Summary
A proliferation inducing ligand (APRIL or TNFSF13) shares receptors with B-cell activation factor of the TNF family (BAFF) on B and T cells. Although much is known about the function of APRIL in B cells, its role in T cells remains unclear. Blocking both BAFF and APRIL suggested that BAFF and/or APRIL contributed to collagen-induced arthritis (CIA), however the role of APRIL alone in CIA remained unresolved. We show here that, in vitro, our newly generated APRIL−/− mice exhibited increased T cell proliferation, enhanced Th2 cytokine production under non-polarizing conditions, and augmented IL-13 and IL-17 production under Th2 polarizing conditions. Upon immunization with OVA and aluminum potassium sulfate (Alum), APRIL−/− mice responded with an increased antigen specific IgG1 response. We also show that in APRIL−/− mice, the incidence of CIA was significantly reduced compared to WT mice in parallel with diminished levels of antigen specific IgG2a autoantibody and IL-17 production. Our data indicate that APRIL plays an important role in the regulation of cytokine production, and that APRIL-triggered signals contribute to arthritis. Blockade of APRIL thus may be a valuable adjunct in the treatment of rheumatoid arthritis (RA).
Keywords: TNF, T cells, Cytokines, Autoimmunity
Introduction
APRIL, a proliferation inducing ligand, also called TNFSF13, TALL-2, TRDL-1 or CD256 is a member of TNF superfamily [1, 2]. APRIL and B-cell activation factor of the TNF family (BAFF, also named TNFSF13B, BLyS, TALL-1, THANK, zTNF4 or CD257) share two receptors, the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI, also called TNFRSF13B or CD267) and B cell maturation antigen (BCMA, also called TNFRSF17 or CD269) [2]. BAFF in addition specifically binds BAFF receptor (BAFF-R, also named TNFRSF13C, BR3 or CD268) [2]. Proteoglycans have been identified as APRIL-specific binding partners [3, 4]. Recently, a weak binding of a shorter variant of APRIL to BAFF-R was found in the murine system [5].
Both APRIL and BAFF are expressed in monocytes, macrophages, dendritic cells, T cells, B cells, osteoclasts, as well as airway and intestinal epithelial cells [2, 6–11]. APRIL was also found in tumor tissues [1]. TACI, BCMA, and BAFF-R, receptors for BAFF and APRIL, are expressed on B cells [12]. While BAFF-R is also found on T cells [2], reports on TACI expression on T cells showed conflicting results [13]. APRIL-specific binding partners are expressed on B cells, T cells, plasma cells, nonhematopoietic cell lines, and tumor cell lines [3, 4].
Roles of APRIL in tumor development, B and T cell immunity and autoimmunity have been reported [1, 2, 4–8, 11–21]. Studies on APRIL function in T cells have focused on T cell co-stimulation, proliferation and survival [4, 13–15, 20, 21], however, the biological function of APRIL in T cell immunity remains unclear.
It has been reported that APRIL−/− mice were viable with normal T- and B-cell development and antibody responses [14]. However, in a second report [15], APRIL−/− mice had impaired IgA class switch, increased numbers of CD44hiCD62LloCD4+ effector/memory T cells, and increased IgG responses to T dependent antigens. To clarify these discrepancies, further characterization of APRIL−/− mice will be helpful.
Whether APRIL participates in rheumatoid arthritis (RA) and other autoimmune diseases is unclear. Collagen induced arthritis (CIA) is an animal model for RA, and requires both humoral and cellular (CD4+ T cell) immune responses [17, 22]. Collagen type II (CII)-specific autoantibody of the IgG2 isotype is crucial to initiate CIA [23, 24]. In addition, IL-17 producing Th17 CD4+ T cells are very important [25–27]. A TACI-Fc fusion protein, blocking both BAFF and APRIL, substantially inhibited mouse CIA [17], indicating that APRIL and/or BAFF contribute to CIA. Comparing the effects of BCMA-Ig and BAFF-R-Ig, BAFF appeared to be a key factor for the progression of CIA in mice [7]. Accumulating evidence showing elevated APRIL or APRIL/BAFF heterotrimers in sera and elevated APRIL in joints of RA patients suggests that APRIL may also play a role in RA [18, 19, 28, 29]. Clearly, to better distinguish the function of APRIL and BAFF in RA, CIA studies under APRIL deficient conditions are needed.
As reported here, we show that APRIL−/− mice have a bias towards Th2 response, diminished susceptibility to arthritis, diminished CII specific IgG2a autoantibody levels and IL-17 production, and reduced IgA levels, indicating that APRIL is an important factor in T cell cytokine regulation and in autoimmune arthritis.
Results
Generation of APRIL−/− mice
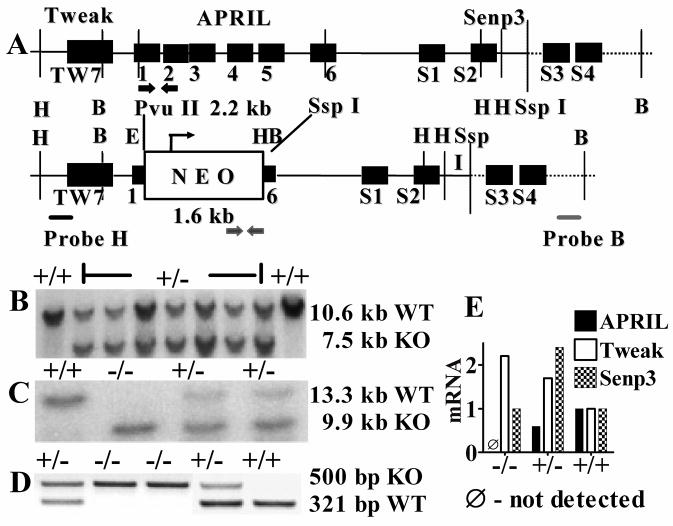
APRIL deficient mice were created by replacing exons 2–5 and the majority of exon 1 and 6 of the APRIL gene with a neomycin cassette (Fig 1A) by homologous recombination in embryonal stem cells and blastocyst injection. Homozygous APRIL−/− mice showed complete absence of APRIL mRNA in splenocytes (Fig. 1E) while mRNA for TNF-related weak inducer of apoptosis (Tweak, or TNFSF12) and Sentrin specific peptidase 3 (Senp3), two genes located 794 bp and 731 bp up- and down-stream, respectively, of APRIL were expressed normally.
