Abstract
The osteo-anabolic effects of intermittent parathyroid hormone (PTH) treatment require insulin-like growth factor (IGF) signaling through the IGF-I receptor. A major downstream target of the IGF-I receptor (via Akt) is the mammalian target of rapamycin (mTOR), a kinase involved in protein synthesis. We investigated whether the bone-building effects of intermittent PTH require functional mTOR signaling. Mice were treated with daily PTH 1-34 (0, 10, 30, or 90 μg/kg) for 6 weeks in the presence or absence of rapamycin, a selective inhibitor of mTOR. We found that all PTH doses were effective in enhancing bone mass, whether rapamycin was present or not. Rapamycin had little to no effect on the anabolic response at low (10 μg) PTH doses, small effects in a minority of anabolic measures at moderate doses (30 μg), but the anabolic effects of high dose PTH (90 μg) were consistently and significantly suppressed by rapamycin (~4-36% reduction). Serum levels of Trap5b, a marker of resorption, were significantly enhanced by rapamycin, but these effects were observed whether PTH was absent or present. Our data suggest that intermittent PTH, particularly at lower doses, is effective in building bone mass in the presence of rapamycin. However, the full anabolic effects of higher doses of PTH are significantly suppressed by rapamycin, suggesting that PTH might normally activate additional pathways (including mTOR) for its enhanced high-dose anabolic effects. Clinical doses of intermittent PTH could be an effective treatment for maintaining or increasing bone mass among patients taking rapamycin analogs for unrelated health issues.
Keywords: parathyroid hormone, mTOR, bone anabolism, Akt, osteoporosis
Introduction
Bone loss due to osteoporosis is a major health concern with an estimated 10 million people affected by the disease in the United States alone. Currently, the only FDA-approved anabolic therapy for osteoporosis is teriparatide, the 1-34 fragment of human parathyroid hormone (PTH). Intermittent PTH is known to increase bone mass and strength, and to decrease the risk of fractures (Neer et al., 2001; Verhaar and Lems, 2009).
Despite its well-documented anabolic effects on bone, little is known regarding how intermittent PTH works to activate cells, though some key signaling components have been identified. An important finding that has emerged recently, regarding the signal transduction pathways used by anabolic PTH treatment, is the requirement of insulin-like growth factor-1 (IGF-I) and its receptor IGF-IR, for the bone-building effects of PTH (Bikle et al., 2002; Wang et al., 2007; Yamaguchi et al., 2005).
A major downstream target of activated IGF-IR is the phosphatidylinositol-3-kinase (PI3k) pathway, which results in the activation of Akt and subsequently, the mammalian target of rapamycin (mTOR), a key enzyme in protein synthesis and cell proliferation (Hay and Sonenberg, 2004). Activated mTOR phosphorylates p70S6-kinase 1 (p70S6k1) and 4E binding protein 1 (4E-BP1), leading to translation of proteins involved in cell proliferation and growth (Mamane et al., 2006; Manning and Cantley, 2007). Because PTH effects in bone involve increased protein synthetic activity (bone matrix production) and enhanced cell (osteoblast) proliferation—two functions mediated by mTOR—and because mTOR is downstream of a known modulator (IGF-IR) of anabolic PTH signaling, we sought to determine whether the anabolic effects of intermittent PTH, which are known to work through IGF-IR, are subsequently channeled through mTOR.
To address this issue, we treated mice with daily PTH injections for 6 weeks, in the presence or absence of a compound (rapamycin) that selectively inhibits mTOR, and measured the effects on bone metabolism. We hypothesized that the anabolic actions of intermittent PTH are mediated by the mTOR pathway. We found that low dose PTH (10 μg/kg/day) was fully anabolic whether rapamycin (3 mg/kg/day) was present or not. However, trabecular bone mass accrual induced by high-dose PTH (90 μg/kg/day) was significantly impaired by rapamycin, suggesting that some of the anabolic effects of high dose PTH are dependent on additional pathways (beyond those activated at lower yet anabolic doses), including mTOR.
