Abstract
Poor folate status is associated with cognitive decline and dementia in older adults. Although impaired brain methylation activity and homocysteine toxicity are widely thought to account for this association, how folate deficiency impairs cognition is uncertain. To better define the role of folate deficiency in cognitive dysfunction, we fed rats folate-deficient diets (0 mg FA/kg diet) with or without supplemental L-methionine for 10 wk, followed by cognitive testing and tissue collection for hematological and biochemical analysis. Folate deficiency with normal methionine impaired spatial memory and learning; however, this impairment was prevented when the folate-deficient diet was supplemented with methionine. Under conditions of folate deficiency, brain membrane content of the methylated phospholipid phosphatidylcholine was significantly depleted, which was reversed with supplemental methionine. In contrast, neither elevated plasma homocysteine nor brain S-adenosylmethionine and S-adenosylhomocysteine concentrations predicted cognitive impairment and its prevention by methionine. The correspondence of cognitive outcomes to changes in brain membrane phosphatidylcholine content suggests that altered phosphatidylcholine and possibly choline metabolism might contribute to the manifestation of folate deficiency-related cognitive dysfunction.
Introduction
Poor folate status is increasingly recognized as an important and potentially modifiable risk factor for age-related cognitive decline and impairment in the elderly (1). Low circulating folate is significantly associated with mild cognitive impairment and depression in non-demented populations (2,3), it predicts increased risk of cognitive decline in longitudinal studies (4), and it is more frequently found in demented and impaired subjects than in unimpaired controls (5–8).
Poor folate status affects an estimated 5–10% of middle-aged and elderly adults in populations exposed to food folate fortification and its prevalence may be higher than 30% in unfortified populations (9). Folate status remains an important predictor of cognitive impairment and depression among elderly adults, even in populations exposed to food folate fortification (10,11). Its importance for cognition in the elderly is highlighted by a recent placebo-controlled, randomized clinical trial, which found that 3 y of folic acid (FA)3 supplementation delayed memory loss in elderly adults who had mildly elevated plasma total homocysteine (tHcy) and normal cognition at baseline (12). However, the relation of folate to cognition may depend on other nutritional factors, including vitamin B-12 (13,14).
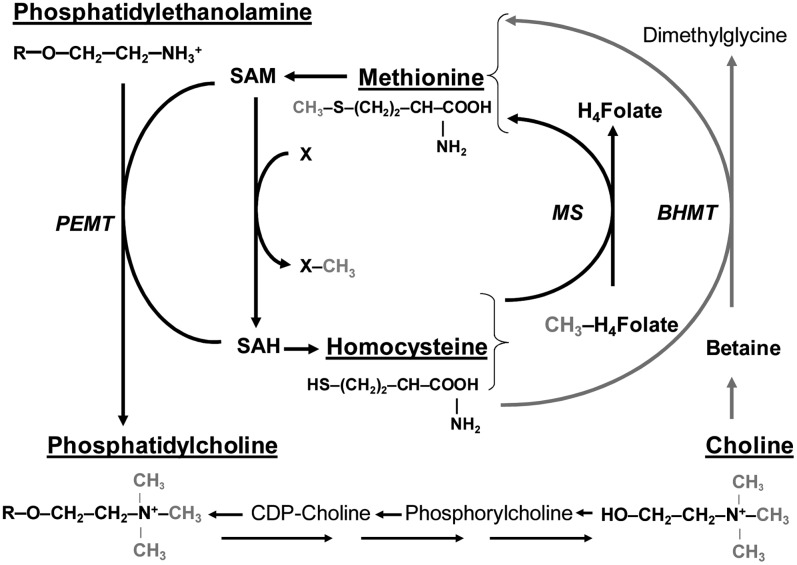
The brain's high requirement for methylation activity is thought to underpin the apparent sensitivity of cognitive function to folate status (15,16). Folate serves as a carrier of 1-carbon groups for the methylation cycle (Fig. 1). In this cycle, the methyl group of methionine is activated by adenosine triphosphate to form S-adenosylmethionine (commonly abbreviated as SAM or AdoMet). SAM is the universal methyl donor in a multitude of methyl-transfer reactions including many that are of vital importance to central nervous function, such as the synthesis of the key cell membrane component phosphatidylcholine from phosphatidylethanolamine. Through the transfer of its methyl-group, SAM is converted to S-adenosylhomocysteine (SAH), which is hydrolyzed to homocysteine. Homocysteine can regenerate methionine for an additional methylation cycle by acquiring a new methyl group from 5-methyltetrahydrofolic acid in a reaction that is catalyzed in all tissues by methionine synthase (17). Alternatively, in liver and kidney, choline can provide labile methyl groups for the regeneration of methionine from homocysteine by betaine-homocysteine methyltransferase (BHMT). This alternative pathway is an important means of maintaining methylation capacity under conditions of folate, vitamin B-12, and methionine deficiency (18). Excess intracellular homocysteine can also be removed from the methylation pathway by conversion to cystathionine in the transsulfuration pathway or through export into circulation (17).
FIGURE 1 .
Integration of folate and choline metabolism. Figure illustrates the close relation of folate methionine and choline in the methylation cycle. The methyl (-CH3) groups of methyl-folate and choline are used to regenerate methionine from homocysteine by methionine synthase (MS) in all tissues and BHMT in liver. SAM is then synthesized from methionine, providing the methyl group for numerous methylation reactions (including the SAM-dependent synthesis of phosphatidylcholine, by PEMT) that also produce SAH and homocysteine. Homocysteine may then accept a new methyl group from folate or choline to begin a new cycle. R, The choline or ethanolamine alcohols of phosphatidylcholine and phosphatidylethanolamine are linked via a phosphodiester bond to a glycerol esterified to 2 fatty acids. X, Methyl accepting substrates; X–CH3, methylated products; CH3-H4-folate, 5′methyl-tetrahydrofolate; H4-folate, tetrahydrofolate.