Fig. 1. Generation of APRIL−/− mice.
A. Upper panel: Genomic locus of murine APRIL. 1–6: Exons of APRIL gene. TW7: Exon 7 of Tweak. S1–S4: Exons of Senp3. Lower panel: APRIL gene targeting construct. H: Hind III, E: EcoR I, B: BamH I. The positions of probes used for Southern blots are indicated by black and gray bars. The positions of the PCR primer sets for genotype analysis are indicated by black (WT) and gray (deletion mutant) arrow pairs. B. Southern blot analysis of DNA derived from several transfected ES cell clones, digested by Hind III. C. Southern blot analysis of tail-derived genomic DNA digested by BamH I. D. PCR analysis of tail-derived genomic DNA. E. Real time RT-PCR analysis of APRIL, Tweak and Senp3 cDNA from splenocytes. The APRIL+/+ sample was used as the calibrator (relative mRNA expression =1).
Decreased serum total IgA levels in naive APRIL−/− mice and increased antigen specific IgG1 responses in immunized APRIL−/− mice
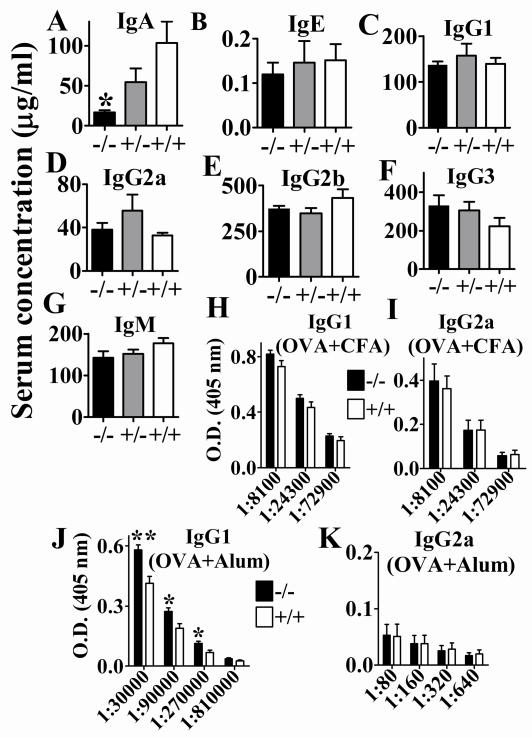
As reported previously [15], we confirmed that APRIL−/− mice have reduced IgA levels in serum (Fig. 2A) while other immunoglobulin isotype levels were normal when compared to APRIL+/+ litter mates (Fig. 2B–G). A gene dose effect of APRIL for IgA production is inferred from the intermediate IgA levels in heterozygous mice.
Fig. 2. APRIL−/− mice had reduced IgA levels without and increased IgG1 levels with immunization.
A – G. Antibody isotype levels in sera of unimmunized mice as detected by ELISA. * p= 0.0171, APRIL−/− vs APRIL+/+, n=4; unpaired t test. H, I. Normal IgG1 or IgG2a levels upon immunization with OVA/CFA on day 0 and OVA/IFA on day 14. n=12, data from 4 independent experiments (n=1–6 each) were combined. J, K. Increased antigen specific IgG1 levels in APRIL−/− mice upon immunization with OVA/Alum on day 0 and day 14. *, p<0.05; **, p<0.01; n=5–6, data from 2 independent experiments (n=2–3 each) were combined; unpaired t test.
To analyze the effect of APRIL on the Th1 mediated antibody response (IgG2a), we immunized mice with OVA emulsified in CFA. No difference in OVA specific IgG2a levels was observed between APRIL−/− and APRIL+/+ mice (Fig. 2H, I), indicating that APRIL does not affect Th1 antibody responses. To assess the role of APRIL on the Th2 mediated antibody response (IgG1), we immunized mice with OVA precipitated with aluminum potassium sulfate (Alum). A significant increase in the OVA specific IgG1 levels was apparent in APRIL−/− mice (Fig. 2J, K), indicating that the presence of APRIL suppresses Th2 antibody responses.