Materials and Methods
Animals and pellet implantation
Eighty two female C57BL/6J mice were purchased from the Jackson Laboratory. The mice were 8 weeks old at arrival. Sixty-four mice were selected for the PTH experiment, and 18 mice were used to verify the efficacy of the rapamycin pellets used to inhibit mTOR signaling (see below). All mice were subcutaneously implanted with either a 45 day sustained-release rapamycin pellet (rapamycin: LC Laboratories, Woburn, MA; pellets: Innovative Research of America, Sarasota, FL) or a placebo pellet (methylcellulose). Pellet implantation was performed under isoflurane anesthesia (2% @1.5 L/min) using a 10 gauge trochar to position the pellet subcutaneously in the right dorsal shoulder/cervical region. Rapamycin pellets were designed to deliver 3 mg/kg/day based on a 20 g mouse (2.7 mg per 45 day pellet). All procedures performed in accordance with guidelines set by the Institutional Animal Care and Use Committee.
Verification of rapamycin efficacy in vivo
In order to test whether the rapamycin pellets delivered a sustained, pharmacologically potent dose of the compound into the general circulation, we performed a standard insulin–skeletal muscle test. This is a reliable in vivo assay to assess rapamycin-induced blockade of mTOR function. Insulin normally stimulates the PI3k-Akt-mTOR-p70s6K pathway, but in the presence of rapamycin, which binds and inhibits mTOR, upstream components of the insulin cascade (e.g., Akt) are activated but elements downstream of mTOR (mTOR, p70s6K) are not activated. Eighteen mice (not involved in the PTH study) were implanted with either a rapamycin pellet (n=9) or a vehicle pellet (n=9) as described above. Three weeks later, the mice were administered IP injections of insulin (5 U/kg; Humulin® R, Eli Lilly, Inc.) or saline. Fifteen minutes after insulin or saline injection, the mice were sacrificed and the tibialis anterior muscle was removed and immediately frozen in liquid N2 for protein extraction and western blotting.
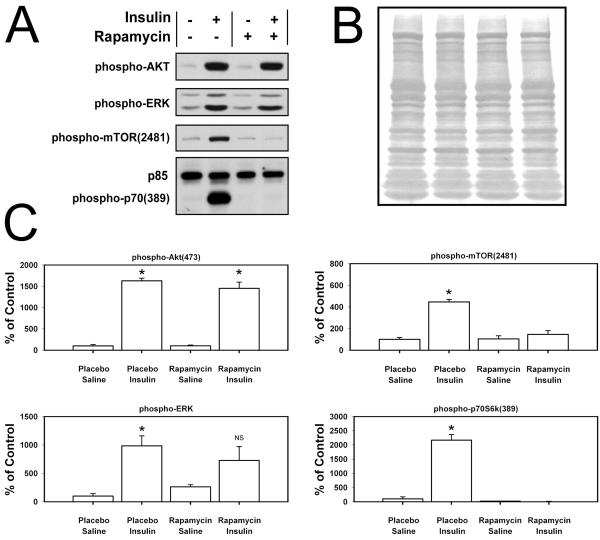
Muscle samples were prepared as previously described (Hornberger and Chien, 2006). Briefly, frozen muscles were homogenized in a protein lysis buffer, assayed for protein concentration, and electrophoresed on 7.5% acrylamide gels using equal protein loading in each lane. Proteins were transferred to PVDF membranes and probed for phospho-Erk1/2, phospho-Akt, phospho-mTOR, and phospho-p70S6k, followed by Coomassie Blue staining of the membranes to verify equal protein loading (Fig 1C). Densitometric measurements were collected using ImageJ.
Figure 1.
(A) Rapamycin efficacy was tested in vivo by injecting pellet-harboring mice with insulin or saline, then 15 min later, harvesting and extracting protein from the tibialis anterior muscle. Western blots from electrophoresed protein extracts reveal robust activation (phosphorylation) of the PI3k–mTOR–p70S6k pathway upon insulin stimulation (lane 2) compared to saline injection (lane 1). The presence of a rapamycin pellet nullified the insulin effect on mTOR and p70S6k activation (lane 4) compared to the saline injection (lane 3), while leaving the upstream targets of the insulin receptor, PI3k and Erk, intact. The phospho-p70S6k antibody also recognizes p85, which is not affected by insulin treatment, and consequently, serves as an internal loading control. (B) Following image capture of phosphoprotein blots, the membranes were stained with Coomassie Blue to further verify equal protein loading in all lanes. (C) Quantification of phosphorylated proteins from Western blots shown in panel A, with n≥4 animals per treatment. * = p<0.05 compared to corresponding saline injection; NS = not significant.
