure.
Implicit association task (IAT)
An Asian-White IAT and a Black-White IAT were administered to each participant after the ERP recording session was complete. The IAT is based on the principle that people can respond faster when stimuli that are similar in valence are assigned to the same response key than stimuli that differ in valence. Participants were not told about the specifics of the IAT until the ERP study was complete. The IAT administration followed the procedure suggested by Greenwald, Nosek, and Banaji (2003) (see Table 1 in Greenwald et al., 2003, for more details). The faces used were color pictures of five Asian, five African American, and five White faces (in each case, three male and two female faces) from the FERET database (Phillips et al., 1998; 2000) that were cropped so that only the area of eyes and nose were showing. Cropping was done to prolong the processing time, and a large range of response times (RTs) was obtained using the conventional IAT procedure (see https://implicit.harvard.edu/implicit/demo/). The positive words used were “happy, joy, love, lucky, peace”, and the negative words used were “death, devil, pain, terrible, war”. All were short and frequently-used words. In different blocks, participants pressed one key for same-race faces and positive words (or negative words) and another key for other-race faces and negative words (or positive words). Accuracy and RTs were recorded and used to compute an IAT score. The adjusted average RT in the block in which same-race faces were associated with positive words was subtracted from the adjusted average RT in the block in which other-race faces were associated with positive words, divided by the standard deviation of the RTs in the two blocks (for more detailed information, see Table 4 in Greenwald et al., 2003). A higher positive IAT score indicates that the task was more difficult when positive words were assigned the same key as other-race faces. An IAT score of zero reflects a neutral implicit racial association and a positive IAT score indicates more positive evaluative associations for Whites than for another race (Blacks or Asians).
EEG recording
The electroencephalogram (EEG) was recorded simultaneously from 32 scalp locations using tin electrodes embedded in a fabric cap (Electrode Caps International, Ohio). The electrode locations included the 10/20 system and additional sites. Additional channels recorded the electrooculogram (EOG) from two electrodes attached beside each eye and one underneath the right eye, in order to monitor horizontal and vertical eye movements and blinks. With the exception of these EOG channels, all other scalp electrodes were recorded against a common reference placed on the participant’s nose. A ground electrode was placed on the participant’s forehead. The EEG was amplified by an EEG amplifier with a gain of 20,000 and a bandpass of 0.1-100 Hz. Impedance of each electrode on the electro-cap was below 5 kΩ.
The EEG was acquired continuously at a sampling rate of 250 Hz (4 ms per data point) and logged to a disk file using custom software. Digital codes corresponding to stimulus condition were written to a separate digital channel that was sampled simultaneously with the EEG.
EEG processing
A 60 Hz digital notch filter was applied offline to eliminate residual line noise from the recorded EEG. Epochs beginning 100 ms before and extending to 1000 ms after each stimulus code were then extracted from the continuous EEG. Epochs with EOG artifacts (eye movements and eye blinks) were discarded using the variance of the EOG channels as criteria. Six of the original 27 participants had fewer than 75% of trials left after EOG artifact rejection and were excluded from further analysis. The mean amplitude of the interval from 100 ms before to 52ms after each face onset was subtracted from every time point of the epoch to remove any pre-stimulus EEG baseline differences. The artifact free epochs were averaged separately for each of the three races for each participant and grand-averaged across all participants. The average amplitude of prominent ERP peaks (see results) were measured for each participant over a time window beginning 20 ms before to 20 ms after the average peak latency. The average amplitudes for particular ERP components were compared across stimulus conditions using repeated-measures ANOVA and planned paired t-tests. Color-coded topographic maps were computed to represent the scalp distribution of ERP amplitudes. In addition to topographic maps, the sLORETA technique (low resolution brain electromagnetic tomography, Pascual-Marqui, Michel, & Lehmann, 1994) was applied to these data to search in an exploratory manner for plausible cortical sources for the observed scalp-recorded ERPs.
The average ERP amplitudes over the +-20 ms time range for each major component was separately correlated with each of the two IAT scores, Asian-White and Black-White. As discussed further below, follow-up correlations were then carried out across the entire ERP waveform to better characterize the temporal intervals over which the IATs influenced ERP amplitudes.
RESULTS
Gender identification task
Analyses were performed on responses from the gender identification task. The analyses only include data from 20 participants because one participant’s data were lost due to a computer error. Response times (those longer than 10,000 ms were removed) to decide whether the person was a man or woman did not differ significantly for faces from different races. Accuracy of identification of gender was greater for Male faces (99.00%) than for Female faces (94.73%, t(19) = 4.63, p < .001), and accuracy in judging the gender of faces varied as a function of Race (F(2, 38) = 3.67, p < .05). Participants were more accurate judging the gender of White faces (97.50%) than Asian faces (95.90%, t(19) = 2.18, p < .05), but there was no difference between White and Black (97.20%) faces.
IAT
The mean Asian-White IAT score was .192, which was significantly larger than zero (t(20) = 2.73, p < .05); the Black-White IAT mean of .233 was marginally different from zero (t(20) = 1.73, p = .10). Scores on the IAT-Asian were not significantly correlated with scores on the IAT-Black. Neither IAT was significantly correlated with the self-reported number of other-race friends or performance on the gender identification task.
ERPs
The peaks chosen for measurement were based upon the prior ERP findings reviewed in the introduction and included P100, N170, P200, N200 and LPC. These components all showed sensitivity to race and will be discussed below in temporal sequence. A summary of the findings is provided in Table 1.
Table 1.
The correlations between same-race (White) and other-race (Asian, Black) ERP amplitude differences and IAT measures at the time range of the ERP components of interest (A indicates Asian, B indicates Black, W indicates White, and SR and OR indicate same-race and other-race).
