Abstract
INTRODUCTION
Arterial spin labeling (ASL) is a safe, noninvasive imaging method for evaluating cerebral blood flow (rCBF). The purpose of this article is to present ASL imaging features of 38 elderly cognitively normals (CN) with their rCBF values and an averaged profile of targeted anatomic regions rCBF values.
METHODS
Thirty-eight CN underwent MR imaging especially ASL with voxel morphometric techniques fusing the MR anatomical and ASL images to a standard reference brain (colin27). The ASL images were fused to echo planar images, which were then coregistered to high-resolution anatomical SPGR images. rCBF was calculated per region of interest using a modified continuous arterial spin labeling (CASL) convolutional method. Anatomical regions were selected and identified by the Talairach atlas in SPM2.
RESULTS
We identified areas of decreased and increased perfusion (compared to the averaged rCBF of all 38 CN) corresponding to decreased and increased quantified rCBF. The most common sites for decreased perfusion were precuneus (53%), superior temporal (48%), and orbitofrontal (37%), and for increased perfusion the caudate (39%), posterior cingulate (34%), anterior cingulate (32%), and amygdala (32%).
CONCLUSION
There are regional variations in rCBF both increased and decreased with the posterior cingulate and precuneus cortex showing the highest averaged values and signal intensity (bright spots). These variations represent the normal profile of a CN elderly brain, with higher perfusion in areas associated with cognition, memory, and behavior. It is necessary to understand these normal variations in order to determine if there are perfusion changes in ASL detected in neurodegenerative disorders such as Alzheimer’s disease.
Keywords: Arterial spin labeling, MRI, normal aging brain, neurodegenerative disease, cerebral perfusion, cerebral blood flow
Introduction
Arterial spin labeling (ASL) is a safe, noninvasive imaging procedure that assesses cerebral perfusion, and can generate regional cerebral blood flow (rCBF) values. Perfusion describes the volume of blood delivered to brain tissue at the capillary level, which correlates with brain metabolism. Although there is no direct correlation of brain function to perfusion imaging except in task-specific fMRI imaging such as blood oxygen level dependency (BOLD), perfusion, measured as regional cerebral blood flow and volume, does reflect the state of health of the brain. ASL uses an individuals own water molecules in blood as a tracer agent by energizing them with an adiabatic RF pulse to invert the water molecules or tag them. When these tagged molecules reach the capillary bed they exchange with brain tissue and by the property of magnetic transfer impart their energy to brain tissue. This alteration in brain tissue signal is enhanced by a subtraction technique in order to produce an image.
ASL has been utilized as a research tool in multiple disease processes such as neuropsychiatric disorders, cerebrovascular disease, brain tumors, epilepsy, affective disorders, anxiety and stress, substance abuse, HIV, and in particular in the evaluation of Alzheimer’s disease (AD).1 There is no experience in the literature utilizing ASL images as a clinical tool for making the diagnosis of neurodegenerative disorders. The imaging profile of CN-aged individuals studied by ASL has not been presented before either. It is our hypothesis that the visual images reflect the quantified rCBF data, and presents the normal profile of regional variations of blood flow in the CN brain. It is the purpose of this article to present the visual ASL imaging findings in 38 aged cognitive normals, as well as their quantified rCBF by regions of interest (targeting key areas of cognition and memory).
Methods
Thirty-eight elderly CN individuals (mean age 82.2 ± 3.7 years) were selected from the Cardiovascular Health Study Cognition Study, which is a continuation of the CHS Dementia Study (CHS-CS) group of patients, which also includes AD and MCI patients, having been studied by MR and ASL.2–4 Their clinical and demographic data including their MMSE scores are presented in Table 1. Of the 927 participants identified in 1998–1999, a total of 532 normal, AD, and MCI subjects were available for study in 2002–2003. All of these subjects had complete neurological and neuropsychological examinations in 1998–1999, and 2002–2003, and an MRI of the brain in 1992–1994.4 Of these 38 elderly age-matched patients studied by ASL without hypertension, or MR evidence of white matter/ischemic changes provided a standard reference for the cerebral blood flow for a similar number of AD patients. These 38 had no technical errors encountered in their scanning or evidence for stenotic carotid artery on accompanying MR angiograms. There were no MR structural lesions such as tumor, nor evidence of prior surgery. Standard MR as well as 3-dimensional volumetric images in these 38 showed no evidence of any periventricular or subcortical white matter or ischemic lesions. None had a history of stroke, atherosclerotic disease, head trauma, or had consumed caffeine 8 hours prior to imaging. This project was approved by the CHS-CS and local IRB.
