Abstract
Diabetes may adversely affect cognitive function, but the underlying mechanisms are unknown. To investigate whether manipulations that enhance neurotrophin levels will also restore neuronal structure and function in diabetes, we examined the effects of wheel running and dietary energy restriction on hippocampal neuron morphology and BDNF levels in db/db mice, a model of insulin resistant diabetes. Running wheel activity, caloric restriction, or the combination of the two treatments increased levels of BDNF in the hippocampus of db/db mice. Enhancement of hippocampal BDNF was accompanied by increases in dendritic spine density on the secondary and tertiary dendrites of dentate granule neurons. These studies suggest that diabetes exerts detrimental effects on hippocampal structure, and that this state can be attenuated by increasing energy expenditure and decreasing energy intake.
Keywords: Diabetes, dendrite, hippocampus, exercise, dietary restriction
Due to the ready availability of calorie-dense foods and decreased opportunities for physical activity, the prevalence of diabetes and obesity is rapidly increasing in Western societies (Reaven, 2005). Alterations in metabolic efficiency are not unique to peripheral tissues, as there have been some suggestions that changes in energy availability and expenditure will also impact the central nervous system. Specifically, individuals with insulin resistant diabetes may exhibit accelerated cognitive deficits in middle age (Messier, 2005; Sandeep et al., 2004). Moreover, in animal models of diabetes, there is abundant evidence for behavioral and biochemical deficits, particularly in the hippocampus, a brain region important for learning and memory (Biessels et al., 1996; Li et al., 2002; Stranahan et al., 2008a; Stranahan et al., 2008b; Stranahan et al., 2008c).
Dendritic spines are the primary sites of excitatory neurotransmission in the adult brain. Although previous studies have shown reductions in hippocampal synaptic density in insulin deficient diabetes (Martínez-Tellez et al., 2005; Zhou et al., 2007), far less is known about dendritic changes in the hippocampus of insulin resistant rodents. Using a diet-induced insulin resistance model, we have demonstrated reductions in dendritic spine density in hippocampal area CA1 (Stranahan et al., 2008c). Other studies have shown reductions in presynaptic marker expression in whole-hippocampal homegenates from genetic models of insulin resistance (Ahima et al., 1999). However, no studies to date have characterized dendritic spine density or neuronal morphology in the dentate gyrus of the hippocampus in insulin resistant diabetes.
The consequences of diabetes for hippocampal neuronal structure are qualitatively similar to the effects of restricting brain-derived neurotrophic factor (BDNF) signaling. BDNF levels in the hippocampus are responsive to alterations in glucose levels (Anson et al., 2003; Duan et al., 2003), and BDNF plays a role in cellular metabolism (Burkhalter et al., 2003; Yeo et al., 2004). BDNF is also particularly abundant in the dentate gyrus, relative to the CA1 subfield (Friedman et al., 1991). Functionally, dentate gyrus BDNF signaling determines antidepressant efficacy, suggesting a role in anxiety and mood regulation (Adachi et al., 2008). This indicates that correlated alterations in dentate gyrus BDNF signaling and neuronal structure may be associated with the changes in anxiety-like behavior that have been reported in rodent models of insulin resistance (Asakawa et al., 2003).
Voluntary wheel running and caloric restriction increase levels of BDNF in the hippocampus (Mattson et al., 2004a; Mattson et al., 2004b; Neeper et al., 1996; Ding et al., 2006) and enhance peripheral metabolism. Accumulating evidence suggests that the enhancement of peripheral metabolism is accompanied by alterations in central metabolic markers, with consequences for neuronal function (Vaynman et al., 2006; Gomez-Pinilla et al., 2008). Mice selected for high levels of wheel running have improved peripheral metabolism and exhibit greater exercise-induced upregulation of hippocampal BDNF (Johnson et al., 2003). This ‘metabotrophic hypothesis’ for the effects of exercise and caloric restriction on hippocampal structure and biochemistry has potential relevance for the treatment and prevention of neurodegenerative disease.
The coincident roles of BDNF in energy metabolism and spinogenesis prompted us to intially characterize differences in hippocampal neuronal morphology in an animal with deficient metabolic function. The db/db mouse carries a mutation that inactivates the leptin receptor, producing an animal that is obese and insulin resistant (Hummel et al., 1966). We observed reduced hippocampal BDNF and loss of dendritic spines in db/db mice, and therefore investigated the consequences of wheel running and caloric restriction – two ethologically relevant manipulations of energy availability. These manipulations enhanced dendritic spine density and hippocampal BDNF expression in wild type mice, and partially reversed abnormalities in db/db mice. These findings suggest that the adverse effects of diabetes on hippocampal structural plasticity can be ameliorated by increasing energy expenditure and decreasing energy intake.
Materials and Methods
Animals and activity monitoring
Animal care and experimental procedures followed NIH guidelines and were approved by the National Institute on Aging Animal Care and Use Committee. Male leptin receptor mutant (db/db, n=24) mice, bred on a C57Bl/6 background, were purchased from Jackson Laboratories. Age-matched male C57Bl/6 mice (wild type, n=24) were used as controls. Animals were one month old at the start of experiments. Half of the mice from each genotype (n=12 db/db, n=12 wild type) were kept in individual cages containing a running wheel equipped with an automated monitoring system attached to a Dell computer. The running wheel was continuously available. The number of wheel rotations per day for each mouse was recorded using MedSci Behavior monitoring software (Columbus Instruments, Columbus, OH).
Caloric restriction
During the initial two weeks of the experiment, all mice were fed ad libitum, and food weights were recorded daily by experimenters. Dietary restriction feeding levels were calculated by feeding the animals at sixty percent of their individual mean food intake. The vivarium was maintained on a twelve hour light/dark cycle; all mice assigned to the caloric restriction diet were fed once daily at the onset of the dark period (18:00). After group assignment (sedentary/ad libitum, sedentary/caloric restriction; runner/ad libitum, runner/caloric restriction; n=6 mice from each genotype per group), we recorded body weights on two successive days per week.