| P100 | N170 | P200 | N200 | Other | LPC | ||
|---|---|---|---|---|---|---|---|
| Peaking time | 116 ms | 160 ms | Difference ~192 ms | 244 ms | 500 ms | 592 ms | |
| Largest at | Oz | T6 | Cz | FC4 | F3 | T6 | |
| Amplitude differences | B > W > A | A > W | B > W | W > B | A > W (p = .07) | SR > OR | |
| Asian | Time range of (p < .05) | ~ 72 – 160 ms | ~ 100 – 200 ms | ~ 220 – 280 ms | ~ 172 – 280 ms | ~ 480 – 580 ms | ~ 540 – 620 ms |
| Median r | (.11) | (.28) | (-.09) | (.007) | .56 | (-.19) | |
| p | (>.50) | (.20) | (>.50) | (>.50) | <.01 | (.40) | |
| Black | Time range of (p < .05) | ~ 72 – 160 ms | ~ 100 – 200 ms | ~ 220 – 280 ms | ~ 172 – 280 ms | -- | ~ 540 – 620 ms |
| Median r | (.14) | (.27) | .50 | .52 | -- | (.34) | |
| p | (>.50) | (>.20) | < .03 | < .02 | -- | (>.10) |
P100
A P100 was observed with maximal amplitude at Oz and with a peak latency of 116 ms from face onset. Figure 2A shows the grand average ERPs elicited by Asian, Black and White faces at Oz where a main effect of race was observed (F(2,40) = 13.18, p < .001). Subsequent contrasts showed a larger amplitude P100 evoked by Black faces (7.12 uv) than by White faces (5.71 uv, F(1,20) = 6.02, p < .05), and a larger amplitude P100 evoked by White faces than by Asian faces (4.45 uv, F(1,20) = 6.75, p < .05). In addition to Oz, there were significant amplitude differences in P100 between Black and White, or between Asian and White, at several nearby electrode locations (Black-White: O1, p < .05; Asian-White: O2, T6, TP8, p < .05) (see Figure 2B), all following the same rank ordering of amplitude as at Oz. There was no significant correlation between other-race/same-race difference for P100 and IAT scores at Oz or any other electrode site. A larger P100 was elicited by male faces compared to female faces (Oz: p = .06; O2: p < .05).
Figure 2.
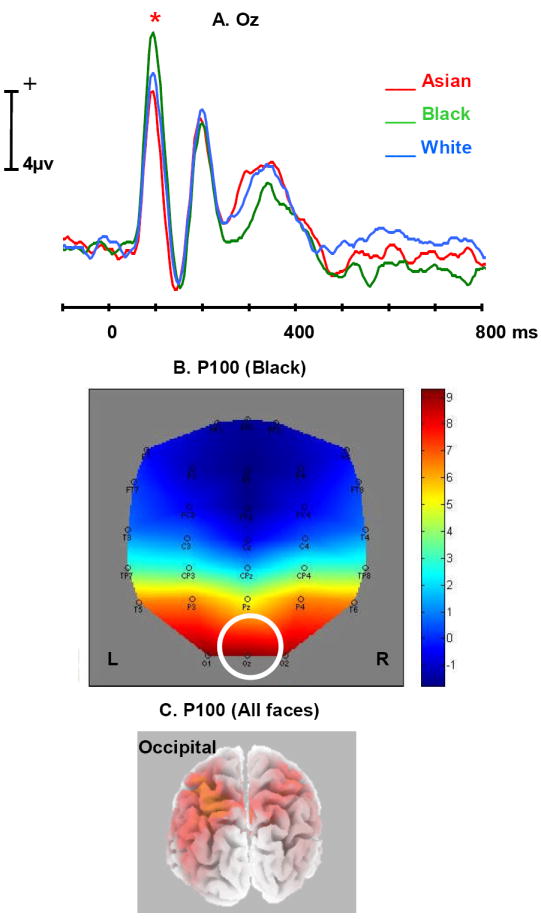
A. Average ERP time waves for Asian (red), Black (green), and White (blue) at the Oz electrode, illustrating the difference in P100 amplitude (indicated by *).
B. Topographic map illustrating the scalp distribution of the ERP amplitude elicited by Black at the peaking time of P100 (More positive: Red, More negative: Blue). Oz is highlighted in the figure.
C. Source modeling results from sLORETA at the peaking time of P100 (all faces included in the analysis).
Note: Time waves in all figures were smoothed by averaging the amplitudes of 2 points before/after each particular time point (8 ms before and after).
N170
An N170 was observed with a peak latency of ~160 ms over right temporal electrode sites for faces of all races. Figure 3A shows the grand average ERPs elicited by Asian, Black and White faces at T6 where the amplitude of the N170 elicited by Asian faces (-3.06 uv) was significantly larger than for White faces (-2.03 uv, t(20) = 2.65, p < .05). Larger N170 amplitude to Asian as compared to White faces was also observed at several other right temporal and occipital electrode locations, including P4, TP8, O2, and Oz (all ps < .05, see Figure 3A. No significant N170 amplitude differences were found between Black and White faces. Across races, the N170 was largest at T6. The topographic map (Figure 3B) showed that the largest Asian-White N170 amplitude difference was also found at T6. There was no correlation between the Asian-White N170 amplitude difference and the IAT-Asian at T6 or any other electrodes. Figure 3C illustrates the results from sLORETA which suggest that possible sources of N170 include left and right posterior lateral temporal regions, with the activation larger and more superior on the right. There was no significant difference related to gender of the faces for N170.
Figure 3.
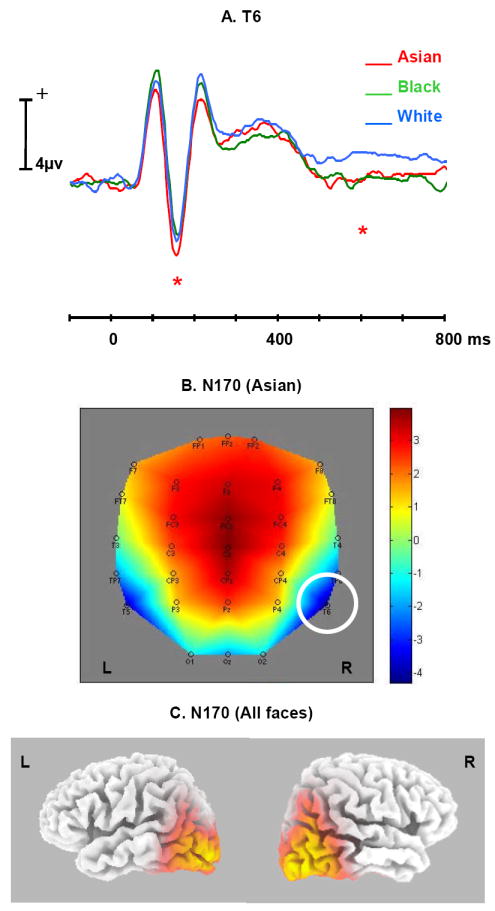
A. Average ERP time waves for Asian (red), Black (green), and White (blue) at the T6 electrode, illustrating the difference in N170 and LPC amplitudes (indicated by *).
B. Topographic map illustrating the scalp distribution of the ERP amplitude elicited by Asian at the peaking time of N170 (T6 highlighted in the figure).