Parathyroid hormone (PTH) treatment and fluorochrome administration in vivo
Sixty-four mice were divided into 8 groups of 8 mice such that each group had equal mean and standard deviation for body weight. At 9.5 weeks of age, half of the groups were implanted with rapamycin pellets and the other half were implanted with placebo pellets (methylcellulose). Two days later, daily PTH/vehicle treatment began and continued 7 days/week for 6 weeks.
Each pellet group (rapamycin or placebo) was subdivided into 4 groups of 8 mice for the three different concentrations (10, 30, or 90 μg/kg/day) of human PTH 1-34 (Bachem, Torrance, CA) or vehicle control (0 μg/kg/day). PTH concentrations were adjusted weekly based on weekly body mass measurements. The fluorochrome label oxytetracycline (25 mg/kg) was injected intraperitoneally three days prior to start of PTH injections. Calcein green (20 mg/kg) was injected intraperitoneally five days prior to sacrifice. Mice were sacrificed 44 days after start of PTH injections.
Dual energy x-ray absorptiometry (DEXA)
Mice were anesthetized with isoflurane (2% @ 1.5 liters/min) and placed in a prone position with limbs outstretched on a GE Lunar PIXImus dual energy x-ray absorptiometer. Whole-body scans were collected prior to pellet implantation (baseline) and at 6 weeks. From the whole body scans, areal bone mineral density (aBMD) and bone mineral content (BMC) were calculated for the post-cranial skeleton.
Micro-computed tomography (μCT)
After sacrifice, the distal femoral metaphyses were scanned on a desktop μCT (μCT 20; Scanco Medical AG, Bassersdorf, Switzerland) to measure 3-dimensional morphometric properties in the trabeculae as previously described (Sawakami et al., 2006). The following parameters were calculated from each reconstructed stack of 225 slices through the metaphysis: bone volume (BV/TV), trabecular number (Tb.N), trabecular separation (Tb.Sp), and trabecular thickness (Tb.Th).
Peripheral quantitative computed tomography (pQCT)
The harvested right femurs were centered in the gantry of a Norland Stratec XCT Research SA+ pQCT (Stratec Electronics, Pforzheim, Germany). A midshaft slice was collected at a distance 50% of the total femur length. A distal femur slice was taken at a distance 17% of the total femur length from the distal end. Bone mineral content (BMC) and volumetric bone mineral density (vBMD) were calculated for each slice using the Stratec software as described previously (Sawakami et al., 2006).
Serum collection and resorption measurements
Serum samples were collected via tail bleeds prior to pellet implantation (baseline), at 3 weeks, and at 6 weeks. Blood was collected in 3 non-heparinized capillary tubes, allowed to clot for 30 minutes, and then separated via centrifugation. Serum was stored at −80°C. Serum concentration of tartrate-resistant acid phosphatase (Trap5b) was measured by ELISA, using a commercially available kit (MouseTrap, IDS Diagnostics, Inc.). Serum samples at both time points were measured in triplicate and averaged.
Bone histomorphometry
Histomorphometric indices of bone formation were measured from the fluorochrome labeling. All fluorochrome measurements were collected on a Nikon Optiphot fluorescence microscope (Nikon USA, Garden City, NJ, USA) using the Bioquant semiautomatic digitizing system (R & M Biometrics, Nashville, TN, USA). Femurs were fixed in 10% neutral buffered formalin for 48 hrs, dehydrated in graded ethanols, and embedded undecalcified in methylmethacrylate. Midshaft femoral sections were cut in the transverse plane (~60 μm thick) using a diamond-embedded wire saw (Delaware Diamond Knives, Wilmington, DE)). Periosteal bone formation parameters were collected and calculated by measuring the extent of single labeled perimeter (sL.Pm), double labeled perimeter (dL.Pm), and the area between the two labels. Derived histomorphometric parameters included mineralizing surface (MS/BS, %), a measure of active bone-forming surface, calculated as follows: the [½ sL.Pm +dL.Pm]/Tt.Pm * 100; mineral apposition rate (MAR, μm/day), a measure of the rate of radial expansion of new bone, calculated as follows: dL.Ar/dL.Pm/[days between labels]; and bone formation rate (BFR, μm3/μm2/year), an overall measure of bone formation that combines MS/BS and MAR, calculated as follows: MS/BS * MAR * 3.65.