Open field activity
We measured open field activity within the same group of mice at two different points during the diurnal cycle. First we evaluated open field activity at the onset of the dark period (18:00) and again during the initial portion of the light phase (07:00). Open field activity was measured using an open field activity system (ENV 515-16, size: 44 × 44 cm) and Activity software (Version 4.36; Med Associates Inc., St. Albans, VT). Mice were placed in the upper right corner of the open field box, and activity was recorded for 5 minutes. The center of the open field was defined as a square positioned in the center of the arena, 6.5 cm from the outside wall. Percent time in center and total distance were recorded as a measure of anxiety and locomotor activity.
Glucose and 3-hydroxybutyrate measurements
For measurement of fasting glucose levels, food was removed from the cages of the mice on the ad libitum diet, and the daily allotment of food was withheld from mice on the caloric restriction diet. The following morning, animals were briefly restrained, and glucose levels were measured following tail-nick using a Therasense handheld analyzer (Therasense, Alameda, CA). Postprandial glucose and 3-hydroxybutyrate levels were measured in serum collected following euthanasia. Serum glucose was measured using a glucose assay kit (catalog no. 235-60, Diagnostic Chemicals Limited, Oxford, CT), on a Roche Cobas Fara II robotic chemical analyzer according to the manufacturer’s specifications. DC-Cal reagent (catalog no. SE-035C2, Diagnostic Chemicals Limited, Oxford, CT) was used to calibrate the instrument, and DC-Trol level 1 & 2 control reagents (catalog no. SM-052 and SM-056, Diagnostic Chemicals Limited, Oxford, CT) were used to insure accuracy between assay runs.
Serum total ketone bodies (acetoacetone and 3-hydroxybutyrate) were measured using a total ketone bodies kit (catalog no. 415-73301 & 411-73401, Wako Diagnostics USA, Richmond VA), on a Roche Cobas Fara II robotic chemical analyzer according to the manufacturer’s specifications. The total ketone body calibrator set (catalog no. 412-73791, Wako Diagnostics USA, Richmond VA) was used to produce the standard curve and the total ketone body control reagent (catalog no. 418-73891, Wako Diagnostics USA, Richmond VA) was used to insure accuracy between assay runs.
Insulin and leptin ELISA
Insulin and leptin concentrations in these serum samples were determined by ELISA (Crystal Chem., Inc., Downers Grove, IL). These assays were performed according to the manufacturer’s instructions. Briefly, a microtiter plate coated with mouse anti-insulin or guinea pig anti-leptin antibody was washed three times with wash buffer [50 mM Tris-buffered saline (TBS) containing Tween 20]. Five microliters of diluted standards and serum samples were added to wells in duplicate. Detection antibodies conjugated to the appropriate species were applied, and the plate sealed and incubated for 2 hours while shaking. The wells were then washed and enzyme solution was incubated for 30 min. After washing, wells were reacted with substrate solution (o-phenylenediamine). Once the color developed sufficiently (15 min), stop solution (1N sulfuric acid) was added and the plates read at 490 nm on an automatic plate reader (Perkin Elmer HTS 7000 Plus Bio Assay Reader, Perkin Elmer, Waltham, MA).
Corticosterone radioimmunoassay
Corticosterone levels were measured using a commercially available kit (Diagnostic Products Corp., Los Angeles, CA). In brief, serum was separated from trunk blood by centrifugation at 14,000 rpm for two minutes. Serum samples were stored at -20°C prior to analysis. Samples and corticosterone standards were thawed at room temperature and added to antibody-coated tubes in duplicate. We then added 1.0ml of [I-125]-labeled corticosterone and vortexed each tube before incubating the reaction for two hours at room temperature. Tubes were then decanted and counted using a gamma counter.
BDNF ELISA
Mice were euthanized during the light phase, within three minutes of cage disturbance. The mice were anesthetized with Isoflurane, decapitated, and the brain was dissected on ice. Half of each brain was immediately moved into Golgi-Cox solution and the other half was dissected to isolate the hippocampus. Dissected regions were snap-frozen on dry ice and stored at -80°C prior to protein extraction. For extraction, hippocampi were homogenized in lysis buffer (137 mm NaCl, 20 mm Tris, 1% NP-40 detergent, 10% glycerol, 1 mm phenylmethylsulfonylflouride, 10 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.5 mm sodium orthovanadate; pH 7.2) at 4°C.
Homogenates were centrifuged at 14,000 rpm for 15 min (4°C), and supernatants were used for ELISA analysis according the manufacturer’s instructions (Promega Corp., Madison, WI). Briefly, ninety-six-well plates were coated with a mouse monoclonal BDNF antibody overnight. Samples (300 μg protein) were added in duplicate onto each plate and serial dilutions of BDNF standard (0–500 pg/ml) were added to generate a standard curve. Plates were incubated for 2 hours, washed five times in Tris-buffered saline with Tween-20 (TBST), and reacted for an additional 2 hours in a solution containing a rabbit polyclonal BDNF antibody. Wells were washed five times with TBST, and a hydrogen peroxide solution was added together with a peroxidase substrate. Reactions were stopped by adding 1N hydrochloric acid and the absorbance was measured at 450 nm.
Golgi impregnation and analysis
For Golgi impregnation we used a commercially available kit (FD Neurotechnologies kit #SS201) according to the manufacturer’s instructions. Following two weeks incubation in Golgi-Cox solution, the tissue was sectioned on the transverse plane (100 μm) using a Vibratome. After visualizing impregnated cells, the sections were dehydrated in increasing concentrations of ethanol, cleared in Histoclear, and coverslipped under Permount. Cells were selected for analysis as described (Stranahan et al., 2007). Briefly, cells were required to exhibit a fully impregnated cell body, and dark brown or black dendrites, with sufficient separation from other labeled cells, and no apparent severed dendrites. Dendritic segments selected for analysis of spine density were on second-or third-order dendrites.