C. Source modeling results from LORETA at the peaking time of N170 (all faces included in the analysis).
P200
P200 and N200 were largest in amplitude over fronto-central scalp sites. Figure 4A shows the grand average ERPs elicited by Asian, Black and White faces at the Cz fronto-central electrode. P200 was broad in shape and the peak latency of the difference between Black and White faces in P200 (192ms) was later than the absolute 168 ms peak of P200. P200 amplitude was thus measured over the 192 +- 20 ms range. A main effect of race in P200 was observed at Cz, (F(2, 40) = 6.03, p < .01), with subsequent contrasts showing larger amplitude for Black (4.15 uv) than for White (2.08 uv) faces (F(1, 20) = 11.18, p < .01), but no significant difference between White and Asian (2.64 uv) faces. In addition to Cz, the amplitude of P200 elicited by Black faces was significantly larger than that elicited by White faces at several nearby frontal electrodes including Fz, FCz, CPz, F3, F4, FC3, FC4, C3, and CP3 (All ps < .05; for FCz, CPz, all ps < .01), with all the differences in the same direction as at Cz. The topographic map (Figure 4B) representing the scalp distribution of this Black-White difference at 192ms showed that the largest difference was at the electrode Cz.
Figure 4.
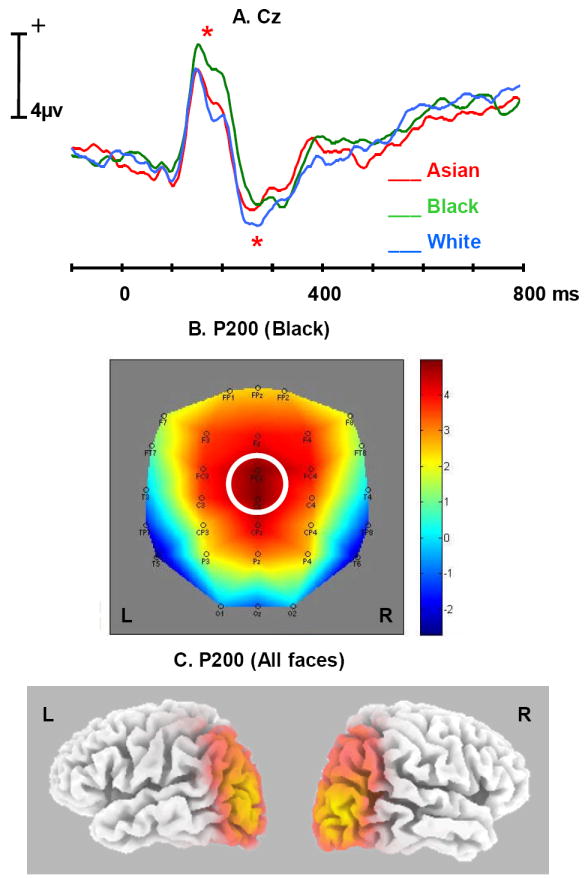
A. Average ERP time waves for Asian (red), Black (green), and White (blue) at the Cz electrode, illustrating the difference in P200 and N200 amplitudes (indicated by *).
B. Topographic map illustrating the scalp distribution of the ERP amplitude elicited by Black at the peaking time of P200 (Cz highlighted in the figure).
C. Source modeling results from LORETA at the peaking time of P200 (all faces included in the analysis).
The Black-White ERP amplitude differences were significantly correlated with IAT-Black at Cz beginning at 220 ms and persisting until 280 ms (all ps < .05). The correlation between Black-White ERP differences and IAT-Black will be discussed in more detail later. Other fronto-central electrode sites also showed a significant correlation with IAT-Black and will be discussed with the N200 results below. There was no significant correlation of Asian-White with IAT-Asian at the time range of P200. There was no significant difference related to gender of the faces for P200.
N200
An N200 was observed with maximal amplitude at FC4 and a peak latency of 244 ms (the ERP wave at FC4 was similar to that at Cz, shown in Figure 4A). A significant amplitude difference between White and Black in N200 was observed at FC4, with the amplitude of N200 elicited by White faces (-1.78 uv) significantly larger than the amplitude of N200 elicited by Black faces (-.07 uv, t(20) = 2.47, p < .05). In addition to FC4, this White>Black N200 amplitude difference was observed at most frontal scalp locations including FPz, Fz, FCz, Cz, FP2, F3, F4, and FC3 (all ps < .05). There were no amplitude differences between Asian and White faces.
There was a significant correlation between the Black-White amplitude difference at FC4 and the IAT-Black, starting from 172ms and remaining significant until 288 ms, which covered the latency range of both P200 and N200. Figures 5C and D present the correlation of Black-White ERP amplitude differences with IAT-Black as scatter plots with trend lines added at frontal electrode site FC4 and temporal electrode site T6. The largest correlation between Black-White amplitude difference and IAT-Black at FC4 was observed at 228+- 20 ms (r = .63, p < .003) (Figure 5C), and the largest correlation at T6 was at 224+- 20 ms, (r = .57, p < .01) (Figure 5D). There was no significant correlation between the Asian-White amplitude difference and the IAT-Asian at this time range. There was no significant difference related to gender of the faces in N200.
Figure 5.
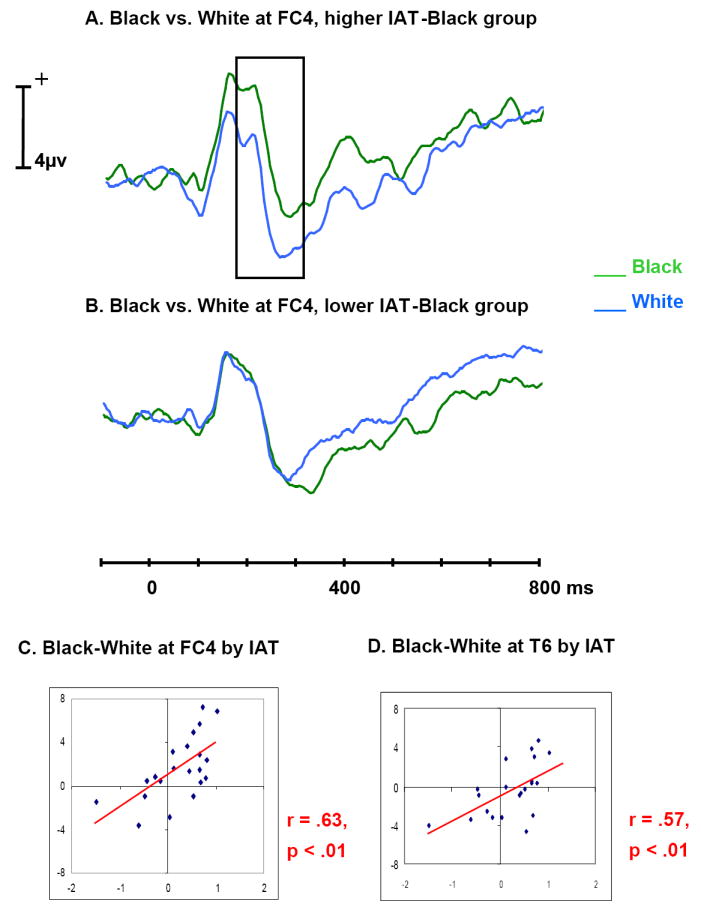
A. Average ERP time waves for Black (green) and White (blue) at frontal electrode FC4, in the group of participants with IAT-Black scores higher than the median IAT-Black. The difference between Figure A and B in ERP amplitude is highlighted in Figure A. Participants with more negative associations to Black faces appear to have larger P200 to Black and larger N200 to White.