From these pathways, folate deficiency can generally be predicted to limit methionine synthesis and thereby SAM availability and to cause SAH to accumulate in tissues and homocysteine to accumulate in blood (hyperhomocysteinemia). At high concentrations, homocysteine might be directly toxic to cerebral blood vessels, but it is unclear whether mild elevations are similarly harmful. At the same time, rate-limiting concentrations of SAM together with feedback inhibition of some methyltransferase enzymes by elevated SAH concentrations would be expected to deplete the methylated products of such reactions. Nevertheless, these predictions do not always hold true, as the specific manifestations of folate deficiency differ by tissue (i.e. liver vs. brain), severity, and duration of the deficiency (19,20) and they can be modified by other metabolic factors, such as the availability of methionine and choline (21).
This complexity confounds the simple interpretation of epidemiological associations between cognitive impairments with folate, homocysteine, and other closely related metabolites and has impeded progress in clarifying the mechanisms that actually account for the epidemiological associations, whatever their theoretical plausibility (4,22,23).
Recent studies that have combined folate deficiency with other metabolic perturbations to expose genetic and pharmacological models of neurodegenerative disease to hyperhomocysteinemia have found that such treatments can exacerbate neurodegenerative or cognitive outcomes in models with preexisting neurological insults (24–26). However, such studies do not tell us whether the exacerbation of brain dysfunction is due to folate deficiency, hyperhomocysteinemia, or other factors (4,27). Earlier studies that specifically examined the effect of folate deficiency in rodents showed inconsistent effects on methylated derivatives of biogenic amine and cholinergic neurotransmitters (28–31). Other findings in folate-deficient rodents include ultrastructural abnormalities in rat brain capillaries (32) and slowed neurogenesis in mouse brain (33). Although folate-dependent changes in all these outcomes could ultimately lead to cognitive dysfunction, none of these studies report on the association of the neurochemical or structural impairments with cognitive outcomes.
In this study, we examined cognitive function in rats fed folate-deficient diets with and without supplemental methionine and determined the extent to which cognition in these rats corresponded to diet-induced changes in indicators of methylation activity: circulating homocysteine, liver and brain SAM and SAH, and brain membrane phosphatidylcholine and phosphatidylethanolamine.
Methods
Rats and diets.
All animal procedures were approved by the Tufts-New England Medical Center and Jean Mayer USDA Human Nutrition Research Center on Aging Institutional Animal Care and Use Committee. Young male Sprague Dawley rats were systematically assigned to groups of similar mean body weight (∼85 g), housed individually, and fed their assigned diet for 10 wk. Diets formulated with AIN 93M vitamin-free, ethanol-precipitated, casein-based basal mix (TD 03595, Harlan Teklad) and an appropriate vitamin mix to distinguish between outcomes associated with different perturbations of homocysteine metabolism. The AIN 93M diet, which we and others have used previously in studies of homocysteine metabolism in young mice and rats, contains less methionine, folate, and vitamin B-12 than the AIN 93G diet and in our experience provides useful control conditions for experimental manipulation of dietary folate and methionine (34). The control diet (C) contained both normal folate (2 mg FA/kg diet, vitamin mix TD 94047) and the normal methionine concentration of ∼0.35% in dietary protein. One folate-deficient diet (FD) contained 0 mg FA/kg diet (vitamin mix TD 95052) and normal methionine concentration. A second folate-deficient diet (FDM) was supplemented with 10 g l-methionine/kg diet (1% wt:wt). The diets also contained 1% sulfathiazole (Sigma), a nonabsorbed sulfa drug that inhibits folate formation by gut bacteria, to ensure that the animal's only source of available folate was from diet. Rats were provided with free access to water and were group pair-fed to ensure comparable food intake by all rats (35). In this procedure, the group of rats that initially consumed the least food determines the amount of food provided to all other groups. During this experiment, additional groups of rats were provided with 2% supplemental l-methionine. The 2% l-methionine diets suppressed food intake in association with slower growth than the other groups and are therefore not described here. Nevertheless, the intake of rats fed 2% l-methionine determined the amount of food provided to all other groups, which was 10 g/d food per rat. Data reported here are derived from a total of 30–31 rats per group that were raised in 5 repeated cohorts (no. of rats allocated to each specific outcome measure are given in Tables 1–3).
TABLE 1.
The effect of diet on Morris water maze and psychomotor performance1
| Morris water maze performance (d 4 reversal task) | Diet |
|||
|---|---|---|---|---|
| C | FD | FDM | P | |
| n | 21 | 20 | 20 | |
| Mean escape latency, s | 16.6 (7.3)a | 24.2 (11.6)b | 14.8 (7.8)a | 0.003 |
| Probe trial escape latency | 5.9 (4.4) | 9.1 (8.2) | 5.6 (4.9) | 0.127 |
| Probe trial swim speed, m/s | 0.24 (0.03) | 0.24 (0.03) | 0.24 (0.03) | 0.839 |
| Probe trial swim distance, m | 14.5 (1.8) | 14.3 (1.7) | 14.6 (1.7) | 0.840 |
| Visible platform trial escape latency, s | 10.1 (7.3) | 10.3 (7.9) | 11.9 (10.7) | 0.761 |
| Psychomotor performance | ||||
| Accelerating rotarod latency, s | 40.8 (29.9) | 32.1 (20.2) | 46.4 (37.8) | 0.312 |
| Wire suspension latency, s | 20.6 (10.9) | 28.1 (16.9) | 25.6 (18.1) | 0.308 |
Values are means ± SD. Means in a row with superscripts without a common letter differ, P < 0.05.
TABLE 2.
The effect of diet on plasma folate, vitamin B12, vitamin B6 and tHcy1
| Diet |
||||
|---|---|---|---|---|
| C | FD | FDM | P | |
| n | 10 | 11 | 10 | |
| Folate, nmol/L | 148 (24)a | 7 (2)b | 6 (2)b | <0.0001 |
| Vitamin B-12, pmol/L | 414 (63) | 324 (98) | 410 (91) | 0.050 |
| Vitamin B-6, nmol/L | 675 (136) | 619 (212) | 585 (163) | 0.581 |
| tHcy, μmol/L | 4.2 (1.8)a | 31.3 (8.3)b | 31.2 (6.9)b | <0.0001 |
Values are means ± SD. Means in a row with superscripts without a common letter differ, P < 0.05.
TABLE 3.
