Abstract
The planar cell polarity (PCP) signaling pathway is essential for embryonic development because it governs diverse cellular behaviors, and the “core PCP” proteins, such as Dishevelled and Frizzled, have been extensively characterized1–4. By contrast, the “PCP effector” proteins, such as Intu and Fuz, remain largely unstudied5, 6. These proteins are essential for PCP signaling, but they have never been investigated in a mammal and their cell biological activities remain entirely unknown. We report here that Fuz mutant mice display neural tube defects, skeletal dysmorphologies, and Hedgehog signaling defects stemming from disrupted ciliogenesis. Using bioinformatics and imaging of an in vivo mucociliary epithelium, we establish a central role for Fuz in membrane trafficking, showing that Fuz is essential for trafficking of cargo to basal bodies and to the apical tips of cilia. Fuz is also essential for exocytosis in secretory cells. Finally, we identify a novel, Rab-related small GTPase as a Fuz interaction partner that is also essential for ciliogenesis and secretion. These results are significant because they provide novel insights into the mechanisms by which developmental regulatory systems like PCP signaling interface with fundamental cellular systems such as the vesicle trafficking machinery.
PCP signaling is essential for a variety of vertebrate developmental events, including morphogenesis of the neural tube, heart, kidney, and ear. Components of the pathway govern a wide array of polarized cellular behaviors, including cell intercalation and migration, cell division, and ciliogenesis1, 2. In Drosophila and Xenopus, the “PCP effector” proteins, including Fuz, act together with the “core” components such as Dishevelled (Dvl)5, 6. The PCP effectors have received little attention, being the subject of only a single study in vertebrate animals6, whereas the core proteins have been the subject of intense study1, 2, 7–9. Fuz is essential for ciliogenesis in Xenopus6, but its precise molecular function, like that of all intracellular PCP proteins, remains very poorly understood.
We asked if PCP effectors were essential for mammalian development by obtaining murine ES cells with a gene-trap inserted into the second of eleven exons in Fuz, the mouse orthologue of Drosophila Fuzzy and Xenopus Fuz. This gene trap is predicted to disrupt the transcription of the Fuz gene. These cells were used to generate mice carrying the inactive Fuz allele. Litters from heterozygous matings produced no viable full-term homozygous mutant pups, as the small litters failed to follow expected genotypic ratios upon analysis. Homozygous fetuses were obtained at E18, and these mice displayed a wide range of developmental defects (Fig. 1 and Supp. Fig. 1).
Figure 1.
Mice lacking a functional Fuz gene display multiple developmental defects. (a) Control mouse, E18.5 and (b) Fuzgt/gt mouse. Skeletal preparation of (c) control hindlimb and (d) Fuzgt/gt hindlimb. Inset shows a paw with extreme polydactyly from a Fuzgt/gt mouse. (e, f) Sternum preparations from control and Fuzgt/gt mouse. (g, h) Confocal projections of Meckels’ cartilage stained with acetylated tubulin (red) and DAPI (blue) exhibits diminished primary cilia in Fuzgt/gt mouse sections. Scale bars = 5μm. Mean cilia length +/− SEM = 1.73+/−0.06μm in WT (n=70) and 0.87 +/−0.04μm in Fuzgt/gt (n=52); p<0.001. (i) Dissected heart from a control mouse, arrows indicate outflow tracts. (j) Dissected heart from a Fuzgt/gt mouse, arrowhead indicates single outflow tract. (k, l) Expression of Nkx2.2 (green) in the ventral neural tube is diminished in Fuzgt/gt mice (red arrowhead). (m, n) Expression of FoxA2 (green) in the ventral neural tube is lost in Fuzgt/gt mice (red arrowhead).
All homozygous mutant mice displayed severe developmental defects, including craniofacial malformations and incompletely penetrant rostral neural tube closure defects, such as exencephaly and encephaloceles (Fig. 1B, Supp. Fig. 1D, E). Some Fuz mutant mice displayed normal neural tube closure despite having severe craniofacial and ocular defects (Supp. Fig. 1F). However, even mice with mild overt neural tube closure defects displayed severe internal hydrocephalus (Supp. Fig. 1H). Fuz mutant mice consistently displayed polydactyly on all limbs (Fig. 1D), and we observed widespread defects in skeletal development and organogenesis, including malformed sternum, ribs, and long bones, as well as severely hypoplastic lungs and conotruncal defects (Fig. 1C–F, I, J; Supp. Fig. 1I–L). This spectrum of defects reflects the phenotype of mice with defects in ciliogenesis10, 11, and is also reminiscent of the defects in human patients with ciliopathic syndromes such as Bardet-Biedl Syndrome 12, 13, Meckel-Gruber syndrome14 or Jeune’s asphyxiating thoracic dystrophy15
Collectively, these malformations are consistent with a failure of cilia-mediated Hedgehog signaling in Fuz mutant mice, so we next examined the expression of Hedgehog target genes in the spinal cord6, 10. We found that while Nkx2.2 and FoxA2 were robustly expressed in the ventral spinal cord of control mice, these expression domains were almost entirely absent in Fuz mutant mice (Fig. 1K–N). Finally, we found that Fuz mutant mice displayed defects in primary ciliogenesis. Immunostaining for acetylated tubulin revealed that primary cilia in the Fuz mutant mice were significantly shorter than cilia of wild-type mice (Fig. 1G–H). Despite the extremely significant difference in average length, the effect on cilia length was variable, and cilia of nearly normal length were occasionally observed in Fuz mutant mice (Supp. Fig. 1B), consistent with the result of Fuz knockdown in Xenopus6. Moreover, this finding is consistent with the Fuz mutant mouse embryonic phenotypes; Fuz mutants resemble single BBS mutations or hypomorphic alleles of IFT genes, in which cilia are present but defective10, 11. By contrast, Kif3a null mice lack cilia entirely and display far more severe embryonic phenotypes16.
In addition to these defects in Hedgehog signaling, Fuz mutant mice also displayed defects consistent with a failure of PCP signaling. For example, the homozygous Fuz mutant mice displayed a kinked or curly tail (Fig. 1B), a phenotype that is consistently associated with heterozygous mutations in core PCP proteins such as Dvl or Vangl28, 9. The homozygous Fuz mutant mice also displayed cardiac defects, including single outflow tracts and ventral septal defects (Fig. 1J; Supp. Fig. 1L), similar to those observed in mouse models lacking core PCP genes2, 8. The pattern of congenital malformations in the Fuz mutant mice is thus entirely consistent with that found in Xenopus embryos following Fuz knockdown6. Fuz morphant Xenopus embryos and Fuz mutant mice each display comparatively mild PCP defects together with more severe defects in cilia-mediated developmental events.