Increased in vitro T cell proliferation as well as Th1 and Th2 cytokine production in APRIL−/− mice
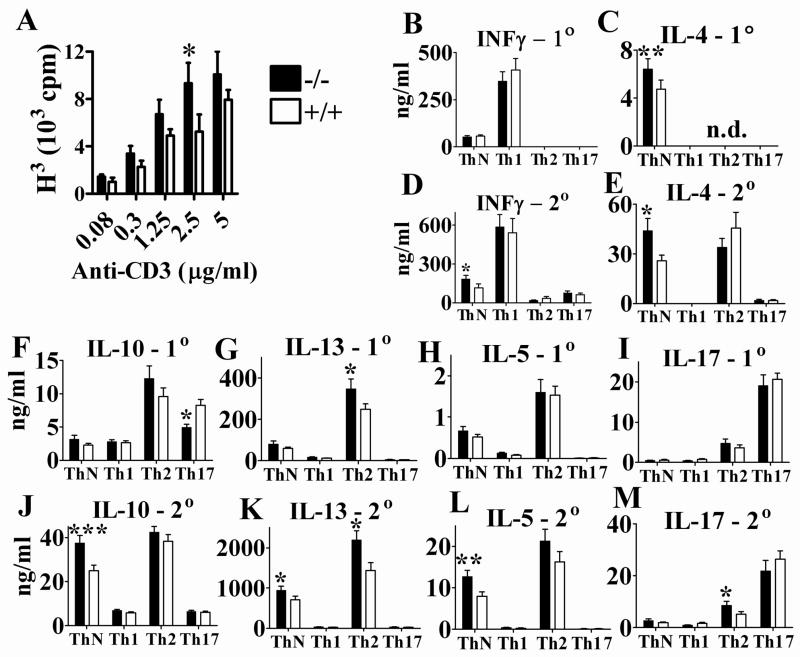
APRIL binds to the receptors BCMA and TACI expressed on B and T cells. T cell responses to APRIL however have not been analyzed extensively. Sensitive indicators for T cell function are changes in proliferation and in cytokine production. APRIL−/− mice exhibited moderately increased T cell proliferation which was significant only at a concentration of 2.5μg/ml of anti- CD3 stimulation (Fig. 3A).
Fig. 3. Increased T cell proliferation and cytokine production in APRIL−/− mice.
A. T cell proliferation was measured by [3H] thymidine incorporation. Splenocytes were cultured at 1×106 cells/ml and stimulated with 0–5μg/ml of anti-CD3 for 3 days. Representative data represent 7 pairs of mice from 5 independent experiments; * p=0.0401; n=7; paired t test. B – M. Cytokine production under ThN, Th1, Th2 and Th17 conditions upon primary (1°) and secondary (2°) stimulation, respectively. For details see Material and Methods. *, p<0.05; **, p<0.01; ***, p<0.001; n=10; paired t test. Data from 6 independent experiments (n=1–3 each) were combined. n.d.- Not done.
Cytokine production by purified, unpolarized and polarized CD4+ T cells showed significant changes in APRIL−/− mice. Under Th neutral (ThN), non-polarizing conditions, IL-4 was upregulated in primary (1°) stimulation (Fig. 3C), while all Th2 cytokines and IFN-γ were increased upon secondary (2°) stimulation of APRIL−/− CD4+ T cells (Fig. 3D, E, J–L). Increased IFN-γ production was also observed in naive APRIL−/− splenocytes stimulated with anti-CD3 (data not shown). Analyzing various polarizing conditions, APRIL deficiency had no detectable effect on Th1 polarized cells (Fig 3B–M). Under Th2 polarizing conditions, APRIL−/− CD4+ T cells secreted increased IL-13 in 1° and 2° stimulation (Fig. 3G, K) and significantly more IL-17 in 2° activation (Fig. 3M) than APRIL+/+ CD4+ T cells. Under Th17 polarizing conditions, APRIL−/− CD4+ T cells produced significantly less IL-10 upon 1° stimulation (Fig. 3F) while IL-17 secretion was not affected after 1° or 2° stimulation (Fig. 3I, M). For easier orientation, the effects of APRIL deficiency on cytokine production under polarizing and non-polarizing conditions are also tabulated in Table 1. The analysis indicates that APRIL has the capacity to regulate cytokine production in unpolarized and in Th2 and Th17 polarized cells but not in Th1 polarized cells (Table 1). APRIL mediated differences in cytokine secretion seen in non-polarizing conditions, but not in polarizing conditions, suggests that cytokines, added to achieve polarization in vitro, can override the absence of APRIL.
Table 1.
Effect of APRIL deficiency on cytokine production by unpolarized (ThN), Th1, Th2, or Th17 polarized CD4+ T cells after primary (1°) and secondary (2°) stimulation
| Polarization | Cytokine regulation in APRIL−/− versus APRIL+/+ CD4+ T cellsa | |||||
|---|---|---|---|---|---|---|
| IFN-γ | IL-4 | IL-5 | IL-10 | IL-13 | IL-17 | |
| ThN | Up, 2° | Up, 1°+ 2° | Up, 2° | Up, 2° | Up, 2° | none |
| Th1 | none | none | none | none | none | none |
| Th2 | none | none | none | none | Up, 1°+ 2° | Up, 2° |
| Th17 | none | none | none | Down, 1° | none | none |
‘Up’ and ‘Down’ indicates statistically significantly higher or lower cytokine levels, respectively, secreted by APRIL−/− CD4+ T cells compared to APRIL+/+ CD4+ T cells. 1° or 2° indicates change only after primary or secondary stimulation, respectively; 1° + 2° indicates change after either stimulation.