The effect of diet on brain and liver SAM, SAH and membrane lipids1
| Diet |
||||
|---|---|---|---|---|
| C | FD | FDM | P | |
| Tissue SAM and SAH | n = 10 | N = 11 | n = 10 | |
| Liver SAM, nmol/g | 30.7 (4.6)a | 12.6 (3.3)b | 11.4 (3)b | <0.0001 |
| Liver SAH, nmol/g | 11.9 (2.4)a | 20.8 (5.3)b | 24.4 (4.3)b | <0.0001 |
| Liver SAM:SAH | 2.7 (0.7)a | 0.7 (0.3)b | 0.5 (0.1)b | <0.0001 |
| Brain SAM, nmol/g | 13.3 (0.6)a | 12.1 (0.7)b | 11.8 (0.8)b | <0.0001 |
| Brain SAH, nmol/g | 1.7 (0.3) | 2 (0.5) | 2.2 (0.5) | 0.13 |
| Brain SAM:SAH | 7.8 (1.3)a | 6.5 (1.6)ab | 5.8 (1.6)b | 0.02 |
| Brain membrane lipids, nmol Pi/mg protein | n = 8 | n = 9 | n = 8 | |
| Phosphatidylcholine | 187.8 (43.6)a | 93.1 (17.0)b | 191.8 (51.1)a | <0.0001 |
| Phosphatidylethanolamine | 55.1 (10.6)a | 163.8 (88.3)b | 165.9 (97.6)b | <0.05 |
| PC:PE ratio | 3.5 (0.8)a | 0.7 (0.3)b | 1.5 (0.7)c | <0.0001 |
Values are means ± SD. Means in a row with superscripts without a common letter differ, P < 0.05.
Cognitive and psychomotor testing.
Spatial learning and memory were evaluated by the Morris water maze and psychomotor function was evaluated by the accelerating Rotarod and wire suspension tests (36) during the 10th and last week of the experiment. Using these tests, we have shown that in mice, combined folate, vitamin B-12, and vitamin B-6 deficiency causes specific cognitive deficits on the Morris water maze but not in psychomotor performance (37).
Morris water maze.
The Morris water maze is a well-validated and highly sensitive test of rodent cognitive function and is particularly sensitive to hippocampal dysfunction. The maze requires a rat to use spatial learning to find a hidden platform submerged below the surface of the water in a circular pool filled with water. The rat must use distal cues placed outside of the maze to effectively locate and remember the location of the escape platform from previous trials. Accurate navigation is rewarded with escape from the water onto the hidden platform. In this study, we used a 4-d testing protocol as previously described (36). Briefly, rats underwent 3 d of training in the maze of 6 trials each day, followed by a fourth and final day of testing in which the position of the escape platform was changed, thereby requiring the rat to retain the learned escape strategy and to quickly learn the new escape position. Trial 6 on d 2–4 was a probe trial in which the platform was removed and the rat was allowed to swim for 60 s to assess spatial strategies before the trial was stopped. On d 4, following the probe trial, rats were tested on their ability to escape to a visible platform raised above the water's surface to ensure that differences in performance were not the result of differences in visual acuity. Performances were videotaped and analyzed with image tracking software (HVS Image). Performance variables including escape latency for the training trials, latency to the escape position for the probe trials, swim speed, and swim path length were automatically generated by the software.
Wire suspension.
The wire suspension assay measured muscle strength and the prehensile reflex, an animal's ability to grasp a taut horizontal wire with its forepaws and to remain suspended. Rats were held gently by the tail and the forepaws were placed on a suspended wire 2 mm in diameter and 62 cm above a cushioned surface. The latency to let go was measured using a stopwatch, with shorter latency to drop indicating reduced strength and/or reflex ability. If a rat did not let go of the wire within 60 s, it was removed from the wire and a latency of 60 s was assigned to that measurement. Each animal was used only once in the wire suspension assay.
Rotarod.
The Rotarod assay measures motor coordination and balance. Rats were placed on a rotating 7-cm-diameter cylinder (Ugo Basile). Each rat was placed on the rod at 2 rpm until it maintained its grip and orientation without assistance. The rod then accelerated steadily for 5 min (by 2 rpm every 30 s) until it reached 20 rpm. Latency to fall (maximum = 300 s) was recorded, with shorter latencies indicating impaired performance.
Blood and tissue assays.
Rats were food-deprived overnight, decapitated, and trunk blood and tissues were rapidly harvested. EDTA plasma was separated within an hour of collection and frozen at −80°C for further analysis. Folate and vitamin B-12 were measured using the Quantaphase II radioassay kit (Bio-Rad Laboratories). Pyridoxal 5′-phosphate (vitamin B-6) was determined by the tyrosine decarboxylase apoenzyme method (38). tHcy was determined by HPLC (39).
Brains were rapidly harvested and snap-frozen in liquid nitrogen. Frozen whole brain tissue (including cerebrum, cerebellum, and medulla) was ground to a fine powder in liquid nitrogen. Tissue SAM and SAH levels were determined by liquid chromatography MS (40). Brain membranes were isolated by ultracentrifugation (41), followed by separation and quantification of membrane phospholipids (42). Briefly, phospholipids were separated by 2-dimensional TLC using a serum lipid mixture as a standard to identify individual phospholipids and which are quantified by their inorganic phosphorus (Pi) content, expressed as nmol Pi/mg protein (43).
Data analysis.
Behavioral and biochemical data were analyzed using 1-way ANOVA with diet as a between-groups measure. Morris water maze data were analyzed using 3-way ANOVA (day × trial × diet) with day of testing and trial as repeated measures, diet as a between-groups measure, and with post-hoc Tukey's honestly significant difference tests as appropriate. Descriptive statistics are expressed as means ± SD and α was set at 0.05 throughout.
Results
All rats gained weight after receiving the diets and had no morbidity. At the end of the study, the body weight of FD rats (225.5 ± 8.7 g) was ∼3% greater than of FDM rats (217.8 ± 12.3 g) (P < 0.05), but neither differed from the C group (220.4 ± 8.8 g). Brain wet weight was not affected by diet and was 1.71 ± 0.04 g for all rats combined.
Cognitive and psychomotor testing.