The evolutionarily conserved role for Fuz from frogs to mammals provides us an opportunity to exploit the tremendous wealth of bioinformatics data in mammalian systems to help us elucidate the mechanisms of action for the novel Fuz protein. We first queried the human interactome for potential Fuz-interacting proteins. We noted that high-throughput yeast two-hybrid screening17 suggested a weak interaction between human Fuz and the protein encoded by human Chromosome 1 Open Reading Frame 89 (Chr1orf89). BLAST predicted this gene to encode a small GTPase similar to REM2 and the vesicle-targeting Rab proteins (Supp. Fig. 2A). Based on this homology, we propose renaming C1orf89 as Rem/Rab-Similar GTPase 1, RSG1. Co-immunoprecipitation confirmed that RSG1 associates with Fuz (Supp. Fig. 2B).
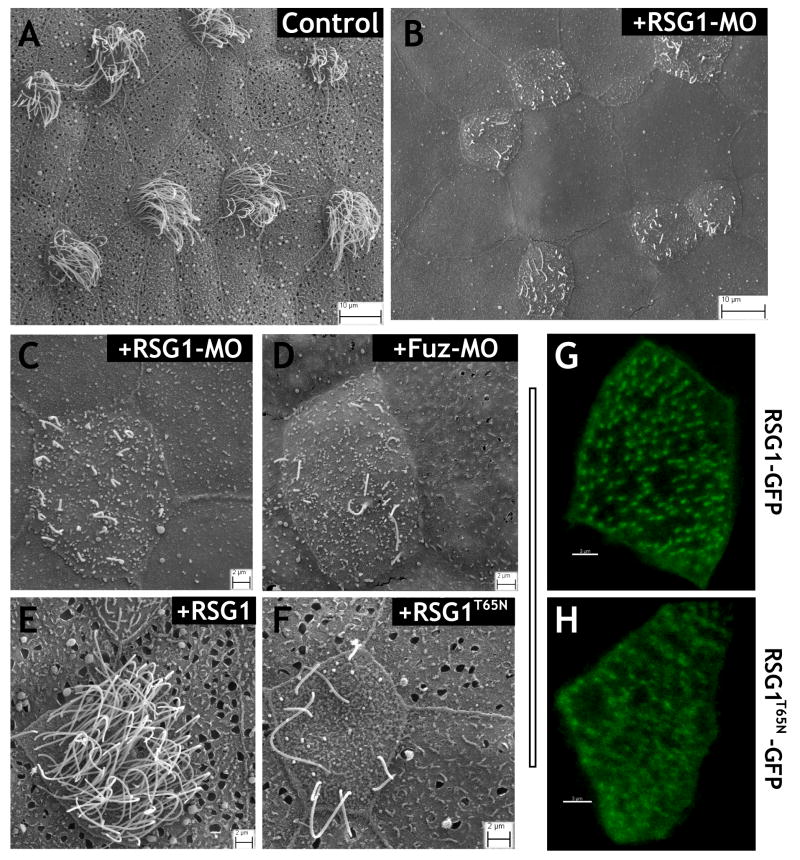
To assess the function of RSG1, we designed an antisense morpholino-oligonucleotide (MO) to block translation of the protein. Dorsally-targeted injection of this MO resulted in defects in rostral neural tube closure, similar to the defects observed in Fuz morphants6 and in Fuz mutant mice (Supp. Fig. 2D, F). Co-injection of GFP-RSG1 mRNA suppressed the open neural tube phenotype of RSG1 morphants in a dose-dependent manner, demonstrating that the effect of the MO was specific (Supp. Fig. 2D, G). To examine the function of RSG1 during ciliogenesis, we made use of the Xenopus embryonic epidermis, which is a highly tractable and easily imaged in vivo model for mucociliary epithelial development18. The MO was targeted specifically to the epidermis by ventral injection, thus circumventing the neural tube phenotype. In these morphants, SEM revealed severe defects in ciliogenesis in the epidermal multi-ciliated cells (Fig. 2A–C). This phenotype was very similar to that of Fuz knockdown (Fig. 2D).
Figure 2.
RSG1 controls ciliogenesis and secretion. (a) SEM of intact, control Xenopus ciliated epidermis reveals multi-ciliated cells and surrounding mucous secreting cells (b) RSG1 morphants display defects in ciliogenesis and absence of mucous granules and exocytic pits. (c) Higher-magnification view of RSG1 morphant ciliated epidermis displaying diminished cilia numbers and lengths and a decrease in exocytic pits in neighboring secretory cells. (d) Fuz morphants also display diminished cilia numbers and lengths and a decrease in exocytic pits in secretory cells. (e, f) Epidermal targeted over-expression of RSG1T65N, but not wild type RSG1, results in defects in ciliogenesis as well as decreases in mucous granules and exocytic pits in secretory cells. (g, h) GFP-RSG1 (low-level expression) localizes to the basal body region of multi-ciliated cells, whereas GFP-RSG1T65N (low-level expression) in multi-ciliated cells is diffuse and not tightly associated with basal bodies. Observations of fluorescence levels following expression of GFP-RSG1 and GFP-RSG1T65N mRNA were comparable, suggesting similar rates of translation for the two proteins.
RSG1 contains the invariant serine/threonine residue whose mutation to asparagine has been shown in other GTPases to alter the guanine nucleotide binding affinity and to generate a dominant-negative protein (Supp. Fig. 2C yellow/pink residues). We therefore mutated this residue and expressed high levels of RSG1T65N in Xenopus embryos. Overexpression of RSG1T65N resulted in defective ciliogenesis in multi-ciliated cells of the epidermis, while we observed no effect from overexpression of wild-type RSG1 (Fig. 2E, F). These experiments confirm the results of RSG1 knockdown and suggest that the GTPase activity of RSG1 is essential for ciliogenesis.
Finally, we examined RSG1 subcellular localization by expression of low levels of GFP-RSG1, and we observed that it localized strongly to the vicinity of basal bodies in multi-ciliated cells (Fig. 2G). This localization is very likely accurate, since GFP-RSG1 can rescue the phenotype of RSG1 morphants (Supp. Fig. 2D, G). By contrast, the GFP-RSG1T65N localized only very poorly to basal bodies (Fig. 2H). Together, the results of knockdown, expression of the dominant-negative, and localization of the GFP-fusion proteins, suggest a role for the RSG1 GTPase in ciliogenesis. These results are consistent with a role for this protein in mediating Fuz function.
