Abstract
Adoptive transfer of anti-tumor T cells is a promisingly effective therapy for various cancers, but its effect on endogenous anti-tumor immune mechanisms remains largely unknown. Here we demonstrate that the administration of naïve T cells de novo primed for only 7 days against tumor antigens resulted in the durable rejection of otherwise lethal ovarian cancers when coupled with the depletion of tumor-associated immunosuppressive dendritic cells (DCs). Therapeutic activity required tumor antigen specificity and perforin expression by the adoptively transferred T cells, but not IFN-γ production. Importantly, these shortly primed T cells secreted large amounts of CCL5, which was required for their therapeutic benefit. Accordingly, transferred T cells recruited CCR5+ DCs into the tumor, where they showed distinct immunostimulatory attributes. Activated CCR5+ host T cells with anti-tumor activity also accumulated at tumor locations, and endogenous tumor-specific memory T cells remained elevated after the disappearance of transferred lymphocytes. Therefore, persistent, long-lived anti-tumor immunity was triggered by the administration of ex vivo activated T cells, but was directly mediated by immune cells of host origin. Our data unveil a CCL5-dependent mechanism of awakening endogenous anti-tumor immunity triggered by ex vivo expanded T cells, which is augmented by tumor-specific targeting of the cancer microenvironment.
INTRODUCTION
Adoptive cell transfer therapy (ACT), the ex-vivo activation, expansion, and subsequent administration of tumor-reactive T cells, is a vastly successful therapy against certain cancers. In fact ACT is currently the most effective therapy against metastatic melanoma, with objective regressions reported in 50% of patients [1–4]. The therapeutic effects of ACT are commonly attributed to the in vivo expansion and anti-tumor activity of transferred lymphocytes. Correspondingly, major efforts have been focused on promoting long-term persistence of adoptively transferred T cells [5]. Thus, recent studies indicate that compared with more differentiated effector lymphocytes, memory T cells or early effectors have a higher capacity for in vivo expansion, which is associated with enhanced therapeutic effects against melanoma [6].
Most ACT studies in mouse cancer models are performed with transgenic T cells [7] and their effect on activating endogenous (host-derived) protective immune mechanisms is not usually addressed. However, although their function in the tumor microenvironment is suboptimal without treatment, anti-tumor T cells exert spontaneous immune pressure against cancer progression [8]. Upon pharmacological inhibition of tumor-associated immunosuppressive leukocytes endogenous T cells spontaneously and therapeutically expand, become activated [9], and contribute to the control of cancer after conventional chemotherapy [10]. Correspondingly, changes in the tumor microenvironment caused by ACT and/or lymphodepletion may also release endogenous anti-tumor immune mechanisms from suppression. Therefore, the relative contribution of enhanced endogenous anti-tumor immunity to the persistent, long-lived therapeutic benefit elicited by ACT remains unknown.
Despite inducing major clinical responses in melanoma patients, ACT strategies attempted against significantly more lethal epithelial tumors have not yet yielded similar positive results. Recent studies in ovarian cancer, one of the most aggressive and frequent forms of epithelial cancer, indicate that although their persistence is short-lived, large numbers of tumor-reactive T cells can be safely given to patients [11]. Still, there is strong rationale for developing ACT strategies against ovarian cancer, which may be applicable to other aggressive epithelial tumors. Firstly, chemotherapies implemented in the last 30 years have led to a 5-year survival rate of 30%, at best, for patients with metastatic ovarian carcinoma, the stage at which most cases are diagnosed [12]. As a result, epithelial ovarian cancer will claim the lives of more than 15,000 women in the United States alone, in 2008 [12]. Secondly, seminal studies by Coukos and colleagues [13, 14] demonstrated that the infiltration of ovarian tumor islets by T cells strongly predicts a better clinical course which was supported by subsequent studies that restricted the favorable prognostic effect to intraepithelial CD8+ cytotoxic T cells [15, 16]. Thirdly, several cancer antigens recognized by antibodies and/or CD8 T cells have been found in many ovarian cancers [17–20] and could be harnessed for potent therapies.
To design clinically effective immunotherapies against aggressive epithelial tumors, multiple immunosuppressive networks orchestrated by regulatory T cells (Treg) and myeloid-derived suppressor cells [9, 21–23] need to be overcome. Additionally, regulatory MHC-II+DEC205+CD11c+CD14− dendritic cells (DCs), the most abundant leukocyte subset in solid ovarian cancer specimens [24] contribute to tumor vascularization and immunosuppression [24, 25]. Thus, DCs in ovarian cancer present an additional barrier to successful immunotherapies and their elimination from ovarian cancer-bearing mice significantly delays cancer progression by boosting anti-tumor immunity [24].
Here we demonstrate that the adoptive transfer of de novo primed tumor-reactive T cells coupled with depleting immunosuppressive DCs results in durable regression of established ovarian cancer. Interestingly, this combination therapy triggered the sustained activation of endogenous, host-derived anti-tumor T cells in a CCL5-dependent manner. Our data provide a mechanistic rationale for differential targeting of the microenvironment of distinct aggressive epithelial cancers for enhanced immunotherapies, and demonstrate that adoptively transferred T cells induce a previously unrecognized, therapeutically relevant, tumor-specific host response.
RESULTS
Optimization of a clinically relevant system for de novo priming of tumor-reactive T cells
We first developed a clinically relevant system for briefly arousing polyclonal naïve anti-tumor T cells, exploiting the increased capacity of early effector T cells for in vivo expansion [6]. Splenic lymphocytes were stimulated with DCs pulsed with ultraviolet plus gamma irradiated ID8-Defb29/Vegf-a cancer cells, which generate aggressive and widely disseminated ovarian tumors [26]. After only 7 days of de novo priming, more than 68% of (naïve) splenic T cells from healthy mice showed activation markers (Fig. 1A), with a final CD8:CD4 ratio of 3:2 (Fig. 1B). Surprisingly, similarly activated splenic T cells from tumor-bearing mice, did not show stronger activation, higher cytotoxic:helper T cell ratio nor superior therapeutic effects (Fig. 1A–B; Fig. S1A). Subsequent studies were performed with naïve lymphocytes as this approach was more clinically reproducible with peripheral blood T cells from cancer patients.