Decreased incidence of CIA in APRIL−/− mice
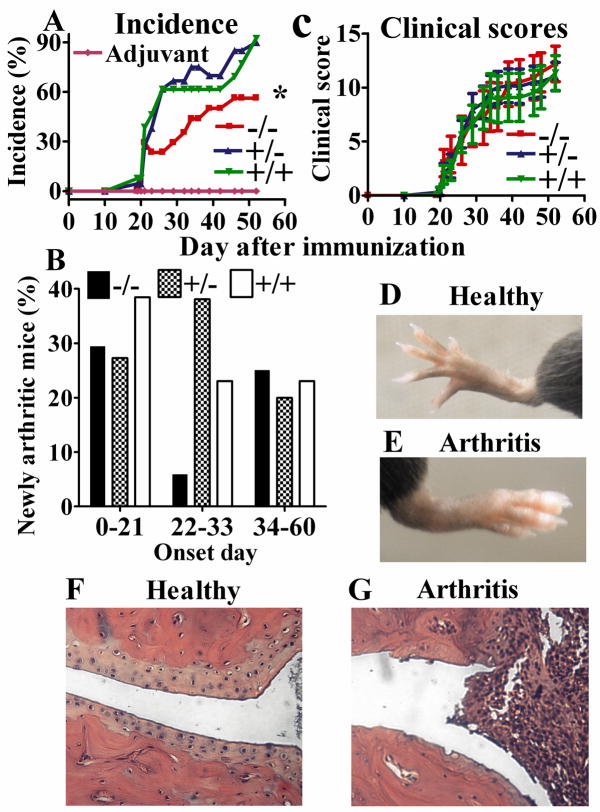
To determine whether the effect of APRIL on cytokines was of patho-physiological importance, we subjected APRIL−/− mice to CIA, a classical animal model for RA. APRIL−/− showed a significantly decreased incidence (mean of −21.32%, 95% confidence interval −27.6 to −15%; P<0.0001) of arthritis upon immunization with chick CII in the presence of Freund’s adjuvant, when compared to APRIL+/+ or heterozygous litter mates (Fig. 4A). The induction of arthritis was dependent on collagen II, since no arthritis occurred in the group injected only with adjuvant (Fig. 4A). Analysis of the percentage of mice becoming newly arthritic at different periods shows a sharp decline of disease onset in APRIL−/− mice during the 22–33 day period after initial challenge (Fig. 4B). However, the clinical scores (Fig. 4C) of APRIL−/− mice that did become arthritic were the same as that of arthritic APRIL+/+ litter mates, suggesting that disease severity is identical, once the threshold of disease induction has been passed. Likewise, disease pathology, as determined by decalcification and histopathology of affected and unaffected joints, was identical in APRIL sufficient mice (Fig. 4D–G) and deficient mice (data not shown).
Fig. 4. Reduced incidence of arthritis in APRIL−/− mice.
A. Incidence: APRIL−/−: 56.3 % (n=16), APRIL+/−: 90.0% (n=20), APRIL+/+: 92.3% (n=13), Adjuvant controls: 0% (n=9). p=0.044 (−/− vs +/+), p=0.049 (+/− vs −/−) and p=1.000 (+/− vs +/+), Fisher’s exact test; B. Percentage of newly arthritic mice during different onset days. C. Clinical scroes (disease severity) of affected mice. APRIL−/−: n=10, APRIL+/−: n=18, and APRIL+/+: n=12 (C); Data from 4 independent experiments were combined (A–C). D, E. Normal and CIA paws of APRIL+/+ mice. D. Normal paw of an adjuvant control mouse. E. CIA paw with erythema and swelling of a CII injected mouse. F, G. Histological assessment of paw joints of APRIL+/+ mice. F shows an adjuvant control joint with even and clear joint space and smooth articular cartilage. G shows a CIA joint with infiltration of inflammatory cells, cartilage destruction and bone erosion. H&E, Original magnification: 20x.
Diminished IL-17 production in in vivo CII primed T cells of APRIL−/− mice
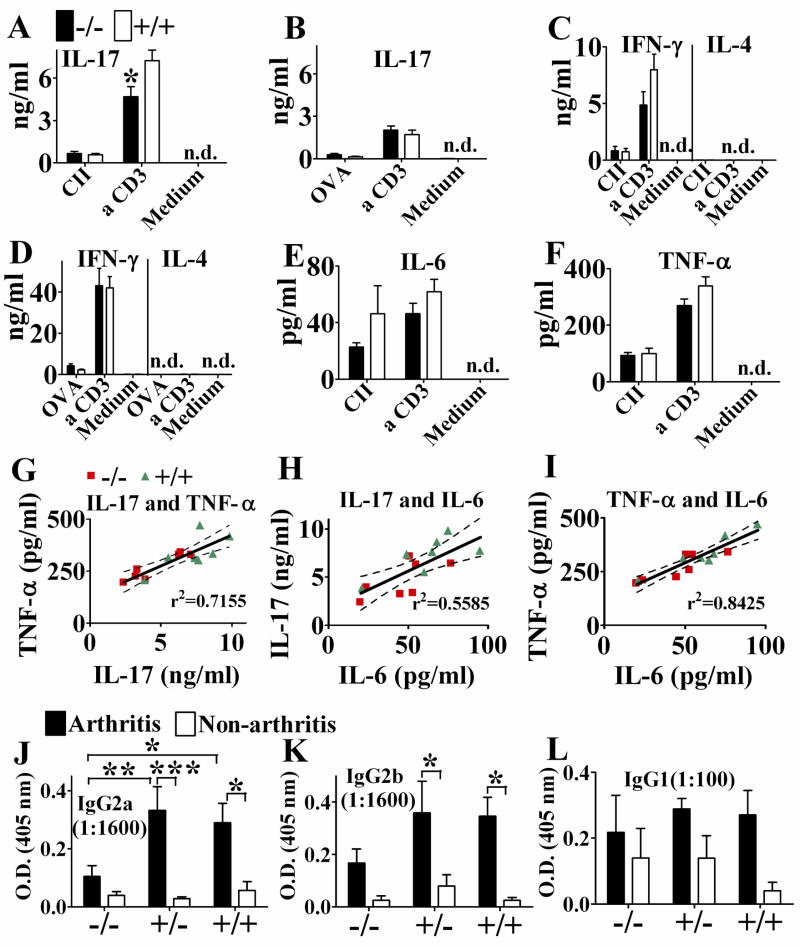
Arthritis in the CIA model is known to be associated with increased IL-17 production [25–27]. Accordingly, inguinal LN cells isolated from APRIL−/− mice one week after a single chick CII immunization, prior to disease onset, showed significantly diminished IL-17 production upon anti-CD3 restimulation in vitro in comparison to immunized APRIL+/+ mice (Fig. 5A). The IL-17 production by T cells was induced by collagen immunization, since splenocytes from mice immunized with OVA and CFA/IFA or from naive mice, stimulated with anti-CD3, produced low levels of IL-17 which showed no difference between APRIL−/− and APRIL+/+ mice (Fig. 5B and data not shown). While T cell proliferation of CII primed inguinal LN cells was enhanced in APRIL−/− mice (data not shown), IFN-γ production was very low and showed no significant difference between APRIL−/− and APRIL+/+ mice, and IL-4 was undetectable (Fig. 5C). Our data show that CII/CFA induced a Th17 dominated immune response. In contrast, OVA/CFA/IFA induced a Th1 dominated immune response, according to the relatively high levels of INF-γ, relatively low levels of IL-17, and undetectable IL-4 production (Fig. 5B, D). TGF-β, IL-6, TNF-α and IL-1 β are important for Th17 induction and IL-23 for maintenance. We also analyzed the in vitro production of these cytokines in CII primed inguinal LN cells. IL-6 and TNF-α showed a trend to diminution in APRIL KO mice (Fig. 5E, F) while TGF-β, IL-23 and IL-1 β were not detectable by ELISA (data not shown). The values for IL-17, IL-6 and TNF-α showed similar pattern (Fig. 5A, E, F) and were significantly correlated to each other as indicated by linear regression analysis (Fig. 5J, K, L). The values of APRIL−/− mice tended to be clustered towards the lower end of the scale while the values for APRIL+/+ mice clustered towards the higher end supporting the finding of attenuated production of inflammatory cytokines in APRIL−/− mice.