To acquire images for analysis of dendritic spine density, we scanned dendritic segments using a 63× oil-immersion objective on a Zeiss LSM 510 confocal microscope. In order for images to appear against a white background, we used Argon and HeNe lasers, with visible light channeled through the rhodamine path. Image stacks were acquired at 4.0× optical zoom with a 1.0μm step size. For analysis of dendritic length and arborization, we acquired images through the 25× objective, using a 0.7× optical zoom and a 2.0 μm step size. Image stacks were exported as .tiff files and imported into Reconstruct (freely available at http://synapses.bu.edu). We used Reconstruct to analyze the density of dendritic spines on five segments per cell, from five cells per mouse. Segments used for analysis of dendritic spine density were approximately 10μm long.
We also used the Z-trace feature in Reconstruct to measure the three-dimensional length of the dendritic arbor, from five cells per mouse. Within each cell, we separately traced first-order dendrites arising from the cell body, followed by second-order dendrites arising following the first bifurcation, followed by third-order dendrites and so on.
Statistics
Hippocampal BDNF levels, dendritic lengths, and spine densities were compared across groups using a 2 × 2 × 2 ANOVA (genotype × diet × physical activity). Serum parameters (insulin, corticosterone, glucose, 3-hydroxybutyrate) were analyzed using a similar statistical design. Planned post hoc comparisons were used to evaluate the effects of diet and physical activity within a given genotype, and also to compare these parameters between sedentary animals maintained on the ad libitum diet across genotypes. Run data were analyzed using a 2 × 2 repeated-measures ANOVA (genotype × diet). Open field activity at each of the two time points was analyzed using a 2× 2 × 2 repeated measures ANOVA (genotype × diet × physical activity). Dendritic lengths at different branch orders were also measured using 2 × 2 × 2 repeated-measures ANOVA. Analyses were conducted using SPSS version 11.0 with significance set at p<0.05.
Results
Reduced BDNF levels and dendritic spine density in db/db mice
To evaluate whether basal levels of neurotrophin expression were altered by insulin resistance, we measured levels of BDNF using ELISA in whole-hippocampal homogenates from wild type and db/db mice. Under sedentary, ad libitum diet conditions, BDNF concentrations were significantly lower in insulin resistant mice (t7= 2.51, p=0.04; pg/μg protein, wild type = 8.12 ± 1.56, db/db = 3.67 ± 0.21). Reductions in hippocampal BDNF levels were accompanied by reduced dendritic spine density on the secondary and tertiary dendrites of dentate gyrus granule neurons (t7=4.98, p=0.002; spines/10μm, wild type=19.96 ± 0.74, db/db =15.57 ± 0.27). These observations led us to question whether voluntary exercise and dietary restriction, two manipulations known to enhance peripheral glucose metabolism and central BDNF levels, could ameliorate structural and biochemical abnormalities in db/db mice.
Effects of caloric restriction on running wheel activity in db/db and wild type mice
We analyzed the number of wheel turns, as well as the circadian patterns of wheel running, in wild type and db/db mutant mice. db/db mice fed ad libitum exhibit low levels of running wheel activity, relative to wild type mice (for the effect of genotype, F1,13=5.86, p=0.03; Figure 1A, 1C). Caloric restriction increased wheel running in db/db mice (for the effect of diet in db/db mice, F1,11=2.92, p=.002; Figure 1C). Caloric restriction did not influence the amount of wheel running in wild type mice (Figure 1A), consistent with previous data suggesting that C57Bl6 mice are not sensitive to food-restriction induced hyperactivity in the running wheel (Gelegen et al., 2006).
Figure 1. Effect of diabetes and caloric restriction on the amount and pattern of wheel running.
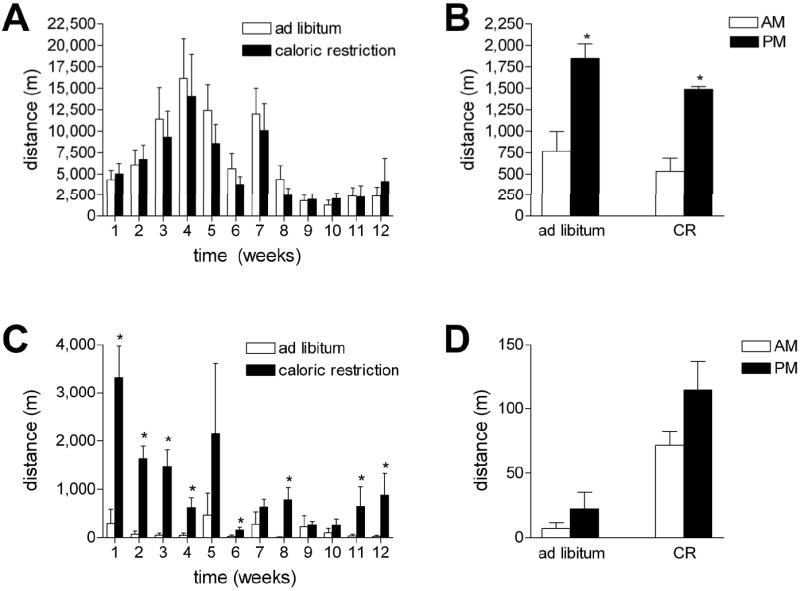
(A) Wild type mice increased their average daily running distance over the first four weeks of the experiment, then gradually reduced their mean distances over the subsequent weeks. There was no significant effect of caloric restriction on running wheel activity. (B) Wild type mice run significantly more at night, and there was no effect of caloric restriction on this pattern. (C) db/db mutant mice run significantly less than wild type mice. In addition, caloric restriction enhanced the amount of daily wheel running in db/db mice. (D) There was no effect of the diurnal cycle on the mean distance in db/db mice fed ad libitum. With caloric restriction, we observed a trend for increased activity during the dark phase (p=0.11). Asterisk (*) reflects significance at p<0.05 following 2-way repeated measures ANOVA (A and C) or 2-way ANOVA (B and D).