Table 1.
Characteristics of the Participants (%)
| Number of Participants | 38 |
| Age | 82.4 ± 3.6 |
| Gender: | |
| Males: | 16 (42) |
| Females: | 26 (58) |
| Education level, >high school | 23 (68) |
| Race: | |
| Whites: | 30 (75) |
| African-Americans | 9 (25) |
| Modified mini-mental state examination | 94.6 ± 5.7 |
| Heart disease* | 5 (14) |
| Diabetes mellitus** | 3 (8) |
| Hypertension*** | 18 (50) |
History of angina, CHF, or myocardial infarction.
American Diabetes Association criteria.
Diagnosed by patient’s physician.
CASL-MRI Imaging
All 38 CN were scanned on a GE Signa 1.5T MR unit. Continuous arterial spin labeling (CASL) was used, but with modification using a 3.7-seconds pulse train at a 92% cycle with ramp-sampled echo planar imaging (EPI), thus requiring only 1 transmit/receive coil. (TE = 60 seconds, TE = 21 ms with ramp sampling, and receiver bandwidth (rBW) of 76 kHz) The pulse sequence was repeated 50 times for signal averaging alternating between a single and a double adiatic inversion of the signal. This modified CASL sequence yields a higher S/N ratio compared to a PASL sequence, and more slices could be obtained. Multiple repetitions at a 92% cycling allows the advantages of CASL imaging to be utilized, but keeps the SARS limit down. Labeling occurred 10 cm below the skull base as magnetic gradient was also applied in the z-axis direction, and sufficient delay before imaging (t = 700 ms). Nineteen EPI contiguous slices were obtained (64 × 64 matrix, 20 cm FOV, 5 mm thick slices no gaps, 21 ms echo time, 700 ms transit delay, and 90° flip angle. Images were acquired sequentially from vertex down to skull base, or from superior to inferior so as not to perturb the labeled spins, which flow from an inferior to superior direction.
EPI images were constructed off-line from the raw k-space data using a reconstruction program written in C. EPI and SPGR images were converted to the Analyze format for processing with Statistical Parametric Mapping (SPM2 Wellcome Department of Imaging Neurosciences). EPI gray and white matter masks were created from segmentation of SPGR images into gray matter, white matter, and CSF/ventricles. By creating a gray perfusion mask issues of volumetric atrophy can be addressed and partial volume correction can be made. The SPGR images were coregistered to a stereotactic reference brain (colin27 brain). The ASL images were generated by the EPI, which was coregistered the SPGR images. SPM2 image realignment corrected physiologic motion between acquisitions. Nonoverlapping volumes, which normally appear on the superior slice or the inferior slice (slice 19), were discarded in the realignment process. The superior slice was usually discarded due to lack of gray matter, since there was a limit to number of slices utilizing the ASL sequence. The normalized gray matter ASL images were smoothed using a 6 mm Gaussian kernel. MR angiography eliminated about one-fourth of the patients scanned if greater than 20% flow difference was calculated related to stenotic disease suggesting carotid stenosis. Thus all 38 had symmetrical flow velocities in their carotid arteries.
Coronal T1W spoiled gradient-recalled echo (SPGR) images were obtained covering the entire brain so as to generate high-resolution structural images (124 slices with a 256 × 192 matrix, 1.5–mm-thick slices(zero spacing) with a TE = 60 seconds, TR = 25 ms, and FOV of 24 × 18 cm, with rBW = 16 kHz).
The rCBF was calculated using a CASL-based convolutional method as proposed by Buxton5:
A global cerebral blood flow (gCBF) was calculated from the averaged values of all regions (gCBF = rCBF thalamus plus rCBF amygdala, etc.). Only select anatomical region rCBF were recorded in Table 2. The rCBF for each of the identified regions was then corrected for age, gender, and hypertension. Linear regression was then applied to Table 2 for age, gender, and hypertension with resulting f-ratios and their P-values. Decreased perfusion was identified as one standard deviation (SD) below or more than the averaged rCBF for that specific region, and increased perfusion as one SD above or more.