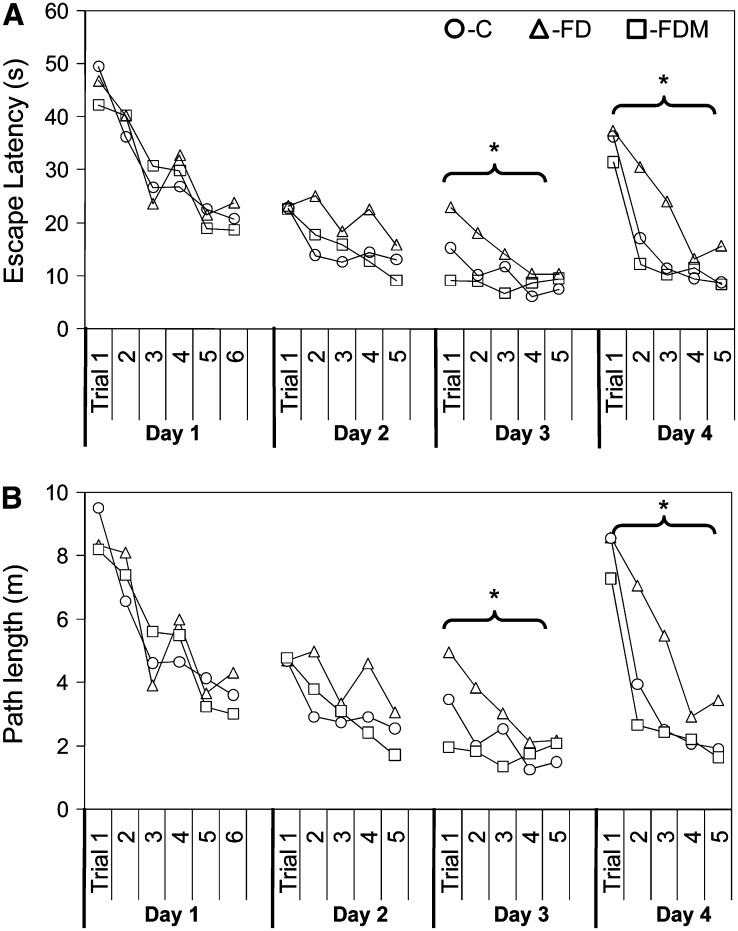
Performance on the wire suspension task and the Rotarod task (did not vary as a function of diet (Table 1). However, in the Morris water maze, the performance of the folate-deficient rats was significantly impaired (Fig. 2). When escape latencies for all trials were compared, all rats acquired the task during the first 3 d of training (P < 0.001). However, when comparing trials for each day, FD rats had a slower learning curve on d 2 and 3 of training and were slower to escape to the hidden platform compared with the other 2 groups by d 3 of training (P < 0.05). Furthermore, FD rats were impaired in their ability to retain the escape strategy and relearn the new escape position when the escape platform was repositioned on the d 4 reversal trial (P < 0.005). Methionine supplementation prevented the folate deficiency-induced impairment so that FDM rats performed at control levels.
FIGURE 2 .
Morris water maze learning curves for daily escape latencies (A) and swim path lengths (B) for each of 4 daily trials in rats fed C, FD, or FDM diets. Values are means, n = 20 except group C, n = 21. *FD differs from C and FDM, P < 0.005.
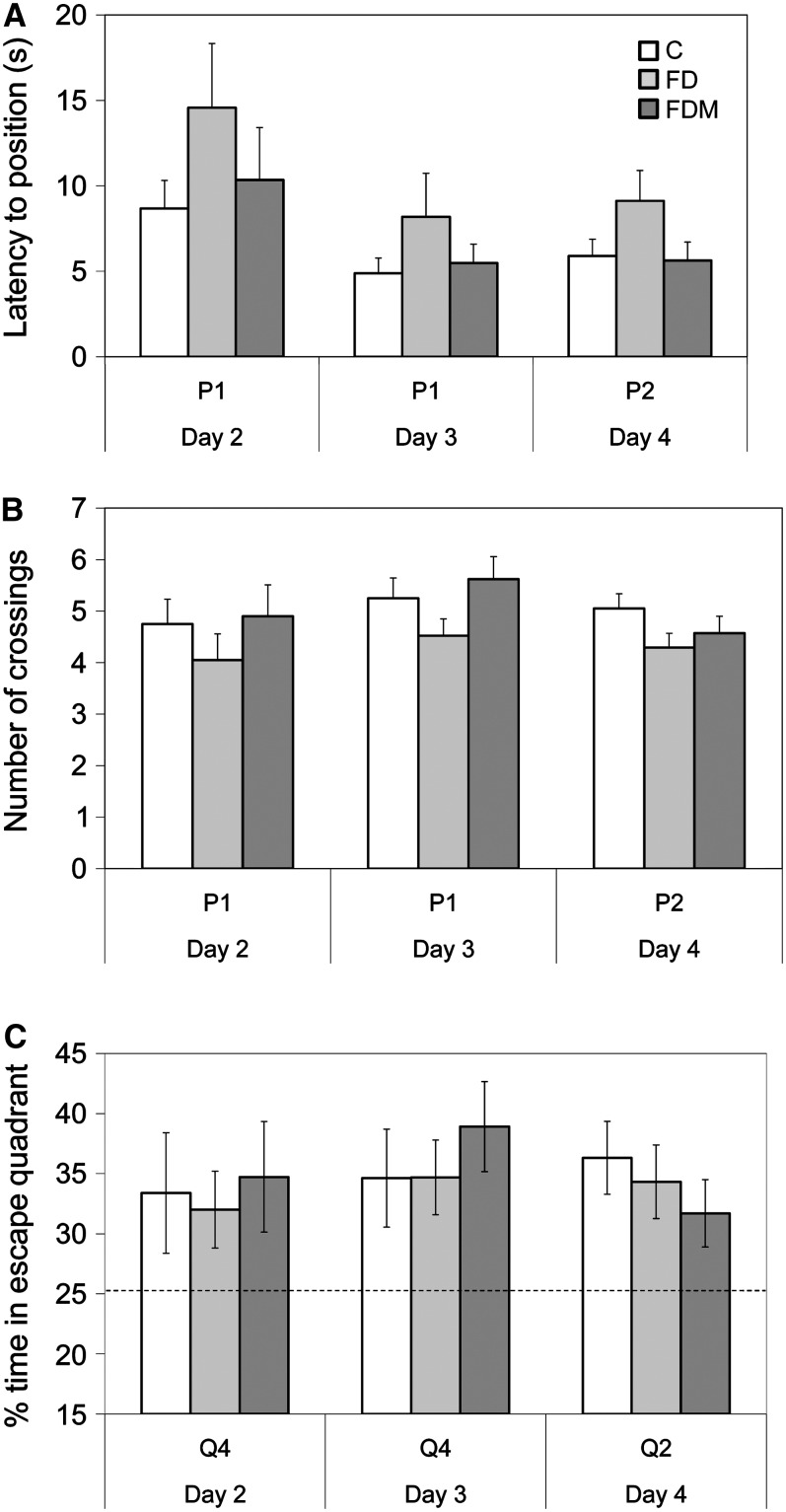
The diets did not significantly affect probe trial performance. Nevertheless, folate deficiency nearly doubled (P, d 4 = 0.127) the mean time required by the FD rats to reach the escape position, whereas FDM rats performed as well as controls (Table 1; Fig. 3A). During the training (d 2 and 3) and reversal probe trials (d 4), FD rats crossed the escape platform position slightly less often (P, d 4 = 0.199) than the other groups (Fig. 3B); however, all groups spent significantly more time swimming in the correct escape quadrant than would be expected by chance (i.e. 25% of trial time).