Wild type mice ran significantly more during the dark period, relative to the light phase, irrespective of diet (for the effect of day vs. night, F1,20=39.22, p<0.001; Figure 1B). In contrast, the effects of the diurnal cycle on the amount of wheel running were not significant in db/db mice on either diet (F1,20=1.76, p=0.11, Figure 1D). This suggests that in addition to running less, the db/db mice also have disordered patterns of wheel running. This is consistent with the disruption of circadian rhythmicity reported in db/db mice by Lapolsky et al. (2008).
Effects of running and caloric restriction on open field exploration
To investigate whether diabetes-induced alterations in the amount and pattern of wheel running were specific to wheel-oriented locomotion, we measured open field activity at two time points during the diurnal cycle. We also used this behavioral measure as an index of anxiety. During both testing sessions, db/db mice exhibit reduced locomotion, relative to wild type mice (for the effect of genotype, F1,37=115.858, p<0.001; Figure 2A-B). Running and caloric restriction influenced open field locomotion, but only in db/db mice, with complex effects that were specific to time of testing.
Figure 2. Caloric restriction and running enhance exploratory behavior in db/db mice.
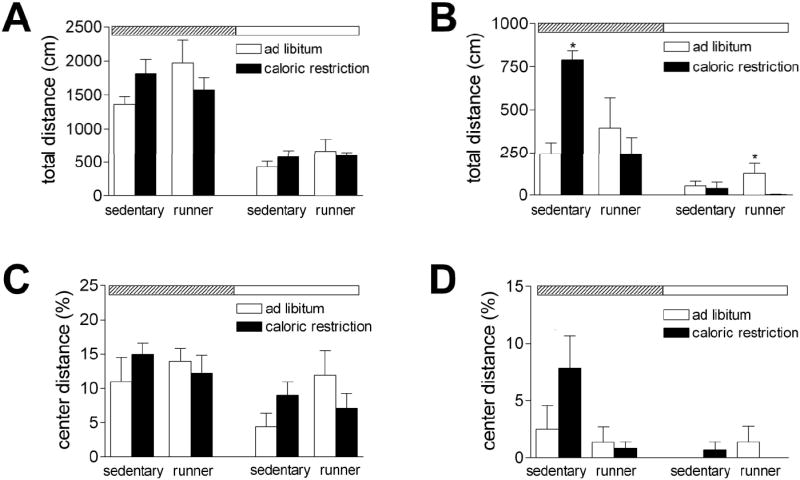
For all graphs, time of testing is indicated by the rectangle at the top of the graph. Testing at the onset of the dark phase is indicated by a diagonally striped rectangle, while testing at the onset of the light phase is shown with a white rectangle. (A) In wild type mice, there were no significant effects of exercise or caloric restriction on open field activity. (B) db/db mice exhibit lower levels of open field activity than wild type mice. Caloric restriction enhances open field exploration in sedentary db/db mice during the dark phase, while running wheel activity promotes locomotion in db/db mice on the ad libitum diet during the light phase. (C), There was no significant effect of running or caloric restriction on the proportion of time spent in the center of the open field in wild type mice. (D), db/db mice spent less time in the center of the open field than wild type mice, but there was no significant effect of running or caloric restriction. For all graphs, asterisk (*) indicates significance at p<0.05 following 2× 2 × 2 repeated measures ANOVA.
db/db mice exhibit greater locomotion in the open field during the dark phase (for the effect of time of testing, F1,17=54.43, p<0.001; Figure 2B). There was also a significant interaction between diet, running, and circadian phase (F1,17=8.19, p=0.01). During the nocturnal testing session, sedentary db/db mice maintained on caloric restriction explored more than all other groups (F1,17=14.73, p=0.001). In contrast, when we tested during the light phase, db/db runners maintained on the ad libitum diet showed the highest levels of locomotor behavior (F1,17=4.66, p=0.04). Wild type mice also showed lower levels of activity during the light phase, relative to testing at night (F1,20=112.92, p<0.001; Figure 2A). There were no significant interactions between diet, physical activity, and circadian phase in wild type mice (F1,20=2.01, p=0.16). These results suggest that there is an overall dampening of activity levels in db/db mice, which can be partially reversed by voluntary exercise and dietary restriction.
The proportion of time spent in the center of the open field is a measure of anxiety-like behavior in rodents. db/db mice spent less time in the center of the open field than wild type mice (for the main effect of genotype, F1,37=47.46, p<0.001; Figure 2C-D). However, there was no significant effect of running or caloric restriction, either in wild type mice (effect of diet, F1,20=0.05, p=0.82; effect of running, F1,20=3.27, p=0.08) or in db/db mice (effect of diet, F1,17=0.43, p=0.52; effect of running, F1,17=2.11, p=0.16).
Alterations in serum chemistry following voluntary exercise and caloric restriction
We monitored fasting glucose levels once every four weeks, and even at the earliest time point, db/db mice were hyperglycemic relative to wild type mice (F1,36=437.71, p<0.001; Figure 3A-B). The degree of hyperglycemia increased steadily during the course of the study in db/db mice fed ad libitum, irrespective of physical activity (for the effect of physical activity in db/db mice, F2,34=0.77, p=0.48). Fasting hyperglycemia was attenuated in db/db mice on caloric restriction (F1,17=13.41, p=0.002). Caloric restriction also reduced fasting glucose levels in wild type mice (F1,19=10.85, p=0.004). In the postprandial state, although the db/db mice had significantly higher serum glucose concentrations relative to wild type mice, there was no effect of running or caloric restriction on glucose levels (data not shown).