Table 2.
rCBF by Anatomic Regions (mL/100 g/min). Adjusted for Age, Gender, and Hypertension. Averaged Values from 38 CN Individuals
| Unadjusted | Adjusted | F-Ratio | P-value | |
|---|---|---|---|---|
| Posterior cingulate** | 54.27 | 53.42 | 2.63 | .02 |
| Anterior cingulate* | 41.94 | 40.86 | 3.07 | .01 |
| Precuneus** | 56.78 | 56.69 | 3.93 | .003 |
| Superior parietal* | 45.28 | 44.45 | 4.43 | .001 |
| Caudate | 39.14 | 37.05 | 4.07 | .002 |
| Globus pallidus | 36.63 | 35.30 | 5.15 | <.001 |
| Thalamus* | 41.14 | 40.05 | 2.27 | .06 |
| Hippocampus | 39.96 | 37.49 | 8.77 | <.001 |
| Amygdala | 37.19 | 36.62 | 1.97 | .09 |
| Orbitofrontal** | 52.31 | 50.58 | 3.07 | .01 |
| Lateral frontal (preF)* | 42.28 | 41.98 | 3.28 | .009 |
| Superior temporal* | 44.62 | 44.65 | 3.11 | .01 |
| Standard deviation | ± 6.71 | ± 7.01 |
Anatomical regions of interest were identified by the Talairach map in SPM2. (Fig 1) In particular, those regions with increased perfusion or hot spots were first revealed by the SPM2 analysis (Table 3). SPM2 identified those regions with abnormal perfusion/rCBF as well as the cluster size in terms of voxel comparing each region to an averaged rCBF for each region in the same locations for all 38 individuals (Table 3). The larger the cluster size, the lower or more significant was the P-value. Both the Gaussian filtered gray scale and the red or hot mode (SPM2) images were reviewed. The color scale in terms of signal intensity is included in Figure 2. Bright spots seen as yellow or white on the hot made were seen in the gray scale images as regions of brighter intensity, and thus do not represent pseudo-coloring scaling. Furthermore, these bright spots corresponded to regions of higher quantified rCBF identified by SPM2 (Fig 3) (Table 4). Normal rCBF ranged from 35 to 45 mL/100 g/min and presented as dark to light red, whereas bright spots had rCBF greater than 45 mL/100 g/min and appeared as yellow or white colors. Because the emphasis is on visual rating for clinical application the bright spots were graded as mild (dot-like), moderate (less than 5 mm in size), and marked (greater than 5 mm in size). The total number of bright spots for each targeted region was then divided by the total number of individuals (n = 38) in order to demonstrate those regions with the highest and lowest numbers of either increased or decreased regional cerebral blood flow values. (Table 4).
Fig 1.

Targeted regions.
Table 3.
Summary of SPM2 Cluster-Level Statistics for Regions of Hypoperfusion in 38 CN
| Anatomical Region | Cluster Size | Cluster P-Values |
|---|---|---|
| Posterior cingulate | 312 | .01 |
| Anterior cingulate | 4,763 | <.001 |
| Caudate | 12,099 | <.001 |
| Hippocampus | 226 | .01 |
| Globus pallidus | 1,589 | <.001 |
| Precuneus | 3,092 | <.001 |
| Superior parietal | 312 | .01 |
| Lateral frontal (PreF) | 2,238 | <.001 |
| Superior temporal | 1,356 | <.001 |
| Orbitofrontal | 605 | .003 |
| Amydgala | –(none) | – |
| Thalamus | – | – |
Fig 2.