Our bioinformatic approach successfully revealed novel aspects of the molecular network in which Fuz functions, so we extended this approach to investigate the cell biological function of the novel protein encoded by the Fuz gene. We turned to mouseFUNC, a large-scale community effort to systematically predict mouse gene function using the consensus of diverse computational approaches19. MouseFUNC predicted a central role in vesicle trafficking for Fuz (Supp. Fig. 3A). This suggestion was supported by results of iterative BLAST searches (PSIBLAST), which identified modest similarity between Fuz and the yeast vacuolar fusion protein Mon1 (data not shown).
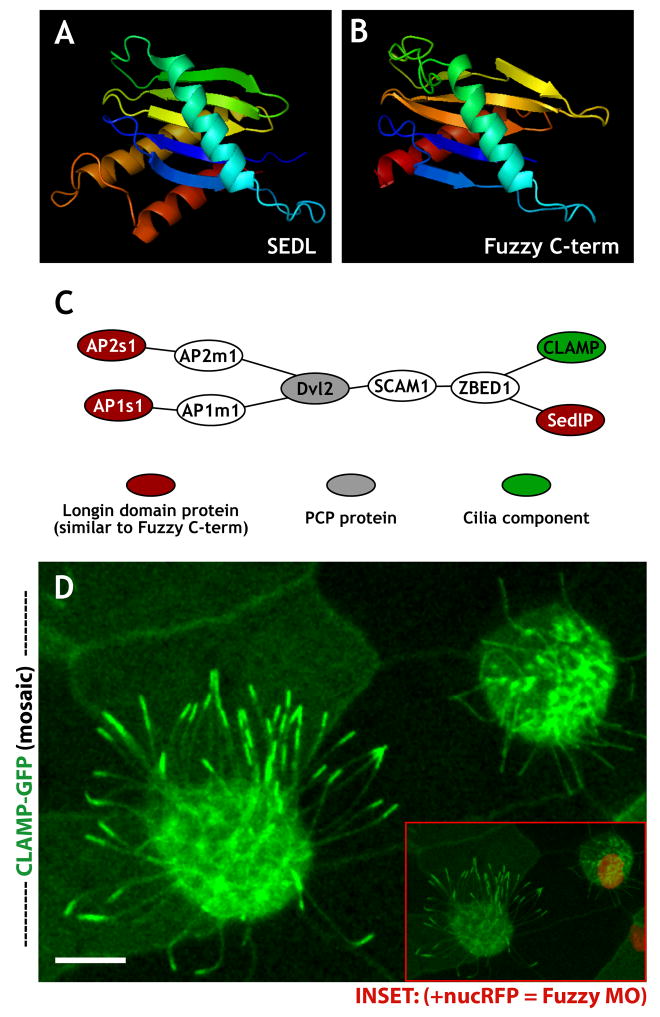
We next used homology modeling20, 21 to predict the structure of the Fuz protein. Using mGENTHREADER (see Supplemental Methods), we predicted that the C-terminus of the Xenopus Fuz protein should fold into a series of five β-sheets flanked by α-helices (Fig. 3A, B; Supp. Fig. 3C, D). This structure, known as a longin-domain22, is consistent with a vesicle trafficking function for Fuz. Indeed, the longin-domain is present in the structure of SEDL, a subunit of the vesicle-tethering TRAPP complex that is associated with skeletal dysmorphogenesis23, 24. Moreover, the longin-domain is shared by several other vesicle trafficking proteins, including subunits of the AP clathrin adaptor complexes25 and the membrane-fusing SNARE proteins, sec22b, VAMP7, and Ytk6p (Refs. 22, 26).
Figure 3.
Homology modeling, network predictions, and experimental validation suggest a trafficking function for Fuz. (a, b) Rendered protein models (Open-Source PyMOL 0.99rc6 software). (a) Sedl N-terminal domain (pdb:1H3Q). (b) 1H3Q based homology threaded model of the C- terminal -aa(s) 287–419 - of Xenopus Fuz protein. (c) Illustration of experimentally-derived protein-protein interactions (see Supplemental Methods) linking Dvl2 with longin-domain proteins AP1s, AP2s, and SedlP as well as CLAMP. (d) Mosaic imaging of live embryo expressing CLAMP-GFP, which localizes to ciliary axonemes and apical tips. A nucRFP marks the nuclei, serving as a lineage tracer for co-injected Fuz MO. The confocal slice reveals a loss of apical localized CLAMP-GFP at ciliary tips in FUZMO cells (inset shows merge of (e) and a more basal slice to display nucRFP signal). Scale bar in (e) = 10uM.
These longin-domain containing proteins are all tightly linked to one another in probabilistic networks of human, mouse, and yeast genes, and this node within the gene networks is tightly linked to other core vesicle-trafficking proteins (Supp. Fig. 4; and see Supplemental Methods). However, the scale of these linkages was too large to generate easily testable hypotheses. We therefore returned to the physical interactome data, looking this time for relationships between longin-domain containing proteins with structural similarity to the Fuz C-terminus. We found that the longin-domain proteins AP2σ, AP1σ, and SEDL were all linked by physical associations to the PCP protein Dvl2 (Fig. 3C), which interacts genetically with Fuz5, 6. More importantly, AP2σ, AP1σ, SEDL and Dvl2 were all linked by physical association to the CaLponin Homology and Microtubule-associated Protein (CLAMP, also called spef1; Fig. 3C), a microtubule-bundling protein that is a known component of cilia and flagella7, 27,28.
Since Fuz is essential for ciliogenesis, we asked if Fuz may play a role in CLAMP localization. In living Xenopus embryos, CLAMP-GFP labeled the axonemes of cilia in multi-ciliated cells (Fig. 3D, left), as has been reported previously for sperm flagella28. In addition to the axonemal labeling, however, we also observed an obvious enrichment at the apical tips of cilia (Fig. 3D, left). To test the effect of Fuz knockdown, we used targeted injection to generate in vivo mosaic epidermis, where control and morphant cells are intermingled. In these mosaics, the morphant cells are indicated specifically by co-injected mRNA encoding histone 2B-RFP (nucRFP; Fig. 3D, inset). In nucRFP-positive Fuz morphant cells, CLAMP-GFP was visible in the shortened and dysmorphic cilia, but the normal accumulation of CLAMP-GFP at the apical tips was entirely absent (Fig. 3D, right).