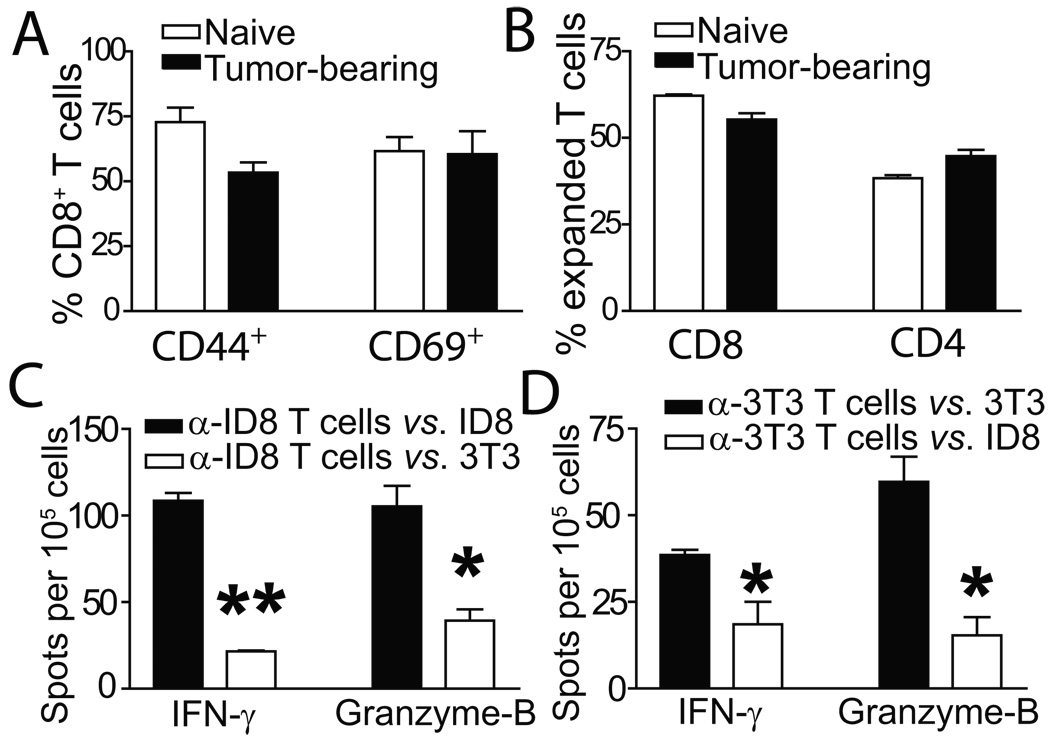
Figure 1. Phenotypic characterization of tumor-reactive and irrelevantly primed T cells.
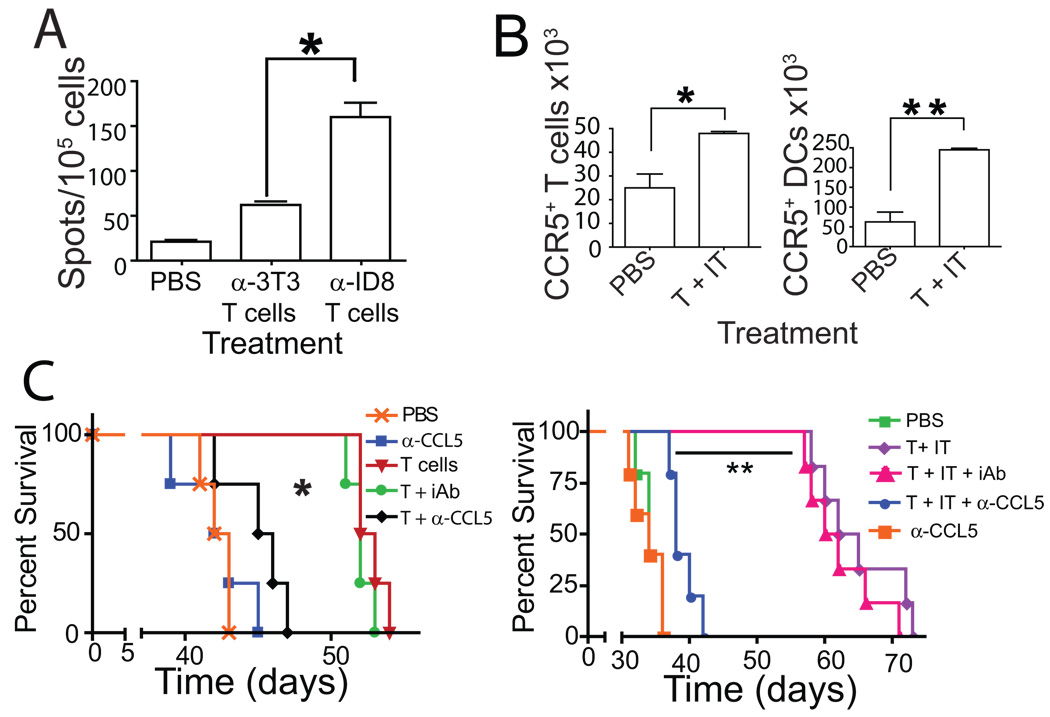
(A) Activation status of T cells from either healthy (naive) or ID8-Defb29/Vegf-a tumor-bearing (tumor-bearing) mice primed to ID8-Defb29/Vegf-a antigens. Representative of 3 independent experiments. (B) T cells expanded from tumor-bearing or healthy mice show comparable proportions of CD8 vs. CD4 T cells. (C) IFN-γ and granzyme-B ELISPOT analyses of naïve T cells primed to ID8-Defb29/Vegf-a antigens, in response to their cognate tumor antigen (α-ID8 T cells vs. ID8), or NIH-3T3 fibroblasts (α-ID8 T cells vs. 3T3). Representative of 2 independent experiments (n=6/group, total). (D) Similar ELISPOT analysis performed with naïve T cells primed to irrelevant NIH-3T3 fibroblasts (*- P<0.05; **- P<0.01).