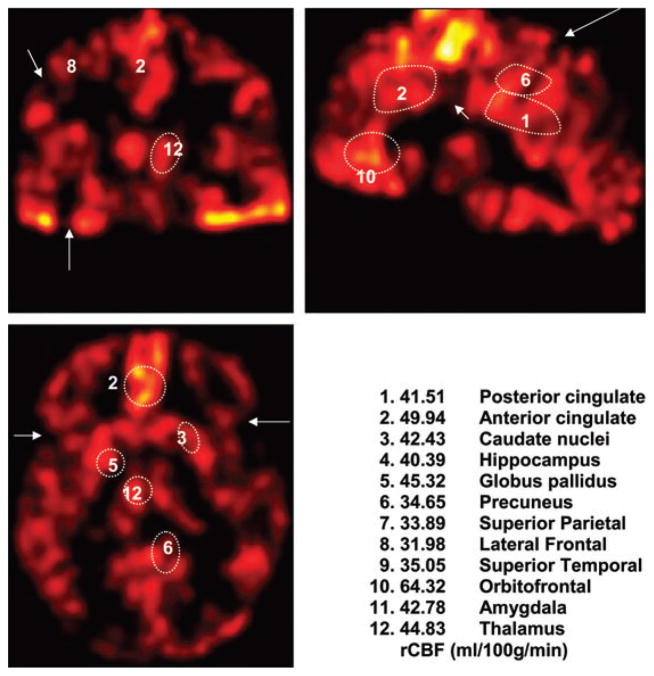
Cognitively normals (CN) with robust cortex. Orbitofrontal bright spot (10) has rCBF = 59.56. Posterior cingulate (1) has rCBF = 61.49. Similar areas of increase signal can be observed on the gray scale images (not included). The intensity scale is included, which also correlates with rCBF values.
Fig 3.
SPM2 analysis showing regions of increased perfusion in the posterior cingulate (top two) and again in the posterior cingulate but also in the thalamus and the left orbitofrontal cortex as well as lateral temporal cortex (bottom 2). These correspond to bright spots on ASL images.
Table 4.
Areas of Decreased or Increased Perfusion from Quantified rCBF
| Anatomical Site | Decreased Perfusion | Increased Perfusion |
|---|---|---|
| Posterior cingulate | 9/38 = 24% | 13/38 = 34% |
| Anterior cingulate | 7/38 = 18% | 12/38 = 32% |
| Caudate nucleus | 6/38 = 16% | 15/38 = 39% |
| Precuneus | 20/38 = 53% | 6/38 = 15% |
| Superior parietal | 9/38 = 24% | 12/38 = 32% |
| Lateral prefrontal | 13/38 = 34% | 11/38 = 29% |
| Superior temporal | 18/38 = 47% | 6/38 = 16% |
| Orbitofrontal | 14/38 = 37% | 10/38 = 26% |
| Amygdala | 8/38 = 21% | 12/38 = 32% |
| Thalamus | 10/38 = 26% | 11/38 = 29% |
Decreased perfusion is one standard deviation (SD) below or greater than the averaged rCBF for that specific region. Increased perfusion is one SD or greater. This corresponded to the bright spots. The number of anatomical bright spots for each region of interest was summated and then divided by the total population of 38 CN elders.
Results
The rCBF is usually reported related to a specific region of the brain. When the blood flows in all of these regions are averaged together this yields a total or gCBF. The gCBF in these 38 CN averaged from the entire automated sampled R.O.I. was 43.35 mL/100 g/min ± 7.12 compared to the reported gCBF in young healthy brains of 55–65 mL/100 g/min. (Table 2) There is variation of the rCBF from region to region among various structures including cortex and basal ganglia. The averaged rCBF in our 38 CN ranges from a low of 35.43 mL/100 g/min (globus pallidus), 36.62 mL/100 g/min (amygdala), 37.05 mL/100 g/min (caudate), and 37.49 mL/100 g/min (hippocampus), to a high of 51.82 mL/100 g/min (orbitofrontal), 53.42 mL/100 g/min (posterior cingulate), and 56.69 mL/100g/min (precuneus) (Table 2).
The averaged rCBF by targeted regions are presented in Table 2. There was a wide range of rCBF values among the individual patients manifest mainly by decreased values compared to the averaged rCBF of all 38 individuals. The cluster-level statistics for regions of hypoperfusion by SPM2 are given in Table 3 (Fig 3). Less often areas of increased rCBF occurred. Several bright spots (white or yellow) occurred in characteristic sites on both the gray scale as well as the hot color mode ASL images. The regions of quantified hypoperfusion and hyperperfusion are presented in Table 4 (Figs 2, 4, 5). The regions with the highest number of hypoperfusion (≥10 mL/100 g/min decrease below averaged value) were located in the precuneus, superior temporal lobe, and orbitofrontal based on decreased rCBF. The regions with the highest number of hyperperfusion (≥10 mL/100 g/min increase above averaged value) were located in the posterior and anterior cingulate, caudate, superior parietal, and amygdala.