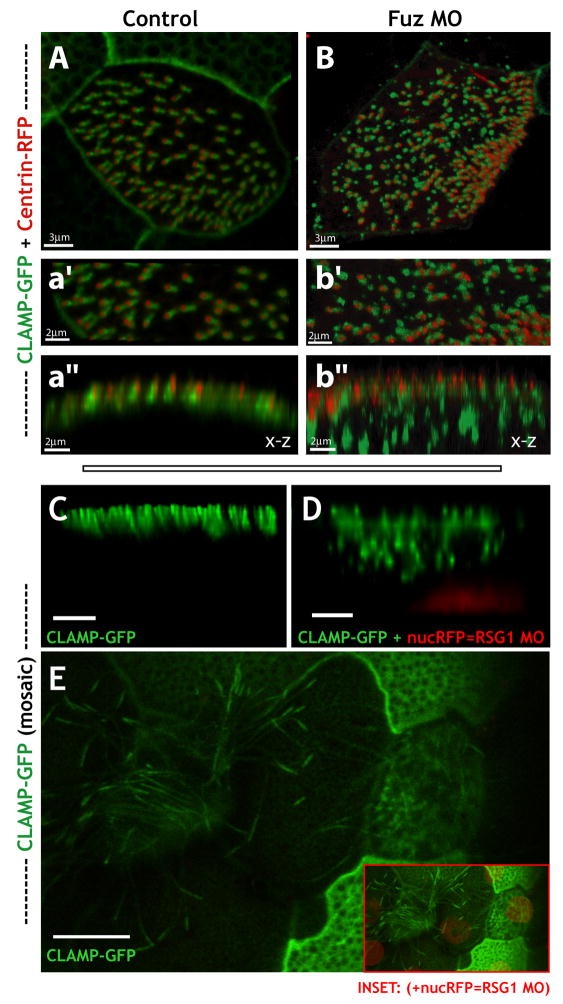
This role for Fuz in CLAMP localization was of particular interest because we previously found that CLAMP also co-localizes with Dvl2 in the vicinity of the ciliary rootlet7, which is a known nexus for vesicle trafficking to cilia7, 29, 30. In control embryos, CLAMP-GFP is restricted to the apical cell surface, where it formed a well-defined, linear structure adjacent to the apically-docked basal bodies indicated by co-expressed centrin-RFP (Fig. 4A, a′, a″ and see Refs. 7, 18). In contrast to controls, the CLAMP-GFP signal in Fuz morphant embryos was present in larger, irregularly-shaped foci. Z-projections revealed that many of these irregular foci were well below the apical cell surface (Fig. 4B, b′, b″), though centrin-RFP formed an even line at the apical surface of these cells. This result indicates that Fuz, unlike Dvl (Ref. 6), is not essential for apical docking of basal bodies, but is essential for apical trafficking of CLAMP. The dual localization of CLAMP-GFP at the basal apparatus and at the apical tip is reminiscent of IFT88 and IFT52 in mammalian cells31, and our data suggest that Fuz is required for accumulation of CLAMP at both sites.
Figure 4.
Fuzzy and RSG1 control trafficking to basal bodies as well as to the tips of cilia. (a, b) Fuz knockdown disrupts localization of CLAMP-GFP to the ciliary rootlet. Confocal stacks of formaldehyde fixed Xenopus epidermis expressing centrin-RFP and CLAMP-GFP mRNAs. (a) Multi-ciliated cell in x-y view from an uninjected control embryo exhibits elongated CLAMP-GFP signal extending from relatively evenly spaced basal bodies (centrin-RFP). (a′) Higher magnification view of the x-y section from [a]. (a″) X-Z projection of the stack shown in [a′] displays apical co-localization of centrin-RFP and CLAMP-GFP. (b) Ciliated cell in an x-y view of the apical surface in a Fuz morphant reveals defects in the spacing of centrin-RFP signal and defects in elongated CLAMP-GFP signal. Additionally CLAMP-GFP signal is not faithfully co-localized with centrin-RFP signal in many cases. (b′) Higher magnification view of the x-y section from [b]. (b″) X-Z-projection of the stack shown in [b′] reveals apical alignment of the centrin-RFP signal however in many cases the CLAMP-GFP signal is below the apical surface in large punctae. (c–e) Mosaic imaging of live agarose embedded embryo. CLAMP-GFP highlights a variety of epidermal structures. RFP-Histone 2B (nuc-RFP) serves as a lineage tracer for morpholino-injected cells. (c, d) 3D projections (x-z). RSG1 MO (+ nucRFP cells) display defects in normal CLAMP-GFP localization along the apical surface. (e) Confocal slice (x-y) exhibiting a loss of apical localized CLAMP-GFP to ciliary tips in RSG1 MO cells (inset shows merge of (e) and more basal slice to display nucRFP signal). Scale bars in (c, d) are 3uM; scale bar in (e) is 10μm.
Because the RSG1 GTPase binds to Fuz and is essential for ciliogenesis, we predicted interference with RSG1 function would elicit a similar CLAMP trafficking phenotype. Indeed, in mosaic embryos, nucRFP-positive RSG1 morphant cells displayed defects in the trafficking of CLAMP-GFP to the apical cell surface, while nearby control cells displayed no such defects (Fig. 4C, D). In addition, expression of RSG1T65N also severely disrupted apical trafficking of CLAMP-GFP, while expression of wild-type RSG1 had little effect (Supp. Fig. 5). Finally, RSG1 knockdown also eliminated the accumulation of CLAMP-GFP to the apical tips of cilia in multi-ciliated cells (Fig. 4E).