Although the priming regimen lasted only 7 days, T cells specific to ID8-Defb29/Vegf-a tumor cells were selectively activated, as significantly more lymphocytes produced interferon-γ (IFN-γ) and Granzyme-B in response to their cognate antigen than to non-specific NIH-3T3 fibroblasts (Fig. 1C; P<0.01, P<0.05). Correspondingly, significantly fewer T cells similarly expanded to irrelevant NIH-3T3 cell antigens produced IFN-γ and Granzyme-B in response to ID8-Defb29/Vegf-a tumor antigens, compared to their cognate NIH-3T3 cell antigen (Fig. 1D; P<0.05). Importantly, T cells primed to these irrelevant antigens showed comparable CD8:CD4 ratios and activation phenotype (Fig. S1B & Fig. S1C), demonstrating the feasibility of selectively priming resting antigen-specific T cells within 7 days.
Eliminating CD11c+ regulatory DCs enhances the therapeutic effects of adoptively transferred tumor-reactive T cells
Since eliminating ovarian cancer-associated DCs boosts anti-tumor immunity [24], we hypothesized that their depletion from tumor locations could augment the effect of ACT. Thus, ID8 ovarian cancer bearing mice were treated with an anti-CD11c immunotoxin (IT). As we published, this DC depleting tool consists of a mouse CD11c-specific single-chain antibody fused to a Pseudomonas exotoxin-A lacking the cell-binding domain [24] and elicits its lethality to the cytoplasm of tumor-associated CD11c+ cells, but not to other cells or splenic DCs [24]. Confirming our previous observations, mice depleted of tumor-associated DCs showed a significant lifespan increase of 34% without additional treatment (Fig. 2A–B; P<0.05). ACT alone led to a similar therapeutic benefit, but did not produce durable curative responses (Fig. 2B; P<0.05). In contrast, eliminating regulatory DCs prior to ACT (ACT + IT) led to the elimination of any obvious disease in 50% of treated mice, which remain healthy >485 days later (Fig. 2B; P<0.01). Accordingly, ACT + IT significantly diminished tumor burden after 8 weeks of cancer progression (Fig. S1D; Quantification – right; P<0.01). Therefore, eliminating regulatory tumor-associated DCs transforms the administration of shortly primed anti-tumor T cells from a simply palliative intervention to a durable curative approach.
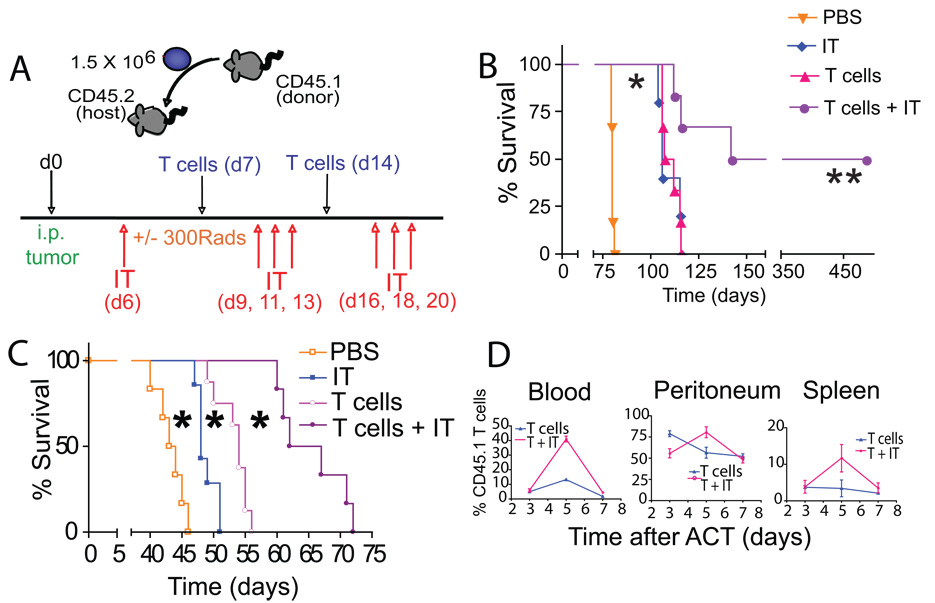
Figure 2. Elimination of CD11c+ cells enhances the efficacy of ACT.
(*- P<0.05; **- P<0.01; T cells - T cell transfer; IT - anti-CD11c immunotoxin). (A) Experimental design. CD45.2+mice were inoculated i.p. with tumor and treated on day 7 and day 14 of tumor progression with 1.5×106 CD45.1+ tumor-primed T cells (T cells). When relevant, tumor-bearing mice were irradiated on the first day of T cell transfer. Tumor-bearing mice were depleted of CD11c cells with the anti-CD11c immunotoxin (IT) the day prior to the initial T cell transfer and thrice weekly for two weeks thereafter. (B) ACT plus anti-CD11c immunotoxin, but not individual treatments, induced the regression of established intraperitoneal ID8-luciferase tumors (n=12 per group in 2 independent experiments). (C) ID8-Defb29/Vegf-a tumor-bearing mice receiving ACT plus IT survived significantly longer than untreated mice, or mice receiving individual treatments (n=24 mice/group, total in 4 independent experiments). (D) In vivo proliferation of transferred congenic (CD45.1+) T cells with (T+IT) or without (T) immunotoxin administration. The percentage of transferred cells at specific locations over time was determined (n=8 mice/group in 2 independent experiments).
