Abstract
Stress strongly inhibits proliferation of granule cell precursors in the dentate gyrus, while voluntary running has the opposite effect. Few studies, however, have examined the possible effects of these environmental manipulations on the maturation and survival of young granule cells. We examined number of surviving granule cells and the proportion of young neurons that were functionally mature, as defined by seizure-induced immediate-early gene expression, in 14 and 21 day-old granule cells in mice that were given access to a running wheel, restrained daily for 2 hours, or given no treatment during this period. Importantly, treatments began two days after BrdU injection, to isolate effects on survival from those on cell proliferation. We found a large increase in granule cell survival in running mice compared with controls at both time points. In addition, running increased the proportion of granule cells expressing the immediate-early gene Arc in response to seizures, suggesting that it speeds incorporation into circuits, i.e., functional maturation. Stressed mice showed no change in Arc expression, compared to control animals, but, surprisingly, showed a transient increase in survival of 14-day-old granule cells, which was gone 7 days later. Examination of cell proliferation, using the endogenous mitotic marker proliferating cell nuclear antigen (PCNA) showed an increase in cell proliferation after 12 days of running but not after 19 days of running. The number of proliferating cells was unchanged 24 hours after the 12th or 19th episode of daily restraint stress. These findings demonstrate that running has strong effects on survival and maturation of young granule cells as well as their birth and that stress can have positive but short-lived effects on granule cell survival.
Keywords: dentate gyrus, running, adult neurogenesis, immediate-early gene, Arc
Introduction
Adult neurogenesis produces a substantial number of new granule neurons in the mammalian dentate gyrus. Recent ablation studies have implicated adult-born neurons in several hippocampal-dependent behaviors including spatial learning (Snyder et al., 2005), contextual fear conditioning (Saxe et al., 2006; Winocur et al., 2006) and depressive behaviors (Santarelli et al., 2003). Additionally, correlative studies have identified numerous environmental factors that regulate adult neurogenesis and also affect behavior, possibly via neurogenesis-dependent mechanisms. Chronic stress, for example, induces depressive behavior and also reduces the proliferation of new neurons (Drew and Hen, 2007). In contrast, running has antidepressive effects and increases the proliferation of new neurons (Ernst et al., 2006).
The majority of studies examining neurogenesis in the context of stress and exercise have examined effects on cell proliferation and the fate of cells born during treatment. In contrast, very few stress studies, and no running studies, have examined the effects of these factors on the rate of survival of new neurons in a straightforward way, isolating effects on survival from those on cell proliferation and BrdU incorporation by starting treatment at least 2 days after BrdU injection. In addition, it is interesting to consider possible effects of these factors on the synaptic integration of the new neurons. Most adult-born neurons begin to receive excitatory synapses between 2 and 4 weeks of age (Esposito et al., 2005; Ge et al., 2006), and during this period are more likely to show long-term potentiation (LTP) than their mature counterparts (Ge et al., 2007). However, many 2–4 week-old cells are not yet integrated and are therefore unable to exhibit synaptic plasticity and contribute to behavior. Thus, understanding the effects of these factors on the integration of new neurons into the hippocampal circuitry, and the relationship between synaptic integration and survival at different time points, would provide a better picture of the effects of running and stress on adult neurogenesis.
We therefore examined the effects of voluntary wheel running or daily restraint stress, compared with control treatment, on survival and maturation of 14 and 21 day-old cells. We injected BrdU two days before treatments began to examine cell survival in the three treatment groups. The synaptic activity-dependent immediate-early genes (IEGs) zif268 and Arc were used to examine seizure-induced activation of young neurons as an indication of their functional maturity (Guzowski et al., 1999). Finally, the endogenous mitotic marker PCNA (Mandyam et al., 2004; Olariu et al., 2007) was used to examine cell proliferation.