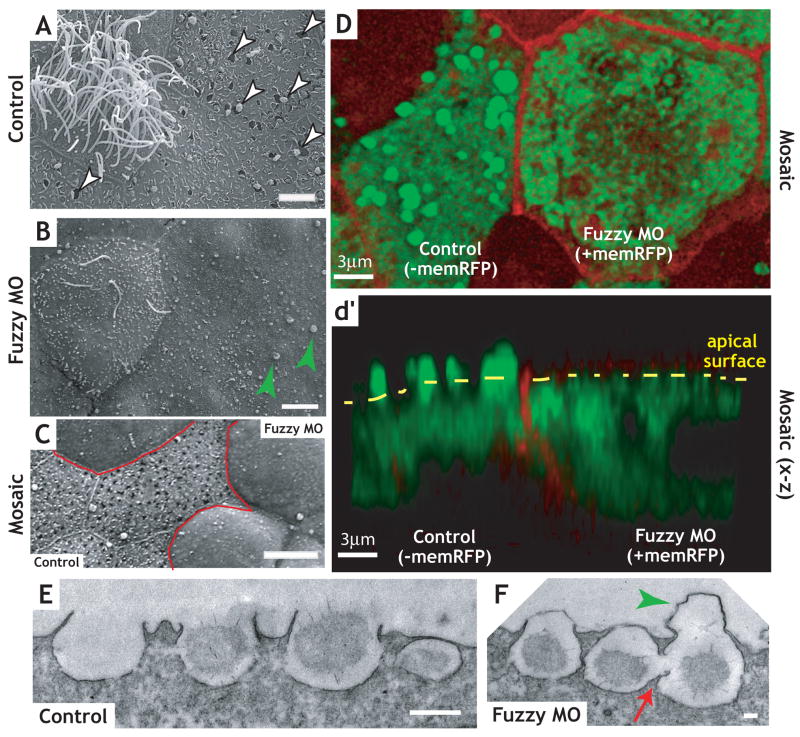
Our data thus demonstrate that Fuz and RSG1 act to regulate trafficking during ciliogenesis. Because longin-domain proteins participate in many fundamental vesicle trafficking events22, we next asked if Fuz might play a broader role in trafficking than is reflected by the phenotype in multi-ciliated cells. We examined the mucus-secreting goblet cells that surround the multi-ciliated cells in the Xenopus epidermis18. Scanning electron microscopy (SEM) demonstrated that the apical surface of the goblet cells in controls were decorated with numerous open exocytic vesicles, many of which were actively releasing mucus granules (Fig. 5A). By contrast, open exocytic vesicles were extremely rare and few mucus granules were visible on goblet cells in Fuz morphants (Fig. 5B). We also observed that either knockdown of RSG1 or overexpression of RSG1T65N elicited defects in exocytosis in mucus-secreting cells (Fig. 2C, F), suggesting that this GTPase is a key effector of Fuz function in multiple cell types.
Figure 5.
Knockdown of Fuz disrupts exocytosis in mucus-secreting cells. (a) Wild type multi-ciliated cell (left) flanked by secretory cells in Xenopus mucociliary epidermis. Control cells have an average of over 90 open exocytic pits per cell. (b) Fuz morphants display defects in ciliogenesis in multi-ciliated cell (left) and failure of exocytosis in mucus-secreting cells (note the absence of exocytic pits indicated by white arrowheads in [a]). Green arrowheads indicate apical membrane blebs (see also panel F, below). In a representative experiment, Fuz morphant cells had fewer than 5 exocytc pits per cell on average (difference from control is significant by the Mann-Whitney U-test; p<0.0001). Scale bars in A, B = 5μm (c) Mosaic epidermal tissue, with morphant cells outlined in red. Scale bar = 10μm (d) Confocal section (x-y) of mosaic embryo in which membrane-RFP (memRFP, red) mRNA was co-injected with Fuz morpholino and processed for Xeel (interlectin-2) antibody (green). Cell expressing a high level memRFP (right) lacks apical Xeel antibody staining comparable to the neighboring cell (right). (d′) Confocal projection (x-z) of [d] illustrates the loss of apical Xeel staining (green) in the Fuz morphant cell correlated with apical memRFP expression (right). Scale bars in D, d′ = 3μm (e) TEM of section of control Xenopus epidermis shows empty and mucus granule-containing vesicles docked at the apical surface, whereas Fuz morphants (f) display a defect of vesicle fusion with the plasma membrane release, as illustrated by large membrane protrusions (green arrows in [b, f]). Additionally, frequent homotypic vesicle fusion events were observed in secretory cells of Fuz morphants (red arrow). Scale bar in E = 500nm; Scale bar in F= 100nm.
To confirm the failure of secretion in Fuz morphant goblet cells, we turned again to mosaic epidermis where we examined immunostaining for Intelectin2, a major component of the secreted Xenopus epidermal mucus18, 32. In control cells of these mosaics (indicated by an absence of membrane-RFP co-injected with the MO), Intelectin2 in exocytosing mucus granules was visible as discrete foci, at or above the apical surface (Fig. 5D, d′). By contrast, Intelectin2 signal was present only below the apical cell surface in neighboring morphant cells (indicated by the presence of co-injected membrane-RFP in Fig. 5D, d′). The failure of exocytosis in morphant cells in these mosaic epithelia was also confirmed by SEM (Fig. 5C).
The joining of membrane compartments proceeds through discrete steps of transport, tethering, and fusion33. Our bioinformatic analyses suggested a possible relationship between Fuz and either vesicle tethering or membrane fusion processes (Supp. Fig. 4), and electron microscopy of Fuz morphants supports the latter relationship. TEM revealed that morphant goblet cells often were decorated by large apical membrane blebs atop putative exocytic vesicles (Fig. 5F, green arrowhead) and this phenotype was obvious in SEM (Fig. 5B, green arrowheads). Such apical membrane blebs were also apparent in RSG1 morphants (Fig. 2B, C). Many mucus-filled vesicles in Fuz morphants appeared to be tethered to the apical plasma membrane, though very few had fused (Fig. 5F).
The finding that mucus-filled vesicles in Fuz morphant cells tether to, but fail to fuse with, the apical plasma membrane might suggest a general role for Fuz in governing vesicle fusion. However, secretion in Xenopus goblet cells can proceed by compound exocytosis, in which secretory vesicles fuse with one another as they approach the plasma membrane3, 34–36. We also observed such homotypic fusion of mucus-containing vesicles in Fuz morphants, despite the failure of nearby, apparently tethered, vesicles to fuse with the plasma membrane (Fig. 5F, red arrow; Supp. Fig. 6B, C). In some cases, vesicles were observed that had fused to one another, but had not yet tethered to the apical plasma membrane (Supp. Fig. 6B, arrows). These data demonstrate that Fuz is essential for mediating only a specific subset of membrane fusion events.
The PCP signaling cascade is broadly required for development of vertebrate embryos. However, studies to date have focused on only a small number of the known PCP genes, such as Dvl and Vangl2 (Refs. 2, 7–9). Here, we have demonstrated that the largely un-studied PCP effector protein Fuz is fundamentally necessary for embryonic development in vertebrates. Our data suggest a central role for Fuz in regulating targeted membrane fusion events, and this cellular function can explain the phenotype of embryos lacking Fuz function.