FIGURE 3 .
Spatial performance on Morris water maze probe trials on d 2–4 with respect to latency to the escape position (A), number of position crossings (B), and percent of total trial time spent searching for the platform in the escape quadrant (C). Values in A and B are means ± SEM and in C, means ± 95% CI, n = 20 except group C, n = 21. P1 and Q4 designate the escape platform position and quadrant on training d 2 and 3. P2 and Q2 designate the escape platform position and quadrant on d 4.
The effects of diet on water maze performance could not be attributed to physical impairments given that diet: 1) did not affect swim speed or path length when the escape platform was removed from the pool on the probe trial; 2) did not affect escape latencies when the escape platform was raised above the surface of the pool in full view of the rats; and 3) did not affect strength and psychomotor performance on the accelerating Rotarod and wire suspension tasks (Table 1).
Blood chemistry.
Food-deprived plasma folate concentrations were <5% of control values for FD rats. Consistent with this finding, plasma tHcy in FD rats was more than 6-fold higher than in control. The FDM diet did not alter the severity of folate depletion or hyperhomocysteinemia induced by the FD diet (Table 2).
Tissue SAM and SAH.
Liver metabolism was significantly affected by the experimental diets. Folate deficiency caused a significant ∼60% decrease in food-deprived SAM concentrations and almost doubled liver SAH concentrations irrespective of dietary methionine. Conversely, dietary methionine did not change food-deprived liver SAM or SAH concentrations, regardless of folate status (Table 3).
In contrast to liver, brain SAM and SAH concentrations were resistant to the peripheral folate-induced metabolic perturbations. A minor but significant reduction of brain SAM concentrations was induced by folate deficiency, but the absolute mean concentrations were no greater than 1.2–1.5 μmol (8–10%) lower in FD and FDM rats than in C rats. Brain SAH concentrations were not affected by any diet and the SAM:SAH ratio tended to be lower in the FDM rats than in controls (P = 0.02; Table 3).
We also measured liver and brain SAM and SAH in tissues that were harvested from rats that were not food deprived before they were killed. Methionine supplementation of the FDM diet significantly elevated both fed SAM and SAH concentrations compared to both C and FD diets with normal methionine. However, brain SAM and SAH concentrations were almost the same in the food-deprived and fed states regardless of diet. Moreover, in the fed state, the SAM:SAH ratios in both liver and brain did not differ by diet group (data not shown).
Brain membrane phospholipids.
While brain membrane phosphatidylcholine concentration in rats fed the FD diet (93.1 ± 17.0 nmol Pi/mg protein) was one-half that of C rats (187.8 ± 43.6 nmol Pi/mg protein), the supplemental methionine in the FDM diet maintained brain membrane phosphatidylcholine at the control level (191.8 ± 51.1 nmol Pi/mg protein). Regardless of whether the diets contained normal or high methionine, brain membrane phosphatidylethanolamine content was ∼300% higher in rats fed folate-deficient diets than in controls.
These changes significantly affected membrane composition expressed as the ratio of brain membrane phosphatidylcholine to phosphatidylethanolamine. In C rats, brain membranes contained 3.5 times as much phosphatidylcholine than phosphatidylethanolamine (PC:PE = 3.5). In FD rats, this ratio was reversed such that membrane phosphatidylethanolamine was 43% higher than membrane phosphatidylcholine content (PC:PE = 0.7). High methionine only partially mitigated this abnormality, restoring membrane phosphatidylcholine content to the same level as that of control rats but retaining the high concentration of phosphatidylethanolamine. The PC:PE ratio under these conditions was 1.5 (Table 3).
Discussion
The present study shows that in rats, diet-induced folate deficiency perturbs not only SAM, SAH, and homocysteine metabolism but also depletes brain membrane phosphatidylcholine. This folate-dependent depletion of brain membrane phosphatidylcholine was prevented by supplementing the FD diet with l-methionine. We also show that folate deficiency impairs cognition as indicated by poorer performance on a sensitive test of spatial learning and memory. As with phosphatidylcholine, this folate-dependent cognitive impairment was prevented by supplementing the FD diet with l-methionine. The observed association of depleted brain membrane phosphatidylcholine with cognitive impairment in folate deficiency and their concurrent prevention by methionine suggests that abnormal membrane phospholipid composition may play an important role in folate-related cognitive dysfunction.
The impaired capacity of the folate-deficient rats to retain the task and escape from the maze by locating the escape platform when it was repositioned on d 4 was similar to that observed in apolipoprotein E-deficient mice (44) and wild-type mice (37), where 1-carbon metabolism was disrupted by a combined folate-vitamin B-12 and vitamin B-6 deficiency. The main effect of folate deficiency was to impair the rate of learning, particularly on the reversal task, whereas the capacity to utilize spatial strategies to find the platform was only weakly affected. This learning decrement is reminiscent of cholinergic impairment in rats with controlled cortical injury, where the damage initially manifests as slower learning and only months later becomes evident in impaired spatial performance on the probe trial (45). It is also similar to the learning deficit in aged rats that also have difficulty learning a new platform location during reversal training (46), although typically, aging also impairs spatial preference on the probe trial (47,48). The apparent dissociation of folate's effect on the rate of learning and spatial performance suggests that it affects specific aspects of cognitive function (49,50). Furthermore, the finding that dietary methionine mitigated the folate-induced impairment points to the importance of the dietary and metabolic balance of additional methyl donors, including methionine and possibly choline in determining the cognitive impact of poor folate status.