Fig 4.
Cognitively normal with minor cortical defects.
Fig 5.
CN with significant hypoperfusion and loss of posterior cingulate bright spot, thus resembling the ASL image of AD. There are multiple small and large focal gaps in the cortex, and the bilateral thalami and left globus pallidus (asymmetrical) are smaller in size but not hypoperfused based on rCBF alone. So hypoperfusion may be due to overall decrease in size of structure perfused as well.
Discussion
ASL, which utilizes endogenous magnetically labeled water molecules of flowing blood, is noninvasive energizing blood at the labeling plane at the cervicomedullary junction (4–5 cm), or circle of Willis (0 cm). By fusing the ASL images to a standardized brain (colin27), stereotactic localization of specific anatomic sites is reproducible, and repeat or longitudinal exams are possible, aiding in the understanding of the pathology of AD. An ASL image is produced in which signal intensity resulting from magnetization transfer of the labeled spin in arteries to brain tissue and requiring a subtraction technique of a control tag image is utilized to calculate the cerebral blood flow. ASL has been utilized to evaluate AD. However, very little has been written about the appearance of ASL images in CN, or about the regional variations in blood flow in the brain.
Regions of decreased and increased perfusion were encountered, which represent the normal profile of cerebral blood flow variations. It is necessary to understand that these variations normally occur in order to assess changes in perfusion encountered in neurodegenerative disorders, most notably AD’s.
Imaging Findings
ASL of normal young brain shows a robust uniform thick cortical mantle without focal gaps as shown by Jahng and Weiner.6 The ASL image in CN also shows robust and symmetrical and uniform perfusion of the cortical mantle and in the lentiform nuclei (globus and putamen), caudate, and thalamus (Fig 2). The signal intensity of the ASL perfusion map of the cortical mantle matches that of the basal ganglia in normals.
The cortex is of uniform signal intensity but does show brighter areas in characteristic sites–inferior/medial frontal or sometimes referred to as orbitofrontal, anterior, and posterior cingulate regions which also show the highest rCBF (>50 mL/100 g/min). The significance of the presence or absence of bright spots (increased perfusion) as well as decreased perfusion, and the temporal sequence that they occur may aid in the diagnosis of neurodegenerative disorders. Comparison of the rCBF values in key anatomical areas should correlate well with the signal intensity on the ASL since it is the pixel signal intensity that is used to generate the rCBF using the modified Buxton equation. These regional variations in rCBF may be seen on the gray scale images, but the differences are better highlighted by color modes.
The bright spots or regions of increased perfusion are most notably related to the anterior cerebral artery vascular territory including the orbitofrontal, and the anterior and posterior cingulate bright spots, supplied by the proximal anterior cerebral artery, and the anterior and posterior pericallosal artery. The lateral temporal, frontal, and parietal regions are supplied by the middle cerebral arteries, whereas the medial temporal and hippocampal structures are supplied by the posterior cerebral artery. The basal ganglia on the other hand have multiple vascular supplies from the middle and posterior cerebral arteries.
Elderly cognitively normals did not always show a normal pattern of perfusion exhibiting minor foci of cortex thinning, and even focal gaps, mostly small (Fig 4) but occasionally larger with at times total lobar signal absence. But some showed significant hypoperfusion with marked thinning of cortex and decrease in size as well as perfusion of the lentiform, caudate, and thalamic nuclei to the point that they resembled the ASL MR features seen in AD, and yet they were cognitively normal (Fig 5). Hypoperfusion has been reported in neurodegenerative disorders most notably AD7–20 (Fig 6). Hyperperfusion may not be a normal finding, and may represent a compensatory mechanism.
Fig 6.
AD patient with marked thinning of bilateral frontal and especially posterior parietal cortex, typical for AD as shown on PET scans. There are large gaps of hypoperfusion in the cortex. Absence of bright spot in posterior cingulate and of orbitofrontal cortex compared with Figure 2.