Methods
Animal treatments
Thirty-nine adult male C57/Bl6 mice were used in the following experiments (NCI-DCT; Frederick, MD). Mice arrived at 6 weeks of age and were individually housed on a 12:12 light:dark schedule (lights on at 6:00 am) with ad lib access to food and water. All mice used for cell counts were received at the same time and began treatment at the same time. To label adult-born granule neurons, mice were given two injections of 5-bromo-2’-deoxyuridine (BrdU; Roche), spaced eight hours apart (200 mg/kg/injection, 10 mg/ml in saline with 0.007 N NaOH), one week after arriving in the facility. Mice were left untreated for two days after BrdU injection, in order to prevent any effects of treatment on proliferation of BrdU-labeled cells. Following this, mice were divided into three treatment groups: running, stress, and controls. Each of these treatment groups was divided into subgroups perfused either 14 or 21 days after BrdU injection, to examine survival and maturation effects on granule cells at different stages of development. Runners were given unlimited access to a running wheel from day 2 after BrdU until the time of perfusion. Stressed mice were restrained in 50 ml conical tubes (with the tips removed to allow air inflow) in a brightly lit room for 2 hr each day until the day before perfusion (Days 2–13 after BrdU injection for the 14-day group and days 2–20 for the 21-day group). This form of restraint has previously been shown to increase corticosterone levels to greater than 300 ng/ml for at least 14 days (Stewart et al., 2008). Mice were not stressed on the final day of the experiment to prevent effects of acute stress on seizure-induced IEG expression. Control mice remained in their cages throughout the duration of the experiment, with no experimental manipulation between BrdU injection and kainate injection. Two hours prior to perfusion, all mice were injected with kainate (Tocris; 35 mg/kg; IP) to induce seizures as a way to strongly activate the dentate gyrus and induce expression of IEGs such as zif268 and Arc, to provide a measure of functional integration of new neurons into the circuitry (Cole et al., 1990). Initial seizure activity consisted of episodes of stillness. Stage 5 seizures, which were behaviorally characterized by episodes of rearing and falling (Racine, 1972), developed 20–30 min after injection and were stopped 30 min after their onset by injection of the GABA agonist sodium pentobarbital (50 mg/kg; IP). Animals were perfused 90 min after stage 5 seizure onset. It is assumed that the brief, 120 minute, window between kainate injection and perfusion is too short to cause any cell loss that could alter the survival measurements.
Histology
All animals were perfused with 4% paraformaldehyde in phosphate buffered saline (PBS; pH 7.4). Brains remained in fixative overnight, after which they were transferred to 20% sucrose for at least 24 hours and then sectioned coronally on a freezing microtome at a thickness of 40 μm.
To assess cell proliferation at the time of death, a 1:12 series of sections spanning the entire dentate gyrus from each brain was stained for the endogenous cell proliferation marker PCNA. Free floating sections were heated in citric acid (0.1 M, pH 6.0, 90°C) for 10 minutes for antigen retrieval, incubated in blocking solution (0.5% tween20 + 3% goat serum) for 20 min, incubated with mouse anti-PCNA antibody (1:20,000 in blocking solution; Santa Cruz) at 4°C for 2 days, and then incubated in biotinylated goat anti-mouse immunoglobulin G (1:200; Sigma, St. Louis, MO) at room temperature for 1 hour. Immunostaining was then visualized using an avidin-biotin-horseradish peroxidase kit (Vector Laboratories, Burlingame, CA) and cobalt-enhanced DAB (Sigma Fast tablets). Light gray cellular background staining eliminated the need for counterstaining, so sections were simply mounted onto slides, dehydrated, and coverslipped under Permount.
For the survival analysis, a 1:12 series was mounted onto slides, heated in citric acid (0.1 M, pH 6.0, 90°C) for 10 minutes for antigen retrieval, permeabilized with trypsin for 10 minutes, and denatured in 2 N HCl for 30 minutes. Sections were then incubated in blocking solution (0.5% tween20 + 3% goat serum) for 20 min and then incubated with mouse anti-BrdU antibody (1:100 in blocking solution; Becton-Dickinson, San Jose, CA) at 4°C overnight. Sections were then processed for DAB visualization as above, counterstained with cresyl violet acetate, dehydrated, and coverslipped under Permount.
The maturity of BrdU+ cells was examined using simultaneous fluorescent double labeling for BrdU and NeuN with one of the IEGs. Free-floating sections were treated with 2 N HCl for 1 hour, incubated in blocking solution (0.5% tween20 + 3% donkey serum) for 20 min, and then incubated in primary antibodies diluted in blocking solution for 3 days at 4°C. Primary antibodies used were: rat anti-BrdU antibody (1:1000 in blocking solution, Accurate, OBT0030), rabbit anti-zif268 (1:1000 in blocking solution, Santa Cruz, sc-189), rabbit anti-Arc (1:1000 in blocking solution, Synaptic Systems, 156003), mouse anti-NeuN (1:250 in blocking solution, Chemicon, MAB377). Sections were then incubated for 90 min at room temperature in donkey anti-rat Alexa488, donkey anti-mouse Alexa633, and donkey anti-rabbit Alexa555 antibodies (all 1:250 in PBS, Molecular Probes, Eugene, OR). Sections were then mounted onto slides and coverslipped under Immumount (Thermo Scientific).