First, vesicle trafficking to the basal body and within axonemes is essential for ciliogenesis (e.g. Refs. 7, 29–31), and the phenotypes observed in Xenopus or mouse embryos lacking Fuz function reflect those observed in mice lacking key ciliogenesis factors, such as the BBS or IFT proteins10, 11. A key role of the cilium in development is thought to be the transduction of Hedgehog signaling10, so it is relevant that Hedgehog target gene expression is lost in Fuz morphant Xenopus embryos6 and in Fuz mutant mice (Fig. 1). Secondly, defective secretion in cells lacking Fuz function may also contribute to the embryonic phenotype. We observed severe skeletal defects in vertebrate embryos lacking Fuz function (Fig. 1 and Ref 6); skeletal defects are likewise observed in humans or zebrafish with mutations in Sec23A, a subunit of the COPII complex37, 38 and in humans with mutations in the TRAPP complex subunit, SEDL (Ref. 24).
Our data therefore place Fuz and the interacting GTPase RSG1 at the interface of developmental regulatory systems (e.g. PCP signaling) and fundamental cell biological processes (e.g. ciliogenesis, secretion). In combination with the finding that Dvl mediates vesicle association with basal bodies in ciliated cells7, the data here suggest that coordination of vesicle trafficking may be a unifying mechanism by which PCP signaling can control so many diverse cellular behaviors during embryonic development.
Materials and Methods
The Materials and Methods used in this work are described in the Supplemental Information.
Supplementary Material
Supp. Figure 1 PCR genotyping, cilia length defects, and variably penetrant neural tube closure defects and organogenesis defects in Fuz mutant mice. (a) Agarose gel electrophoresis results of FuzGt1(neo) knockout mouse PCR genotyping (DNA extracted from tails of fetuses at E18). PCR with primers to detect the wild type allele (Fuz forward & Fuz reverse primers - see Supplemental Methods) produces a 295 bp product, which was detectable in both +/+ and +/gt mice (left). PCR with primers detecting the mutant allele (Fuz forward & LTR reverse - see Supplemental Methods) produces a 220 bp product, which was detected in +/gt and gt/gt mice (right). (b) Graph of primary cilia length in chondrocytes of Meckel’s cartilage in wild type and Fuz mutant mice, as determined by the length of the acetylated tubulin signal following immunostaining (See Fig. 1G, H). E18.5 mice Fuzgt/gt showing variable NTDs. (c) Control mouse. (d) Fuzgt/gt mouse displaying excencephaly. (e) Fuzgt/gt mouse displaying encephalocoele (red arrow). (f) Fuzgt/gt mouse displaying normal neural tube closure (note reduced eyes and jaw). (g) Thick section of control brain. (h) Thick section of Fuzgt/gt brain from a fetus with an encephalocele (red arrow). (i, j) Fuzgt/gt mice display severely hypoplastic lungs. (k) Section through control heart. (l) Section through Fuzgt/gt heart with ventriculoseptal defect (arrow).
Supp. Figure 2 Human chromosome 1 open-reading frame 89 encodes a novel Rab-Similar GTPase (RSG) that is a Fuz interacting protein and dorsally targeted RSG1 MO results in anterior neural tube closure defects that are rescued by co-injection of a GFP-RSG mRNA. (a) Neighbor joining tree of human GTPase proteins with RhoT1 and RhoA serving as outgroups. RSG1 forms a clade with REM2 as its closest protein homolog. Parentheses indicate percent amino acid identities to RSG1. (b) Co-immunoprecipitation of FLAG-RSG protein (green band at ~27kD in Exp. Elute lane), by pull-down of MYC-FUZ with anti-MYC beads (red band at ~57kD in Exp. Elute lane). Whereas embryo lysates expressing only FLAG-RSG protein exhibit no interactions with anti-MYC beads (Ctl. Elute). Both products are present in raw lysates. (c, d) Rendered protein models (Open-Source PyMOL 0.99rc6 software). (c) Predicted model of RSG1 (cyan) threaded on the REM2 structure (green) (pdb:3CBQ). (d) Predicted model of RSG1 (green) threaded on the Rab1a structure (cyan) (pdb:2RHD). Contrasting colored amino acid (i.e. yellow or magenta) in each structure reflects the location of the conserved threonine residue mutated in our study (T65 in RSG, mutated to N; see main text for discussion). (d) RSG morphants exhibit significant defects in anterior neural tube closure compared to uninjected or GFP-RSG1 injected sibling embryos (P< 0.001). (e) Representative uninjected stage 20 embryo. (f) Representative RSG morphant embryo displaying a severe anterior neural tube closure defect. (g) Representative GFP-RSG1 (750pg) rescue embryo displaying a subtle but significant decrease in severity of the anterior neural tube defect. * P <0.05 and *** P < 0.001 versus RSG morpholino injection embryos or control as indicated by the line. n = 3 independent replicate experiments. All P values were analyzed by one-way ANOVA with Bonferoni correction. Data are shown as means ±SEM.
Supp. Figure 3 Structure modeling of the Fuz protein. (a) MouseFUNC predicts a vesicle trafficking function for Fuz. The description column defines the Gene Ontology descriptors for Fuz function ranked in order of combined score (blue column). Specific Gene Ontology identifiers (GO id’s) are listed in the leftmost column. The combined score represents the overall prediction of GO id by all algorithms generated in the MouseFUNC competetion19. The columns at right (B–H) are the relative scores for each GO id that were predicted by individual algorithms. The Type column indicates the parent GO hierarchy for the annotations (cc, cellular compartment; bp, biological process). (b) The primary sequence of Fuz is predicted to contain a single transmembrane-spanning domain in the N-terminus (MEMSAT3) and a putative longin-domain in the C-terminus (mGENTHREADER). (c) Comparison of secondary structures for Xenopus Fuz and three longin-domain-containing proteins, Ykt6 (3bw6A0), SEDL (1hgA0), and AP2σ (1vg1S0). The β-sheets and α-helices predicted for Xenopus Fuz are indicated by the boxes (see labels above each box). The sheets and helices of the other three proteins are indicated by blue arrows and red barrels, respectively. Critical residues in the Fuz C-terminus, which are conserved in the other proteins, are indicated by the vertical grey bars. (d) Rendered protein models of AP-2σ (left), and homology threaded model of the C -terminus of Xenopus Fuz (middle) and homology-threaded model of the C-terminus of human Fuz (right).