To confirm the therapeutic potential of combining ACT with regulatory DC depletion in a more aggressive model of ovarian cancer, we next treated mice growing intraperitoneal ID8-Defb29/Vegf-a tumors. These tumors show enhanced angiogenesis, immunosuppression and accelerated progression [26]. To boost the homeostatic expansion of adoptively transferred T cells, mice were sublethally irradiated prior to treatment. Confirming our previous results, mice depleted of tumor-associated DCs showed a modest but significant lifespan increase of 11%, while ACT alone statistically significantly enhanced survival by 22% in this aggressive model (Fig. 2C; P<0.05 for both). Importantly, in agreement with previous reports [27], irradiation did not delay tumor progression of untreated or individually treated mice (Fig. S2A). Furthermore, eliminating tumor-associated regulatory DCs promoted increased in vivo expansion of subsequently transferred tumor-reactive T cells (Fig. 2D; P<0.05;) and induced dramatically increased survival compared to individual treatments and a 53% increase in lifespan compared to untreated mice (Fig. 2C; P<0.01 for both).
The therapeutic effects of ACT are mediated by cytotoxic T cells homing to tumor locations in an antigen-specific, IFN-γ-independent manner
Since administered T cells were derived from de novo primed lymphocytes, it was possible that the therapeutic effects resulted from antigen-independent, non-specific secretion of pro-inflammatory factors. To define whether transferred T cells required tumor-specific priming, splenic T cells from healthy, congenic (CD45.1) mice were expanded against either irradiated ID8-Defb29/Vegf-a cells or irradiated irrelevant NIH-3T3 fibroblasts, then transferred into ID8-Defb29/Vegf-a-tumor-bearing mice. Interestingly, irrelevantly- primed T cells exerted some therapeutic activity in this system (Fig. 3A; P <0.05), comparable to that induced by naïve lymphocytes (not shown). Nevertheless, tumor-primed T cells significantly prolonged survival compared to irrelevantly-primed (NIH-3T3) T cells (Fig. 3A; P <0.05), supporting the requirement for antigen-specificity in eliciting robust therapeutic effects.
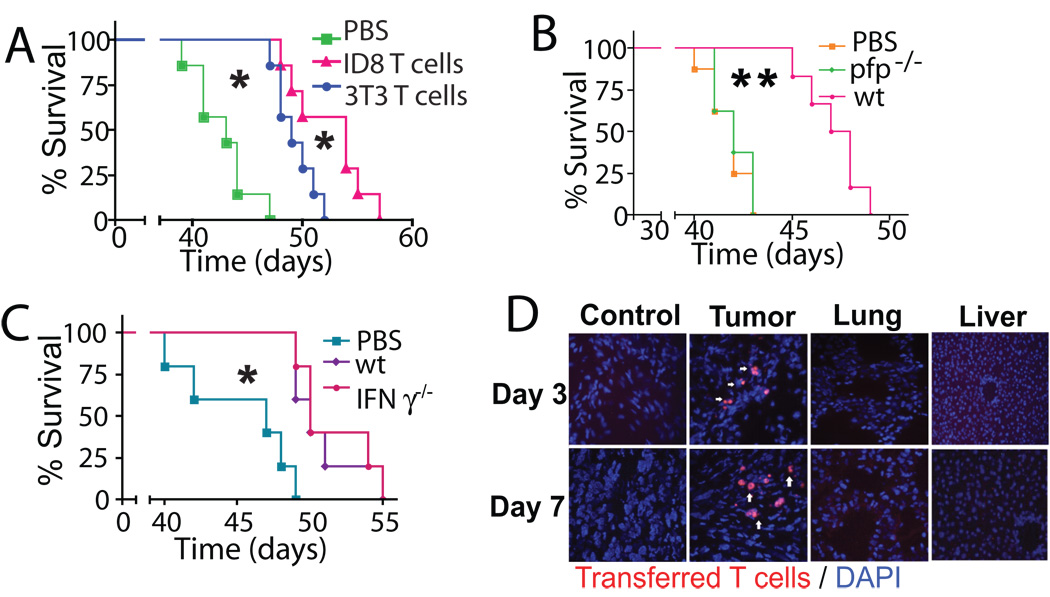
Figure 3. The therapeutic effects of ACT require perforin and antigen specific priming but not IFNγ.
(*- P<0.05; **- P<0.01). (A) Survival of ID8-Defb29/Vegf-a tumor–bearing mice receiving T cells primed either against ID8-Defb29/Vegf-a tumor cell antigens (“ID8 T cells”) or irrelevant NIH-3T3 fibroblasts (“3T3 T cells”) was established (n=8 mice/group). (B) ID8-Defb29/Vegf-a–tumor expanded splenic T cells from CD45.2+ perforin deficient (pfp−/−) or wild-type (wt) mice were transferred into ID8-Defb29/Vegf-a tumor-bearing, congenic (CD45.1) mice (n=16 mice/group in 2 independent experiments). Survival was determined. (C) Survival induced by IFN-γ deficient (IFN-γ−/−) T cells (n=16 mice/group in 2 independent experiments) was compared in an identical experiment. Mice were not irradiated in (B) or (C). (D) Mice bearing 14-day old flank ID8-Defb29/Vegf-a tumors received ID8-Defb29/Vegf-a primed red fluorescent T cells intraperitoneally and immunofluorescent microscopy was performed. (magnification - 200 X; n=6 mice/group).
Transferred T cells also required tumor-specific cytotoxicity for successful ACT as expanded perforin-deficient T cells did not prolong survival in tumor-bearing hosts with (Fig. S2B; P<0.05) or without (Fig. 3B; P<0.01) IT. The absence of effect was not caused by a functional deficit in perforin-deficient T cells, since they secrete similar amounts of IFN-γ as wild-type T cells when cultured with tumor cells [28]. In contrast, administering IFN-γ-deficient T cells prolonged survival in tumor-bearing mice as effectively as wild-type T cells, with (Fig. S2C; P<0.05), or without (Fig. 3C; P<0.05) IT.
De novo activated tumor-reactive T cells specifically homed to tumor locations, as tumor-primed fluorescently labeled T cells administered intraperitoneally, accumulated at distal (axilar) ID8-Defb29/Vegf-a tumors but were undetectable in other non-immune organs at different temporal points (Fig. 3D). Together, these data indicate that the therapeutic activity of transferred T cells required selective tumor homing, tumor-specific priming and perforin secretion but not IFN-γ production.