Figure 3. Effects of energy deficit induced by caloric restriction alone or in combination with running wheel activity on serum glucose and 3-hydroxybutyrate concentrations.
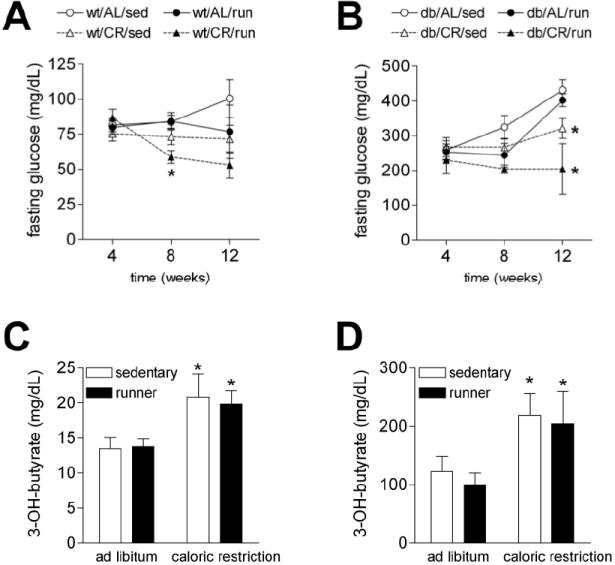
(A) Fasting glucose measurements were made every four weeks throughout the experiment. Wild type runners on caloric restriction exhibit reduced fasting glucose levels during the eighth week of the experiment. (B) db/db mice were hyperglycemic (relative to wild type animals), even at the earliest time point. However, caloric restriction attenuated fasting hyperglycemia in these animals. (C) Serum ketone body (3-hydroxybutyrate) concentrations were elevated by caloric restriction in both sedentary and running wild type mice. (D) Although serum 3-hydroxybutyrate levels were elevated in sedentary db/db mice fed ad libitum, the db/db mice retain the capacity for caloric restriction-induced enhancement of serum ketone concentrations. Asterisk (*) indicates significance at p<0.05 following 2-way repeated measures ANOVA (A and B) or 2-way ANOVA (C and D).
While exercise and caloric restriction promote physiological enhancement of ketone metabolism, diabetes has been associated with pathological ketoacidosis (Mitchell et al., 1995). To determine whether ketone metabolism was altered by running and caloric restriction in wild type and db/db mice, we also measured serum ketone body concentrations. Levels of 3-hydroxybutyrate were elevated in db/db mice, relative to wild type mice (for the effect of genotype, F1,34=42.96, p<0.001; Figure 3C-D). When we examined the consequences of caloric restriction and running wheel activity exclusively in db/db mice, we observed that mice on caloric restriction had significantly higher levels of 3-hydroxybutyrate than mice fed ad libitum (F1,14=7.85, p=0.02; Figure 3D). We observed the same relationship in wild type mice; caloric restriction increased levels of 3-hydroxybutyrate in both sedentary and running animals (F1,18=8.75, p=0.008; Figure 3C). These results suggest that despite elevation of circulating 3-hydroxybutyrate levels at baseline in db/db mice, these animals still retain their sensitivity to the effects of caloric restriction on ketone levels.
Effects of running and caloric restriction on body weight and hormone concentrations
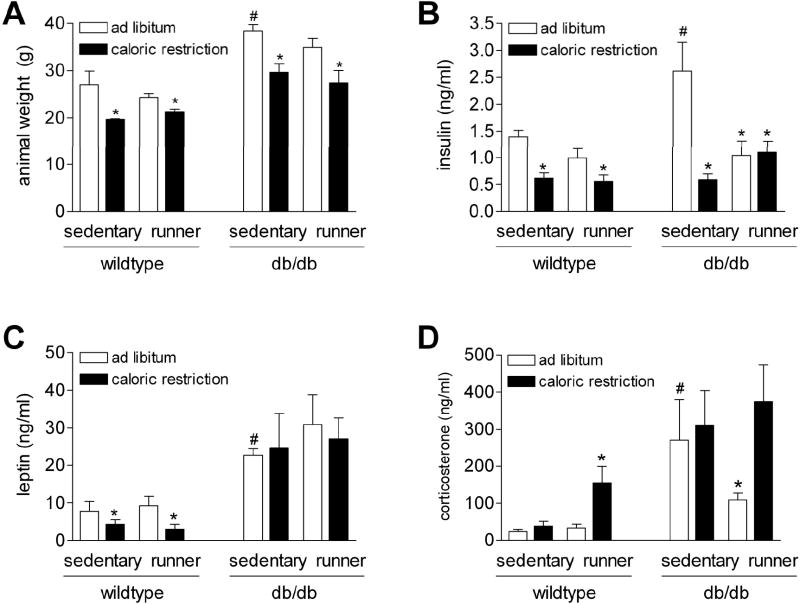
Under ad libitum, sedentary conditions, db/db mice were heavier than wild type animals (F1,47=59.52, p<0.001; Figure 4A). Caloric restriction reduced body weight in both wild type and db/db mice (F1,47=29.17, p<0.001). Type 2 diabetes is characterized by hyperinsulinemia, and this was true in sedentary db/db mice fed ad libitum (F1,21=2.16, p=0.04; Figure 4B). However, running, caloric restriction, or combined running and caloric restriction all reduced serum insulin concentrations in db/db mice (F1,20=5.21, p=0.03). In fact, serum insulin levels in these conditions were reduced to levels that were comparable to those of wild type mice. When we looked exclusively at the wild type mice, we found that caloric restriction reduced insulin levels, irrespective of physical activity (F1,26=6.94, p=0.02).
Figure 4. Effects of reduced energy intake and voluntary exercise on body weights and hormone concentrations in wild type and db/db mice.