Data Analysis
Stereological counts of DAB-labeled BrdU+ and PCNA+ cells were performed using a 40x objective on a 1 in 12 series of sections through the dentate gyrus. All BrdU+ cells located in the granule cell layer or within 20 im of the granule cell layer (in the subgranular zone) were counted.
IEG levels were measured in BrdU+ cells that were also NeuN+, to ensure neuronal identity. The majority of BrdU+ cells (~90%), even at 14 days post-BrdU injection, expressed NeuN. IEG expression was measured in 25–35 BrdU+/NeuN+ cells in the dentate gyrus (infrapyramidal and suprapyramidal blades) in each animal. To objectively quantify IEG immunostaining intensity, while taking into account varying levels of specific and background staining, confocal z-stacks at 1 um intervals were acquired using a 60x oil immersion lens (N.A. 1.25). IEG immunostaining fluorescence intensity was measured within the nucleus in the central confocal plane, where the diameter of the BrdU+ nucleus was largest. This value was compared to the fluorescence intensity of a nearby region in the hilus, within the same image, that did not contain any cells or appear to contain specific IEG staining. Cells that had IEG expression at least 2x background levels were counted as positive. This intensity criterion facilitated quantification of cells with weak IEG expression by eliminating false positives caused by nearby staining from adjacent cells without excluding cells that appeared positive for specific IEG expression by eye. This method was particularly useful for zif268 since tissue stained for this marker had higher levels of non-specific background fluorescence. Statistical comparisons were made using 2-way Analysis of Variance (ANOVA), with treatment and time point as factors. Significant main effects were further examined using Bonferroni post-hoc tests.
Results
Effects of running and chronic stress on proliferation of granule cell precursors
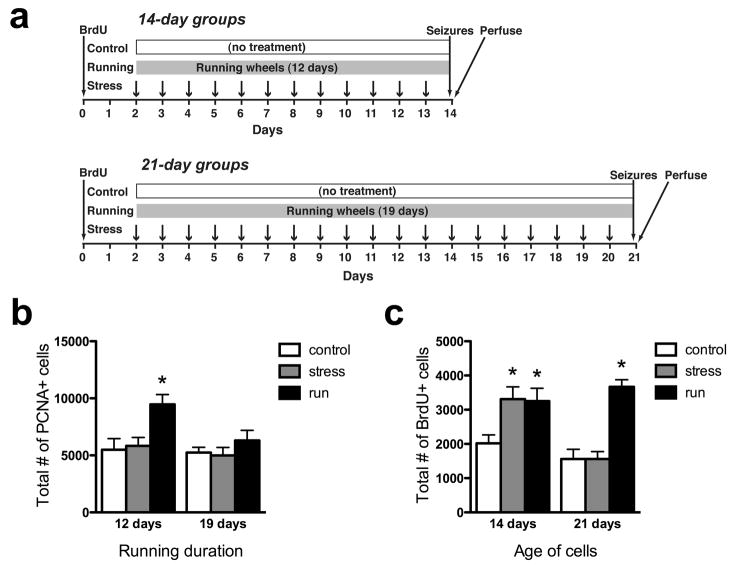
We first examined the effects of running and chronic restraint stress on cell proliferation in the dentate gyrus, by comparing the numbers of PCNA-labeled dividing precursors across treatment groups (Fig. 1a, 2b). A 2-way ANOVA found significant main effects of treatment (P<0.0047) and time (P=0.0366), with no significant treatment x time interaction (P=0.1794). Running animals had 62–72% higher mean PCNA+ cell counts than the other treatment groups 12 days after treatment began, a difference that was statistically significant by post-hoc analyses (P<0.01). The increase in cell proliferation observed after 12 days of running appears to be transient, as it was no longer apparent 19 days after the mice were given the running wheels.
Figure 1.