Adoptive administration of shortly primed tumor-reactive T cells boosts persistent tumor-specific host immune responses that are further augmented by depleting tumor-associated DCs
Although early effector T cells persist the longest in vivo [6], shortly primed tumor-reactive T cells started contracting after only 5 days in intraperitoneal ovarian cancer-bearing hosts, supporting previous reports [11]. Administered lymphocytes were undetectable at lymphatic locations, in the tumor or bone marrow after 12 days (Fig. S2D). We therefore hypothesized that the persistent, long-lived therapeutic benefit elicited by ACT could be mediated by endogenous, host-derived anti-tumor immune responses, rather than only resulting from direct killing by administered T cells.
Supporting this proposition, the number of endogenous (CD45.2+), host-derived Granzyme B (P<0.01) and IFN-γ (P<0.05 at day 2; P<0.01 at day 4) producing T cells was significantly higher in ACT treated mice than untreated mice (Fig. 4A–B). Surprisingly, the number of endogenous (CD45.2+) T cells producing IFN-γ and Granzyme B in response to tumor antigens was significantly higher than their ex vivo primed transferred (CD45.1+) counterparts at early timepoints (Fig. 4A–B; P<0.05). The activation of host anti-tumor T cells occurred within only 2 days of ACT, suggesting that release from tumor-induced immunosuppression (awakening), rather than de novo priming, prompted these effects. Notably, exogenous and endogenous anti-tumor lymphocytes remained concurrently activated at tumor locations for at least 4 days after ACT. Most importantly, depletion of CD11c cells before ACT induced significantly higher numbers of endogenous (CD45.2+) T cells producing IFN-γ and Granzyme B in response to tumor antigens as early as 2 days after treatment (Fig. 4A–B; P<0.05).
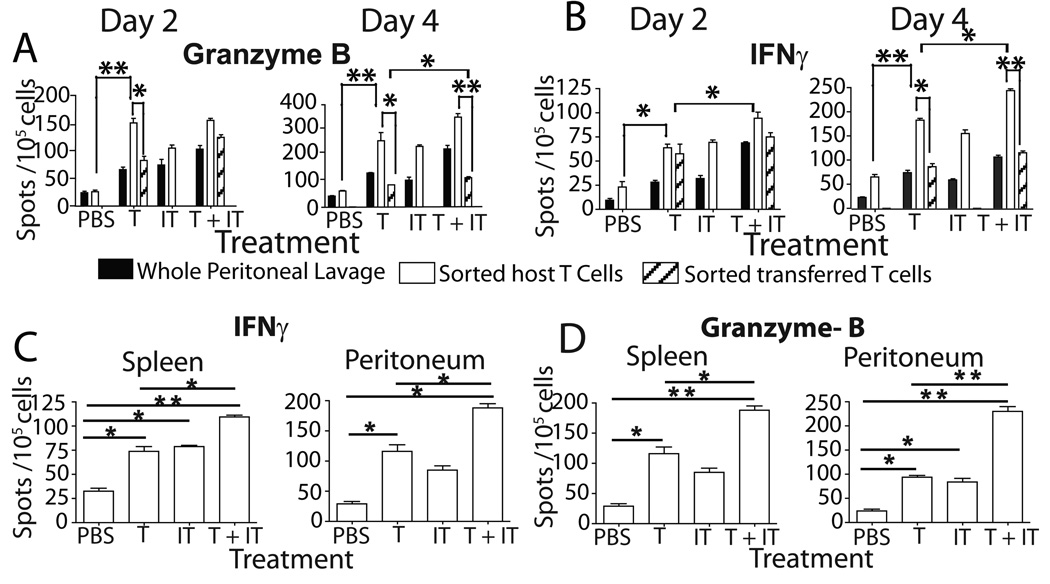
Figure 4. Adoptive T cell therapy induces host immune responses that are augmented by the elimination of CD11c+ cells.
(* - P<0.05; **- P<0.01; T- T cell transfer; IT- anti-CD11c immunotoxin). (A) Beginning on day 2 after T cell transfer, peritoneal host (CD45.2) and transferred (CD45.1) T cells were independently FACS sorted. Whole peritoneal wash samples, sorted host cells and sorted transferred cells (n=6/group in 2 independent experiments) were then analyzed by ELISPOT for secretion of granzyme-B (left) and IFN-γ (right) in response to tumor antigen. (B) IFN-γ (left) and granzyme-B (right) ELISPOT analyses were performed on peritoneal wash samples and spleens obtained from mice at day 26 of tumor progression, when transferred T cells are no longer detectable (n=9/group in 3 independent experiments).
This awakening of endogenous anti-tumor immunity persisted longer than the direct effect of exogenous tumor-reactive T cells in tumor-bearing mice, because 18 days after their adoptive administration, after their elimination from the host, the number of endogenous, host-derived (tumor-associated and splenic) lymphocytes producing IFN-γ and Granzyme-B in response to tumor antigens remained significantly increased, compared to untreated mice (Fig. 4C–D; P<0.05). Depleting immunosuppressive tumor-associated DCs magnified this endogenous anti-tumor immune response elicited by ACT, as demonstrated by IFN-γ and Granzyme B production 18 days after ACT (Fig. 4C–D; P<0.01).
Confirming this awakening of endogenous anti-tumor immunity by ACT, tetramer analyses demonstrated significantly increased proportions of host-derived (CD45.2+), activated (CD69+), antigen-experienced (CD44+) T cells specifically recognizing an H-2Db-restricted mesothelin epitope expressed by ID8 tumor cells [29] at splenic and tumor locations of ACT treated mice and further increased by prior elimination of regulatory DCs (Fig. S3A–B; P<0.05 for both). Furthermore, the proportion of endogenous (CD45.2+), tumor antigen (mesothelin)-specific T cells exhibiting a central memory (CD44+CD62L+) phenotype in the bone marrow was significantly increased in ACT treated mice compared to untreated mice (Fig. S3C; P<0.05), and was augmented by depleting DCs (P<0.05). Therefore, transferred T cells trigger durable and potent anti-tumor host responses which persist after their initial cytotoxic effect and subsequent disappearance, are partly mediated by endogenous memory T cells and are significantly enhanced by regulatory DC depletion, resulting in prolonged survival.