B. Average ERP time waves for Black and White at frontal electrode FC4, in the group of participants with IAT-Black scores lower than the median IAT-Black.
C. Scatter plot of the mean Black-White amplitude difference at around 228 ms over frontal scalp by IAT-Black for each participant, with a trend line added to show the correlation
D. Scatter plot of the mean Black-White amplitude difference at around 224 ms over temporal scalp by IAT-Black for each participant, with a trend line added to show the correlation.
Note: Correlations in the figures are the largest correlations in that time range. For median correlations, see Table 1.
Late positive component, LPC
Figure 3A shows the averaged ERP amplitudes across time for all three conditions at T6. Beginning at ~550 ms, a slow positive component was larger for White than for either Black or Asian faces. There was a main effect of race on the amplitude of LPC (peaking at 592 ms) at T6, (F(2, 40) = 3.49, p < .05), with subsequent contrasts showing larger amplitude for White (1.71 uv) than for Black (.01 uv, F(1, 20) = 5.12, p < .05), and for White than for Asian faces (.11 uv, F(1, 20) = 4.56, p < .05), but no difference between Black and Asian faces. Paired t-tests showed that the differences in LPC for both Black and Asian faces relative to White faces were significant at T5 as well (p < .05). There was no significant correlation between these amplitude differences and IAT measures found at this time range. In addition, there was a positive-going potential around 500~600 ms which was larger for Male faces compared to Female faces at multiple left frontal-temporal electrode sites (at 580+- 20 ms, FPz, FP2, F7, FT7, T3: p < .05).
Correlational Timeline
As reported above, there were significant correlations of Black-White ERP amplitude difference with IAT-Black observed in some of the time periods corresponding to the ERP components that were selected for study on the basis of our literature review. However, as discussed above, the maximal amplitude differences between Black and White faces for the P200 component did not correspond precisely to the peak amplitude of P200. Thus, in order to fully investigate the timeline of potential IAT influences, we conducted additional correlations across the entire time range of the difference waveforms. See Table 1 for a summary of correlations between ERP differences and IATs. In order to test whether the correlation for Black faces was significantly stronger than the correlation for Asian faces at the time range of P200 and N200, the r(Asian) and r(Black) at 272±20 ms at Cz were compared using a method suggested by Chen and Popovich (2002) and the results showed that the correlation for Black (.50) was significantly greater than the correlation for Asian (-.09, z = 1.98, p < .05).
In addition to the correlations over the P200-N200 interval described above, Black-White ERP amplitude differences were significantly correlated with IAT-Black in a later 300-400 ms time period. The largest correlation was found at FC4 (frontal), at (348+- 20 ms, r = .55, p = .01). Although the individual participant ERP differences between Black and White faces correlated with IAT, there was no overall significant difference in amplitude between ERPs evoked by Black and White faces in this time range.
The Asian-White ERP amplitude differences were significantly correlated with IAT-Asian in a period around 500 ms, with maximal correlations observed at F3 (frontal) and T5 (temporal). There was a larger negative ERP component for Asian as compared to White in this time range, showing a trend level of significance (p = .07 at F3, p = .08 at T5). The magnitude of this negative component (or the absolute voltage compared to the baseline) was used to quantify the ERP difference between Asian and White. Figure 6C and D present the correlation of Asian-White ERP component size difference with IAT-Asian as scatter plots with trend lines added at F3 and T5. The largest correlation between Asian-White amplitude difference and IAT-Asian at F3 was observed at (512+- 20 ms), (r = .67, p = .001) (Figure 6C), and the largest correlation at T5 was at (496+- 20 ms), (r = .48, p < .03) (Figure 6D).
Figure 6.
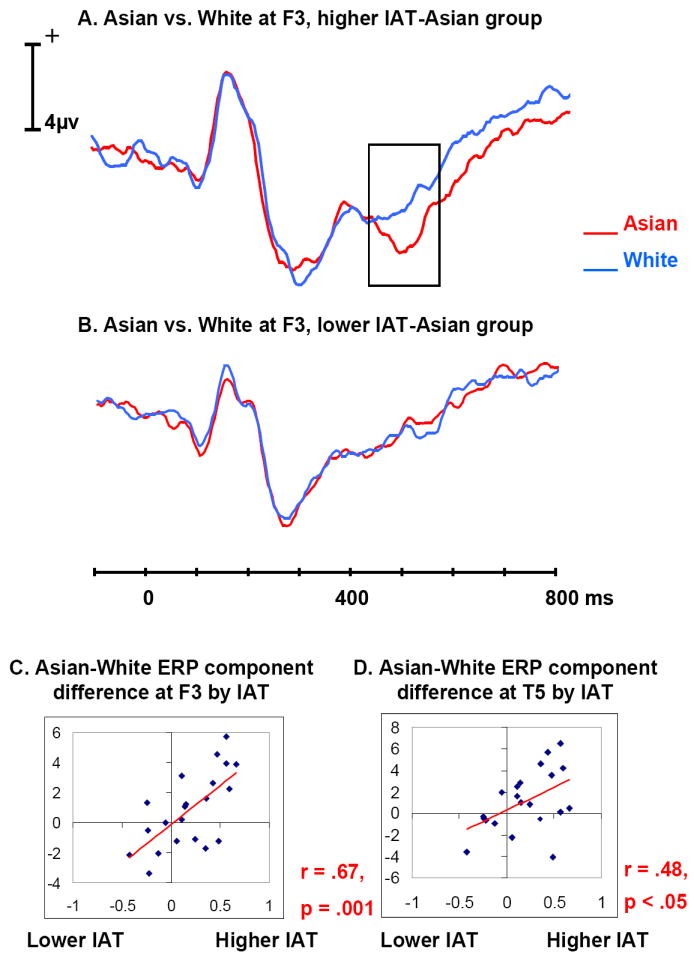
A. Average ERP time waves for Asian (red) and White (blue) at frontal electrode F3, in the group of participants with IAT-Asian scores higher than the median IAT-Asian. The difference between Figure A and B in ERP amplitude is highlighted in Figure A. Participants with more negative association to Asian faces appear to have larger negative ERP component at around 500 ms for Asian.