Statistical Analysis
Statistical analysis was computed using JMP (SAS Institute, Inc. Version 4.0). The data was fitted to a standard least squares model with main effects of PTH dose (0, 10, 30, or 90 μg), pellet type (rapamycin or placebo), and interaction between the PTH and pellet type. This model determined if pellet type affected the outcome measurements, if PTH affected the outcome measurements, and if there was a significant interaction between these two variables that would suggest that rapamycin is affecting PTH action. Because we were interested in determining whether rapamycin changed the way PTH affected bone gain, we focused our analyses on interactions between these two main effects, rather than on each main effect individually. When a significant interaction was found, a least squares mean contrast test (PLSD) within a PTH dose determined where significant differences between pellet types occurred. Statistical significance was taken at p < 0.05. Data are presented as mean +/− SEM.
Results
Rapamycin pellets inhibit the PI3k-Akt-mTOR pathway in vivo
To verify that the rapamycin pellets inhibited mTOR and its downstream targets in vivo, mice implanted with rapamycin or placebo pellets were injected with insulin or saline, and the activation of different nodes along the PI3k-mTOR pathway were monitored in skeletal muscle (tibialis anterior). This procedure is a sensitive kinase assay to test for circulating levels of rapamycin. Insulin activates the insulin receptor (IR), and quickly induces sequential phosphorylation of PI3k, Akt, mTOR (at serine 2481), and p70S6k (at serine 389). Fifteen minutes after insulin administration, phosphorylation of mTOR and p70S6k was prominent in placebo pellet mice, but in mice harboring rapamycin pellets, mTOR and p70S6k phosphorylation were not different from controls (Fig 1A & 1B). Signaling components upstream of mTOR were unaffected by rapamycin, as indicated by the enhanced phosphorylation of Akt. Erk phosphorylation, another target of the activated insulin receptor, was enhanced by insulin regardless of pellet type, but statistical significance could not be achieved in the rapamycin-treated mice (Fig 1A & 1B). Taken together, these data demonstrate that the pellets were effective in delivering potent doses of rapamycin to the circulation, reaching remote tissues to inhibit mTOR (and consequently, downstream targets of mTOR), while upstream targets remained intact (capable of activation).
Rapamycin does not alter the effects of PTH on body mass or longitudinal bone growth
Body weight was dose-dependently increased by PTH treatment, but rapamycin had no significant effects on body weight at the various PTH doses (Table 1). Conversely, both PTH and rapamycin had significant effects on femur length; PTH significantly increased femur length, while rapamycin significantly decreased femur length (Table 1). No interaction between PTH and rapamycin was detected for bone length.
Table 1.
Body mass, femoral length, peripheral quantitative computed tomography (pQCT) measurements of femoral mineral density and content, and midshaft femur histomorphometric measurements of the periosteal surfacea
| Femur | Midshaft Femur pQCT | Distal Femur pQCT | Midshaft Femur Histomorphometry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body Mass |
Length |
vBMD (mg/cm3) |
BMC (mg) |
vBMD (mg/cm3) |
BMC (mg) |
MS/BS (%) |
MAR (μm/day) |
BFR/BS (μm3/(μm2/yr) |
||
| PTH Dose |
Initial (g) |
Final (g) |
(mm) |
|||||||
| 0 μg PTH/kg/day | ||||||||||
| Placebo | 17.4 ±0.2 | 20.0 ±0.3 | 15.3 ±0.07 | 613.3 ±9.2 | 1.15 ±0.02 | 425.6 ±6.3 | 1.36 ±0.02 | 37.4 ±1.7 | 1.3 ±0.1 | 176.6 ±19.6 |
| Rapamycin | 17.3 ±0.2 | 20.5 ±0.2 | 15.1 ±0.04 | 604.2 ±10.1 | 1.06 ±0.02 | 406.7 ±6.7 | 1.25 ±0.02 | 35.8 ±2.3 | 0.9 ±0.0 | 117.7 ±10.5 |
| 10 μg PTH/kg/day | ||||||||||
| Placebo | 17.3 ±0.2 | 20.7 ±0.2 | 15.5 ±0.08 | 628.3 ±9.4 | 1.22 ±0.03 | 512.5 ±17.2 | 1.68 ±0.06 | 40.6 ±0.9 | 1.7 ±0.1 | 248.9 ±18.5 |