Biochemically, our findings are consistent with evidence of a close metabolic linkage of folate, choline, and methionine in 1-carbon transfer reactions and synthesis in vivo. Phosphatidylcholine can be synthesized from choline by the cytidylcholine pathway or from the methylation of phosphatidylethanolamine. The latter is catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) (51). The PEMT reaction is the only known pathway for the de novo synthesis of choline. It occurs extensively in liver but is also important in brain. In contrast, the cytidine (5′)-diphosphocholine pathway merely redistributes preexisting choline moieties between different phospholipid molecules (52). In rats, choline deficiency depletes not only liver choline, betaine, and phosphatidylcholine but also methionine, SAM, and folate, whereas folate deficiency has been shown to deplete choline and phosphocholine (53) and limit phosphatidylethanolamine methylation in liver, increasing its membrane concentration at the expense of phosphatidylcholine (54,55). However, these effects were not found in brain. Similarly, in humans, low folate intake significantly reduces plasma choline and phosphatidylcholine levels (56).
Functionally, abnormal membrane phospholipid composition can be predicted to adversely affect neurotransmission. Membrane phospholipid composition is an important modulator of cellular signal transduction (57). It influences receptor function through membrane-protein interactions and determines the availability of phospholipid degradation products that function as second messengers (51,58–60) and some of these processes may be regulated by the PEMT-catalyzed synthesis of phosphatidylcholine (60,61). Finally, in cholinergic neurones, membrane phosphatidylcholine may serve as a choline reservoir to ensure its rapid availability for neurotransmitter synthesis (62). If the demand for choline exceeds supply, then cell membranes may be degraded to liberate choline (63). Thus, it is possible that in addition to the absolute membrane content of phosphatidylcholine, the observed shift in the ratio of phosphatidylcholine: phosphatidylethanolamine from a preponderance of phosphatidylcholine to a preponderance of phosphatidylethanolamine in cognitively impaired rats represents 2 functionally important membrane anomaly. Such changes are most likely to be evident in cholinergic structures, such as the nucleus basalis, or in brain regions with a high requirement for methylation, such as striatum. Identification of regional effects of folate deficiency on PEMT activity, membrane phospholipid composition, and acetylcholine content might help to test these predictions.
Interestingly, in postmortem brain tissue from Alzheimer's disease patients, brain phosphatidylcholine content and PEMT activity in the frontal cortex have been found to be decreased compared to controls (52).
It is unlikely that homocysteine mediated the observed folate deficiency-induced cognitive impairment. If hyperhomocysteinemia were fully responsible for the folate-induced cognitive impairment, then cognition should not have improved upon methionine supplementation. Although it is possible that homocysteine could directly harm the brain or its vasculature under some conditions (26,32,37), the observed dissociation of hyperhomocysteinemia from cognitive impairment is important because it indicates that conditions exist where the association of hyperhomocysteinemia with cognitive impairment is insufficient as the causal mechanism.
We also examined the hypothesis that the association of poor folate status with cognitive dysfunction and decline is mediated by inhibited brain methylation capacity, under the assumption that low folate limits the synthesis and availability of brain methionine and SAM. Our finding that supplemental methionine prevents cognitive impairment in the face of folate deficiency seems consistent with this hypothesis. However, it does not easily account for the resistance of brain SAM and SAH to perturbation by folate deficiency. Indeed, there was no significant difference in the SAM:SAH ratio (often referred to as the “methylation potential”) did not differ in the brain of cognitively intact control rats and in cognitively impaired rats fed the folate-deficient diet with normal methionine. Conversely, the SAM:SAH ratio was significantly lower in the brain of cognitively intact rats fed folate-deficient, high-methionine diets. Nor can absolute brain SAM concentrations account for the cognitive impairment, because they were similarly decreased in rats fed the folate-deficient diets, irrespective of dietary methionine. Similarly, elevated brain SAH cannot account for the cognitive impairment, because SAH concentrations were not significantly elevated in the cognitively impaired rats. Moreover, the differences in SAM and SAH concentrations between control and folate-deficient, normal-methionine rats were very modest, amounting to an absolute difference of −1.2 μmol/L and +0.3 μmol/L, respectively (the equivalent of an 8% relative reduction in SAM and an 18% increase in SAH). Even if these differences were biologically meaningful, they do not explain the prevention of cognitive impairment by the addition of methionine to the folate-deficient diet, because the addition of methionine did nothing to improve SAM and SAH and in fact increased the differences. To the extent that global brain SAM and SAH concentrations reflect brain methylation capacity and activity, the modest diet induced changes in brain SAM and SAH concentrations do not indicate hypomethylation as a likely explanation for the observed cognitive impairment. However, global brain SAM and SAH concentrations may fail to capture regional differences in brain methylation potential and in the activity of specific methylation reactions.
If impaired methylation activity is nonetheless a mediator of folate deficiency-induced cognitive impairment, then it is likely that inhibition of specific methylation reactions will be more closely related than global methylation capacity to cognitive impairment. The activity of specific methyltransferase enzymes depends less on the SAM:SAH ratio than on the absolute concentrations and the enzyme's Michaelis constant and inhibition constant (Km and Ki) for SAM and SAH, rendering some reactions more vulnerable than others to impaired methylation. Furthermore, SAM and SAH concentrations may reflect the steady state of the substrate and product of methylation reactions but may be less reliable indicators of the rate at which these reactions occur, or of the flux through the cycle. Thus, in theory, while brain SAM is maintained at a relatively stable homeostatic set point, an influx of methionine to brain could boost the availability of labile methyl groups.