The overall global CBF in our population ranged from 45 to 55 mL/100 g/min, which is much lower than the reported value of 55–65 mL/100 g/min. This in part may reflect changes of decreased perfusion, which occurs with aging. Also, loss of tissue volume or atrophy will cause apparent decrease in perfusion, because there is no longer viable tissue requiring the blood flow. This was corrected for by factoring in volumetric tissue loss requiring stripping or segmentation of gray and white matter as well as CSF. Because of the short half-life of the energized proton, there is no white matter penetration or energized protons in the white matter because of the early signal decay.
However, it may also be related to the fact that we chose the plane of spin or tag labeling at 10 cm, which may result in underestimation of perfusion due to the longer transit time from labeling plane to imaging plane in the brain. Alternatively, it may be related to symmetrical carotid stenosis or symmetrical tortuosity of the cervical portions of the carotids.
The main limitation of CASL imaging in assessing cerebral functions of memory and cognition is that it images perfusion dynamics, mainly in the hypoperfusion category. Presence of hypoperfusion does not always translate into functional impairment, because of the inherent brain reserves that vary from patient to patient that prevent full-blown clinical manifestation of dementia. However, the more hypoperfused the brain is, the more likely there are significant cerebral functional deficits. The other limitations are based on the assumptions that the blood brain barrier is intact, and that flow is orthogonal (probably correct in basal ganglia). In the former, arteriosclerosis alters the BBB causing it to be leaky and to become more permeable particularly the end or perforating arterioles, which may lead to apparent hyperperfusion, as there is greater than normal exchange of labeled tracer at the capillary level. But this effect is much less on the quantified rCBF because the tag labeling decays so fast. In the latter, tortuous vessels are no longer orthogonal and increase in the length of the artery translates into increased transit time from the labeling plane up to the imaging plane in the brain. Also, structures at the base of the skull by air containing sinuses such as the inferior temporal and frontal lobes may suffer from susceptibility, which may cause apparent hypoperfusion.21–29
Although we did not have ASL studies on young, healthy individuals,6 the ASL findings of a normal brain could be inferred from this population of CN, and from ASL images in healthy young individuals reported in the literature. Part of this decrease may be volume atrophy age-related with loss of tissue and perfusion, or represent a normal phenomenon of aging in which global brain perfusion decreases with age. Nevertheless, visually there are clearly gaps in the perfusion of the cortex and even deep basal nuclei in the control normal ASL images, which are not accounted for by atrophy or age-related issues.
Both the visual as well as quantified analysis in ASL highlights the posterior cingulate and precuneus not only in the fact that they have the highest averaged rCBF, but also that decreased perfusion is notable in these structures with the precuneus and posterior cingulated showing the largest degree of change (Table 4). The role of the posterior cingulate and development of hypoperfusion in AD has been documented by others.15,16,18,19,23 The role of the precuneus is now just being understood.30 Both structures play a key role in functional circuitry.31,32
One observation that needs verification with a larger sample is the symmetrical appearance of the basal ganglia not only in size, but in the signal intensity, most notably the thalamus, caudate, and lentiform nuclei (globus pallidus and putamen). It is also of note in Table 3, that no regions of hypoperfusion were identified by SPM2 in the thalamus or in the amygala.
This project chose a limited number of what we felt to be key R.O.I.s in the assessment of neurodegenerative disease. Nevertheless, this population of R.O.I.s illustrates that rCBF varies from region to region in CN elders, and probably in healthy younger brains as well. Despite the limited number of slices because of the EPI technique, more images can be obtained in less time, at a far less cost than PET and perhaps even SPECT if a limited MR exam with ASL is performed. It is more facile to generate a rCBF map based on multiple anatomical R.O.I’s with ASL than with older non-MR methods of assessing cerebral perfusion for which mainly time constraints limits the numbers of R.O.I. that can be evaluated.
Despite these limitations, ASL when properly implemented with minor hardware and software alterations (often requiring onsite engineering and computer programming expertise) can prove to be a valuable tool in the assessment of rCBF. However, because of its superior S/N ratio ASL may eventually fall into the realm of the 3T machines, but pulsed arterial spin labeling (PASL) techniques will be required because the CASL techniques will exceed the SARS limit at 3T compared to the 1.5T machines. It may be possible to devise imaging criteria based on ASL visually that may diagnose neurodegenerative disorders. Perhaps the disappearance of characteristically seen bright spots, or the appearance of atypical bright spots or areas of hypoperfusion may suggest a recognizable pattern. Because the ASL image provides a highly accurate map of rCBF, it may be visually used to assess and monitor perfusion changes as full-blown dementia develops. As we are beginning to develop reader criteria for visual analysis of ASL, reproducibility and inter- and intrarater reliability will be generated eventually when we attempt to define reader criteria for neurodegenerative disorders.