Adoptive lymphocyte transfer induces the accumulation of activated host immune cells at tumor locations
To define the mechanisms mediating the host anti-tumor immunogenic boost elicited by ACT, cytokine and chemokine production by tumor-primed T cells were analyzed, and high levels of the pro-inflammatory cytokine CCL5 were observed (Fig. 5A). CCL5 correlates with a better clinical outcome in ovarian cancer patients [14] and recruits lymphocytes and DCs through CCR5 and CCR8. Correspondingly, six-times more host (CD45.2+) T cells accumulated at tumor sites of ACT-treated mice compared to untreated mice (Fig. S4A; P<0.01). Leukocyte recruitment began 2 days after ACT and lasted up to 7 days later. Importantly, the recruitment of host T cells induced by ACT was independent of the depletion of tumor-associated DCs, although the proportion of antigen-experienced (CD44+) cytotoxic T cells in this host-derived population was augmented upon DC depletion (Fig. 5B upper; P<0.05). Additionally, ACT significantly increased the activation of APCs at tumor locations (Fig. 5B lower; P<0.05), specifically MHC-II+CD80+CD11c+ DCs. Thus, tumor-associated DCs expressed significantly higher levels of co-stimulatory CD86, CD70 and CD40 in mice treated with ACT + IT, compared to untreated mice (Fig. S4B; P<0.05, P<0.01). This increased APC maturation was not due simply to the elimination of immature DCs by IT administration as IT administration occurred at least 2 days prior to analysis allowing for repopulation, and did not completely eradicate host DCs [24]. Moreover, the total number of activated APCs was significantly higher in mice receiving ACT + IT (Quantification – Fig. 5B right; P<0.05).
Figure 5. Expanded T cells secrete CCL5 and activate host immune cells.
(*- P<0.05; **-P<0.01, Mann-Whitney) T- T cell transfer; IT - anti-CD11c immunotoxin. (A) Supernatants from naïve splenic T cells primed for 7-days against ID8-Defb29/Vegf-a tumor cell antigens were examined by Luminex assays for cytokine production. (B) (upper) Phenotypic analysis of host (CD45.2+CD3+) T cells accumulated at the tumor site 3 days after ACT. (lower) Expression profile of host CD11c+ (DCs) at tumor sites 3 days after treatment. Values represent mean percentages +/− SEM. (right) Quantification of MHCII+CD80+CD11c+ DCs in the peritoneum of mice treated with ACT and anti-CD11c immunotoxin (T+IT), compared to control mice. (C) Luminex analysis of PMA/Ionomycin-stimulated peritoneal MHCII+CD80+CD11c+ DCs from pre and post-treatment mice. (D) Proliferation of CFSE-labeled magnetically purified (left) allogeneic Balb/c slenocytes (proliferation indices-2.84 (treated) vs 1.07 (untreated)) and (center) CD8+ OT-1 splenocytes (proliferation indices-4.68 (treated) vs 1.56 (untreated)) upon culture with sorted peritoneal MHCII+CD80+CD11c+ DCs from pre (shaded) and post-treatment (unshaded) mice. (right) Granzyme-B ELISPOT analysis of CD3+ splenocytes from untreated tumor-bearing mice cultured with sorted peritoneal MHCII+CD80+CD11c+ DCs from pre and post-treatment mice. n=9 in 3 independent experiments.
Notably, CD45.2+MHCII+CD11c+ DCs sorted from treated mice secreted significantly lower levels of pro-angiogenic (KC, VEGF and PDGF-bb) and immunosuppressive (IL-10) factors compared to untreated mice (Fig. 5C), supporting our previous observations surrounding the critical role of ovarian cancer-associated DCs in maintaining tumor vasculature and immunosuppression [24]. Furthermore, these results suggested that post-treatment tumor-associated DCs are significantly more effective at amplifying immune responses against antigens spread by the direct cytotoxic activity of ex vivo activated T cells. Correspondingly, CD45.2+MHCII+CD11c+ cells sorted from (ACT + IT)-treated mice were significantly more effective than those from untreated mice at inducing the allogeneic expansion of CD3+ splenocytes (Fig 5D; left, P<0.05-proliferation indices). In addition, (host) tumor CD45.2+MHCII+CD11c+ DCs from treated mice induced greater antigen-specific proliferation of OVA-specific transgenic CD8 T cells (Fig 5D; center, P<0.05-proliferation indices). To test the actual therapeutic relevance of these findings, the capacity of sorted pre- and post-treatment CD45.2+MHCII+CD11c+ DCs to directly activate negatively purified CD3 T splenocytes from untreated tumor-bearing mice was tested in ELISPOT analyses. Most importantly, the number of splenic T cells from untreated tumor-bearing mice producing Granzyme-B directly in response to unpulsed (returning) tumor-associated CD45.2+MHCII+CD11c+ DCs from treated mice was dramatically increased, compared to that induced by tumor DCs sorted from control mice (Fig.5D; right). Together, these data indicate that transferring tumor-specific lymphocytes combined with depleting regulatory tumor-associated DCs leads to the recruitment of more immunostimulatory and less pro-angiogenic host DCs that elicit host tumor-reactive T cell activation.