Fig. 5. Diminished IL-17 and IgG2a autoantibody production were associated with reduced arthritis in APRIL−/− mice.
A, C, E, F. Inguinal LN cells, isolated one week after a chick CII/CFA immunization, were cultured at 2×106/ml in the absence or presence of denatured chick CII (100μg/ml) or anti-CD3 (2.5μg/ml) for 4 days. Cytokine production in supernatant was detected by ELISA. *, p=0.0291; n=7; unpaired t test. Data from 4 independent experiments (n=1–2 each) were combined. B, D. Splenocytes, isolated on day 17 after OVA/CFA immunization on day 0 and OVA/IFA immunization on day 14, were cultured at 5×106/ml in the absence or presence of OVA (100μg/ml) or anti-CD3 (2.5μg/ml) for 3 days. Cytokine production in supernatant was detected by ELISA. n=6, from the mice in Fig. 2. H, I. with serum IgG1 and IgG2a tested. G – I. Correlation between each two of the cytokine production of IL-17, IL-6 and TNF-α. n=14, linear regression: r2=0.7155, p=0.0001 (G), r2=0.5585, p=0.0021 (H), r2=0.8425, p<0.0001 (I), J – L. Serum antibody titers to mouse CII on day 34, detected by ELISA. The serum titer shown was within the linear range of antibody dilution; n=5–6 (n=3 for non-arthritis APRIL+/+ mice). *, p<0.05; **, p<0.01; ***, p<0.001; two-way ANOVA followed by Bonferroni post-tests.
Reduced CII specific IgG2a autoantibody levels in APRIL−/− mice
Cross reacting IgG2 autoantibody against mouse CII, induced by chicken CII immunization, has been shown to be essential in CIA induction in addition to the disease association of IL-17. Comparing anti-mouse collagen IgG2a, IgG2b and IgG1 autoantibody levels in healthy and arthritic mice, we found that increased IgG2a (Fig. 5J) and to a lesser extent IgG2b (Fig. 5K) levels were correlated with clinical disease while anti-mouse CII IgG1 levels were not (Fig. 5L). APRIL−/− mice had significantly lower anti-mouse CII autoantibody levels of the IgG2a isotype (Fig. 5J) than APRIL+/+ mice, correlating with and explaining decreased disease incidence. As in APRIL+/+ mice, arthritic APRIL−/− mice tended to have higher IgG2a levels than healthy APRIL−/− mice (Fig. 5J, left columns). Since the antibody levels were very low, it is difficult to detect significant difference. The IgG2a levels of arthritic APRIL−/− mice was about twice of the level of non-arthritic, but challenged APRIL+/+ mice, suggesting that the threshold for CIA induction by IgG2a autoantibody is relatively low.
Discussion
APRIL regulates antibody isotype switching in vivo and cytokine production in vitro under Th1, Th2 and Th17 polarizing conditions. We confirm, as reported previously [15], that APRIL−/− mice have reduced serum IgA levels. The fact that heterozygotes have intermediate IgA levels suggests that the gene dose effect of APRIL may be limiting for IgA production under homeostatic conditions. In addition we found that APRIL deficiency increased the IgG1/IgG2a antibody ratio upon specific immunization with Alum, but not after immunization in Freund’s adjuvant. Alum mediates Th2 polarization of CD4+ T cells resulting in antibody isotypes such as IgG1 that depend on IL-4 as switch factor. APRIL deficiency enhances IL-13 but not IL-4 production in Th2 polarized cells. However it is noteworthy that APRIL−/− CD4+ T cells under ThN condition can produce the same level of IL-4 as Th2 polarized APRIL+/+ CD4+ cells after secondary stimulation. It is likely therefore that uncommitted but activated CD4+ T cells secreting IL-4 in APRIL−/− mice may contribute to Th2 polarization and IgG1 production. Importantly, APRIL had no effect on the IgG1/IgG2a antibody ratio when Freund’s adjuvant was used. Freund’s adjuvant causes Th1 polarization of T cells as we confirmed, and Th1 cytokine production in Th1 polarized T cells, as shown in this study, was not affected by APRIL.