(A) db/db mice were heavier than wild type mice under ad libitum diet, sedentary conditions. Caloric restriction reduced body weights in both wild type and db/db mice. (B) Serum insulin levels were reduced by caloric restriction in wild type mice. While sedentary db/db mice were hyperinsulinemic relative to wild type mice, caloric restriction with or without running wheel activity lowered insulin levels. (C) Serum leptin was depressed by caloric restriction in wild type mice; however, there was no effect of voluntary exercise or dietary restriction on elevated serum leptin levels in db/db mice. (D) Serum corticosterone measurements were made in samples collected during the middle of the resting phase (between 10 AM and 12 PM; lights on at 6 AM). Combined running and caloric restriction elevated serum corticosterone concentrations in wild type mice. In db/db mice, wheel running lowered serum corticosterone levels in animals fed an ad libitum diet. Asterisk (*) indicates significance at p<0.05 relative to animals in the same genotype. Pound sign (#) indicates significant difference between wild type and db/db mice in the sedentary, ad libitum diet condition.
Homozygous db/db mice carry a mutation that inactivates the leptin receptor, producing obesity, insulin resistance and hyperleptinemia. To evaluate whether elevated leptin levels might be influenced by diabetes and caloric restriction in db/db mice, we measured postprandial serum concentrations using ELISA. In wild type mice, caloric restriction reduced serum leptin concentrations, but wheel running had no effect (Figure 4C). db/db mice had higher serum leptin levels, relative to wild type mice, but there was no effect of running or caloric restriction.
The adrenal steroid corticosterone plays a central role in the pathogenesis of diabetes (Stranahan et al., 2008a; Stranahan et al., 2008b; Shimomura et al., 1987). Sedentary db/db mice fed ad libitum had elevated levels of corticosterone, relative to sedentary wild type mice on the same diet (F1,10=2.26, p=0.04; Figure 4D). However, sedentary db/db mice maintained on caloric restriction had lower levels of serum corticosterone than all other groups within their genotype (F1,14=5.88, p=0.03). In wild type mice, concurrent running and caloric restriction elevated serum corticosterone levels (F1,20=7.78, p=0.01).
Running and caloric restriction enhance dendritic spine density in db/db mice
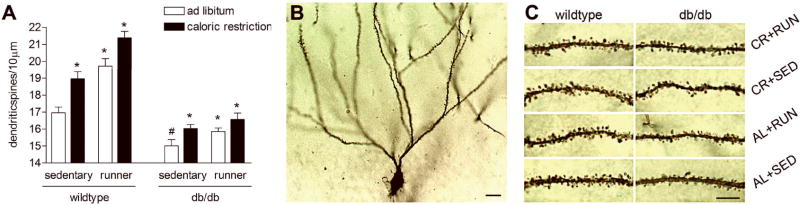
To evaluate the consequences of voluntary exercise and caloric restriction on neuronal structure, we analyzed the density of dendritic spines on the secondary and tertiary dendrites of Golgi-impregnated neurons in the dentate gyrus. Sedentary db/db mice on the ad libitum diet had fewer dendritic spines per ten-micron segment, relative to wild type mice (F1,12=6.73, p=0.0005; Figure 5A-C). When we looked exclusively at the db/db mice, we observed that running, caloric restriction, or the combination of these manipulations increased dendritic spine density (F1,12=27.43,p=0.0002). Wild type mice responded to caloric restriction and exercise with greater increases in dendritic spine density, consistent with their higher levels of activity (F1,12=13.44, p=0.003). These results suggest that diabetes-induced alterations in neuronal structure can be attenuated by manipulations that enhance peripheral metabolism. No significant effects of any treatment were observed on total dendritic length or arborization (Figure 6).
Figure 5. Effects of running and caloric restriction on dendritic spine density in the hippocampus of db/db and wild type mice.
(A) In wild type mice, running, caloric restriction, or both treatments all enhanced dendritic spine density in the dentate gyrus. While sedentary db/db mice fed ad libitum had fewer dendritic spines, relative to wild type mice in the same condition, this deficit could be partially mitigated by running, caloric restriction, or the combination. Asterisk (*) indicates significance at p<0.05 relative to animals in the same genotype. Pound sign (#) indicates significant difference between wild type and db/db mice in the sedentary, ad libitum diet condition. (B), Dentate gyrus granule neuron visualized with Golgi impregnation. Scale bar = 20 μm. (C), Dendritic segments from each of the conditions. Scale bar = 5 μm. Abbreviations: CR = caloric restriction, AL = ad libitum, RUN = runner, SED = sedentary.
Figure 6. Stability of dendritic length and arborization in wild type and diabetic mice following running and caloric restriction.
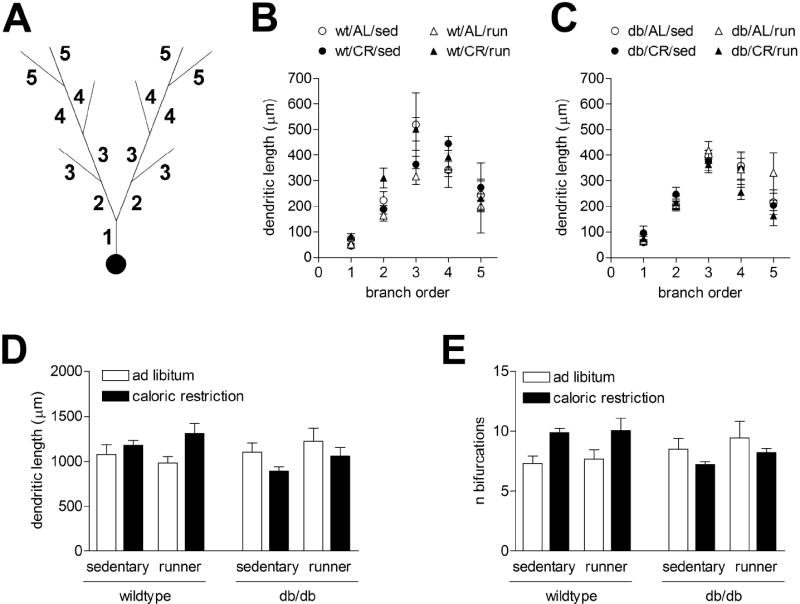
(A), Schematic diagram showing dendritic branch orders. (B), Dendritic lengths are not significantly altered by physical activity or caloric restriction in the dentate gyrus of wild type mice. (C) There was no significant effect of genotype, physical activity, or diet on dendritic lengths in any branch order in db/db mice. (D), Total dendritic lengths were stable following running and caloric restriction in wild type and db/db mice. (E), The number of bifurcations per neuron was also unchanged following diabetes, physical activity, or caloric restriction.