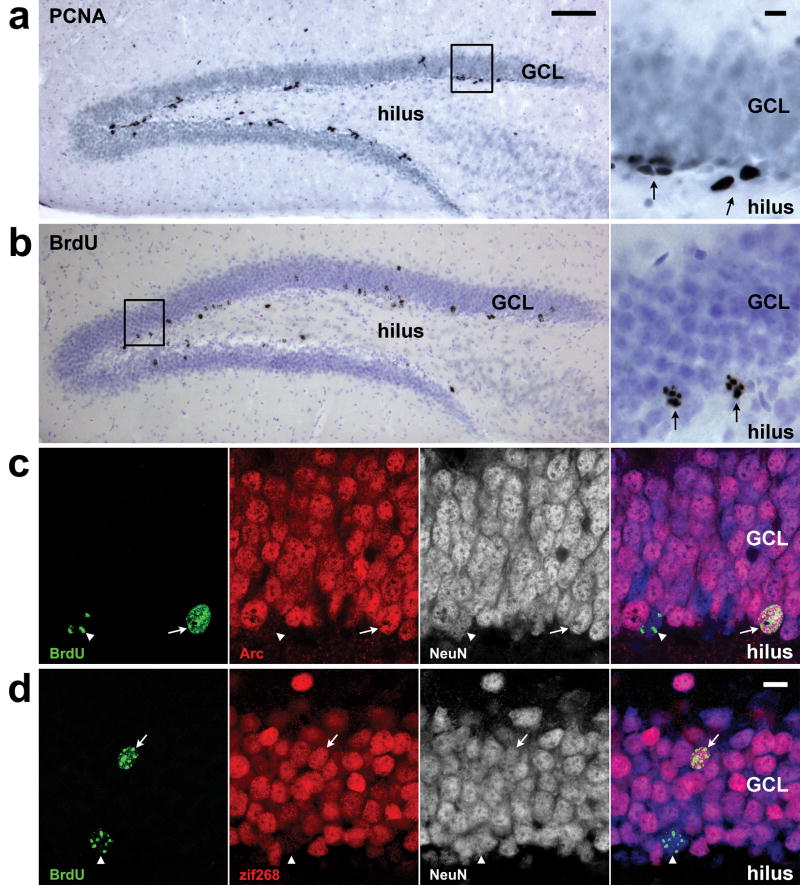
Markers used to measure adult neurogenesis. a) Cobalt-enhanced DAB immunohistochemistry against proliferating cell nuclear antigen (PCNA) was used to identify proliferating precursor cells (dark gray/black). Arrows on inset photo indicate clusters of 4 and 2 cells from left to right. b) Cobalt-enhanced DAB immunohistochemistry against bromodeoxyuridine (BrdU) was used to identify young neurons born two days prior to treatment onset (dark gray/black against purple nuclear counterstain). Arrows on inset photo indicate BrdU+ cells. c) Fluorescent immunohistochemistry against Arc (red), an activity-dependent immediate-early gene, was used to determine whether cells of known age, labeled by BrdU (green) were activated by seizures. The neuron-specific antibody NeuN (white alone or blue in composite) was used to exclude non-neuronal cells from the analysis. Image shows two 21-day-old neurons, one expressing Arc following seizure (arrow) and one showing Arc staining below the threshold (arrowhead). d) Fluorescent immunohistochemistry against zif268 (red), another activity-dependent immediate-early gene, was also used to determine whether BrdU+/NeuN+ neurons of specific ages were activated by seizures. Image shows a 21-day-old neuron staining for zif268 after seizure (arrow) and a second BrdU+ neuron with zif268 staining below the detection criteria (arrowhead). Scale bars = 100 μm for large brightfield images and 10 μm for brightfield insets and fluorescent images. GCL, granule cell layer.
Figure 2.
Effects of stress and running on proliferation and survival. a) Experimental timelines (see methods for details). b) Twelve days of running significantly increased proliferating cell number, an effect that was no longer significant after 19 days of running. c) Both running and restraint stress enhanced the survival of new neurons to 14 days of age. Only running enhanced survival of new neurons to 21 days of age. *p<0.05 versus control at the same time point.