Our data also show that APRIL deficiency is associated with a significantly decreased incidence of arthritis and diminished IgG2a autoantibody levels to murine CII. Since the pathogenesis of arthritis in the murine CIA model is critically dependent on IgG2 autoantibodies to CII, and within a given strain, the levels of antibody correlate well with the presence or absence of arthritis, our data readily explain the diminished incidence of arthritis in APRIL−/− mice as being due, at least in part, to diminished IgG2a autoantibodies. APRIL−/− inguinal LN cells from CII immunized mice, in addition, secreted less IL-17 than LN cells from APRIL sufficient mice upon restimulation with anti-CD3 in vitro, indicating that Th17 polarization is diminished or that IL-17 production is dependent on APRIL costimulation. Since Th17 T cells are known to be very important in the pathogenesis of arthritis, diminished IL-17 production in APRIL−/− is also likely to contribute to diminished incidence of arthritis. The decreased IL-17 production and reduced CII-specific IgG2a antibody level associated with decreased CIA incidence in APRIL−/− mice, fit well with a previous study showing that in IL-17-deficient mice, CIA was suppressed in parallel with reduced anti-CII IgG2a levels while other Ig isotypes were unaffected [27].
It seems puzzling that after CII or OVA immunization in Freund’s adjuvant only IgG2a specific for CII, but not for OVA, was decreased in APRIL−/− compared to APRIL+/+ mice. These two antigens induce very different immune responses. CII/CFA induces a Th17 dominated immune response while OVA/CFA/IFA induces a Th1 dominated immune response. In contrast to OVA, chick CII has cross reacting epitopes with mouse CII. CII-specific autoantibodies thus bind to CII of cartilage in joints and activate the complement system resulting in recruitment of neutrophils and macrophages and in the release of chemokines and proinflammatory cytokines that cause joint inflammation. Under certain conditions, such as low levels of IFN-γ and IL-4 and high levels of TGF-β and IL-6, Th17 cells are induced and osteoclastogenesis occurs, which causes bone erosion [30]. This pathway appears to be costimulated by APRIL. Th17 cells may in turn provide help to B cell to produce more CII-specific autoantibodies [31], which is also supported by the previous finding that IL-17 deficiency suppressed anti-CII IgG2a levels and CIA [27]. Therefore, APRIL deficiency may firstly lead to a defect in IL-17 production, followed by a decreased CII-specific IgG2a autoantibody response.
APRIL deficiency does not affect TH1 responses driven by Freund’s adjuvant. Therefore, it is likely that the effect of APRIL in CIA is primarily mediated by autoantibody binding to CII in the joint, resulting in secondary responses that require APRIL support to maintain IgG2a levels, but in APRIL deficiency lead to lower IgG2a levels and diminished disease.
Further investigation is required to determine at which point APRIL intervenes as costimulus for IL-17 production. The following possibilities could be considered. First, IL-17 production is suppressed by both IFN-γ and IL-4 [26], thus it is possible that increased IL-4 production observed in APRIL−/− mice under non polarizing, homeostatic condition contributes to diminished IL-17 production after collagen challenge. Second, APRIL may regulate Th17 polarization, maintenance or Th17 related cytokine production. Analysis of the in vitro production of TGF-β, IL-6, IL-23, IL-1β and TNF-α of in vivo CII-primed inguinal LN cells showed a trend to diminution of IL-6 and TNF-α in APRIL−/− mice, whereas TGF-β, IL-23 and IL-1β were not detectable by ELISA. A significant correlation was found in production of IL-17, IL-6 and TNF-α, tending to be high in APRIL+/+ and low in APRIL−/− mice. IL-6 is one of the Th17 polarizing cytokines, and TNF-α is a crucial cytokine during CIA onset. Th17 cells can be induced in vitro in the presence of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, together with TGF-β. IL-17 in turn can stimulate the production of inflammatory mediators including TNF-α, IL-6. IL-17 in vitro and in vivo has additive or even synergistic activity with IL-1 and TNF-α in inducing proinflammatory cytokine expression and joint damage [30]. A study using tumor necrosis factor receptor I (TNFRI) and IL-6 double knockout mice showed that blocking of TNFRI and IL-6 synergistically attenuated CIA [32].
The effect of APRIL on IL-17 production was different in vivo from its effect in in vitro assays. In CIA in vivo it is likely that CII-specific autoantibodies bind to CII of the cartilage in joints and cause the secretion of inflammatory cytokines, such as TGF-β and IL-6, which in turn induce IL-17 production. Under in vitro Th17 polarizing conditions induced with TGF-β and IL-6, no significant IL-17 decrease was observed in APRIL−/− mice, suggesting that the function of APRIL may have been overridden by added TGF-β and/or IL-6 in the in vitro polarization assay.
Our data demonstrate that APRIL has significant effects on the pathogenesis of arthritis by modulating autoantibody and cytokine production. Previous studies [7, 17] using TACI-Ig, BCMA-Ig and BAFFR-Ig as ligand blockers have not been able to distinguish between the effects of BAFF and APRIL on RA. The new data allow us to conclude unambiguously that APRIL is a significant factor in the pathogenesis of RA in the murine model, and provide an explanation for the increased APRIL levels observed in human disease.