B. Average ERP time waves for Asian and White at frontal electrode F3, in the group of participants with IAT-Asian scores lower than the median IAT-Asian.
C. Scatter plot of the mean Asian-White amplitude difference at around 512 ms over frontal scalp by IAT-Asian for each participant, with a trend line added to show the correlation
D. Scatter plot of the mean Asian-White amplitude difference at around 496 ms over temporal scalp by IAT-Asian for each participant, with a trend line added to show the correlation.
To help visualize ERP differences associated with the IAT scores, the 21 participants were divided into two 10-participant groups on the basis of their IAT-Black and, separately, for their IAT-Asian scores. The 10 participants who had IAT scores higher than the median IAT score were put into the higher IAT (either IAT-Black or IAT-Asian) group, while the 10 participants who had IAT scores lower than the median IAT score were put into the lower IAT group. The participant with the median IAT score was dropped. The ERPs of these four groups (higher/lower IAT-Asian/IAT-Black groups) were averaged separately. Figures 5A and B show that the P200 was larger for Black than for White and the N200 was larger for White than for Black at the frontal electrode FC4 within the higher IAT-Black group, while the P200 difference disappeared and the N200 was larger for Black than for White within the lower IAT-Black group. This interaction of IAT-group (higher or lower IAT scores) by race (Black or White faces) was significant at 228 ms at FC4, (F(1, 18) = 6.60, p < .05). This is within the time range where the correlation between the Black-White ERP difference and the IAT-Black was observed. Similarly, Figures 6A and B show that the negative going component around 500 ms was larger for Asian than for White faces at the frontal electrode F3 within the higher IAT-Asian group, while this component was smaller for Asian than for White within the lower IAT-Asian group. This interaction of IAT-group by race (Asian vs. White faces) was significant at 512 ms at F3, (F(1, 18) = 5.49, p < .05) which was within the time range where the correlation between the Asian-White ERP difference and the IAT-Asian was observed.
No correlation between the race-associated ERP differences and the measure of cross-race contact (number of the other-race friends) was obtained. No correlations between the gender accuracy and ERP differences were found. However, Dickter and Bartholow (2007) recently reported a larger N200 for both White and Black participants to same-race than to other-race faces was significantly correlated with faster response in judging the gender of same-race faces, and proposed that the ERP amplitude difference might indicate different amounts of attention distributed to processing the gender of the faces as a function of perceived racial cues. To examine this possibility further, we analyzed our response time data, although our participants were instructed not to respond until they saw the response cue and thus, presumably, had time to prepare their responses. Even so, Black-White response time differences in the gender task were correlated significantly with Black-White ERP amplitude differences, with the largest correlation at (332+- 20 ms, r = .50, p < .03) at Cz (before the removal of an extreme data point, r = .61, p < .005). The larger a participants’ negative ERP component for White relative to Black faces at around 332 ms, the faster they were to judge the gender of White faces relative to Black faces.
DISCUSSION
The present study demonstrates that ERPs evoked by faces are sensitive to differences among faces of different races as early as 116 ms after face onset. More important, beginning as early as 172 ms, ERP differences between same-race and other-race faces were strongly correlated with differences in implicit valenced associations as measured by the IAT. No prior study had explored the relationship between ERP differences to faces of other races and response time-based implicit measures of racial associations. The largest and earliest differences occurred between ERPs evoked by White and Black faces, and the earliest relation to implicit associations was also obtained for the IAT-Black. These differences cannot be readily attributed to differences in initial perceptual processing; on the gender identification task, participants did not respond differently to White and Black faces in response latencies or accuracy. Differences between Asian and White faces that correlated with the IAT-Asian were overall smaller in amplitude and later in latency. Finally, at the longest latencies, the same-race White faces were differentiated from both other-race faces, which did not differ from each other. This late difference was not related to either IAT.
The P100 amplitude significantly differentiated the three races which, to our knowledge, has not been previously reported. Most prior studies have not found P100 to be face-specific (e.g., Linkenkaer et al., 1998; Rossion et al., 2003), although Taylor (2002) found P100 latency differences between upright and inverted faces, and a prior MEG study reported a larger M100 to faces than to houses (Liu, Harris, & Kanwisher, 2002). As our study only employed faces, we cannot conclude that these P100 effects were face specific. Because the P100 differences found here were not related to IAT scores, and occurred earlier than the face-specific potential N170, the P100 amplitude differences related to race could have arisen from physical differences among faces such as skin tone and other lower level visual properties, which can be detected quickly without fully processing the stimuli as faces. As variations in P100 amplitude have been reliably associated with the deployment of visual attention (e.g., Eason, Harter & White, 1969; Taylor, 2002), it is tempting to speculate that facial features associated with race differentially capture attention leading to differences in subsequent processing. However, the ordering of P100 amplitude (Black > White > Asian) observed here is not consistent with an argument that supposes that all out-group faces (Black and Asian) initially attract more (or less) attention than in-group faces. The current study did not try to quantify or measure the featural differences across race or to standardize the skin tone among faces from different races because we believe that the skin tone difference is an important factor in naturalistic face processing. However, it would be interesting to determine if such physical differences among faces from different races influence short-latency visual ERPs.
A larger amplitude N170 was elicited by Asian faces than by White and Black faces. An Asian-White N170 difference was recently reported by Herrmann et al. (2007) in a study in which only Asian faces were included as other-race faces. The lack of a N170 difference between Black and White faces in the current study is consistent with Ito et al. (2004) and a subsequent study by Ito and Urland (2005) in which Black and White faces were compared in tasks unrelated to racial categorization as in our study. In contrast, Ito and Urland (2005) did find an increased N170 to White than to Black faces in White participants in a task in which race was an attended feature. If a larger N170 reflects enhanced processing, there is at least an apparent inconsistency between Ito and Urland’s (2005) report of a larger N170 for a White face and the finding of Herrmann et al. (2007) and the present study of a larger N170 for an Asian face. Resolution of this issue remains for future studies. Notably, the Asian-White N170 amplitude differences reported here did not correlate with IAT-Asian scores. Thus, as for P100, it is likely that the N170 was sensitive to structural face features or other lower level visual features that may have differentiated the White and Asian faces. It is possible that the structural features that are most salient and reflected in these early ERP components vary with the overall context (i.e., the types and variety of races being presented).