In light of these considerations, it is not surprising that a neurologically important methylated compound such as phosphatidylcholine has a closer association with cognitive function than global SAM and SAH concentrations. However, as described above, dietary folate and methionine may determine brain phosphatidylcholine content, not only through brain PEMT activity, but also through the reciprocal relation of methionine and choline metabolism in liver. Our finding that folate deficiency resulted in increased membrane phosphatidylethanolamine content is consistent with the postulated inhibition of PEMT activity. Such inhibition would also explain the depletion of membrane phosphatidylcholine in folate deficiency with normal methionine. That the addition of methionine to the folate-deficient diet preserved brain membrane phosphatidylcholine has several possible explanations. One possibility is that methionine supplementation is sufficient to stimulate or restore PEMT activity that is otherwise limited by the secondary, folate-dependent methionine and SAM deficiency. Alternatively, enhanced availability of methionine might downregulate BHMT activity that would otherwise be required to compensate for inhibited folate-dependent methionine synthase activity. This in turn might protect choline, which would otherwise be consumed for methionine synthesis at the expense of phosphatidylcholine (64–66). If brain membrane phosphatidylcholine is preserved by methionine-dependent conservation of choline, then the fact that the BHMT reaction takes place primarily in liver but not in brain would point to a central contribution of liver phospholipid metabolism for brain function. This possibility is particularly intriguing given that the marked perturbation of liver SAM and SAH by folate deficiency was not ameliorated by methionine supplementation (Table 3).
In conclusion, the present study underscores the need for a far more detailed understanding of these metabolic and pathologic relationships than is currently available. Identifying the factors that enhance or mitigate folate- and homocysteine-related cognitive impairment will be crucial to effectively targeting these powerful, prevalent, and potentially modifiable risk factors for cognitive decline in older adults.
Acknowledgments
We thank Bina M. Albuquerque and Sherley Casseus for technical assistance with this study.
Supported by the USDA, Agricultural Research Service, under agreement No. 58-1950-7-707. K. E. D. was supported by the NIH Training grant T32 DK007651. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Author disclosures: A. Troen, W. Chao, N. Crivello, K. D'Anci, B. Shukitt-Hale, D. Smith, J. Selhub, and I. Rosenberg, no conflicts of interest.
Abbreviations used: BHMT, betaine-homocysteine methyltransferase; C, control diet; CDP-Choline, cytidine (5′)-diphosphocholine; FA, folic acid; FD, folate-deficient diet; FDM, folate-deficient, methionine-supplemented diet; PC:PE, phosphatidylcholine:phosphatidylethanolamine ratio; PEMT, phosphatidylethanolamine N-methyltransferase; Pi, inorganic phosphorous; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; tHcy, plasma total homocysteine.
References
- 1.Rosenberg IH, Miller JW. Nutritional factors in physical and cognitive functions of elderly people. Am J Clin Nutr. 1992;55:S1237–43. [DOI] [PubMed] [Google Scholar]
- 2.Bell IR, Edman JS, Selhub J, Morrow FD, Marby DW, Kayne HL, Cole JO. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr Scand. 1992;86:386–90. [DOI] [PubMed] [Google Scholar]
- 3.Riggs KM, Spiro Ar, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the normative aging study. Am J Clin Nutr. 1996;63:306–14. [DOI] [PubMed] [Google Scholar]
- 4.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: Macarthur studies of successful aging. Am J Med. 2005;118:161–7. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed alzheimer disease. Arch Neurol. 1998;55:1449–55. [DOI] [PubMed] [Google Scholar]
- 6.Joosten E, Lesaffre E, Riezler R, Ghekiere V, Dereymaeker L, Pelemans W, Dejaeger E. Is metabolic evidence for vitamin B-12 and folate deficiency more frequent in elderly patients with Alzheimer's disease? J Gerontol A Biol Sci Med Sci. 1997;52:M76–9. [DOI] [PubMed] [Google Scholar]
- 7.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–9. [DOI] [PubMed] [Google Scholar]
- 8.Snowdon DA, Tully CL, Smith CD, Riley KP, Markesbery WR. Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: findings from the nun study. Am J Clin Nutr. 2000;71:993–8. [DOI] [PubMed] [Google Scholar]
- 9.Ganji V, Kafai MR. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: analysis of data from National Health and Nutrition Examination Surveys, 1988–1994, 1999–2000, and 2001–2002. J Nutr. 2006;136:153–8. [DOI] [PubMed] [Google Scholar]
- 10.Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–8. [DOI] [PubMed] [Google Scholar]
- 11.Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento area Latino study on aging. Am J Clin Nutr. 2005;82:1346–52. [DOI] [PubMed] [Google Scholar]
- 12.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the facit trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–16. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–5. [DOI] [PubMed] [Google Scholar]
- 14.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir DG, Scott JM. Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull. 1999;55:669–82. [DOI] [PubMed] [Google Scholar]
- 16.Bottiglieri T. Ademetionine (s-adenosylmethionine) neuropharmacology: implications for drug therapies in psychiatric and neurological disorders. Expert Opin Investig Drugs. 1997;6:417–26. [DOI] [PubMed] [Google Scholar]
- 17.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. [DOI] [PubMed] [Google Scholar]
- 18.Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organisation of the human gene. J Biol Chem. 1999;274:7816–24. [DOI] [PubMed] [Google Scholar]
- 19.Varela-Moreiras G, Pérez-Olleros L, García-Cuevas M, Ruiz-Roso B. Effects of ageing on folate metabolism in rats fed a long-term folate deficient diet. Int J Vitam Nutr Res. 1994;64:294–9. [PubMed] [Google Scholar]
- 20.Varela-Moreiras G, Selhub J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992;122:986–91. [DOI] [PubMed] [Google Scholar]
- 21.Varela-Moreiras G, Ragel C, Pérez de Miguelsanz J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J Am Coll Nutr. 1995;14:480–5. [DOI] [PubMed] [Google Scholar]
- 22.Matthews RG, Elmore CL. Defects in homocysteine metabolism: diversity among hyperhomocyst(e)inemias. Clin Chem Lab Med. 2007;45:1700–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr. 2006;136:S1726–30. [DOI] [PubMed] [Google Scholar]