Our findings reveal novel important functions of ARPIL in T cell immunity, i.e. the regulation of Th2 and Th17 responses in addition to functions on IgA and IgG antibody production. Data from our study indicate that APRIL promotes Th17 response and inhibits Th2 response. The underlying mechanistic details of this finding require further studies. The elucidation of the role of APRIL in the cytokine networks that control Th1, Th2 and Th17 responses will be of great interest in explaining the pathogenesis of autoimmune diseases and of potential clinical importance for treatment.
Materials and Methods
Generation of APRIL−/− mice
An 8.4kb genomic DNA fragment including the mouse APRIL gene, exon 7 of the upstream Tweak gene, and the exons 1–4 of downstream Senp3 gene, was used to insert a neomycin cassette. The targeting construct replaced part of APRIL’s exon 1, exons 2–5 and part of exon 6 with the neomycin cassette. Blastocyst injection and generation of founder mice was done in the transgene core facility of University of Miami. APRIL−/− mice for experiments were backcrossed onto C57BL/6 background for 7–14 generations. Control APRIL+/+ C57BL/6 mice were either APRIL+/+ littermates born in our facility or APRIL+/+ mice purchased from Charles River Laboratories (Wilmington, MA).
Real-time RT-PCR
Total RNA was extracted from splenocytes of naive mice. APRIL, Tweak and Senp 3 primer sets (SuperArray Bioscience Corporation, Frederick, MD) were used for SYBR Green real-time PCR. Standardization was performed with β-actin as the endogenous control and the APRIL+/+ sample as the calibrator. Real-time PCR was analyzed with 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA).
OVA immunization with Alum and Freund’s adjuvant
OVA (Grade V) and Alum were purchased from Sigma (St. Louis, MO). For OVA/Alum immunization, each mouse was i.p. injected with 200μl of OVA (66μg) coprecipitated with Alum (6.6mg) on day 0 and day 14. For OVA/CFA/IFA immunization, each mouse was i.p. injected with 100 μl of OVA (66μg) emulsified with 100 μl of CFA on day 0 and OVA (66μg) emulsified with IFA on day 14. Sera were collected on day 17 for ELISA to detect OVA specific IgG1 and IgG2a. In this study, we analyzed IgG2a instead of IgG2c in C57BL/6 mice, since the cross-reactivity with the anti-IgG2a antibodies reflects most of the IgG2c in sera [33].
Cytokine production and T cell proliferation assays
Inguinal LN cells, isolated one week after a single chick CII/CFA immunization were cultured at 2×106/ml in the absence or presence of denatured chick CII (100μg/ml) or anti-CD3 (2C11, 2.5μg/ml) for 4 days. Splenocytes, isolated on day 17 after OVA/CFA immunization on day 0 and OVA/IFA immunization on day 14, were cultured at 5×106/ml in the absence or presence of OVA (100μg/ml) or anti-CD3 (2.5μg/ml) for 3 days. Cytokine production in supernatant was detected by ELISA.
For T cell proliferation, splenocytes were cultured at 1×106/ml and stimulated with 0–5μg/ml of anti-CD3 for 3 days. [3H] thymidine was added during the last 6-hour incubation, and 3H incorporation was quantitated by using a scintillation counter.
In vitro cytokine production under ThN, Th1, Th2 and Th17 conditions
Purified CD4+ T cells at 1×106/ml were activated in 96-well plates with 2μg/ml coated anti-CD3 mAb (2C11) and 1μg/ml soluble anti-CD28 mAb (37.51, eBioscience, San Diego, CA). Cells were polarized under the following conditions. ThN: 10ng/ml rIL-2 (eBioscience). Th1: 10ng/ml rIL-2, 10ng/ml rIL-12 (eBioscience), and 20μg/ml anti-IL-4 mAb (11B11). Th2: 10ng/ml rIL-2, 30ng/ml rIL-4 (eBioscience), 10μg/ml anti-INF-γ mAb (XMG1.2), and 2μg/ml anti-IL-12 mAb (C17.8, eBioscience). Th17: 10ng/ml rIL-6 (BD Pharmingen, San Diego, CA), 5ng/ml human TGF-β1 (R&D, Minneapolis, MN), 10μg/ml anti-IL-4 mAb (11B11), and 10μg/ml anti-INF-γ mAb (XMG1.2). After 4 days, cells were washed and re-cultured for 2 additional days at 1×106/ml with 2μg/ml plate-bound anti-CD3. Supernatants were collected on day 4 (primary stimulation) and day 6 (secondary stimulation) for ELISA to detect cytokine production.
CIA induction
Mice at 7–9 weeks of age were used in a CIA protocol approved by the University of Miami Animal Care and Use Committee (ACUC). CIA induction was carried out as previously described [22]. Chick CII (Sigma, St. Louis, MO) at 2 mg/ml in 50 μl of 10 mM acetic acid was emulsified in an equal volume of CFA, and injected intradermally (i.d.) into the base of the tail on day 0 and day 21. For controls, CII was substituted by 10 mM acetic acid.
Clinical assessment of arthritis and histological examination of arthritic joints
Mouse paws were scored as described previously [34, 35]. Arthritis was considered positive when two consecutive positive evaluations were obtained [36]. At the end of the experiments, mouse paws were fixed, decalcified, and processed for H&E staining. Slides were examined under the microscope. Mouse paw images were captured using a Canon digital camera (PowerShot S70), and histological images were obtained using a Nikon ECLIPSE E800 digital camera. Brightness/Contrast adjustment and cropping of images were performed using Adobe Photoshop 6.0.