The most striking differences between the ERPs evoked by Black and White faces occurred in the P200-N200 range replicating previous findings by Ito and colleagues (Ito et al., 2004; Ito & Urland, 2003, 2005). Across the P200-N200 latency range, Black faces evoked a more positive ERP than White faces. That is, Black faces evoked a larger P200 than White faces, and White faces evoked a larger N200 than Black faces. These effects occurred over multiple fronto-central electrode sites (Figure 4A). Others have reported differences for these same ERP components for Asian and White faces (James et al., 2001; Willadsen-Jensen & Ito, 2006). While Figure 4A shows a trend in our data for Asian faces to evoke a larger P200 and smaller N200 than White faces, these differences were not statistically significant. In the current study a gender judgment paradigm was used since we were more interested in the influence of implicit attitudes while in Willadsen-Jensen and Ito’s (2006) study the participants were asked to judge the race of the faces. Given the fact that three races were used and the task was to identify gender, we speculate that the current study focused participants less on race, reducing the P200/N200 differences.
Of critical interest for the present study, the Black-White ERP differences over the temporal range of P200-N200 were significantly correlated with implicit racial associations as measured by the IAT-Black. This relationship is evident in the scatter plots presented in Figure 5 and the waveform differences associated with the IAT can be seen in the ERP waveforms presented in Figure 5 that were sorted into high and low IAT-Black groups. Our finding that individuals who have more negative associations to Black faces, show a greater Black-White ERP difference is consistent with the previous findings associating P200 with automatic direction of attention toward negative relative to positive information and increased attention to other-race as compared to same-race members (Bartholow & Dickter, 2007). Significant ERP-IAT correlations for Black-White differences occurred as early as 172 ms, which is similar in latency to the N170 ERP that has been associated with structural encoding of faces (Bentin et al. 1996), although subsequent studies have often found an earlier latency for N170 such as the 160 ms latency reported here. Whether N170 reflects structural encoding or some other aspect of face processing, it is the earliest neural indicator that a face is detected, and thus our results suggest that implicit race associations influence the neural processing of faces almost immediately after identification. Whether such an early effect is specific to face processing is an open question. For example, to distinguish the key processes involved, it would be valuable to investigate whether gender judgments of typical same- and other-race names or with models looking away so that skin tone but not facial features would be recognizable would also show a correlation between the difference between same- and other-race early ERP component(s) and implicit associations.
Although implicit race associations were strongly associated with early face processing of Black faces in our White participants, the results for early processing of Asian faces were less striking. As reviewed above, Asian faces evoked a smaller P100 and larger N170 than White faces, but these differences did not correlate with the IAT-Asian. Small differences in P200-N200 amplitude were observed between White and Asian faces, but these differences were not significant and did not correlate with IAT-Asian. An analysis of the full time-course of Asian-White ERP differences, however, revealed a significant difference in the ERPs at about 500 ms over frontal and temporal scalp that correlated significantly with the IAT-Asian. The fact that ERP correlations with IAT scores were slower to emerge for Asian than Black faces suggests that the attitudes being tapped might be more explicit for white participants in the case of Asians than Blacks. Consistent with this, one prior ERP study (Ito et al. 2004) found that the White-Black amplitude difference of a frontally distributed late positive potential (LPP) (peaked ~520 ms) was correlated with a measure racial attitudes (the Modern Racism Scale) that asks for opinions about complex topics likely to elicit deliberative processing. Alternatively, it could be that identifying Asians as other than White took longer in the context of Black faces than it might have if only Asian and White faces had been shown, or that racial attitudes activated toward Asians are somewhat different in the context of Black and White faces than those activated toward Asians in the context of only White faces. In the gender identification task, the perception of Asian as other-race might be influenced by the salience of Black faces, while the IATs were acquired separately for two other-races which did not have this influence as in a three-race setting. Thus, it might explain why we did not get a stronger ERP difference or initial correlation (around 220 ms) between ERP differences and IAT for Asian as for Black. Including only Asian and White faces or Black and White faces in future research would limit racial context effects and help to assess more directly where, in the temporal stream of face processing, such variations in context have their maximal effects.
Finally, a long duration late positive component (LPC) (peaking near 600 ms) was larger in amplitude to the same-race White faces than to both the Asian and Black faces, which did not differ from each other. To our knowledge, this is the first time this LPC difference associated with race has been reported. Our differences were most evident at lateral temporal electrode sites like T6 (Figure 3A), whereas an LPP difference reported by Ito and colleagues (Ito et al., 2004) was evident at Cz. Our temporal scalp LPC difference was not correlated with IAT scores. As there were no LPC difference between Black and Asian faces, and as the differences in LPC did not correlate with either IAT, we speculate that the LPC might reflect additional processing of the same-race “in-group” faces, in effect, an in-group bias (Mullen, Brown & Smith, 1992). That is, participants may have greater interest in own than other race faces, or more relevant associations may be activated (e.g., that guy looks like my brother). Such preferential processing presumably leads to the well-established phenomenon that people show better short-term and long-term recognition memory for faces from their own race than faces from other less familiar races (Meissner & Brigham, 2001; Slone, Brigham, & Meissner, 2000). This other-race effect (ORE) or own-race bias has been reliably observed using various tasks across cultural and racial groups (Meissner & Brigham, 2001; Ng & Lindsay, 1994; Teitelbaum & Geiselman, 1997). Our results provide evidence that differential processing of same-race “in-group” faces as compared to other-race “out-group” faces begins as early as 500 msec. Because we used two different other races, we speculate that this LPC difference may not be related to a specific other race, but can be generalized to different other races as an “out-group.”
Due to the non-uniqueness of inverse models relating scalp ERP distributions to cortical sources, we cannot make definitive statements regarding the neural sources of these ERP differences due to racial attitudes. However, we employed the sLORETA program (Pascual_Marqui, 2002; Pascual-Marqui et al., 1994) to generate plausible distributed cortical models and we report them here so that they can be tested in future fMRI studies. It is interesting to note that the models for the N170 and P200 are similar and both implicate the lateral occipitotemporal cortex (Figure 3C and Figure 4C). This cortical model of N170 is similar (although slightly more ventral) to that reported by Itier and Taylor (2004) for faces. A more occipital model was obtained for P100 (Figure 2C), which is consistent with our conjecture that the differences among races obtained for P100 may reflect lower level visual attributes of our stimuli.