| Rapamycin | 17.3 ±0.2 | 21.0 ±0.3 | 15.4 ±0.06 | 648.4 ±4.6 | 1.21 ±0.02 | 527.3 ±11.5 | 1.69 ±0.03 | 36.2 ±0.9 | 1.4 ±0.1 | 190.1 ±19.1 |
| 30 μg PTH/kg/day | ||||||||||
| Placebo | 17.4 ±0.2 | 21.2 ±0.3 | 15.4 ±0.07 | 640.1 ±5.9 | 1.31 ±0.01 | 698.4 ±15.1 | 2.41 ±0.05 | 40.0 ±2.7 | 1.9 ±0.2 | 283.9 ±43.2 |
| Rapamycin | 17.3 ±0.2 | 21.2 ±0.3 | 15.3 ±0.08 | 648.4 ±5.7 | 1.25 ±0.01 | 580.1* ±19.0 | 1.93* ±0.07 | 36.5 ±0.4 | 1.7 ±0.1 | 224.6 ±11.9 |
| 90 μg PTH/kg/day | ||||||||||
| Placebo | 17.4 ±0.2 | 21.7 ±0.2 | 15.5 ±0.2 | 700.7 ±8.7 | 1.47 ±0.04 | 736.1 ±14.4 | 2.63 ±0.07 | 47.4 ±3.3 | 2.3 ±0.1 | 393.9 ±31.6 |
| Rapamycin | 17.3 ±0.2 | 22.1 ±0.4 | 15.1 ±0.4 | 671.0* ±5.7 | 1.38 ±0.02 | 649.6* ±18.7 | 2.24* ±0.08 | 39.0 ±1.1 | 1.9 ±0.1 | 265.9 ±19.2 |
| Anova | ||||||||||
| PTH effect | N/A | p <0.0001 | p =0.0012 | p <0.0001 | p <0.0001 | p <0.0001 | p <0.0001 | p =0.004 | p <0.0001 | p <0.0001 |
| Pellet effect | N/A | NS | p =0.014 | NS | p =0.0002 | p <0.0001 | p <0.0001 | p =0.001 | p =0.0002 | p <0.0001 |
| Interaction | N/A | NS | NS | p =0.007 | NS | p <0.0001 | p <0.0001 | NS | NS | NS |
Values shown are mean±SEM. N/A=not applicable; NS=not significant (p >0.05);
=significant difference between rapamycin- and placebo-treated mice detected within PTH dose group (calcualted only when ANOVA generated a significant interaction term).
Rapamycin inhibits PTH-induced bone gain at higher PTH doses
To evaluate the effects of mTOR inhibition on PTH-induced bone gain, we performed whole body DEXA scans at baseline and at sacrifice. After sacrifice, we measured structural properties (μCT) and material properties (pQCT) in the femur. As expected, intermittent PTH resulted in a significant dose-response in DEXA-derived whole body areal bone mineral density (aBMD) and content (BMC) (Fig. 2A & 2B). Rapamycin had no effect on whole-body aBMD, but it significantly suppressed BMC, regardless of whether PTH was absent or present at any dose. No interaction was found between PTH and rapamycin for either DEXA measurement, suggesting that rapamycin did not affect the ability of intermittent PTH to add bone to the whole skeleton when evaluated using a gross measurement (DEXA) of skeletal changes.
Figure 2.
Whole-body DEXA-derived measures of percent change in areal bone mineral density (aBMD; panel A) and bone mineral content (BMC; panel B) from 10 wks of age (beginning of PTH treatment) to 16 weeks of age (end of PTH treatment) reveal that PTH increased BMC and aBMD in a dose-dependent manner. Rapamycin had significant inhibitory effects only on BMC (and not aBMD), independent of the PTH effects, but no interaction between PTH and rapamycin was found for either DEXA-derived variable (n ≥ 7). The p-values shown at the tops of the panels indicate the significance of the PTH effect on aBMD and BMC, the significance of the rapamycin (pellet) effect on aBMD and BMC, and the significance of the interaction.
Intermittent PTH also increased all pQCT-derived quantitative measures of bone mineral at the femoral midshaft (a largely cortical bone site) and distal metaphysis (a trabecular-rich site) in a dose-responsive manner (Table 1). Rapamycin had significant suppressive effects on most of the pQCT parameters examined. More importantly, we found significant interaction terms for mid-femur vBMD, distal femur vBMD, and distal femur BMC. When the interactions were probed further with post-hoc tests, only the higher PTH doses (sometimes 30 μg and always 90 μg doses) showed significant rapamycin-induced suppression of bone gain, while the low dose PTH and vehicle treatments were not affected by rapamycin. The pQCT measurements suggest that rapamycin affected the ability of high-dose PTH to add bone to the skeleton, but low-dose PTH was equally anabolic regardless of whether rapamycin was present or absent.