Supp. Figure 4 Network diagram of functional interactions between other structurally related Fuz like longin-domain containing proteins SEDL, YKT6, SEC22B, VAMP7 and AP2σ (PDB id: 1H3Q, 3BW6, 1IFQ, 2VX8 and 1VGL respectively).
Supp. Figure 5 Localization and function of RSG1 in formaldehyde fixed multi-ciliated epidermal cells. (c) Multi-ciliated cell view (x-y) of uninjected control embryo exhibits elongated CLAMP-GFP signal. (c′) Thin x-y section from [c]. (c″) Z-projection of section [c′] displays apical alignment of CLAMP-GFP. (d) Multi-ciliated cell view (x-y) of wild type RSG1 mRNA injected embryo exhibits subtle defects in the elongation of the CLAMP-GFP signal. (d′) Thin x-y section from [d]. (d″) Z-projection of section [d′] displays apical alignment of CLAMP-GFP with a subtle defect in the resolution of apical punctae. (e) Multi-ciliated cell view (x-y) of RSG1T65N mRNA injected embryo exhibits dramatic defects in the elongation of the CLAMP-GFP signal. (e′) Thin x-y section from [e]. (e″) Z-projection of section [e′] displays dramatic loss of the apical alignment as well as aberrant cytoplasmic punctae of CLAMP-GFP signal.
Supp. Figure 6 Additional TEM analysis of mucus secreting cells in Fuz morphants. (a) Control image showing representative Xenopus thin section epidermis. Generally the vesicles display even spacing of vesicles with no lateral mixing. (b, c) Fuz morphants display multiple lateral mixing events (red arrows and double red arrowheads [b, c]) as well as uneven spacing of the vesicles. (d) Wild-type secretory granule. Red arrowhead indicates a membrane signature that maybe indicative of hemifusion. (e) Fuz morphant secretory granule. Plasma membrane blebs out significantly, which may indicate a lack of complete fusion. A membrane signature (possibly hemifusion) similar to that observed in in controls cells is present (red arrowhead).
Acknowledgments
The ES cell clone for making the Fuz mutant mouse was generously provided by Lexicon Pharmaceuticals. We thank P. Paukstelis for aid with structural modeling, S. Vokes for critical comments on the manuscript, and Wei H. for technical help with histology and immunostaining. Phil Abitua is supported by a Diversity Supplement from the NIH/NIGMS. This work was supported by grants to K.J.L from the Wellcome Trust and the BBSRC; to E.M.M. from the NSF, NIH, Welch Foundation (F-1515), Texas Institute for Drug and Diagnostic Development, and a Packard Fellowship; grants to J.B.W. from the NIH/NIGMS, The March of Dimes, The Burroughs Wellcome Fund, the Sandler Program for Asthma Research, and the Texas Advanced Research Program; and by grants to R.H.F. from the NIH and The Texas A&M Institute for Genomic Medicine.
References
- 1.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15(Spec No 2):R227–234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 2.Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 7.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamblet NS, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 9.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 10.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 11.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 12.Ansley SJ, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 14.Smith UM, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 15.Beales PL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 16.Takeda S, et al. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rual JF, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 18.Hayes JM, et al. Identification of novel ciliogenesis factors using a new in vivo model for mucociliary epithelial development. Dev Biol. 2007;312:115–130. doi: 10.1016/j.ydbio.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena-Castillo L, et al. A critical assessment of Mus musculus gene function prediction using integrated genomic evidence. Genome Biol. 2008;9 (Suppl 1):S2. doi: 10.1186/gb-2008-9-s1-s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginalski K. Comparative modeling for protein structure prediction. Curr Opin Struct Biol. 2006;16:172–177. doi: 10.1016/j.sbi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara H, Aoki T, Ohwada N, Fujimoto T. Development of striated rootlets during ciliogenesis in the human oviduct epithelium. Cell Tissue Res. 1997;290:39–42. doi: 10.1007/s004410050905. [DOI] [PubMed] [Google Scholar]
- 22.Rossi V, et al. Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends Biochem Sci. 2004;29:682–688. doi: 10.1016/j.tibs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Jang SB, et al. Crystal structure of SEDL and its implications for a genetic disease spondyloepiphyseal dysplasia tarda. J Biol Chem. 2002;277:49863–49869. doi: 10.1074/jbc.M207436200. [DOI] [PubMed] [Google Scholar]
- 24.Gedeon AK, et al. Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat Genet. 1999;22:400–404. doi: 10.1038/11976. [DOI] [PubMed] [Google Scholar]
- 25.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 26.Pryor PR, et al. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell. 2008;134:817–827. doi: 10.1016/j.cell.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dougherty GW, et al. CLAMP, a novel microtubule-associated protein with EB-type calponin homology. Cell Motil Cytoskeleton. 2005;62:141–156. doi: 10.1002/cm.20093. [DOI] [PubMed] [Google Scholar]
- 28.Chan SW, Fowler KJ, Choo KH, Kalitsis P. Spef1, a conserved novel testis protein found in mouse sperm flagella. Gene. 2005;353:189–199. doi: 10.1016/j.gene.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Li T. The ciliary rootlet interacts with kinesin light chains and may provide a scaffold for kinesin-1 vesicular cargos. Exp Cell Res. 2005;309:379–389. doi: 10.1016/j.yexcr.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata S, Nakanishi M, Nanba R, Fujita N. Developmental expression of XEEL, a novel molecule of the Xenopus oocyte cortical granule lectin family. Dev Genes Evol. 2003;213:368–370. doi: 10.1007/s00427-003-0341-9. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Billett FS, Gould RP. Fine structural changes in the differentiating epidermis of Xenopus laevis embryos. J Anat. 1971;108:465–480. [PMC free article] [PubMed] [Google Scholar]
- 35.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 36.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 37.Boyadjiev SA, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- 38.Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet. 2006;38:1198–1203. doi: 10.1038/ng1880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure 1 PCR genotyping, cilia length defects, and variably penetrant neural tube closure defects and organogenesis defects in Fuz mutant mice. (a) Agarose gel electrophoresis results of FuzGt1(neo) knockout mouse PCR genotyping (DNA extracted from tails of fetuses at E18). PCR with primers to detect the wild type allele (Fuz forward & Fuz reverse primers - see Supplemental Methods) produces a 295 bp product, which was detectable in both +/+ and +/gt mice (left). PCR with primers detecting the mutant allele (Fuz forward & LTR reverse - see Supplemental Methods) produces a 220 bp product, which was detected in +/gt and gt/gt mice (right). (b) Graph of primary cilia length in chondrocytes of Meckel’s cartilage in wild type and Fuz mutant mice, as determined by the length of the acetylated tubulin signal following immunostaining (See Fig. 1G, H). E18.5 mice Fuzgt/gt showing variable NTDs. (c) Control mouse. (d) Fuzgt/gt mouse displaying excencephaly. (e) Fuzgt/gt mouse displaying encephalocoele (red arrow). (f) Fuzgt/gt mouse displaying normal neural tube closure (note reduced eyes and jaw). (g) Thick section of control brain. (h) Thick section of Fuzgt/gt brain from a fetus with an encephalocele (red arrow). (i, j) Fuzgt/gt mice display severely hypoplastic lungs. (k) Section through control heart. (l) Section through Fuzgt/gt heart with ventriculoseptal defect (arrow).