ELISA
ELISA antibody pairs and standards for IgA, IgG1, IgG2a, IgG2b, IgG3, IgM and IgE, as well as IFN-γ, IL-4, IL-17, TGF-β and IL-6 were purchased from BD Pharmingen, and IL-5, IL-13, IL-10 and IL-23 from eBioscience. Serum total Ig levels, serum CII or OVA specific antibody isotypes and cytokine production in culture supernatant were detected by sandwich ELISA, according to the manufacturer’s instructions. TNF-α and IL-1β ELISA was performed by using mouse TNF-α ELISA Ready-SET-Go (eBioscience) and Mouse IL-1β/IL-1F2 Immunoassay (R&D), respectively. In the detection of serum CII or OVA specific antibody isotypes, 96-well plates were coated with 2μg/ml of mouse CII or 100μg/ml of OVA in PBS, and biotin-conjugated anti-mouse Ig isotypes were used for detection.
Statistical analysis
Unpaired t test, paired t test, and two-way ANOVA followed by Bonferroni post-tests were performed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). P < 0.05 was considered as significant. Data are mean ± SD.
Acknowledgments
These studies were supported by NIH AI061807, CA109094, CA039201, and AI068515.
Abbreviations
- APRIL
a proliferation inducing ligand
- BAFF
B-cell activation factor of the TNF family
- TACI
transmembrane activator and calcium modulator and cyclophilin ligand interactor
- BCMA
B cell maturation antigen
- BAFF-R
BAFF receptor
- CIA
collagen-induced arthritis
- CII
collagen type II
- RA
rheumatoid arthritis
- Alum
aluminum potassium sulfate
- Tweak
TNF-related weak inducer of apoptosis
- Senp3
sentrin specific peptidase 3
- ThN
Th neutral, non-polarizing conditions
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 3.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, Qiang F, Gorelik L, Kalled SL, Acha-Orbea H, Rennert PD, Tschopp J, Schneider P. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, Spaargaren M, Medema JP. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 5.Bossen C, Schneider P. BAFF, APRIL and their receptors: Structure, function and signaling. Seminars in Immunology. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Stein JV, Lopez-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodriguez D, Gomez-Caro R, De Jong J, Martinez AC, Medema JP, Hahne M. APRIL modulates B and T cell immunity. J Clin Invest. 2002;109:1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–265. doi: 10.1159/000082106. [DOI] [PubMed] [Google Scholar]
- 8.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Simonin PY, Feldblum S, Kolb JP. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 9.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–5957. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway Epithelial Cells Produce B Cell-Activating Factor of TNF Family by an IFN-beta-Dependent Mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Jelinek DF, Darce JR. Human B lymphocyte malignancies: exploitation of BLyS and APRIL and their receptors. Curr Dir Autoimmun. 2005;8:266–288. doi: 10.1159/000082107. [DOI] [PubMed] [Google Scholar]
- 13.Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–289. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D, Olsson C, Tom L, Erickson S, French D, Schow P, Grewal IS, Ashkenazi A. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Marsters SA, Baker T, Chan B, Lee WP, Fu L, Tumas D, Yan M, Dixit VM, Ashkenazi A, Grewal IS. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol. 2001;2:632–637. doi: 10.1038/89782. [DOI] [PubMed] [Google Scholar]
- 18.Tan SM, Xu D, Roschke V, Perry JW, Arkfeld DG, Ehresmann GR, Migone TS, Hilbert DM, Stohl W. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003;48:982–992. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- 19.Roschke V, Sosnovtseva S, Ward CD, Hong JS, Smith R, Albert V, Stohl W, Baker KP, Ullrich S, Nardelli B, Hilbert DM, Migone TS. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–4321. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 20.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 21.Medema JP, Planelles-Carazo L, Hardenberg G, Hahne M. The uncertain glory of APRIL. Cell Death Differ. 2003;10:1121–1125. doi: 10.1038/sj.cdd.4401291. [DOI] [PubMed] [Google Scholar]
- 22.Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 24.Nandakumar K, Backlund J, Vestberg M, Holmdahl R. Collagen type II (CII)-specific antibodies induce arthritis in the absence of T or B cells but the arthritis progression is enhanced by CII-reactive T cells. Arthritis Res Ther. 2004;6:R544–550. doi: 10.1186/ar1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 27.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 28.Vallerskog T, Heimburger M, Gunnarsson I, Zhou W, Wahren-Herlenius M, Trollmo C, Malmstrom V. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 31.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi N, Ohshima S, Umeshita-Sasai M, Nishioka K, Kobayashi H, Mima T, Kishimoto T, Saeki Y. Synergistic effect on the attenuation of collagen induced arthritis in tumor necrosis factor receptor I (TNFRI) and interleukin 6 double knockout mice. J Rheumatol. 2003;30:22–27. [PubMed] [Google Scholar]
- 33.Pan M, Kang I, Craft J, Yin Z. Resistance to development of collagen-induced arthritis in C57BL/6 mice is due to a defect in secondary, but not in primary, immune response. J Clin Immunol. 2004;24:481–491. doi: 10.1023/B:JOCI.0000040919.16739.44. [DOI] [PubMed] [Google Scholar]
- 34.Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR, Firestein GS. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–130. doi: 10.1016/S0002-9440(10)64356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005;17:325–333. doi: 10.1093/intimm/dxh212. [DOI] [PubMed] [Google Scholar]
- 36.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–368. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]