Effects of running and chronic stress on the survival of new neurons
The effects of running and stress on the number of surviving young granule cells, labeled with BrdU 2 days prior to the beginning of treatment, were examined (Fig. 1b, 2c). A 2-way ANOVA found significant main effects of treatment (P<0.0001) and time (P=0.03) and a significant treatment x time interaction (P=0.005). Both runners and stressed mice had approximately 60% more BrdU-labeled cells than controls 14 days post-BrdU injection (stressed mice: P<0.01, runners: P<0.05). The enhanced survival seen at 14 days in stressed mice was absent by 21 days: stressed mice had significantly fewer cells than at 21 days (P<0.01) and were not different from controls (P>0.05). In contrast, the number of BrdU-labeled cells in runners did not change between 14 and 21 days (P>0.05), giving runners a significant, 135% increase in surviving cells relative to controls at the 21-day time point (P<0.001). The mean number of BrdU-labeled cells in control mice was 21% lower at 21 days than at 14 days after BrdU injection, but post-hoc tests revealed that this difference was not significant (P>0.05).
Effects of running and chronic stress on the functional maturation of new neurons
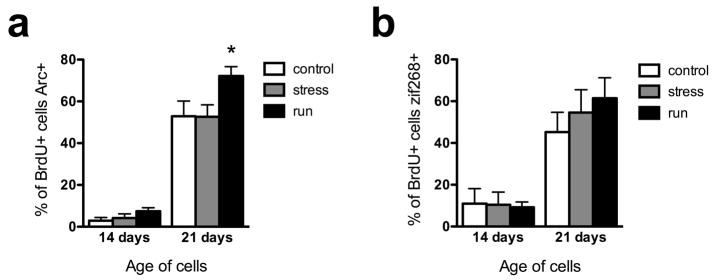
To compare the maturation of new neurons across treatment groups, the proportion of young cells showing seizure-induced IEG expression was determined, as a measure of the proportion of neurons integrated into the hippocampal circuitry. The first IEG examined was Arc, a protein involved in actin polymerization (Bramham, 2007) and AMPA receptor removal from synapses (Tzingounis and Nicoll, 2006). A 2-way ANOVA showed significant main effects of time (P<0.0001) and treatment (P=0.0041) and an interaction between the two factors that showed a trend toward statistical significance (P=0.0629; Fig. 1c, 3a). Very few 14-day-old neurons expressed Arc, even after strong activation by kainate-induced seizures, consistent with electrophysiological studies showing that 14 day-old granule cells do not yet receive significant excitatory inputs (Esposito et al., 2005; but see Ge et al., 2006). The percentage of young granule cells immunostaining for Arc increased greater than 10-fold between 14 and 21 days in all three treatment groups (post-hoc test across time, P<0.001 for each treatment group). Running significantly increased Arc expression in 21-day-old granule cells relative to controls (36% increase, P<0.01), while stress had no effect. Although the mean number of 14 day-old BrdU+/Arc+ cells was twice as high in runners as controls, this difference was not statistically significant (P>0.05 for all), possibly due to the very low proportion of cells activated and consequent high variability at this age.
Figure 3.
Running accelerates the maturation of adult-generated neurons. a) Seizure-induced expression of the immediate-early gene Arc. Running significantly increased the proportion of 21-day-old neurons capable of expressing Arc. b) Seizure-induced expression of the immediate-early gene zif268. Neither stress nor running significantly affected the proportion of young neurons capable of expressing zif268. **p<0.01 compared with control at the same time point.
Zif268 is a transcription factor IEG that is required for late-phase LTP and long-term memory formation (Jones et al., 2001). Like Arc staining, zif268 immunostaining showed a significant main effect of time (P<0.0001) reflecting a greater than 4-fold increase in each treatment group. Unlike Arc staining, zif268 immunostaining showed no main effect of treatment (P=0.6) and no treatment x time interaction (P=0.5). The percentage of cells immunostaining for zif268 was 22% higher in running mice than in controls, suggesting that lack of a significant treatment effect observed with zif268 may be due to higher variability observed with this marker.