Ours is the first study to demonstrate early latency ERP differences among faces of different races that were correlated with response time-based implicit racial associations. Functional MRI studies found that implicit racial associations as measured by the IAT were correlated with differences in amygdala activation, with an increased IAT-Black score predicting increased amygdala activity to unfamiliar other-race Black faces (Cunningham et al., 2004; Phelps et al., 2000). Thus, one possible explanation of the ERP-IAT correlations observed in the current study is that the ERP differences reflect differential activation in amygdala. Cunningham et al. (2004) also found that greater IAT scores were correlated significantly with greater activation in fusiform gyrus to same-race faces as compared to other-race faces. They suggested that people with greater negative other-race associations might process other-race faces more superficially relative to same-race faces. Thus, another possible explanation of the ERP-IAT correlations we observed is that the ERP differences reflect differential activation in fusiform gyrus, or in regions that are strongly connected to the fusiform gyrus. fMRI studies have also found race effects associated with activity in different regions of the frontal lobe. Richeson and colleagues (Richeson, Baird, Gordon, Heatherton, Wyland, Trawalter, & Shelton, 2003) reported that activity in dorsolateral prefrontal cortex was related to an attempt to control racial prejudice. Cunningham et al. (2004) observed increased ventrolateral PFC activation to other-race than to same-race that was correlated with the IAT, and greater dorsolateral PFC activation to other-race than to same-race faces that was correlated with the reduction of the differential B >W amygdala activation from short (30 ms) to long (525 ms) presentation times. The Cunningham et al. (2004) findings thus indicate that the response to same-race and other-race faces may include an automatic component (amygdala activation) that is perhaps related to an emotional response followed by a controlled component that originates in the frontal lobe. Unfortunately, none of these fMRI studies tested Asian faces and so it is not clear whether Asian-White IAT differences would also result in differential amygdala activation or frontal lobe activation. Another interesting point is that the degree of amygdala activation to a particular other race may depend on the overall context, including the distribution of different numbers of different races.
Given the present state of the fMRI literature, it is uncertain whether the ERP-IAT correlations observed here correspond to automatic amygdala activation, increased PFC activation related to the engagement of other processes, or activation in other brain regions. To choose among these and other hypotheses, additional studies in which both fMRI and ERP differences associated with processing of faces of different races and with IAT measures obtained in the same individuals will be required.
The fMRI results, however, provide a possible explanation of a puzzling result of the current study - why are the ERP-IAT correlations for Black and Asian faces obtained over different temporal intervals? We first considered that Asian and White faces may take longer to discriminate from each other than Black and White faces, and the delayed correlation with IAT-Asian reflects this longer processing time. This simple explanation is contradicted by the P100 and N170 data, which shows early and statistically reliable differences between White and Asian faces and thus are inconsistent with a delayed discrimination. With regard to the fMRI literature discussed above, perhaps the ERP-IAT correlation for Black-White beginning at 172 ms reflects a different process than the Asian-White correlation at 500 ms. For example, the early Black-White differences may reflect a more automatic emotional process while the later ERP-IAT correlation for Asian at 500 ms reflects a more extended and/or controlled process (activation of facts or beliefs about Asians), as does the later ERP-IAT correlation for Black faces at 400 ms. Alternatively, the lack of early ERP-IAT correlation for Asian faces might be related to sensitivity – a small P200-N200 amplitude difference for Asian-White was found but was not statistically significant.
In conclusion, the current study observed a differential P100 across Asian, Black and White faces over occipital scalp, indicating that the physical differences across races can be detected as early as 116 ms after face onset, and this difference was associated with each specific race rather than in-group/out-group differentiation. Then the detection of racial categories moved to the lateral temporal scalp electrodes at around 160 ms, shown by a larger N170 to Asian than to White faces, which might reflect differences in the structural encoding of Asian and White faces (Bentin et al., 1996). This difference did not occur for Black faces, however. In addition, larger P200 to Black than to White faces as well as an increased N200 to White than to Black over frontal scalp were observed. Most interesting, these Black-White ERP differences were found to be correlated with an implicit racial attitude measure. These findings indicate that the influence of implicit racial attitudes occurs for Whites’ responses to Blacks as early as around 200 ms – earlier in face processing than previous work has suggested. A correlation between Asian-White ERP difference and IAT-Asian was also observed at a comparatively later time range (500 ms) over frontal scalp. Finally, the late positive component (LPC) was observed to be greater to White than to Black and Asian faces over lateral temporal scalp. This comparatively late same-race versus other-race difference is consistent with preferential processing of same-race faces over other-race faces. Because of the typically better memory for same than other-race faces, we speculate this reflects deeper processing of same-race faces. At the time range of LPC (550 ms~600 ms), the ERP was no longer influenced by the implicit racial evaluation, consistent with generally increased processing of in-group members (e.g., due to idiosyncratic associations, or availability of face schemas) that may be unrelated to attitudes toward race. Finally, the correlation between the ERP amplitude differences across race and the implicit attitude measures for both Asian and Black showed the influence of an evaluative process (probably including both early automatic and later controlled processes) on cross-race face processing. A combination of ERP and fMRI techniques is necessary in order to confirm this hypothesis in the future.
Acknowledgments
This research was supported by NIMH grant MH-05286 to GMc. The authors thank Ms. Rosa Li for technical assistance. Portions of the research in this paper use the FERET database of facial images collected under the FERET program, sponsored by the DOD Counterdrug Technology Development Program Office. The MacBrain Face Stimulus Set used as stimuli in this research was created with support of the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. For more information concerning the stimulus set, contact Nim Tottenham at tott0006@tc.umn.edu.