Structural properties of the distal femoral trabecular bone were measured using μCT to further assess rapamycin effects on PTH efficacy (Fig. 3). Similar to the outcomes from the previous two instruments, μCT-derived trabecular volume (BV/TV), spacing (Tb.S), number (Tb.N), and thickness (Tb.Th) exhibited dose-responsive changes to intermittent PTH. Rapamycin had significant suppressive effects on three of the four μCT parameters examined. However, similar to the pQCT results, we found significant interaction terms for all four of the μCT parameters examined. When the interactions were probed further with post-hoc tests, only the higher PTH doses (sometimes 30 μg and always 90 μg doses) showed significant rapamycin-induced suppression of trabecular bone gain, while the low dose PTH and vehicle treatments were not affected by rapamycin (with the exception of Tb.Th, which did yield a significant rapamycin effect at the 10 μg dose of PTH). Like the pQCT results, the μCT measurements suggest that rapamycin affected the ability of high-dose PTH to add bone to the skeleton, but low-dose PTH was equally anabolic regardless of whether rapamycin was present or absent.
Figure 3.
(A) Representative images of μCT reconstructions (anterior portion removed in silico to reveal trabecular bone) from the distal femur of animals implanted with either a placebo pellet (top) or a rapamycin sustained-release pellet (bottom). The PTH dose increases from left to right. Note the expected increase in trabecular bone with increasing PTH dose, but among high dose (90 μg) PTH-receiving animals, a visible decrease in trabecular prevalence and thickness in the rapamycin-treated animal, compared to its respective placebo-treated control. (B) Analysis of quantitative measurements from the trabecular compartment yielded significant interaction terms between PTH and rapamycin for all four outcomes. Post-hoc tests revealed that the interactions were fueled largely by significant differences among the higher dose (30 and 90 μg) groups, though the mice treated with the 10 μg dose were significantly different from their rapamycin-treated controls for Tb.Th (n = 8). * = p<0.05 compared to corresponding placebo pellet. The p-values shown at the tops of the panels in (B) indicate the significance of the PTH effect on μCT parameters, the significance of the rapamycin (pellet) effect on μCT parameters, and the significance of the interaction.
Rapamycin has minimal effect on PTH-induced changes in resorption
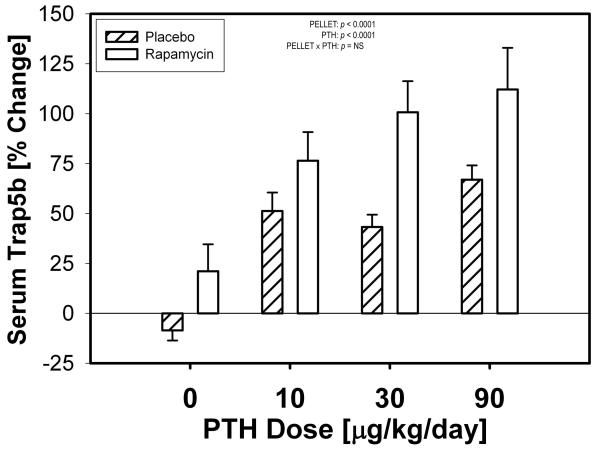
To gain insight into whether mTOR inhibition alters the bone resorption response to PTH, we collected serum samples at baseline and after 3 weeks of PTH treatment for measurement of serum Trap5b levels. As expected, after 3 weeks of treatment, PTH increased serum Trap5b levels among all mice that received the hormone. Rapamycin significantly increased serum Trap5b beyond that induced by PTH (25-57% increase, Fig 4) regardless of whether PTH was absent or present at any dose. No interaction was found between PTH and rapamycin for serum Trab5b, suggesting that rapamycin did not alter the ability of PTH to induce bone resorption.
Figure 4.
Percent change in serum Trap5b levels at 3 weeks compared to baseline. Serum samples were measured for each mouse at both time points in triplicate then averaged (n ≥ 4). PTH significantly increased the serum Trap5b concentration, and by inference, the osteoclast-mediated resorption rate. Rapamycin treatment significantly increased the serum Trap5b concentration beyond that induced by PTH, but this increase was independent of PTH (i.e., no interaction was found).