Discussion
In this study we compared the effects of two environmental manipulations, chronic stress and running, on the survival and maturation of new neurons in the adult mouse dentate gyrus as well as on the proliferation of granule cell precursors. We found that voluntary running transiently increases the number of PCNA+ dividing precursor cells after 12 days but not 19 days of running. We also found a large (135%) increase in survival of new granule cells in running mice, contrary to previous findings suggesting that running has no effect on survival of young granule cells (discussed below). Finally, voluntary running increased the proportion of young granule cells expressing the immediate-early gene Arc in response to seizures, suggesting that it speeds incorporation into circuits, i.e., functional maturation, of young granule cells. Fewer effects were found with daily restraint stress than with running. The number of PCNA+ proliferating cells was unchanged by this stress paradigm, as was the expression of immediate-early genes induced by seizures. Somewhat surprisingly, however, restraint stress caused a transient increase in survival of new granule cells up to 14 days old, which was gone by the time the young cells reached 21 days of age.
Acute and Chronic Effects on Cell Proliferation
Our finding that 12 days of running increased cell proliferation is consistent with previous studies showing that running increases proliferation, as measured either using a single BrdU injection or endogenous markers of proliferating cells (Eadie et al., 2005; Kronenberg et al., 2006; Stranahan et al., 2006). The finding that proliferation returns to control levels after 19 days of running is also consistent with a previous study in mice showing increased proliferation in the dentate gyrus after 3 and 10 days, but not 32 days, of voluntary running (Kronenberg et al., 2006). The transient enhancement of proliferation could potentially reflect a decrease in time spent running seen in C57Bl/6 mice over the first 3 weeks of voluntary running, though this decrease was relatively small (Jung et al., 2006; Turner et al., 2005). The idea that proliferation is proportional to the amount of running is supported by findings that weanling mice given running wheels show increased running for at least 3 weeks after being given a running wheel (Call et al., 2008) and have increased cell proliferation even after 3 weeks of voluntary running (Kitamura et al., 2003). In contrast to the changes observed during the first few weeks, voluntary running for several months appears to restore enhanced proliferation in mice (Kronenberg et al., 2006) and overcome the corticosteroid-mediated decrease in proliferation due to running in socially isolated rats (Stranahan et al., 2006). Thus, there appear to be complex interactions between the duration of running and its effects on cell proliferation, mediated by changes in running behavior and glucocorticoids, and likely other factors as well.
Acute stress has been shown to decrease granule cell precursor proliferation in a wide variety of paradigms and species (Mirescu and Gould, 2006). At least three rat studies (Czéh et al., 2002; Heine et al., 2004; Pham et al., 2003) have found decreased proliferation following chronic stress, in contrast to the current study, which found no stress-induced change in cell proliferation. The most likely explanation for the lack of effect on cell proliferation in the current study is the 24-hour delay between the final stress treatment and sacrifice, when expression of PCNA was measured. Many studies using a chronic stress paradigm inject BrdU or sacrifice shortly after the final stress treatment (Czéh et al., 2002; Lee et al., 2006; Námestková et al., 2005), making it unclear whether changes in proliferation are due to effects of chronic stress or due to acute effects of the last stress episode. Prolonged effects of stress on cell proliferation have been observed (Heine et al., 2004; Malberg and Duman, 2003; Pham et al., 2003), but these studies used stressors of longer duration and/or higher intensity, both of which are thought to play a role in determining how long effects on proliferation are maintained (Mirescu and Gould, 2006). Another possible reason we did not see reduced proliferation after chronic restraint in the current study is the use of mice instead of rats; acute stress has been shown to increase cell proliferation in mice, opposite to the effect observed with identical treatment in rats (Bain et al., 2004).