- 24.Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in app-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28:1195–205. [DOI] [PubMed] [Google Scholar]
- 25.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem. 2002;80:101–10. [DOI] [PubMed] [Google Scholar]
- 26.Shea TB, Ortiz D, Rogers E. Differential susceptibity of transgenic mice lacking one or both apolipoprotein alleles to folate and vitamin E deprivation. J Alzheimers Dis. 2004;6:269–73. [DOI] [PubMed] [Google Scholar]
- 27.Troen AM. The central nervous system in animal models of hyperhomocysteinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1140–51. [DOI] [PubMed] [Google Scholar]
- 28.Botez MI, Bachevalier J, Tunnicliff G. Dietary folic acid and the activity of brain cholinergic and gamma-aminobutyric acid (gaba) enzymes. Can J Neurol Sci. 1980;7:133–4. [DOI] [PubMed] [Google Scholar]
- 29.Botez MI, Young SN, Bachevalier J, Gauthier S. Folate deficiency and decreased brain 5-hydroxytryptamine synthesis in man and rat. Nature. 1979;278:182–3. [DOI] [PubMed] [Google Scholar]
- 30.Butler IJ, Rothenberg SP. Dietary folate and biogenic amines in the CNS. J Neurochem. 1987;49:268–71. [DOI] [PubMed] [Google Scholar]
- 31.Gospe SMJ, Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, Ellis WG, Clifford AJ. Behavioral and neurochemical changes in folate-deficient mice. Physiol Behav. 1995;58:935–41. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Lee H, Chang N. Hyperhomocysteinemia due to short-term folate deprivation is related to electron microscopic changes in the rat brain. J Nutr. 2002;132:3418–21. [DOI] [PubMed] [Google Scholar]
- 33.Kruman II, Mouton PR, Emokpae R Jr, Cutler RG, Mattson MP. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055–9. [DOI] [PubMed] [Google Scholar]
- 34.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76a diet. J Nutr. 1997;127:S838–41. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Pae M, Ren Z, Guo Z, Smith D, Meydani SN. Dietary supplementation with white button mushroom enhances natural killer cell activity in c57bl/6 mice. J Nutr. 2007;137:1472–7. [DOI] [PubMed] [Google Scholar]
- 36.Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male fischer 344 rats. Exp Gerontol. 1998;33:615–24. [DOI] [PubMed] [Google Scholar]
- 37.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA. 2008;105:12474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin YS, Rasshofer R, Friedrich B, Endres W. Pyridoxal-5′-phosphate determination by a sensitive micromethod in human blood, urine and tissues; its relation to cystathioninuria in neuroblastoma and biliary atresia. Clin Chim Acta. 1983;127:77–85. [DOI] [PubMed] [Google Scholar]
- 39.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. [DOI] [PubMed] [Google Scholar]
- 40.Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin Chem. 2004;50:365–72. [DOI] [PubMed] [Google Scholar]
- 41.Crivello NA, Rosenberg IH, Dallal GE, Bielinski D, Joseph JA. Age-related changes in neutral sphingomyelin-specific phospholipase c activity in striatum, hippocampus, and frontal cortex: implication for sensitivity to stress and inflammation. Neurochem Int. 2005;47:573–9. [DOI] [PubMed] [Google Scholar]
- 42.Denisova NA, Strain JG, Joseph JA. Oxidant injury in PC12 cells: a possible model of calcium “dysregulation” in aging. II. Interactions with membrane lipids. J Neurochem. 1997;69:1259–66. [DOI] [PubMed] [Google Scholar]
- 43.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–6. [DOI] [PubMed] [Google Scholar]
- 44.Troen AM, Shukitt-Hale B, Chao WH, Albuquerque B, Smith DE, Selhub J, Rosenberg J. The cognitive impact of nutritional homocysteinemia in apolipoprotein-e deficient mice. J Alzheimers Dis. 2006;9:381–92. [DOI] [PubMed] [Google Scholar]
- 45.Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–22. [DOI] [PubMed] [Google Scholar]
- 46.Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–8. [DOI] [PubMed] [Google Scholar]
- 47.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–60. [DOI] [PubMed] [Google Scholar]
- 48.Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. [DOI] [PubMed] [Google Scholar]
- 49.Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–23. [DOI] [PubMed] [Google Scholar]
- 50.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–70. [DOI] [PubMed] [Google Scholar]
- 51.Vance DE. Boehringer Mannheim award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990;68:1151–65. [DOI] [PubMed] [Google Scholar]
- 52.Guan ZZ, Wang YN, Xiao KQ, Hu PS, Liu JL. Activity of phosphatidylethanolamine-n-methyltransferase in brain affected by Alzheimer's disease. Neurochem Int. 1999;34:41–7. [DOI] [PubMed] [Google Scholar]
- 53.Kim YI, Miller JW, da Costa KA, Nadeau M, Smith D, Selhub J, Zeisel SH, Mason JB. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr. 1994;124:2197–203. [DOI] [PubMed] [Google Scholar]
- 54.Akesson B, Fehling C, Jägerstad M, Stenram U. Effect of experimental folate deficiency on lipid metabolism in liver and brain. Br J Nutr. 1982;47:505–20. [DOI] [PubMed] [Google Scholar]
- 55.Akesson B, Fehling C, Jägerstad M. Lipid composition and metabolism in liver and brain of vitamin B12-deficient rat sucklings. Br J Nutr. 1979;41:263–74. [DOI] [PubMed] [Google Scholar]
- 56.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–7. [DOI] [PubMed] [Google Scholar]
- 57.Hirata F, Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980;209:1082–90. [DOI] [PubMed] [Google Scholar]
- 58.Roth GS, Joseph JA, Mason RP. Membrane alterations as causes of impaired signal transduction in Alzheimer's disease and aging. Trends Neurosci. 1995;18:203–6. [DOI] [PubMed] [Google Scholar]
- 59.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 60.Axelrod J, Hirata F. Phospholipid methylation and membrane function. Ann N Y Acad Sci. 1981;373:51–3. [DOI] [PubMed] [Google Scholar]
- 61.Hirata F, Strittmatter WJ, Axelrod J. Beta-adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci USA. 1979;76:368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blusztajn JK, Liscovitch M, Richardson UI. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci USA. 1987;84:5474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wurtman RJ. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992;15:117–22. [DOI] [PubMed] [Google Scholar]
- 64.Horne DW, Cook RJ, Wagner C. Effect of dietary methyl group deficiency on folate metabolism in rats. J Nutr. 1989;119:618–21. [DOI] [PubMed] [Google Scholar]
- 65.Zeisel SH, Zola T, daCosta KA, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem J. 1989;259:725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]