Rapamycin has minimal effect on PTH-induced changes in cortical bone formation
To address whether mTOR inhibition alters PTH-induced cortical bone formation, we measured periosteal bone formation in the midshaft femur using fluorochrome labels given prior to sacrifice. PTH treatment resulted in a dose-related increase in midshaft periosteal apposition. Rapamycin significantly inhibited periosteal bone formation across all PTH/vehicle groups (Table 1). The reduction in periosteal BFR/BS (21-32% decrease) among the rapamycin-treated mice was driven by significant reductions in both mineralizing surface (4-18% decrease) and mineral apposition rate (12-30% decrease). The lack of interaction between PTH and rapamycin among the histomorphometric measures of bone formation suggests that rapamycin did not alter the ability of PTH to induce cortical bone formation on the periosteal surface.
Discussion
We set out to investigate whether mTOR activation was required for the anabolic response to intermittent PTH. Our inquiry was fueled by the published observations that the anabolic effects of PTH require autocrine/paracrine signaling through the IGF receptor. Because mTOR is one of the main downstream targets of the activated IGF receptor (via Akt), we sought to determine whether the PTH-induced IGF-receptor signal is subsequently transduced through mTOR. Our data revealed that the trabecular bone-building effects of high-dose PTH are hampered by rapamycin-induced blockade of mTOR, but the effects of low dose PTH (10 μg) are not; i.e., gains in bone mass driven by low-dose PTH were equally efficacious in the presence or absence of rapamycin (with one exception - trabecular thickness in the distal femur). Thus, our data suggest that at low yet anabolic doses of PTH, mTOR signaling is not required. However, at higher doses, PTH anabolic effects appear to rely on the activation of additional pathways, including the mTOR pathway.
The anabolic effects of PTH were detected at both cortical sites and at trabecular-rich sites, but we found that rapamycin interacted with PTH efficacy only at trabecular-rich sites. Rapamycin did have significant suppressive effects at cortical sites, but those appeared to be independent of PTH, i.e., we observed equal suppression of anabolic markers in vehicle-treated as well as PTH-treated mice. It is unclear why trabecular sites were particularly vulnerable to the suppressive effects of rapamycin on the bone-building effects of high-dose PTH. mTOR is a central molecule in cell proliferation, and the trabecular compartments would be the sites most affected if rapamycin was suppressing mTOR-mediated proliferation of osteoprogenitor cells and preosteoblasts induced by PTH. Alternatively, cortical vs. trabecular difference might be related to the observation that PTH is known to have more pronounced effects in trabecular bone than in cortical bone (Sugiyama et al., 2008). Furthermore, the anabolic effects of PTH might be regulated by different pathways at different sites. For example, Raggat et al found that the anabolic effects of PTH were abolished in trabecular bone but not in cortical bone among interleukin-18 mutant mice (Raggatt et al., 2008).
Rapamycin has been described previously as a “bone-sparing” immunosuppressant compared to other compounds in its class (e.g., cyclosporin A, FK506 (Goodman et al., 2001; Joffe et al., 1993; Romero et al., 1995)). The majority of our data support that claim. Among mice not given PTH (0 μg PTH + rapamycin pellet vs. 0 μg PTH + placebo pellet), we found no difference in whole-body aBMD, mid- and distal-femur vBMD, distal-femur BMC, and all four μCT measurements of the metaphyseal trabeculae. We did, however, find a significant effect of rapamycin alone for whole body BMC, mid-femur BMC, serum Trap5b, and cortical bone formation. The lack of significant interaction between PTH and rapamycin in serum Trap5b measurements suggests that the rapamycin-induced reduction in trabecular bone accrual among high-dose PTH treated mice was not fueled by enhanced resorption, but, rather by inhibition of trabecular formation. Rapamycin also had independent suppressive effects on periosteal cortical bone formation (Table 1). These data are consistent with in vitro experiments in MC3T3-E1 osteoblasts and primary bone marrow stromal cells, in which rapamycin was found to have significant suppressive effects on osteoblast differentiation and proliferation (Singha et al., 2008).
It should be noted that mTOR can be found in two different complexes, TorC1 and TorC2 (Loewith et al., 2002; Wullschleger et al., 2006). TorC1 is inhibited by rapamycin, contains the protein raptor (which associates with p70S6k1 and 4E-BP1), and its upstream effectors are well characterized. In contrast, TorC2 is not inhibited by rapamycin, contains the protein rictor, and its upstream effectors are unknown, though it has a role in actin organization. Thus, if PTH effects are further mediated via TorC2, our study would not be able to differentiate this as we only inhibited TorC1.