Supp. Figure 2 Human chromosome 1 open-reading frame 89 encodes a novel Rab-Similar GTPase (RSG) that is a Fuz interacting protein and dorsally targeted RSG1 MO results in anterior neural tube closure defects that are rescued by co-injection of a GFP-RSG mRNA. (a) Neighbor joining tree of human GTPase proteins with RhoT1 and RhoA serving as outgroups. RSG1 forms a clade with REM2 as its closest protein homolog. Parentheses indicate percent amino acid identities to RSG1. (b) Co-immunoprecipitation of FLAG-RSG protein (green band at ~27kD in Exp. Elute lane), by pull-down of MYC-FUZ with anti-MYC beads (red band at ~57kD in Exp. Elute lane). Whereas embryo lysates expressing only FLAG-RSG protein exhibit no interactions with anti-MYC beads (Ctl. Elute). Both products are present in raw lysates. (c, d) Rendered protein models (Open-Source PyMOL 0.99rc6 software). (c) Predicted model of RSG1 (cyan) threaded on the REM2 structure (green) (pdb:3CBQ). (d) Predicted model of RSG1 (green) threaded on the Rab1a structure (cyan) (pdb:2RHD). Contrasting colored amino acid (i.e. yellow or magenta) in each structure reflects the location of the conserved threonine residue mutated in our study (T65 in RSG, mutated to N; see main text for discussion). (d) RSG morphants exhibit significant defects in anterior neural tube closure compared to uninjected or GFP-RSG1 injected sibling embryos (P< 0.001). (e) Representative uninjected stage 20 embryo. (f) Representative RSG morphant embryo displaying a severe anterior neural tube closure defect. (g) Representative GFP-RSG1 (750pg) rescue embryo displaying a subtle but significant decrease in severity of the anterior neural tube defect. * P <0.05 and *** P < 0.001 versus RSG morpholino injection embryos or control as indicated by the line. n = 3 independent replicate experiments. All P values were analyzed by one-way ANOVA with Bonferoni correction. Data are shown as means ±SEM.
Supp. Figure 3 Structure modeling of the Fuz protein. (a) MouseFUNC predicts a vesicle trafficking function for Fuz. The description column defines the Gene Ontology descriptors for Fuz function ranked in order of combined score (blue column). Specific Gene Ontology identifiers (GO id’s) are listed in the leftmost column. The combined score represents the overall prediction of GO id by all algorithms generated in the MouseFUNC competetion19. The columns at right (B–H) are the relative scores for each GO id that were predicted by individual algorithms. The Type column indicates the parent GO hierarchy for the annotations (cc, cellular compartment; bp, biological process). (b) The primary sequence of Fuz is predicted to contain a single transmembrane-spanning domain in the N-terminus (MEMSAT3) and a putative longin-domain in the C-terminus (mGENTHREADER). (c) Comparison of secondary structures for Xenopus Fuz and three longin-domain-containing proteins, Ykt6 (3bw6A0), SEDL (1hgA0), and AP2σ (1vg1S0). The β-sheets and α-helices predicted for Xenopus Fuz are indicated by the boxes (see labels above each box). The sheets and helices of the other three proteins are indicated by blue arrows and red barrels, respectively. Critical residues in the Fuz C-terminus, which are conserved in the other proteins, are indicated by the vertical grey bars. (d) Rendered protein models of AP-2σ (left), and homology threaded model of the C -terminus of Xenopus Fuz (middle) and homology-threaded model of the C-terminus of human Fuz (right).
Supp. Figure 4 Network diagram of functional interactions between other structurally related Fuz like longin-domain containing proteins SEDL, YKT6, SEC22B, VAMP7 and AP2σ (PDB id: 1H3Q, 3BW6, 1IFQ, 2VX8 and 1VGL respectively).
Supp. Figure 5 Localization and function of RSG1 in formaldehyde fixed multi-ciliated epidermal cells. (c) Multi-ciliated cell view (x-y) of uninjected control embryo exhibits elongated CLAMP-GFP signal. (c′) Thin x-y section from [c]. (c″) Z-projection of section [c′] displays apical alignment of CLAMP-GFP. (d) Multi-ciliated cell view (x-y) of wild type RSG1 mRNA injected embryo exhibits subtle defects in the elongation of the CLAMP-GFP signal. (d′) Thin x-y section from [d]. (d″) Z-projection of section [d′] displays apical alignment of CLAMP-GFP with a subtle defect in the resolution of apical punctae. (e) Multi-ciliated cell view (x-y) of RSG1T65N mRNA injected embryo exhibits dramatic defects in the elongation of the CLAMP-GFP signal. (e′) Thin x-y section from [e]. (e″) Z-projection of section [e′] displays dramatic loss of the apical alignment as well as aberrant cytoplasmic punctae of CLAMP-GFP signal.
Supp. Figure 6 Additional TEM analysis of mucus secreting cells in Fuz morphants. (a) Control image showing representative Xenopus thin section epidermis. Generally the vesicles display even spacing of vesicles with no lateral mixing. (b, c) Fuz morphants display multiple lateral mixing events (red arrows and double red arrowheads [b, c]) as well as uneven spacing of the vesicles. (d) Wild-type secretory granule. Red arrowhead indicates a membrane signature that maybe indicative of hemifusion. (e) Fuz morphant secretory granule. Plasma membrane blebs out significantly, which may indicate a lack of complete fusion. A membrane signature (possibly hemifusion) similar to that observed in in controls cells is present (red arrowhead).