Interestingly, irrelevantly (NIH-3T3 cells) primed T cells produced similar levels of CCL5 (Fig. S5A), and initially recruited comparable numbers of T cells and DCs (Fig. S5B). However, their ability to upregulate the co-stimulatory CD80 on recruited CD11c+MHC-II+ host DCs after ACT was significantly impaired, compared to mice receiving tumor-primed T cells (Fig. S5C; P<0.05 for both). Correspondingly, mice receiving irrelevantly primed T cells had significantly diminished numbers of endogenous (CD45.2+) tumor-associated T cells producing IFN-γ in response to tumor antigens compared to mice treated with tumor-reactive T cells (Fig. 6A; P<0.05). Together, these data indicate that adoptively transferred T cells need to be primed specifically against tumor antigens for the awakening of host-dependent anti-tumor immune mechanisms, which are mediated by the accumulation of antigen-experienced T cells and activated APCs at tumor locations.
Figure 6. Tumor specific priming and CCL5 are required for successful ACT.
(A) ELISPOT analysis of the number of sorted endogenous (CD45.2+) T cells producing IFN-γ in response to tumor antigen 7 days after ACT with tumor-specific or irrelevantly primed T cells (n=6 mice/group, total for A – B in 2 independent experiments). (B) Adoptive transfer of tumor-reactive T cells following anti-CD11c immunotoxin administration (T+IT) increases the total number of host CCR5+ (CD45.2+) CD3+ T cells and CD11c+ DCs (n=6 in 2 independent experiments). (C) Administration of a neutralizing antibody to CCL5 concurrently with ACT, but not an irrelevant IgG, diminished the ability of transferred T cells to increase survival of tumor bearing mice in the presence (right) or absence (left) of CD11c cell depletion. (P<0.05, n=12 in 2 independent experiments).
CCL5 is required for the therapeutic effects elicited by ACT
Since adoptively transferred T cells produced high levels of CCL5 and induced leukocyte recruitment, we next confirmed that most endogenous (CD45.2+) tumor-associated lymphocytes and DCs in mice receiving ACT expressed surface CCR5 (Fig. S5D). Additionally, ACT + IT significantly increased the number of host (CD45.2+) T cells and DCs at tumor locations specifically expressing CCR5, compared to control mice (Fig. 6B; P<0.05 and P<0.01, respectively).
Since transferred T cells produce large amounts of CCL5 and induced the accumulation of CCR5+ target cells, we hypothesized that CCL5 may be also required for the therapeutic effects triggered by ACT. Correspondingly, a neutralizing anti-CCL5 antibody injected concurrently with ACT significantly impaired the therapeutic effect (Fig. 6C; P<0.05), while identical concentrations of an irrelevant IgG did not influence the effect of ACT (Fig. 6C; P>0.05). Importantly, neither the neutralizing anti-CCL5 antibody nor the irrelevant IgG affected the survival of intraperitoneal ID8-Defb29/Vegf-a tumor-bearing mice not receiving ACT (Fig. 6C and data not shown). Notably, this CCL5-dependent effect was also absolutely required for the beneficial effects observed with ACT combined with regulatory DC depletion, as eliminating CCL5 abrogated its therapeutic effect. Together, these data suggest that CCL5 produced by the transferred T cells, rather than endogenous CCL5, is at least partially required for the accumulation of host lymphocytes, which cooperatively function in delaying tumor progression.
DISCUSSION
Here we demonstrate that depleting regulatory DCs from the tumor microenvironment transforms tumor-reactive T cell adoptive therapy from a palliative to a curative strategy in mice bearing ovarian cancers. Most importantly, long-term anti-tumor protection was mediated by exogenous CCL5-dependent host immune mechanisms, which remained activated after the disappearance of exogenously administered T cells.
The clinical effects elicited by adoptively transferred tumor-specific T cells in melanoma patients are usually attributed to their expansion and capacity to persist in vivo, but their ability to arouse host-derived protective immune mechanisms has not been addressed. Our ovarian cancer systems recapitulate recent findings suggesting that tumor-reactive T cells do not persist in patients with aggressive epithelial cancers, despite the fact that large numbers of lymphocytes can be safely administered [11]. Strategies that maximize the awakening of host-mediated anti-tumor immunity, rather than the direct anti-tumor activity of transferred T cells, could be more valuable against these tumors.
Early effector T cells are more effective for in vivo tumor treatment, than more differentiated effector T cells [6]. Aiming to optimize a system for the early priming of tumor-reactive T cells that can be replicated using DCs generated from peripheral blood from ovarian cancer patients, we used dendritic cells pulsed with doubly irradiated cancer cells. We hypothesized that polyclonal T cells would be better equipped to prevent the development of T cell-resistant tumor variants through immunoediting, than lymphocytes engineered to react against a single antigen. Surprisingly, we found that shortly primed lymphocytes from healthy or tumor-bearing mice induced comparable therapeutic effects, suggesting that naïve peripheral blood T cells from ovarian cancer patients could also be converted into effective anti-tumor effectors through de novo priming.
Adoptively administered T cells rapidly expanded upon elimination of tumor-associated DCs, but disappeared within 2 weeks. They required perforin - but not IFN-γ - for their therapeutic effects, supporting the requirement for cytotoxic killing. Shortly primed anti-tumor T cells also required tumor-specific recognition, as irrelevantly-primed T cells induced reduced survival increases, despite producing comparable levels of measured cytokines/chemokines. The therapeutic effects induced by ACT were abrogated by CCL5 neutralization. Correspondingly, tumor-reactive T cells induced rapid accumulation of CCR5+ antigen-experienced (CD44+) T cells and antigen-presenting cells. Importantly, both tumor-specific and irrelevantly primed T cells produced similar levels of CCL5. However, the latter were inefficient at activating DCs, translating to reduced accumulation of T cells at tumor sites and, correspondingly, their inability to stop tumor progression.
The blatant activation of host cells after ACT, as presented here, has never been previously reported. Anti-tumor T cells primed as described produced pro-inflammatory cytokines that recruit host immune cells to the tumor site. Tumor-reactive T cells, but not T cells expanded to irrelevant antigens, also induced the in situ maturation of host DCs and accumulation of antigen-experienced lymphocytes, as seen by their increased CD44 expression and production of IFN-γ and granzyme-B. Combining ACT with regulatory DC depletion recruited antigen-presenting cells capable of stimulating T cell expansion and their production of the cytotoxic granzyme-B. Most importantly, host-derived lymphocytes acquired a memory phenotype and their activity persisted after the elimination of exogenous lymphocytes. Therefore, long-term protection is mediated by host-dependent immune mechanisms, rather than by the persistent effect of transferred T cells.