Survival Time Points Versus Survival Effects
This study is the first to show increased survival of young granule cells by running or stress. Several previous running studies have included a “survival” measure, determining the number of cells remaining in runners and controls several weeks after BrdU injections (Brandt et al., 2003; Farmer et al., 2004; Holmes et al., 2004; Trejo et al., 2001; van Praag et al., 1999a; van Praag et al., 1999b). But because animals in all of these studies were given running wheels before BrdU injections began, treatment effects observed at long survival time points reflect a combination of effects on cell proliferation and survival. All of these studies found increased numbers of BrdU-labeled cells at these “survival” time points, but comparisons between short and long time points could not clearly distinguish effects of running on survival from those on proliferation. The current study is the first in which BrdU was given before animals were given running wheels, that is, prior to any differential treatment. Because of this experimental design, the increase in the number of BrdU-labeled cells in the current experiment cannot be due to differences in proliferation and BrdU uptake, and must instead be due to an increase in the rate of granule cell survival in runners. There are several possible reasons that clear survival effects of running have not been apparent in previous studies. First, in several previous studies, including nearly all studies in mice, BrdU was injected over a period of 7 to 12 days (Brandt et al., 2003; Farmer et al., 2004; Trejo et al., 2001; van Praag et al., 1999a; van Praag et al., 1999b). Since many new granule cells die during the week after their birth (Brandt et al., 2003; Hayes and Nowakowski, 2002), short (often referred to as “proliferation”) survival times after many days of injections actually reflect a combination of proliferation effects and survival effects on the cells labeled by early injections. It may be that differential effects are not observed between short and long time points because the survival effects are already reflected at short time points. Second, it is possible that running differentially affects the survival of cells born during running and cells born prior to the start of running. For example, since the size of cohorts of cells born during running is larger, homeostatic regulation of overall neurogenesis may act to decrease the survival of cells within these cohorts. This explanation is supported by previous findings that changes in BrdU-labeled cell number due to estrogen and stress effects on cell proliferation are lost after several weeks, suggesting that cell survival rates may adjust to normalize the surviving neuron number, possibly via competition for trophic support (Tanapat et al., 1999; Tanapat et al., 2001).
These issues related to combined effects on cell proliferation and cell survival seen with long survival times after BrdU apply equally well to many studies showing decreased neurogenesis with chronic stress (Koo and Duman, 2008; Mineur et al., 2007; Westenbroek et al., 2004). However, four studies have examined the effects of stress specifically on survival, giving BrdU before the beginning of treatment. Two of these studies found no effects of chronic unpredictable stress or daily restraint (Heine et al., 2004; Pham et al., 2003), but both of these studies looked at cell survival 22 days after BrdU injection, and so are consistent with the lack of effect observed at 21 days in the current study. Two additional studies found 20–30% decreases in granule cell survival following 21 days of chronic mild stress (Lee et al., 2006) or 18 days of resident intruder stress (Czéh et al., 2002). These decreases in survival are consistent with a finding that daily corticosterone injections reduce survival during the first 18 days after BrdU injection (Wong and Herbert, 2004) but differ from the current finding. It may be important that all of these studies were done in rats, in contrast to the current study, which used mice. However, this difference may also relate to the magnitude and duration of corticosterone increases produced by the specific paradigms used. Chronic restraint stress, as used in the current study, produces moderate (400 ng/ml) increases in corticosterone that are 50% recovered within 30 minutes of the end of restraint each day (Stewart et al., 2008), while corticosterone injection increase circulating blood levels to >2000 ng/ml and keep them at >450 ng/ml for ≥ 24 hours (Sousa et al., 1998). For similar reasons, more severe stress paradigms, involving multiple stressors per day or actual physical attack may be more likely to decrease granule cell survival, in contrast to the increased survival observed at 14 days in the current study. Although this possibility requires further confirmation, it is consistent with the idea that while chronic stress has generally negative effects, acute stress may positively affect brain function (Brunson et al., 2003; McEwen, 2008). Taken together, these findings point to a dynamic role of stress on granule cell survival and point out the importance of taking temporal factors as well as severity into consideration in trying to understand the relationship between stress and neurogenesis.
Differential survival of 14 and 21-day-old cells
The similar effects of running and stress on the short-term (14-day) survival of new cells, but different effects on survival of older cells (21-day), suggests that young neurons may go through distinct “critical periods” where they are differentially responsive to survival-promoting factors. Very few granule neurons up to 14 days old can be synaptically activated even by strong stimuli, as demonstrated by the current finding that very few cells of this age express IEGs in response to seizures, as well as previous studies showing few 14-day-old granule cells with dendritic spines or excitatory synaptic responses (Esposito et al., 2005; Zhao et al., 2006; but see Ge et al., 2006). This suggests that paracrine or endocrine factors, rather than synaptic-activity dependent trophic factors, are likely to mediate survival during this early period. The similarity between changes observed in stressed and running mice 14 days after BrdU injection suggests that early survival effects may be mediated by corticosterone, since both manipulations increase release of this stress hormone (Droste et al., 2003; Stewart et al., 2008). However, corticosterone has only been demonstrated to have negative effects on granule cell proliferation and survival (Stranahan et al., 2006; Wong and Herbert, 2004). Alternative candidates for mediating survival during this early period include VEGF and IGF-I, which are required for running effects on neurogenesis (Fabel et al., 2003; Trejo et al., 2001), and BDNF, which is increased by running and has been suggested as a mediator of its effects on synaptic plasticity in the hippocampus (Christie et al., 2008).