The IGF-I receptor has been shown to be crucial for PTH-induced bone gain, as revealed by the lack of anabolic effects of PTH in mice harboring a Cre-mediated IGF-IR deletion in osteoblasts (Wang et al., 2007). Furthermore, mouse primary osteoblasts treated with neutralizing antibody to IGF-I fail to exhibit the normal profile of anabolic genes when stimulated with PTH (Locklin et al., 2003). Two major downstream pathways of activated IGF-IR include the PI3k-Akt pathway and the Ras-Raf-MapK pathway. Our data suggest that the mTOR arm of the Akt pathway does not appear to be involved in PTH-induced bone gain at the low dose given in our study. Activation of the Akt pathway could still be required for low-dose PTH-associated anabolism, but if so, it appears likely that the signal is transduced through other targets of Akt that lead to proliferation and cell survival, such as Gsk3β and/or BAD (Manning and Cantley, 2007). Alternatively, the anabolic signal coming through activated IGF-IR could be propogated through the Ras-Raf-MapK pathway. Future experiments will be necessary to elucidate these alternate pathways.
In summary, low-dose PTH induced anabolic effects on the mouse skeleton even in the presence of rapamycin, and those bone-building effects were not compromised by rapamycin until high doses of PTH were administered. Rapamycin is an immunosuppressant prescribed to organ transplant patients for its anti-rejection effects, is used as a coating for vascular stents to reduce stenosis, and is being explored to treat certain cancers in light of its anti-proliferative properties (Kahan, 2000; Lindenfeld et al., 2004; Recher et al., 2005; Stallone et al., 2005; Wan and Helman, 2007; Woods and Marks, 2004). Our data indicate that intermittent PTH might be an effective treatment for maintaining or increasing bone mass among patients taking rapamycin for unrelated reasons, such as transplant, vascular stenting, oncologic, or other reasons.
Acknowledgements
The authors wish to thank Drs. Joseph Bidwell, Keith Condon, and Vince Gattone for help on various portions of the experiments described. This work was supported by NIH grants AR053280 (TAH), AR046530 (CHT), and AR53237 (AGR).
References
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17(9):1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- Goodman GR, Dissanayake IR, Sodam BR, Gorodetsky E, Lu J, Ma YF, Jee WS, Epstein S. Immunosuppressant use without bone loss--implications for bone loss after transplantation. J Bone Miner Res. 2001;16(1):72–78. doi: 10.1359/jbmr.2001.16.1.72. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;97(6):1207–1216. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- Joffe I, Katz I, Sehgal S, Bex F, Kharode Y, Tamasi J, Epstein S. Lack of change of cancellous bone volume with short-term use of the new immunosuppressant rapamycin in rats. Calcif Tissue Int. 1993;53(1):45–52. doi: 10.1007/BF01352014. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356(9225):194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL, 2nd, Kobashigawa J. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation. 2004;110(25):3858–3865. doi: 10.1161/01.CIR.0000150332.42276.69. [DOI] [PubMed] [Google Scholar]
- Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89(1):180–190. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25(48):6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, Qin L, Tamasi J, Jefcoat SC, Jr., Shimizu E, Selvamurugan N, Liew FY, Bevelock L, Feyen JH, Partridge NC. Interleukin-18 is regulated by parathyroid hormone and is required for its bone anabolic actions. J Biol Chem. 2008;283(11):6790–6798. doi: 10.1074/jbc.M709909200. [DOI] [PubMed] [Google Scholar]
- Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, Benzaquen D, Laurent G, Huguet F, Payrastre B. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–2534. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- Romero DF, Buchinsky FJ, Rucinski B, Cvetkovic M, Bryer HP, Liang XG, Ma YF, Jee WS, Epstein S. Rapamycin: a bone sparing immunosuppressant? J Bone Miner Res. 1995;10(5):760–768. doi: 10.1002/jbmr.5650100513. [DOI] [PubMed] [Google Scholar]
- Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103(2):434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43(2):238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Verhaar HJ, Lems WF. PTH-analogs: Comparable or different? Arch Gerontol Geriatr. 2009 doi: 10.1016/j.archger.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12(8):1007–1018. doi: 10.1634/theoncologist.12-8-1007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22(9):1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods TC, Marks AR. Drug-eluting stents. Annu Rev Med. 2004;55:169–178. doi: 10.1146/annurev.med.55.091902.105243. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146(6):2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]