Effects of diet and exercise on hippocampal BDNF levels in wild type and diabetic mice
To determine whether changes in peripheral energy metabolism and locomotor activity were accompanied by alterations in hippocampal neurotrophin levels, we measured BDNF levels in hippocampal homogenates. Sedentary db/db mice fed ad libitum had reduced hippocampal BDNF concentrations, relative to sedentary wild type mice on the ad libitum diet (F1,20=2.82, p=0.04; Figure 7). Caloric restriction, wheel running, or combined caloric restriction and wheel running all enhanced levels of hippocampal BDNF in db/db mice (F1,18=33.19, p<0.001). In wild type mice, we observed additive effects of caloric restriction and running on levels of BDNF in the hippocampus (F1,20=9.65, p=0.005). This suggests that hippocampal BDNF levels are sensitive to peripheral changes in energy metabolism.
Figure 7. Hippocampal BDNF levels are altered by diabetes, running, and caloric restriction.
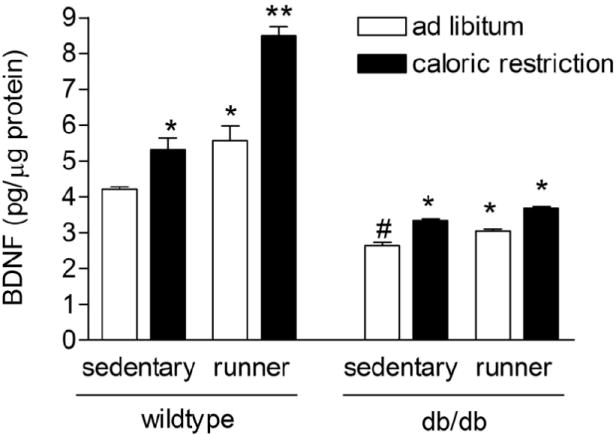
Voluntary running and caloric restriction exert additive effects on hippocampal BDNF levels in wild type mice. db/db mice also respond to running and caloric restriction, but with smaller increases in hippocampal BDNF concentrations. Asterisk (*) indicates significance at p<0.05 relative to animals in the same genotype. Pound sign (#) indicates significant difference between wild type and db/db mice in the sedentary, ad libitum diet condition.
Discussion
The findings of the present study suggest that running and caloric restriction attenuate the adverse effects of diabetes on hippocampal neuronal plasticity. We observed reductions in hippocampal BDNF levels in insulin resistant mice. Impaired BDNF expression was accompanied by reduced dendritic spine density in the hippocampus – an area that has previously been shown to be responsive to dietary restriction and excess (Molteni et al., 2002; Lee et al., 2002; Stranahan et al., 2008c). Reductions in dendritic spine density were attenuated following dietary restriction or wheel running. These results suggest that the hippocampus retains its responsiveness to changes in energy availability in genetically diabetic animals. Moreover, the dendritic alterations that we have observed in insulin resistant mice represent a potential mechanism for the impairment of learning and memory (Li et al., 2002; Stranahan et al., 2008a, Winocur et al., 2005).
The circadian pattern of wheel running and open field exploration was perturbed in db/db mice, as expected based on previous studies in this model (Laposky et al., 2008). db/db mice exhibit reduced activity levels not only in the running wheel, but also in the open field. This suggests that the overall suppression of motor activity generalizes to different environments. In addition to showing less total movement, db/db mice spent less time in the center of the open field, which is suggestive of an increase in anxiety. This result is similar to previous reports in insulin resistant mice (Ahima et al., 1999, Asakawa et al., 2003).
Caloric restriction and running completely reversed hyperinsulinemia in db/db mice. Specifically, levels of serum insulin in db/db mice maintained on caloric restriction, running, or the combined manipulation were reduced to levels that were comparable to sedentary wild type mice fed ad libitum. The reason that we observed reduced insulin levels in diabetic mice following running on the ad libitum diet, but not in wild type mice in that condition, is most likely because young wild type mice already exhibit efficient insulin sensitivity. Therefore, a more drastic manipulation – such as a reduction in caloric intake – is required to influence postprandial insulin concentrations in wild type mice.
Serum corticosterone concentrations in db/db mice were elevated, relative to wild type mice, in agreement with previous reports (Stranahan et al., 2008a; Shimomura et al., 1987). Although we have recently demonstrated that lowering corticosterone levels restores other aspects of hippocampal functioning in insulin resistant animals (Stranahan et al., 2008a), the same mechanism may not be contributing to the effects of caloric restriction in the current report. The only manipulation that reduced circulating corticosterone concentrations in db/db mice was running, on the ad libitum diet. This result was observed during the middle of the resting phase, and we cannot rule out the possibility of circadian-phase-specific alterations in basal corticosterone concentrations, as previously observed in runners under social isolation stress conditions (Stranahan et al., 2006). There may be a potential interaction between running-induced alterations in corticosterone levels and hippocampal BDNF expression, as suggested by previous studies (Adlard and Cotman, 2004).
Serum leptin levels were reduced following caloric restriction in wild type, but not in db/db mice. The reduction in serum leptin following caloric restriction in wild type mice is similar to previous studies demonstrating reduced serum leptin concentrations following caloric restriction in rats (Martin et al., 2008). The absence of any change in serum leptin levels in db/db mice is to be expected, given that db/db mice lack the receptors for leptin (Hummel et al., 1966).