References
- Allison T, Ginter H, McCarthy G, Nobre A, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. Journal of Neurophysiology. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL. Social cognitive neuroscience of person perception: A selective review focused on the event-related brain potential. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: Integrating biological and psychological explanations of social behavior. New York: Guilford Press; 2007. pp. 376–400. [Google Scholar]
- Blair I, Judd C, Fallman J. The automaticity of race and Afrocentric facial features in social judgments. J Pers Soc Psychol. 2004;87(6):763–778. doi: 10.1037/0022-3514.87.6.763. [DOI] [PubMed] [Google Scholar]
- Blair I, Judd C, Sadler M, Jenkins C. The role of Afrocentric features in person perception: judging by features and categories. J Pers Soc Psychol. 2002;83(1):5–25. [PubMed] [Google Scholar]
- Caldara R, Rossion B, Bovet P, Hauert C. Event-related potentials and time course of the “other-race” face classification advantage. NeuroReport. 2004;15:905–910. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- Caldara R, Thut G, Servoir P, Michel CM, Bovet P, Renault B. Face versus non-face object perception and the ‘other-race’ effect: a spatio-temporal event-related potential study. Clinical Neurophysiology. 2003;114:515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- Chen PY, Popovich PM. Sage University Papers Series on Quantitative Applications in the Social Sciences, series no. 07-139. Thousand Oaks, CA: Sage; 2002. Correlation: Parametric and nonparametric measures. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of Black and White faces. Psychological Science. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Dickter CL, Bartholow BD. Racial ingroup and outgroup attention biases revealed by event-related brain potentials. SCAN. 2007;2:189–198. doi: 10.1093/scan/nsm012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason RG, Harter MR, White CT. Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiology and Behavior. 1969;4:283–289. [Google Scholar]
- Ekman P. The argument and evidence about universals in facial expressions of emotion. In: Wagner H, Manstead A, editors. Handbook of social psychophysiology. Vol. 143. Oxford, England: John Wiley & Sons; 1989. p. 164. [Google Scholar]
- Fazio RH, Olson MA. Implicit measures in social cognition research: Their meaning and uses. Annual Review of Psychology. 2003;54:297–327. doi: 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- George N, Evans J, Fiori N, Davidoff J, Renault B. Brain events related to normal and moderately scrambled faces. Brain Res Cogn Brain Res. 1996;4:65–76. doi: 10.1016/0926-6410(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR. Implicit social cognition: Attitudes, self-esteem, and stereotypes. Journal of Personality and Social Psychology. 1995;102:A–21. doi: 10.1037/0033-295x.102.1.4. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The Implicit Association Test. Journal of Personality and Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the Implicit Association Test: I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs. ingroup face stimuli. Neuroreport: For Rapid Communication of Neuroscience Research. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Schreppel T, Jager D, Koehler S, Ehlis A, Fallgatter A. The other-race effect for face perception: an event-related potential study. J Neural Transm. 2007;114(7):951–957. doi: 10.1007/s00702-007-0624-9. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15:1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Ito TA, Thompson E, Cacioppo JT. Tracking the timecourse of social perception: The effects of racial cues on event-related brain potentials. Personality and Social Psychology Bulletin. 2004;30:1267–1280. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85:616–626. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(1):21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- James MS, Johnstone SJ, Hayward WG. Event-Related Potentials, Configural Encoding, and Feature-Based Encoding in Face Recognition. Journal of Psychophysiology. 2001;15:275–285. [Google Scholar]
- Kim JS, Yoon HW, Kim BS, Jeun SS, Jung SL, Choe BY. Racial distinction of the unknown facial identity recognition mechanism by event-related fMRI. Neuroscience Letters. 2006;397:279–284. doi: 10.1016/j.neulet.2005.12.061. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8(2005):720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Palva JM, Sams M, Hietanen JK, Aronen HJ, Ilmoniemi RJ. Face-selective processing in human extrastriate cortex around 120 ms after stimulus onset revealed by magneto- and electroencephalography. Neurosci Lett. 1998;253:147–150. doi: 10.1016/s0304-3940(98)00586-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Stages of processing in face perception: an MEG study. Nat Neurosci. 2002;5:910–916. doi: 10.1038/nn909. [DOI] [PubMed] [Google Scholar]
- McConahay JB. Modern racism, ambivalence, and the modern racism scale. In: Dovidio JF, Gaertner SL, editors. Prejudice, discrimination, and racism. Orlando, FL: Academic Press; 1986. pp. 91–126. [Google Scholar]
- Meissner CA, Brigham JC. A meta-analysis of the verbal overshadowing effect in face identification. Applied Cognitive Psychology. 2001;15:603–616. [Google Scholar]
- Mullen B, Brown R, Smith C. lngroup bias as a function of salience, relevance, and status: An integration. European Journal of Social Psychology. 1992;22:103–122. [Google Scholar]
- Ng W, Lindsay RCL. Cross-race facial recognition: Failure of the contact hypothesis. Journal of Cross-Cultural Psychology. 1994;25:217–232. [Google Scholar]
- Pascual-Marqui RD. Standardized low resolution brain electromagnetic tomography (sLORETA): technical details. Methods & Findings in Experimental & Clinical Pharmacology. 2002;24D:5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology 1994. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji MR. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Moon H, Rizvi SA, Rauss PJ. The FERET Evaluation Methodology for Face Recognition Algorithms. IEEE Trans Pattern Analysis and Machine Intelligence. 2000;22:1090–1104. [Google Scholar]
- Phillips PJ, Wechsler H, Huang J, Rauss P. The FERET database and evaluation procedure for face recognition algorithms. Image and Vision Computing J. 1998;16(5):295–306. [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning.for face, word, and object processing in the visual cortex. Neuroimage. 2003;20:1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Slone A, Brigham J, Meissner C. Social and cognitive factors affecting the own-race bias in Whites. Basic and Applied Social Psychology. 2000;22:71–84. [Google Scholar]
- Smith-McLallen A, Johnson BT, Dovidio JF, Pearson AR. Black and white: The role of color bias in implicit race bias. Social Cognition. 2006;24:46–73. [Google Scholar]
- Taylor MJ. Non-spatial attentional effects on P1. Clinical Neurophysiology. 2002;113:1903–1908. doi: 10.1016/s1388-2457(02)00309-7. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S, Geiselman RE. Observer mood and cross-racial recognition of faces. Journal of Cross-Cultural Psychology. 1997;28:93–106. [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen–Jensen EC, Ito TA. Ambiguity and the Time Course of Racial Perception. Social Cognition. 2006;24(5):580–606. [Google Scholar]
- Willis J, Todorov A. First Impressions: Making Up Your Mind After a 100-Ms Exposure to a Face. Psychological Science. 2006;17(7):592–598. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Young A, McWeeny K, Hay D, Ellis A. Matching familiar and unfamiliar faces on identity and expression. Psychol Res. 1986;48(2):63–68. doi: 10.1007/BF00309318. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time FMRI paradigm control, physiology, and behavior combined with near-real time statistical analysis. Neuroimage. 1999;10(2):91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]