Acknowledgments
The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01-HC-15103 from the National Heart, Lung, and Blood Institute, and grants AG15928 and AG20095 from the National Institute of Aging. ADRC (AG05133). We also thank Dr. Seong-Gi Kim for his assistance.
Footnotes
Conflict of Interest: None.
References
- 1.Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spina labeling: part 2. Applications. J Int Neuropsychol Soc. 2007;13:1–13. doi: 10.1017/S1355617707070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the cardiovascular health study. Stroke. 1998;29:338–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 3.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 4.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly patients. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton RB, Frank LR, Wong EC, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnet Reson Med. 1998;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 6.Jahng GH, Weiner MW, Du A, Schuff N. 4 Tesla Arterial Spin Labeling Perfusion MRI of the Brain. UCSF (U. of California San Francisco) website. Research News. Available at http://www.old.research.ucsf.edu/research/4tesla.shtml.
- 7.De La Torre JC. Alzheimer disease as a vascular disorder–nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 8.Kalaria RN. Vascular factors in Alzheimer’s disease. Int Psychogeriatr. 2003;15:47–52. doi: 10.1017/S1041610203008950. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 10.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hanon O, Forette F. Treatment of hypertension and prevention of dementia. Alzheimer’s Dementia. 2005;1:30–37. doi: 10.1016/j.jalz.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness. The Framingham Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 13.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 14.Leenders K, Perani D, Lammertsma A, Heather J, Buckingham P, Healey M, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks J, Spinks T, Rhodes C, Frackowiak RSJ. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113:24–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Varma AA, Adams W, Lloyd JJ, Carson KJ, Snowden JS, Testa HJ, Jackson A, Neary D. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change in SPECT in young onset patients with Alzheimer’s disease, frontotemporal dementia, and vascular dementia. Acta Neurol Scand. 2002;195:261–269. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkos LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnet Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- 18.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 19.Kogure D, Matsuda H, Ohnishi T, Kumihiro T, Uno M, Takasaki M. Longitudinal evaluation of early Alzheimer disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–1162. [PubMed] [Google Scholar]
- 20.Bradley KM, O’Sullivan VT, Soper NDW, Nagy Z, King EM-F, Smith AD, Shepstone BJ. Cerebral perfusion SPECT correlated with Braak pathological stage in Alzheimer’s disease. Brain. 2002;125:1772–1781. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- 21.Drzezga A, Riemenschneider M, Strassner B, Gimmer T, Peller M, Knoll A, Wagenpfeil S, Minoshima S, Schwaiger M, Kurz A. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64:102–107. doi: 10.1212/01.WNL.0000148478.39691.D3. [DOI] [PubMed] [Google Scholar]
- 22.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Wahlun L-O, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurol. 2002;2(9):1–6. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laasko MP, Hallikainen V, Vanhanen M, Nissinen A, Helkala A-L, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 25.Barbier EL, Lamalle L, Decorps M. Methodology of brain perfusion imaging. An invited review. J Magnet Reson Imag. 2001;13:496–520. doi: 10.1002/jmri.1073. [DOI] [PubMed] [Google Scholar]
- 26.Steger TR, Jackson EF. Experience in implementing continuous arterial spin labeling on a commercial MR scanner. J Appl Clin Med Physics. 2005;6:94–100. doi: 10.1120/jacmp.v6i1.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steger TR, White RA, Jackson EF. Input parameter sensitivity analysis and comparison of quantification models for continuous arterial spin labeling. Magnet Reson Med. 2005;53:895–903. doi: 10.1002/mrm.20440. [DOI] [PubMed] [Google Scholar]
- 28.Petersen ET, Zimine I, Ho Y-CL, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labeling techniques. Brit J Radiol. 2006;79:688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 30.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 31.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry–An update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 32.Morgane PJ, Gatler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;84:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]