Within 21 days of their birth, approximately half of the young granule cells are able to be activated, suggesting that survival effects seen at this time point may reflect synaptic activity-dependent mechanisms. The finding that the early increase in survival was maintained in running animals, which also showed increased functional maturation compared with control and stress animals at 21 days, is consistent with this idea. Further support for an activity-dependent survival mechanism specific to this later phase of granule cell maturation comes from the previous finding that the loss of NMDA receptors decreases granule cell survival between 14 and 21 days after cell birth but has no effect between 7 and 14 days (Tashiro et al., 2006). The increased survival and more rapid functional maturation observed in runners are particularly important to consider in light of the large number of studies of adult neurogenesis that use running wheels as a standard housing feature (Laplagne et al., 2006; Laplagne et al., 2007; Toni et al., 2008; Toni et al., 2007). While the presence of additional cells facilitates physiological experiments, it should be considered that these granule cells in these studies likely have different physiological properties than those in animals housed under standard conditions.
The running-induced increase in activation of 21-day-old granule cells likely reflects an increased number of functional synapses on these cells. An increase in synapse number could be part of a pathway specifically accelerating maturation. Alternatively, increased synapse number could occur through the more global process that increases dendritic spine density in the mature granule cells (as well as other hippocampal and cortical neurons) (Eadie et al., 2005; Stranahan et al., 2007). This latter possibility suggests that these young neurons retain their increased synapse numbers into maturity, resulting in long-lasting changes in both the number and function of adult-born granule cells.
Acknowledgments
This research was supported by the Intramural Program of the National Institutes of Health, National Institute of Mental Health, Z01-MH002784 (HAC).
References
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368(1):7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Control of synaptic consolidation in the dentate gyrus: mechanisms, functions, and therapeutic implications. Prog Brain Res. 2007;163:453–71. doi: 10.1016/S0079-6123(07)63025-8. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24(3):603–13. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27(2):121–36. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, Talmadge RJ, Grange RW. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol. 2008;105(3):923–32. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10(2):47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Abu-Shakra S, Saffen DW, Baraban JM, Worley PF. Rapid rise in transcription factor mRNAs in rat brain after electroshock-induced seizures. J Neurochem. 1990;55(6):1920–7. doi: 10.1111/j.1471-4159.1990.tb05777.x. [DOI] [PubMed] [Google Scholar]
- Czéh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Müller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52(11):1057–65. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS & neurological disorders drug targets. 2007;6(3):205–18. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144(7):3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31(2):84–92. [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25(44):10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134(1–2):77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19(1):131–44. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76(2):216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jung AP, Curtis TS, Turner MJ, Lightfoot JT. Influence of age of exposure to a running wheel on activity in inbred mice. Medicine and science in sports and exercise. 2006;38(1):51–6. doi: 10.1249/01.mss.0000181157.87366.f6. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47(1):55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–13. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Espósito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Kamienkowski JE, Espósito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25(10):2973–81. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Choi SH, et al. Chronic mild stress decreases survival, but not proliferation, of newborn cells in adult rat hippocampus. Exp Mol Med. 2006;38(1):44–54. doi: 10.1038/emm.2006.6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76(6):783–94. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neuroscience. 2007;150(2):251–9. doi: 10.1016/j.neuroscience.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16(3):233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Námestková K, Simonová Z, Syková E. Decreased proliferation in the adult rat hippocampus after exposure to the Morris water maze and its reversal by fluoxetine. Behav Brain Res. 2005;163(1):26–32. doi: 10.1016/j.bbr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501(4):659–67. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130(4):843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794(2):199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Stewart LQ, Roper JA, Young WS, O’Carroll AM, Lolait SJ. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. J Neuroendocrinol. 2008;20(5):597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9(4):526–33. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437(4):496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008 doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MJ, Kleeberger SR, Lightfoot JT. Influence of genetic background on daily running-wheel activity differs with aging. Physiol Genomics. 2005;22(1):76–85. doi: 10.1152/physiolgenomics.00243.2004. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52(3):403–7. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64(4):303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci. 2004;20(10):2491–8. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]