Since CCL5, perforin and specific priming were required for successful ACT, we propose a mechanism whereby transferred lymphocytes briefly primed against tumor antigens mediate the specific lysis of tumor cells. Simultaneously, the release of large quantities of CCL5 by these cells precipitates the recruitment of host anti-tumor T cells and APCs. APCs mobilized to the tumor site become activated by tumor antigens made available by the perforin-mediated lysis, and activate host T cells. Irradiation may further boost this process by facilitating the release of tumor antigen and providing space for tumor-reactive T cell expansion. Although T cells expanded to irrelevant antigens secreted similar levels of CCL5, they were unable to effectively activate tumor-associated APCs. This implies that while CCL5 is crucial for successful ACT, specific lysis of tumor cells is likely required to release antigens necessary to mature accumulated DCs and induce long-lasting antitumor effects.
In summary, our results demonstrate the feasibility of eliciting sustained, host-mediated, protective immunity against ovarian carcinoma by administering previously naïve T cells shortly primed against tumor antigens after depleting tumor-associated regulatory DCs. These findings bring us closer to successfully employing ACT therapies in ovarian cancer and beyond.
MATERIALS AND METHODS
Mice and tumor lines
C57BL/6 (B6) and CD45.1 mice were purchased from the National Cancer Institute (Frederick, MD). B6.129S7-Ifntm/Ts/J (IFN-γ−/−), perforin deficient C57BL/6-Prf1tm/Sdz/J (Pfp−/−) mice and red fluorescent C57BL/6-Tg(ACTB-MAP2K1*K97M)1Stl/J mice were purchased from The Jackson Laboratory. Experiments were conducted in accordance with the Dartmouth Medical School guidelines. ID8-Defb29/Vegf-a, ID8-luciferase, NIH-3T3 and cultured splenocytes and DCs were maintained in RPMI medium containing 10% fetal bovine serum.
T Cell Expansion, Adoptive Immunotherapy and DC Depletion
Bone marrow derived DCs were produced [30], then pulsed overnight (10:1) with irradiated (10, 000 Rads) and UV-light exposed ID8-Defb29/Vegf-a or NIH-3T3 cells. Splenocytes were harvested from CD45.1+ mice and cultured for 7 days with ID8-Defb29/Vegf-a/ NIH-3T3 - pulsed DCs and 10 U/ml recombinant human IL-2 (Peprotech). 6–8 week old mice were injected i.p. with 1.5 ×106 ID8-Defb29/Vegf-a cells and treated on day 7 and 14 with i.p. adoptive transfer of 1.5 ×106 in vitro activated splenic T cells. Lymphopenia was induced by sublethal TBI (300 Rads) of tumor-bearing mice on day 7, 5 hours prior to ACT. DCs were eliminated by i.p. administration of 10 µg/mouse of an anti-CD11c-immunotoxin [24] on the day prior to T cell transfer (day 6) and thrice weekly for two weeks thereafter.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by a 2006 Liz-Tilberis Award; NCI Grants #RO1CA124515 and #R01CA120777; ACS#IRG-82-003-22; and NCRR#2P20RR016437-06. We thank the Immune Monitoring Laboratory for performing the Bioplex experiments; the Irradiation Shared Resource for irradiating the mice and cells, The Englert Cell Analysis Laboratory for cell sorting and the NIH Tetramer Core Facility for providing the tetramer.
ABBREVIATIONS
- IT
Immunotoxin
- ACT
Adoptive Cell Therapy
REFERENCES
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RA, Dudley ME, Wunderlich JR. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunder NN, Wallen H, Cao J. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Klebanoff CA, Palmer DC. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overwijk WW, Theoret MR, Finkelstein SE. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coukos G, Conejo-Garcia JR, Roden RB, Wu TC. Immunotherapy for gynaecological malignancies. Expert Opin Biol Ther. 2005;5:1193–1210. doi: 10.1517/14712598.5.9.1193. [DOI] [PubMed] [Google Scholar]
- 9.Serafini P, Meckel K, Kelso M. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kershaw MH, Westwood JA, Parker LL. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 13.Conejo-Garcia JR, Benencia F, Courreges MC. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Res. 2004;64:2175–2182. doi: 10.1158/0008-5472.can-03-2194. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Conejo-Garcia JR, Katsaros D. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 15.Sato E, Olson SH, Ahn J. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamanishi J, Mandai M, Iwasaki M. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramakrishna V, Ross MM, Petersson M. Naturally occurring peptides associated with HLA-A2 in ovarian cancer cell lines identified by mass spectrometry are targets of HLA-A2-restricted cytotoxic T cells. Int Immunol. 2003;15:751–763. doi: 10.1093/intimm/dxg074. [DOI] [PubMed] [Google Scholar]
- 18.Yen MJ, Hsu CY, Mao TL. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 19.Odunsi K, Jungbluth AA, Stockert E. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 20.Hasegawa K, Koizumi F, Noguchi Y. SSX expression in gynecological cancers and antibody response in patients. Cancer Immun. 2004;4:16. [PubMed] [Google Scholar]
- 21.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 22.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Huarte E, Cubillos-Ruiz JR, Nesbeth YC. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coukos G, Conejo-Garcia JR, Buckanovich R, Benencia F. Vascular leukocytes: a population with angiogenic and immunossuppressive properties highly represented in ovarian cancer. Adv Exp Med Biol. 2007;590:185–193. doi: 10.1007/978-0-387-34814-8_13. [DOI] [PubMed] [Google Scholar]
- 26.Conejo-Garcia JR, Benencia F, Courreges MC. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Finkelstein SE, Klebanoff CA. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 29.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz MB, Kukutsch N, Ogilvie AL. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:277–292. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.