Caloric restriction lowered fasting glucose levels in db/db mice. This is in agreement with previous reports in similar rodent models of type 2 diabetes (Ohneda et al., 1995). Although fasting hyperglycemia was reduced, glucose levels did not approach those of normoglycemic mice. Central infusion of BDNF enhances peripheral glucose sensitivity in db/db mice (Nakagawa et al., 2000), opening the possibility that increases in central BDNF could be a cause, rather than a consequence of reduced fasting glucose levels in serum. However, based on the current report, we cannot conclude that changes in BDNF are driving changes in peripheral metabolic markers and central dendritic spines, or vice versa. An alternative hypothesis would suggest that alterations in the cerebral vasculature could be driving diabetes-induced changes in spines, and alterations in BDNF could be unrelated to dendritic alterations. Future studies will be needed to determine the contributions of changes in hippocampal BDNF levels to alterations in dendritic spine density in db/db mice.
Voluntary running increases the expression of a variety of neurotrophic factors, including vascular endothelial growth factor, nerve growth factor, and insulin-like growth factor-I (IGF-1; for review, see Cotman et al., 2007). Caloric restriction also influences multiple hippocampal signaling mechanisms (Mattson et al., 2004a, Mattson et al., 2004b). While the results of the current study suggest correlated regulation of BDNF and spines following running and caloric restriction, there is no reason to suggest based on the current dataset that this relationship is exclusive. For example, the expression of various neurotrophins, including BDNF and IGF-1, has been shown to be interdependent (Ding et al., 2006). Therefore, correlated regulation of BDNF and spines does not necessarily imply a mutually exclusive mechanistic relationship.
Previous research suggests that there may be a threshold for running-induced enhancement of BDNF levels in wild type mice (Adlard et al., 2005). Because db/db mice ran at lower levels than the threshold for enhancement of BDNF levels in wild type mice, it is possible that db/db mice may have a lower threshold for the positive effects of running on hippocampal BDNF levels. Based on our data, it is apparent that even low levels of activity can mitigate the peripheral consequences of the db/db mouse mutation, in association with a partial rescue of their neuronal phenotype.
Given previously published studies in rats (Eadie et al., 2005; Redila and Christie, 2006; Stranahan et al., 2007), it is surprising that wild type mice in the current study showed no change in neuronal arborization or dendritic length with voluntary running. The duration of running used in those experiments was two (Eadie et al., 2005) or seven weeks (Stranahan et al., 2007), while mice in the current study ran for twelve weeks, opening the possibility that changes in dendritic arborization may be a more acute response to running. The absence of differences in dendritic complexity and total dendritic length in wild type runners is in line with previous studies in mice (Faherty et al., 2003), suggesting that the threshold for changes in dendritic length and complexity may be different for rats and mice.
Inactivity and excessive caloric intake contribute to the development of obesity and diabetes (Reaven, 2005; Messier, 2005), and diabetes induces a neurodegenerative phenotype (Sandeep et al., 2004; Biessels et al., 1996; Li et al., 2002; Stranahan et al., 2008a; Stranahan et al., 2008b; Stranahan et al., 2008c; Martínez-Tellez et al., 2005; Zhou et al., 2007; Winocur et al., 2005). Here we have shown that increased hippocampal neurotrophin levels and dendritic spine density occurs concurrently with attenuation of the peripheral metabolic characteristics of insulin resistance. These results suggest that there is a bidirectional relationship between global somatic metabolism and central plasticity. Based on the extensive literature linking plasticity among spines with synaptic function (Bourne and Harris, 2008), and the relationship between spine and synapse plasticity and hippocampus-dependent memory (Leuner and Shors, 2004), it is possible that our findings may be relevant to hippocampal function.
Acknowledgments
Funding: This research was supported by NIH NRSA F31 AG024690-03 to A.M.S. through Princeton University, and by the National Institute on Aging Intramural Research Program.
Some of the studies in this manuscript were reported in a dissertation thesis submitted to Princeton University by A.M.S.
References
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–9. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–20. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–92. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–62. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications. 2003;17:105–7. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Olson L, Persson H. Cells that express brain-derived neurotrophic factor mRNA in the developing postnatal rat brain. Eur J Neurosci. 1991;3:688–697. doi: 10.1111/j.1460-9568.1991.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Collier DA, Campbell IC, Oppelaar H, Kas MJ. Behavioral, physiological, and molecular differences in response to dietary restriction in three inbred mouse strains. Am J Physiol Endocrinol Metab. 2006;291:574–81. doi: 10.1152/ajpendo.00068.2006. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–87. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–8. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00026.2008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29:117–30. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W, 3rd, Prabhu V, de Cabo R, Maudsley S, Mattson MP. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PLoS ONE. 2008;3:2398. doi: 10.1371/journal.pone.0002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Tellez R, Gómez-Villalobos Mde J, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005;1048:108–115. doi: 10.1016/j.brainres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004a;3:445–64. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004b;1:111–6. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26(Suppl 1):26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18:193–216. [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Ohneda M, Inman LR, Unger RH. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Sandeep TC, Yau JL, MacLullich AM, Noble J, Deary IJ, Walker BR, Seckl JR. 11Beta-hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci U S A. 2004;101:6734–6739. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Bray GA, Lee M. Adrenalectomy and steroid treatment in obese (ob/ob) and diabetic (db/db) mice. Horm Metab Res. 1987;19:295–299. doi: 10.1055/s-2007-1011804. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008a;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem. 2008b;90:479–83. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008c;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–34. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang L, Ling S, Zhang X. Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol. 2007;206:201–208. doi: 10.1016/j.expneurol.2007.04.013. [DOI] [PubMed] [Google Scholar]